Abstract
Alcohol consumption is a major social and forensic issue. It is often the cause of road accidents, industrial accidents, suicides and other crimes. On account of this, it is of fundamental importance in forensic toxicology to correctly quantify blood alcohol concentration (BAC). In this work, a straightforward method for the quantification of ethanol from blood samples by means of headspace gas chromatography with flame ionization detection is presented and validated. For method validation linearity, limit of detection (LOD), lower limit of quantification (LLOQ), accuracy, precision (% CV) and interference studies were carried out. All the validation conditions were satisfied according to the acceptance criteria. Proof of applicability was performed on 50 real blood samples, showing that the method was effective.
1. Introduction
Ethyl alcohol is the oldest and most frequently used psychoactive substance [1]. Alcohol consumption is a major public health problem, classified in Europe as the third risk factor of illness and premature death after smoking and arterial hypertension [2]. As reported by the World Health Organization (WHO) in the “Global status report on alcohol and health”, there are about 2.3 billion people in the world who consume alcohol. The total per capita consumption of alcohol worldwide among those over 15 years is 6.4 L per year, corresponding to 13.9 g of alcohol per day. In 2016, harmful alcohol consumption was responsible for more than 3 million deaths, accounting for 5.3% of all deaths worldwide [3].
The excessive consumption of alcoholic beverages is currently a serious issue worthy of social alarm, especially after the recent spreading of this practice among the younger population. It must be considered a problem of high priority because of its risk of causing road accidents, industrial accidents, suicides and other crimes [4]. The effects of alcohol consumption impact families and communities broadly, resulting in strained personal and work relationships, increased criminal behavior (like vandalism and violence), reduced productivity and higher healthcare costs.
Furthermore, alcohol consumption is an important danger regarding road safety [5]. In Europe and North America, many jurisdictions have adopted a blood alcohol concentration (BAC) limit of 0.5 g/L, above which driving is prohibited [6].
In Italy, various regulatory measures have been promoted in the field of alcohol abuse in relation to driving: the Legislative Decree no. 285/1992 [7], which approved the new Highway Code; the Law no. 41/2016, which introduces new criminal offenses such as road homicide and road personal injury into the Criminal Code; and the D. Lgs. no. 59/2011 [8], which prohibits the issue or renewal of a license to those who are addicted to alcohol or who cannot dissociate driving from alcohol consumption.
The consumption of alcohol in the workplace is an additional risk factor that can affect the health and safety of the worker himself and third parties [9]. According to the WHO, from 10 to 30% of work accidents are related to alcohol consumption [10]. Such conduct is increasingly the focus of attention of institutions that, in this regard, have in recent years encouraged an increasing number of preventive and informative interventions and issued specific legal requirements. In Italy, for example, Law no. 125, which took effect on 30 March 2011 [11], prohibits the consumption and administration of alcoholic beverages and spirits in the workplace.
Investigations involving alcohol are of considerable importance in forensic toxicology, especially because they must be related to important cases of medical–legal interest that consider the recent use of alcoholic beverages [12]. In addition to searching for ethanol, in some circumstances and for specific purposes, it is useful to search for alcohol metabolites, such as ethyl glucuronide in hair (hEtG), to determine chronic abuse [13]. However, the best way to determine a person’s ethanol levels is through the measurement of BAC in samples from living and dead subjects. For an evaluation of acute consumption of alcohol, the sample of choice is blood because it enables a direct determination of ethanol levels. One of the established methods for measuring alcoholaemia is headspace gas chromatography with a flame ionization detector (HS-GC-FID) [14,15].
The aim of this study was to establish and validate a rapid and sensitive HS-GC-FID method for the evaluation and quantification of ethanol in blood using a minimum amount of starting sample (100 µL) without diluting it. In addition, this method was tested using several blood samples from living people and from postmortem samples. Considering that, the applicability of this method for both types of samples guaranteed reliable results even with more complex matrices such as cadaveric blood and low-volume blood samples. The validation of this method produced an objective result and data that are as accurate as possible, allowing for the enforcement of the abovementioned provisions of the law.
2. Materials and Methods
2.1. Reagents
n-Propanol (internal standard), ethanol, distilled water and sodium chloride (NaCl) were purchased from Carlo Erba (Cornaredo, Italy). Aqueous ethanol certified standards were purchased from ACQ SCIENCE (Rottenburg am Neckar, Germany).
2.2. Instrumentation
For quantitative analysis, the Thermo Scientific Trace 1300 gas chromatograph coupled with a flame ionization detector was used. The column was a 30 m × 0.53 mm × 3.00 µm Rtx-BAC1 purchased by Restek (Bad Homburg, Germany). The GC run was set as follows: the starting temperature was 60 °C for 0.3 min; then, it was increased first to 90° C at a rate of 20 °C/min for 4 min; and lastly, it was increased to 120 °C at a rate of 20 °C/min for 4 min. An injection split ratio of 25:1 was used for this type of analysis. The GC inlet was maintained at 180 °C with a constant flow of helium carrier gas at 2 mL/min. The GC detector was maintained at 200 °C. The FID air and hydrogen flows were set at 350 mL/min and 35 mL/min, respectively.
2.3. Preparation of Internal Standard, Calibration and Quality Control Samples
For the internal standard (IS) solution, 0.3 g of n-propanol was added to 1 L of distilled water. Six certified aqueous solutions were used to prepare the calibration and quality control samples, and each had a different ethanol concentration: 0.1 g/L, 0.2 g/L, 0.5 g/L, 0.8 g/L, 1.5 g/L and 3.0 g/L. Calibration and analytical standard samples containing the various concentrations of ethanol were prepared by adding 100 μL of the certified aqueous solutions, 100 μL of the IS solution and 100 mg of NaCl to a 2 mL glass vial. The vial was closed with a screw cap and heated on a heating plate for 30 min at 60 °C to allow the volatile compounds to pass to the gas phase. A total of 1 mL of the gas phase was injected into the HS-GC-FID. Calibration solutions were used for all the validation procedures.
2.4. Sample Preparation
Blood samples from cadavers and living people were collected by means of a dedicated whole blood sampling using a nonalcoholic disinfectant in the sampling area. Blood tubes containing an anticoagulant, EDTA, were used for the collection. Samples were stored in the refrigerator at a temperature of +4.0 °C if analyzed within 24 h of sampling, otherwise they were stored in the freezer at −20.0 °C to prevent bacterial fermentation. For real blood samples, 100 μL of blood, 100 μL of IS and 100 mg of NaCl were added to a 2 mL glass vial. The vials were sealed and heated for 30 min at 60 °C.
After heating, the samples were manually injected into the HS-GC-FID. With a syringe, the septum of the vial was pierced, and 1 mL of the gas present above the sample was withdrawn and injected.
2.5. Quantification
At the end of each chromatographic run, the ratio between the areas of the peaks of ethyl alcohol and the internal standard obtained from the chromatograms was calculated. The alcohol concentration in the sample was calculated by inserting the ratio value into the calibration curve equation.
2.6. Method Validation
The method was validated according to the standards of the American Association of Forensic Sciences [16]. Evaluated parameters included bias, precision (% CV), linearity of calibration model, limits of detection (LODs), lower limit of quantification (LLOQ) and interference studies. All the validation data are shown in Table 1.

Table 1.
R2 (determination coefficient), LLOQ, bias and % CV.
2.6.1. Calibration Model
Calibration samples at six different nonzero concentrations were used to establish the calibration model. The calibration samples were prepared at concentrations of 0.1, 0.2, 0.5, 0.8, 1.5 and 3.0 g/L. Each calibration sample was analyzed once per run across five separate runs. The data from all runs were combined into a single calibration curve.
2.6.2. Limits
Sensitivity evaluation involved determining the limit of detection (LOD) and the lower limit of quantification (LLOQ). The LOD, indicating the minimum measurable concentration for which the analyte’s presence can be reliably inferred with statistical confidence, was established by fortifying samples with decreasing ethanol concentrations and identifying the smallest concentration yielding a positive result. To define the LLOQ, the lowest nonzero calibration approach was used. Three blank matrix samples were fortified with ethanol using the lowest concentration of the calibration curve and analyzed over three runs to prove that all detection, identification, bias and precision requirements were met.
2.6.3. Interference Studies
Interference studies on the matrix and other analytes commonly used in the laboratory were conducted. To assess interference caused by the matrix effect, 10 blank matrix samples were treated using the developed method, but no IS was added. For an evaluation of interference caused by analytes that are commonly used in our laboratory, a mixture of methanol, acetaldehyde, acetone and hexane was prepared and analyzed.
2.6.4. Bias and Precision
Accuracy and precision studies were performed by analysing the quality control samples at three different concentrations in triplicate over five days: low (0.1 g/L), medium (0.8 g/L) and high (3 g/L). Bias, a measure of accuracy, was calculated as the percentage deviation of the mean from the theoretical concentration. Precision, indicated by the coefficient of variation (% CV), was assessed for both intraday and interday variations and was determined as the percent relative standard deviation of the mean at each concentration. Criteria for acceptable bias and % CV were established at ±10%.
2.6.5. Proof of Applicability
The applicability test was performed on 50 blood samples from both deceased (collected during the autopsies) and living people involved in road accidents. In this regard, the University Research Ethics Committee (University of Macerata) established that this study met the ethical requirements and approved it (Prot. no. 0033285–1 March 2023).
3. Results
3.1. Calibration Model
A calibration curve was constructed with a range of linearity between 0.1 g/L and 3 g/L, and the relative regression equation was generated using Microsoft Excel. The coefficient of determination (R2) was over 0.99, with no evidence of random dispersion for each calibration point of the five replicates.
3.2. Limits
The LOD was 0.01 g/L. All detection, identification, bias and precision criteria were met. The results of the LLOQ studies demonstrated that a concentration of 0.1 g/L enabled reproducibility and provided symmetrical peaks while maintaining bias and precision (% CV) at an acceptable ±10% for the LLOQ. Therefore, 0.1 g/L was confirmed as the LLOQ of the method. The concentration of 0.1 g/L was also chosen since it is not possible to distinguish between endogenous and exogenous ethanol below this concentration [14].
3.3. Interference Studies
During the assessment of matrix interference, no interfering signals were detected in the blank matrix samples. To further examine interference from other substances frequently utilized in the laboratory, matrix blank samples fortified with methanol, acetaldehyde, acetone and hexane were analyzed. These compounds had different retention times compared to that of ethanol, so no interference from any compounds commonly used in the laboratory were observed (Figure 1).

Figure 1.
Chromatogram from the interference studies. (a) Methanol, (b) ethanol, (c) acetone, (d) n-propanol and (e) hexane + acetaldehyde. All the compounds commonly used in the laboratory had different retention times compared to those of ethanol and n-propanol.
3.4. Bias and Precision
Bias for each concentration was measured and found to be within ±10%. Precision at each concentration did not exceed ±10%. Precision was evaluated both within the same analytical session (intraday precision) and across different sessions (interday precision) using a one-way ANOVA approach. Low-concentration samples presented the highest % CV, with a within-run precision of 8.04% and a between-run precision of 7.65%, but these values were still in the ±10% range.
3.5. Proof of Applicability
A total of 50 blood samples from both deceased (collected during the autopsies) and living people involved in road accidents were used to perform the proof of applicability tests. Samples that underwent the preparatory procedures described above and were injected into the HS-GC-FID. Of the 50 samples analyzed, 34 tested negative (under the LOD) and 16 tested positive (Figure 2). Each sample was prepared in triplicate, and each replicate was injected. The standard deviation for each sample was lower than ±0.1 g/L. The maximum concentration of ethanol was 4.33 g/L, and the minimum was 0.59 g/L, with a mean concentration of 1.97 g/L.
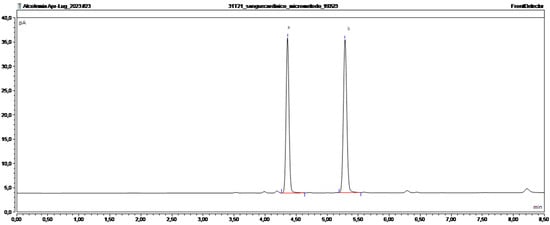
Figure 2.
Chromatogram of a positive real sample. (a) Ethanol and (b) n-propanol.
This method that was developed in our laboratory has also been used by external quality programs for laboratories involved in the quantification of blood alcohol concentration for forensic purposes. The results obtained using this method are in line with the required quality standards of the external quality programs.
4. Discussion
BAC determination is very important in forensic toxicology, as there are numerous legal implications involving alterations in a person’s physical and cognitive abilities resulting from the use or abuse of alcohol and alcoholic substances. Alcohol consumption is a real danger regarding road safety. In the Italian legislature, the issue of alcohol abuse has been broached several times. The penal code expressly refers to the state of drunkenness related to criminal acts, which affects imputability. The D. Lgs. No. 285/1992 in articles 186 and 186b prohibits driving under the influence of alcohol, which confirms that the biological sample of choice to assess the state of intoxication is blood. The blood alcohol concentration is in fact closely related to the impairment of driving ability due to the depression that it causes at the level of the central nervous system.
The penalties for driving under the influence of alcoholic substances, which were updated by Law No. 120 on 29 July 2010, are differentiated according to the gravity of the violation committed and are linked to three blood alcohol bands (0.5–0.8; 0.8–1.5; and >1.5 g/L).
The recent enactment of Article 186b into law makes a blood alcohol content of zero mandatory for newly licensed drivers (those under the age of 21 years or those who have had a license for less than three years) and for workers involved in the transportation of people or goods [7].
It is considered necessary to clarify that in Italy there are very few toxicology laboratories equipped with suitable and validated devices for the detection of alcohol for forensic purposes. Most laboratories, especially those in hospitals, are not provided with equipment capable of achieving a high level of certainty about blood alcohol concentration. This is because they carry out investigations on the serum and use methods that are useful for diagnostic–therapeutic purposes but that are not valid for forensic purposes. In particular, they use indirect enzymatic methods for the detection of alcohol or immunochemical methods that lead to false positive results.
HS-GC-FID is a widely accepted method for determining blood alcohol concentration. A suitable amount of blood is not always available for the analysis, especially if the investigations need to be carried out on postmortem samples. In fact, because of the decomposition process triggered soon after death, and since some traumatic deaths involve substantial blood loss, the time it takes to find the corpse and perform the autopsy has a significant role in determining the status and availability of biological fluids. In this study, a method for the quantification of ethanol in blood using headspace gas chromatography was validated using a minimal amount of blood (100 µL) from both living subjects and cadaveric samples. This approach makes it possible to perform analyses using low-volume blood samples without compromising the results [17]. Sastre et al. (2012) also used a small volume of blood to measure blood alcohol levels, but the sample was diluted with water [18].
In the literature, there are few scientific articles concerning the validation of an analytical method for the determination of ethanol in blood. Even fewer are those that concern the execution of this analysis on cadaveric blood.
In this method, three different calibration samples were used: 0.1 g/L, 0.8 g/L and 3 g/L. The quality control at 0.1 g/L was used to assess the lower limit of quantification of blood alcohol concentration. The quality control at 3 g/L was used to ensure the quality of the results above 0.8 g/L, as increasing penalties are imposed on drivers with a BAC higher than this limit.
The prerequisite for obtaining reliable results for forensic purposes is the application of analytical methodologies validated by the use of suitable equipment. Hence, this work proposes a protocol that is easy to perform with few preparatory steps, which guarantees significant and reliable results that are of a high forensic standard. This work could have very interesting implications in the field of forensic toxicology because it could allow analyses using low-volume blood samples as well as a repetition of the analyses if the need arises during various stages of the legal process.
Author Contributions
Conceptualization, R.F.; methodology, G.M.; validation, G.R. and A.C.; formal analysis, A.C.; investigation, G.R.; resources, M.C. (Marta Cippitelli); data curation, E.B.; writing—original draft preparation, A.C.; writing—review and editing, M.C. (Marta Cippitelli); supervision, M.C. (Mariano Cingolani); project administration, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Union–NextGenerationEU under the Italian Ministry of University and Research (MUR) National Innovation Ecosystem grant ECS00000041–VITALITY–CUP no. D83C22000710005, which can be found at https://www.safina-vitality.it (accessed on 22 May 2024).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Macerata (Prot. no. 0033285–1 March 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Perry, P.J.; Doroudgar, S.; Van Dyke, P. Ethanol Forensic Toxicology. J. Am. Acad. Psychiatry Law 2017, 45, 429–438. [Google Scholar] [PubMed]
- Monteiro, M.G. The road to a world health organization global strategy for reducing the harmful use of alcohol. Alcohol Res. Health 2011, 34, 257–260. [Google Scholar] [PubMed]
- WHO. Global Status Report on Alcohol and Health 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/274603/9789241565639-eng.pdf (accessed on 10 March 2023).
- Savini, F.; Tartaglia, A.; Coccia, L.; Palestini, D.; D’Ovidio, C.; de Grazia, U.; Merone, G.M.; Bassotti, E.; Locatelli, M. Ethanol Determination in Post-Mortem Samples: Correlation between Blood and Vitreous Humor Concentration. Molecules 2020, 25, 2724. [Google Scholar] [CrossRef] [PubMed]
- Alonso, F.; Pastor, J.C.; Montoro, L.; Esteban, C. Driving under the influence of alcohol: Frequency, reasons, perceived risk and punishment. Subst. Abus. Treat. Prev. Policy 2015, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Kugelberg, F.C.; Jones, A.W. Interpreting results of ethanol analysis in postmortem specimens: A review of the literature. Forensic Sci. Int. 2007, 165, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Decreto Legislativo 30 Aprile 1992, n.285—Nuovo Codice Della Strada.
- Decreto Legislativo 18 Aprile 2011, n.59.
- Riboldi, L.; Bordini, L. Abuso acuto e cronico di alcol e lavoro [Acute and chronic alcohol abuse and work]. G. Ital. Med. Lav. Ergon. 2008, 30 (Suppl. 3), 56–66. [Google Scholar] [PubMed]
- Persechino, B. Attività lavorativa ed uso/abuso di alcol: Le problematiche [Work and alcohol abuse: The issues]. G. Ital. Med. Lav. Ergon. 2007, 29, 510–513. [Google Scholar] [PubMed]
- Gazzetta Ufficiale n. 90. 18 Aprile 2001.
- Froldi, R. Lezioni di Tossicologia Forense, 6th ed.; Giappichelli: Torino, Italy, 2024. [Google Scholar]
- Triolo, V.; Spanò, M.; Buscemi, R.; Gioè, S.; Malta, G.; Čaplinskiene, M.; Vaiano, F.; Bertol, E.; Zerbo, S.; Albano, G.D.; et al. EtG Quantification in Hair and Different Reference Cut-Offs in Relation to Various Pathologies: A Scoping Review. Toxics 2022, 10, 682. [Google Scholar] [CrossRef] [PubMed]
- Musile, G.; Pigaiani, N.; Pasetto, E.; Ballotari, M.; Tagliaro, F.; Bortolotti, F. Validation of a New Salt-Assisted HS-GC-FID Method for the Determination of Ethanol in the Vitreous Humor. J. Anal. Toxicol. 2023, 46, e274–e279. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.; Proença, P.; Tavares, C.; Castañera, A.; Real, F.C. Interference of anesthetics in blood alcohol analysis by HS-GC-FID: A case report. Forensic Sci. Int. 2016, 265, 65–69. [Google Scholar] [CrossRef] [PubMed]
- AAFS Standards Board. Standard Practices for Method Validation in Forensic Toxicology; American Academy of Forensic Sciences: Colorado Springs, CO, USA, 2019; Available online: http://www.aafs.org/sites/default/files/media/documents/036_Std_e1.pdf (accessed on 22 May 2024).
- Cingolani, M.; Ferrante, L.; Froldi, R. Valutazione della validità del micrometodo per l’analisi alcolimetrica in head space. Med. Leg. 1991, 13, 163. [Google Scholar]
- Sastre, C.; Baillif-Couniou, V.; Musarella, F.; Bartoli, C.; Mancini, J.; Piercecchi-Marti, M.D.; Leonetti, G.; Pelissier-Alicot, A.-L. Can subclavian blood be equated with a peripheral blood sample? A series of 50 cases. Int. J. Legal Med. 2013, 127, 379–384. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).