Development of Sensitive Electrochemical Sensor Based on Chitosan/MWCNTs-AuPtPd Nanocomposites for Detection of Bisphenol A
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus Nanjing
2.3. Preparation of MWCNTs-AuPtPd
2.4. Preparation of GCE/CS/MWCNTs-AuPtPd
2.5. Analytical Procedure
2.6. Sample Detection
3. Results
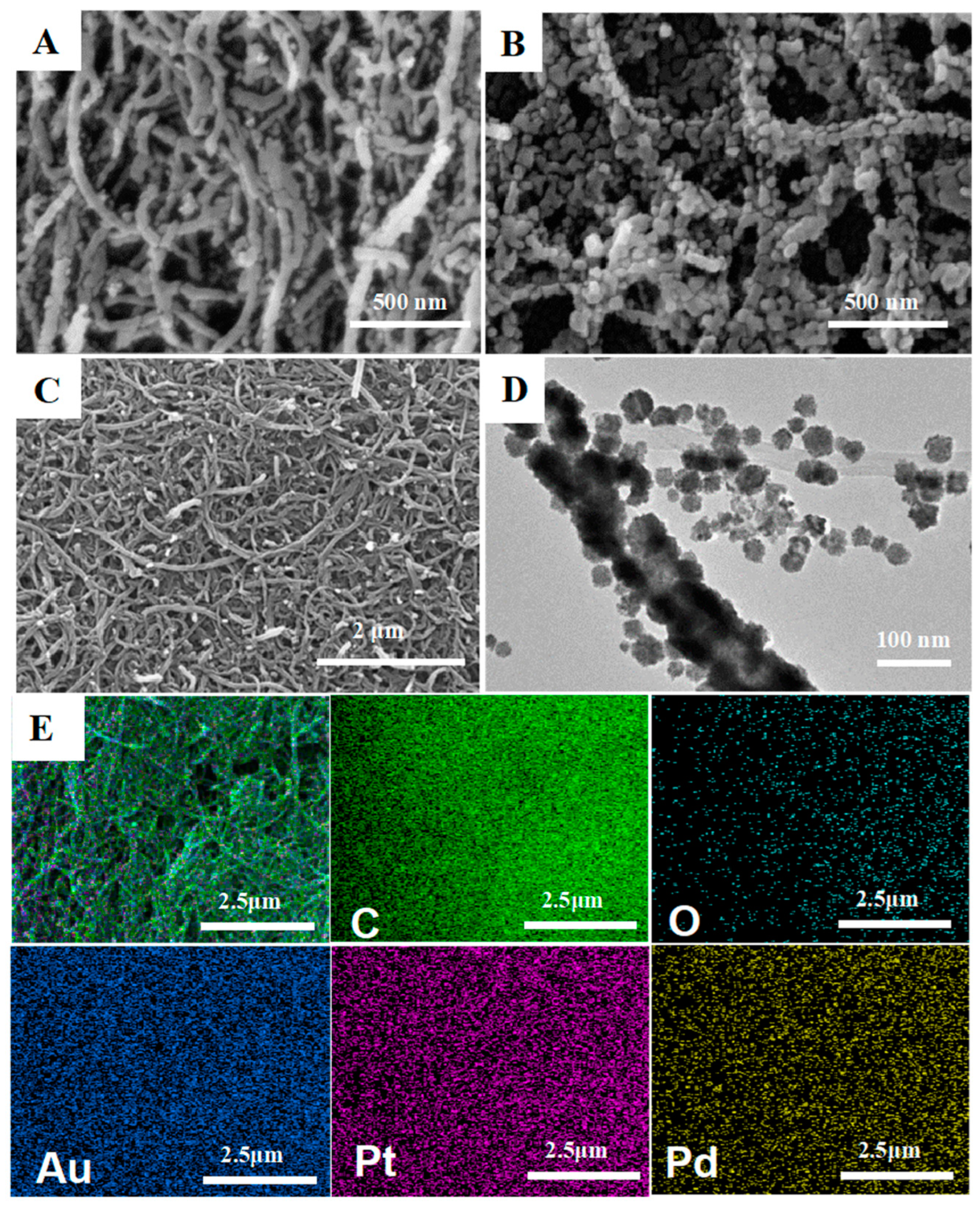
3.1. Characterization of the CS/MWCNTs-AuPtPd Nanocomposites
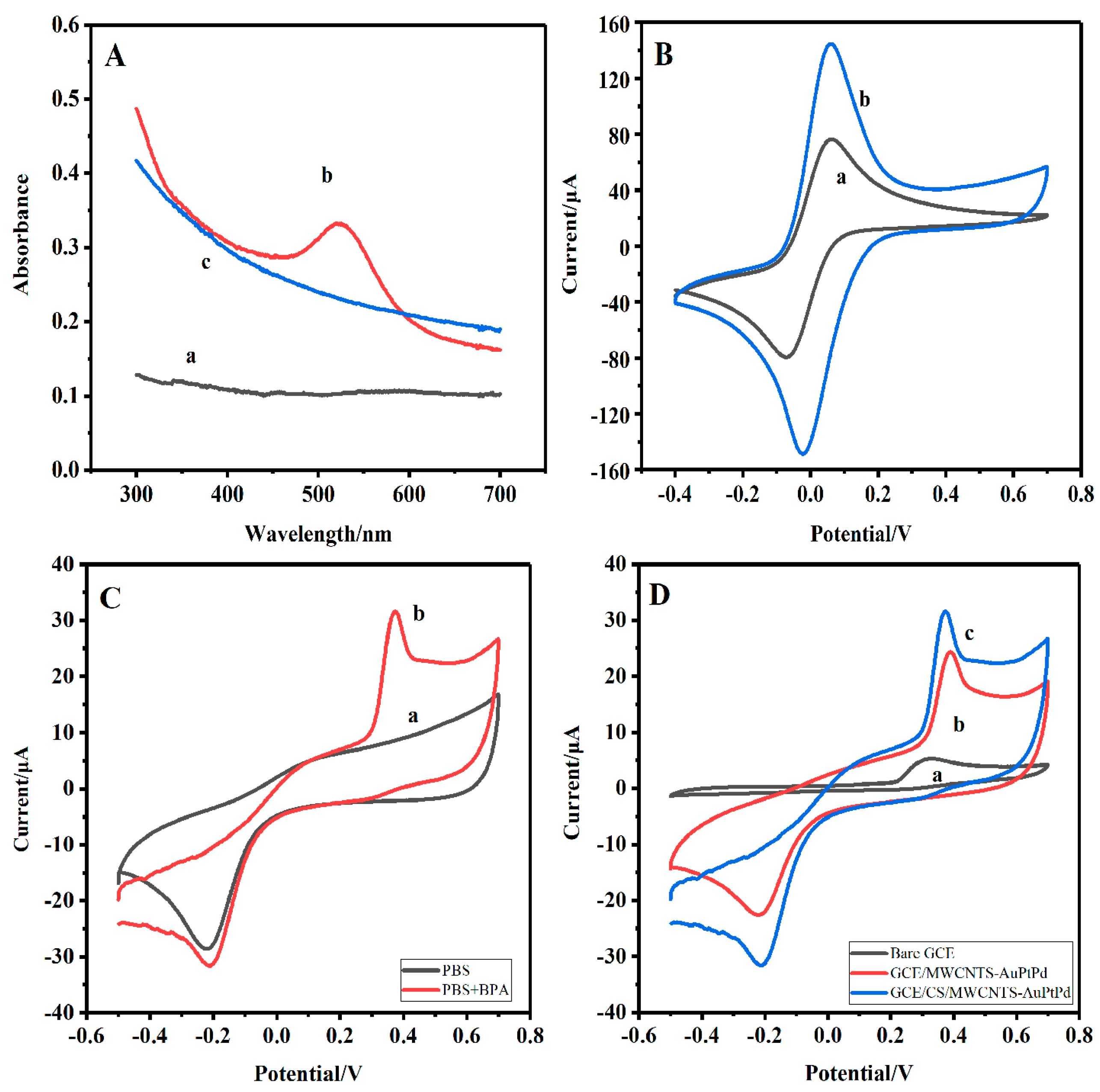
3.2. Enhancement Effect of the Nanocomposite for BPA Detection
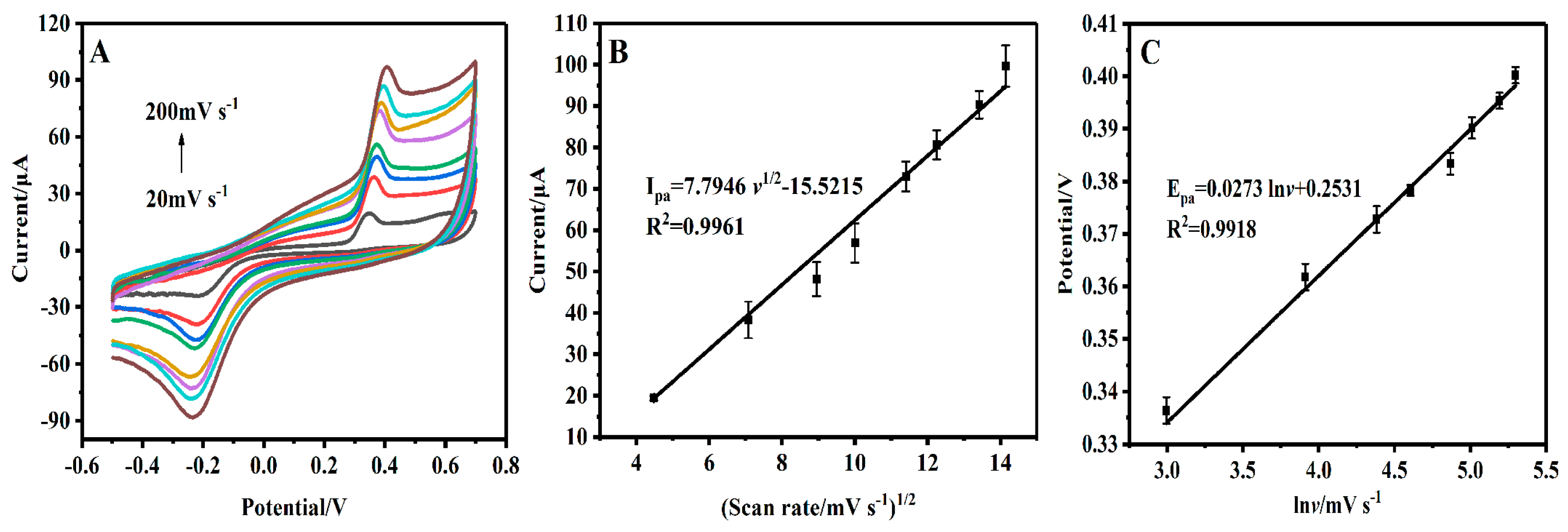
3.3. Kinetic Studies of BPA on GCE/CS/MWCNTs-AuPtPd
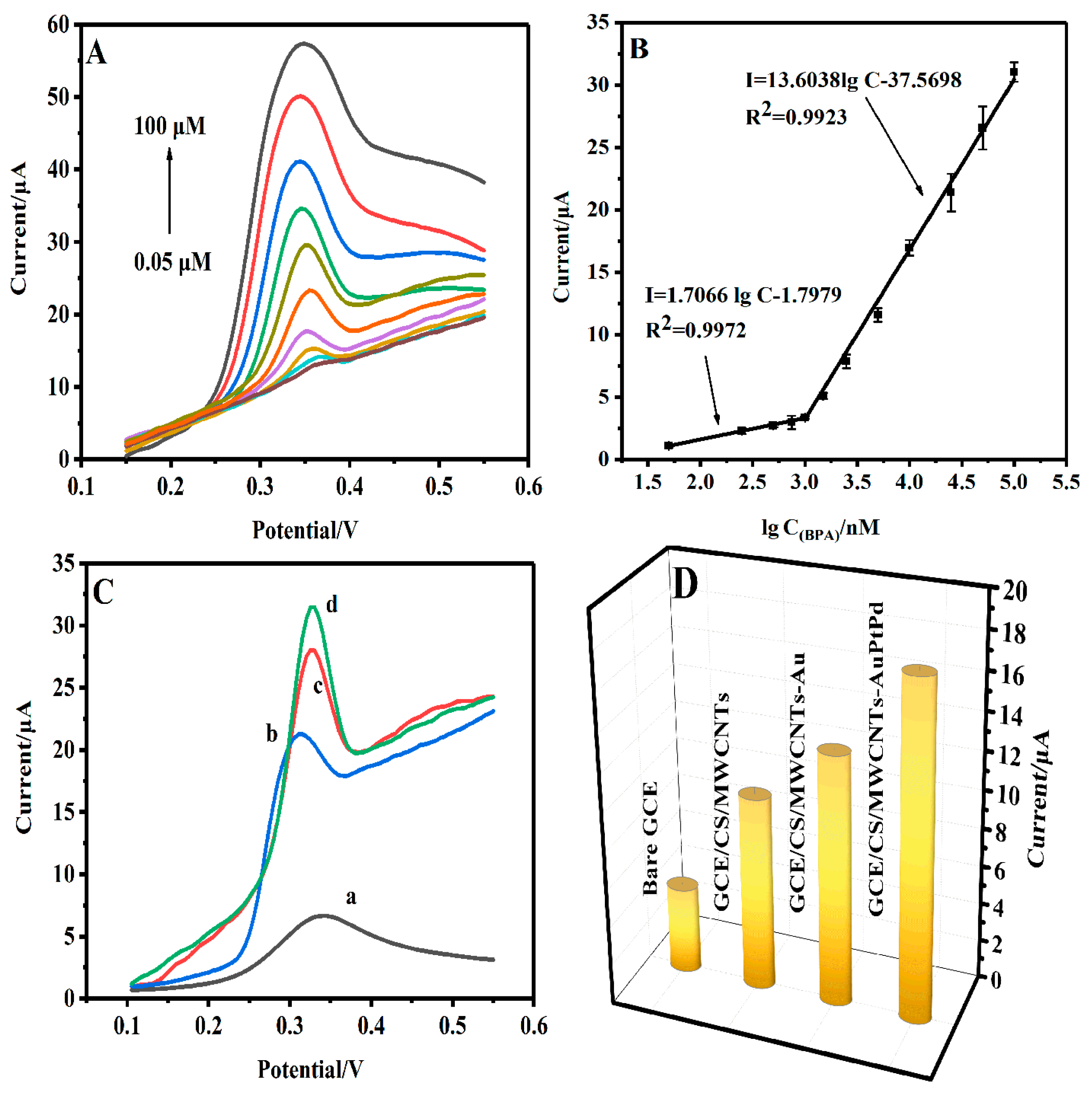
3.4. Optimization of Conditions for BPA Detection
3.5. Electrochemical Detection of BPA
3.6. Reproducibility, Repeatability, Stability and Selectivity
3.7. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.I.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Kim, B.N.; Lim, Y.H. Association of bisphenol A, bisphenol F, and bisphenol S with ADHD symptoms in children. Environ. Int. 2022, 161, 107093–107103. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhang, W.; Han, X.; Zhou, L.; Zhen, H.; Wu, C.; Yu, Q.; Xiu, G. B, N-decorated carbocatalyst based on Fe-MOF/BN as an efficient peroxymonosulfate activator for bisphenol A degradation. J. Hazard. Mater. 2022, 430, 127832–127847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, R.; Shi, W.; Zhou, X.; Sun, S. The association between bisphenol A exposure and oxidative damage in rats/mice: A systematic review and meta-analysis. Environ. Pollut. 2022, 292, 118444–118453. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Adegoke, E.; Pang, M.G. Drivers of owning more BPA. J. Hazard. Mater. 2021, 417, 126076–126092. [Google Scholar] [CrossRef]
- Cao, P.; Zhong, H.N.; Qiu, K.; Li, D.; Wu, G.; Sui, H.X.; Song, Y. Exposure to bisphenol A and its substitutes, bisphenol F and bisphenol S from canned foods and beverages on Chinese market. Food Control 2021, 120, 107502–107510. [Google Scholar] [CrossRef]
- Durovcova, I.; Kyzek, S.; Fabova, J.; Makukova, J.; Galova, E.; Sevcovicova, A. Genotoxic potential of bisphenol A: A review. Environ. Pollut. 2022, 306, 119346–119362. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhou, Q.; Huang, X. Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater. 2020, 384, 121488–121501. [Google Scholar] [CrossRef]
- Gely, C.A.; Huesca, A.; Picard-Hagen, N.; Toutain, P.L.; Berrebi, A.; Gauderat, G.; Gayrard, V.; Lacroix, M.Z. A new LC/MS method for specific determination of human systemic exposure to bisphenol A, F and S through their metabolites: Application to cord blood samples. Environ. Int. 2021, 151, 106429–106438. [Google Scholar] [CrossRef]
- Fernandez, M.A.M.; Andre, L.C.; Cardeal, Z.L. Hollow fiber liquid-phase microextraction-gas chromatography-mass spectrometry method to analyze bisphenol A and other plasticizer metabolites. J. Chromatogr. A 2017, 1481, 31–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, W.; Wang, J.; Yue, X.; Liu, W.; Zhang, Q.; Wang, J. Simultaneous colorimetric determination of bisphenol A and bisphenol S via a multi-level DNA circuit mediated by aptamers and gold nanoparticles. Microchim Acta 2017, 184, 951–959. [Google Scholar] [CrossRef]
- Jia, M.; Chen, S.; Shi, T.; Li, C.; Wang, Y.; Zhang, H. Competitive plasmonic biomimetic enzyme-linked immunosorbent assay for sensitive detection of bisphenol A. Food Chem. 2021, 344, 128602–128610. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chen, Z.; Jiang, X.; Feng, D.Q.; Zhao, J.; Fan, D.; Wang, W. In-situ hydrothermal synthesis of molecularly imprinted polymers coated carbon dots for fluorescent detection of bisphenol A. Sens. Actuators B Chem. 2016, 228, 302–307. [Google Scholar] [CrossRef]
- Lu, H.; Xu, S. Visualizing BPA by molecularly imprinted ratiometric fluorescence sensor based on dual emission nanoparticles. Biosens. Bioelectron. 2017, 92, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Razavipanah, I.; Rounaghi, G.H.; Deiminiat, B.; Damirchi, S.; Abnous, K.; Izadyar, M.; Khavani, M. A new electrochemical aptasensor based on MWCNT-SiO2@Au core-shell nanocomposite for ultrasensitive detection of bisphenol A. Microchem. J. 2019, 146, 1054–1063. [Google Scholar] [CrossRef]
- Wu, L.; Gao, J.; Lu, X.; Huang, C.; Dhanjai; Chen, J. Graphdiyne: A new promising member of 2D all-carbon nanomaterial as robust electrochemical enzyme biosensor platform. Carbon 2020, 156, 568–575. [Google Scholar] [CrossRef]
- Chen, J.; Xu, F.; Zhang, Q.; Li, S. N-doped MoS2-nanoflowers as peroxidase-like nanozymes for total antioxidant capacity assay. Anal. Chim. Acta 2021, 1180, 338740–338752. [Google Scholar] [CrossRef]
- Alizadeh, N.; Salimi, A.; Sham, T.K.; Bazylewski, P.; Fanchini, G.; Fathi, F.; Soleimani, F. Hierarchical Co(OH)2/FeOOH/WO3 ternary nanoflowers as a dual-function enzyme with pH-switchable peroxidase and catalase mimic activities for cancer cell detection and enhanced photodynamic therapy. Chem. Eng. J. 2021, 417, 129134–129146. [Google Scholar] [CrossRef]
- Lu, S.; Guo, X.; Zhang, F.; Li, X.; Zou, M.; Li, L.-L. Bioactivated in vivo assembly (BIVA) peptide-tetraphenylethylene (TPE) probe with controllable assembled nanostructure for cell imaging. Chin. Chem. Lett. 2021, 32, 1947–1952. [Google Scholar] [CrossRef]
- Cai, Y.; Niu, L.; Liu, X.; Zhang, Y.; Zheng, Z.; Zeng, L.; Liu, A. Hierarchical porous MoS2 particles: Excellent multi-enzyme-like activities, mechanism and its sensitive phenol sensing based on inhibition of sulfite oxidase mimics. J. Hazard. Mater. 2021, 425, 128053–128062. [Google Scholar] [CrossRef]
- Song, N.; Chen, S.; Tian, D.; Li, Y.; Wang, C.; Lu, X. Cu2+-doped polypyrrole nanotubes with promoted efficiency for peroxidase mimicking and electrochemical biosensing. Mater. Today Chem. 2020, 18, 100374–100382. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Liu, Q.; Wu, X.; Dong, Y.; Wang, G.L. Smart nanozyme of silver hexacyanoferrate with versatile bio-regulated activities for probing different targets. Talanta 2021, 228, 122268–122277. [Google Scholar] [CrossRef]
- Leau, S.A.; Lete, C.; Lupu, S. Nanocomposite Materials based on Metal Nanoparticles for the Electrochemical Sensing of Neurotransmitters. Chemosensors 2023, 11, 179. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zhong, W.; Wen, M.; Sukhorukov, G.; Shang, L. The density of surface ligands regulates the luminescence of thiolated gold nanoclusters and their metal ion response. Chin. Chem. Lett. 2021, 32, 2390–2394. [Google Scholar] [CrossRef]
- Hussain, I.; Sahoo, S.; Mohapatra, D.; Ahmad, M.; Iqbal, S.; Javed, M.S.; Gu, S.; Qin, N.; Lamiel, C.; Zhang, K. Recent progress in trimetallic/ternary-metal oxides nanostructures: Misinterpretation/misconception of electrochemical data and devices. Appl. Mater. Today 2022, 26, 101297–101328. [Google Scholar] [CrossRef]
- Kang, C.; Ma, L.; Chen, Y.; Fu, L.; Hu, Q.; Zhou, C.; Liu, Q. Metal-organic framework derived hollow rod-like NiCoMn ternary metal sulfide for high-performance asymmetric supercapacitors. Chem. Eng. J. 2022, 427, 131003–131013. [Google Scholar] [CrossRef]
- Chen, M.; Wu, D.; Tu, S.; Yang, C.; Chen, D.; Xu, Y. CRISPR/Cas9 cleavage triggered ESDR for circulating tumor DNA detection based on a 3D graphene/AuPtPd nanoflower biosensor. Biosens. Bioelectron. 2021, 173, 112821–112828. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.X.; Zhou, Y.T.; Zhu, Y.F.; Chen, Z.Y.; Yan, J.M.; Jiang, Q. Tri-metallic AuPdIr nanoalloy towards efficient hydrogen generation from formic acid. Appl. Catal. B Environ. 2022, 309, 121228–121236. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, K.; Li, S.; Wang, X.; Wang, P.; Chen, F.; Yu, H. Mass-transfer control for selective deposition of well-dispersed AuPd cocatalysts to boost photocatalytic H2O2 production of BiVO4. Chem. Eng. J. 2022, 443, 136429–136438. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Feliu, J.M. State of the art in the electrochemical characterization of the surface structure of shape-controlled Pt, Au, and Pd nanoparticles. Curr. Opin. Electrochem. 2020, 22, 65–71. [Google Scholar] [CrossRef]
- Barman, S.C.; Hossain, M.F.; Yoon, H.; Park, J.Y. Trimetallic Pd@Au@Pt nanocomposites platform on -COOH terminated reduced graphene oxide for highly sensitive CEA and PSA biomarkers detection. Biosens. Bioelectron. 2018, 100, 16–22. [Google Scholar] [CrossRef]
- Cen, S.Y.; Ge, X.Y.; Chen, Y.; Wang, A.J.; Feng, J.J. Label-free electrochemical immunosensor for ultrasensitive determination of cardiac troponin I based on porous fluffy-like AuPtPd trimetallic alloyed nanodendrites. Microchem. J. 2021, 169, 106568–106577. [Google Scholar] [CrossRef]
- Qian, Y.; Huang, J.; Chen, J.; Xue, G.; Zhou, Z.; Gao, P. Activation of peracetic acid by RuO2/MWCNTs to degrade sulfamethoxazole at neutral condition. Chem. Eng. J. 2022, 431, 134217–134227. [Google Scholar] [CrossRef]
- Capelari, T.B.; Cássia Mendonça, J.; Rocha, L.R.; Prete, M.C.; Angelis, P.N.; Camargo, L.P.; Dall’Antonia, L.H.; Tarley, C.R.T. Synthesis of novel poly(methacrylic acid)/β-cyclodextrin dual grafted MWCNT-based nanocomposite and its use as electrochemical sensing platform for highly selective determination of cocaine. J. Electroanal. Chem. 2021, 880, 114791–114802. [Google Scholar] [CrossRef]
- Zouaoui, F.; Bourouina-Bacha, S.; Bourouina, M.; Jaffrezic-Renault, N.; Zine, N.; Errachid, A. Electrochemical sensors based on molecularly imprinted chitosan: A review. TrAC-Trend Anal. Chem. 2020, 130, 115982–115998. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, W.; Wu, D.; Yang, Y.; Feng, X.; Gao, C.; Meng, L.; Shen, X.; Zhang, Y.; Tang, X. An electrochemical method for determination of amaranth in drinks using functionalized graphene oxide/chitosan/ionic liquid nanocomposite supported nanoporous gold. Food Chem. 2022, 367, 130727–130735. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Silva, T.A.; Lourencao, B.C.; da Silva, A.D.; Fatibello, O. An electrochemical sensing platform based on carbon black and chitosan-stabilized platinum nanoparticles. Anal. Methods 2023, 15, 1077–1086. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Doan, T.C.D.; Huynh, T.M.; Nguyen, V.N.P.; Dinh, H.H.; Dang, D.M.T.; Dang, C.M. An electrochemical sensor based on polyvinyl alcohol/chitosan-thermally reduced graphene composite modified glassy carbon electrode for sensitive voltammetric detection of lead. Sens. Actuators B Chem. 2022, 345, 130443–130452. [Google Scholar] [CrossRef]
- Ali, M.Y.; Alam, A.U.; Howlader, M.M.R. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319–128328. [Google Scholar] [CrossRef]
- Kim, M.Y.; Park, H.; Lee, J.Y.; Park, D.J.; Lee, J.Y.; Myung, N.V.; Lee, K.H. Hierarchically palladium nanoparticles embedded polyethyleneimine-reduced graphene oxide aerogel (RGA-PEI-Pd) porous electrodes for electrochemical detection of bisphenol a and H2O2. Chem. Eng. J. 2022, 431, 134250–134262. [Google Scholar] [CrossRef]
- Pang, Y.H.; Huang, Y.Y.; Wang, L.; Shen, X.F.; Wang, Y.Y. Determination of bisphenol A and bisphenol S by a covalent organic framework electrochemical sensor. Environ. Pollut. 2020, 263, 114616–114625. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Deng, X.; Sun, S.; Li, Y. A novel dual-signal electrochemical sensor for bisphenol A determination by coupling nanoporous gold leaf and self-assembled cyclodextrin. Electrochim. Acta 2018, 271, 417–424. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Li, S.; Dang, Y. A novel electrochemical sensor for highly sensitive detection of bisphenol A based on the hydrothermal synthesized Na-doped WO3 nanorods. Sens. Actuators B Chem. 2017, 245, 238–246. [Google Scholar] [CrossRef]
- Annalakshmi, M.; Balamurugan, T.S.T.; Kumaravel, S.; Chen, S.M.; He, J.L. Facile Hydrothermal Synthesis of Manganese Sulfide Nanoelectrocatalyst for Highly Sensitive Detection of Bisphenol A in Food and Eco-samples. Food Chem. 2022, 393, 133316–133325. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, T.; Zhao, W.; Zhang, M.; Zhang, Z.; Lai, W.; Kadasala, N.R.; Liu, H.; Liu, Y. Magnetic-Core–Shell–Satellite Fe3O4-Au@Ag@(Au@Ag) nanocomposites fordetermination of trace bisphenol A based on Surface-Enhanced Resonance Raman Scattering (SERRS). Nanomaterials 2022, 12, 3322. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M.; Alfaifi, S.Y. Ultra-sensitive, selective and rapid carcinogenic bisphenol A contaminant determination using low-dimensional facile binary Mg-SnO2 doped microcube by potential electro-analytical technique for the safety of environment. J. Ind. Eng. Chem. 2022, 109, 147–154. [Google Scholar] [CrossRef]
- Tan, S.; Ruan, X.; Shao, J.; Shan, J.; Shan, X.; Ye, H.; Shi, Y. Facile preparation of aluminum nanocomposites and the utilization in analyzing BPA in urine samples. Chem. Pap. 2022, 76, 2029–2039. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Shi, P.; Zhao, G. A Highly Sensitive and Selective Label-free Electrochemical Biosensor with a Wide Range of Applications for Bisphenol A Detection. Electroanalysis 2022, 34, 743–751. [Google Scholar] [CrossRef]




| Sensors | Linear Range (μM) | LOD (nM) | References |
|---|---|---|---|
| SPCE 1/RGA 2−PEI 3−Pd | 1–1000 | 25.5 | [40] |
| GCE/CTpPa-2 4 | 0.1–50 | 20 | [35] |
| SPCE/MWCNTs-βCD 5 | 0.125–30 | 13.76 | [39] |
| GCE/Tyr 6−GDY 7−Chi 8 | 0.1–3.5 | 24 | [15] |
| GE/SH-β-CD 9/NPGL 10 | 0.3–100 | 60 | [42] |
| CPE 11/Na-doped WO3 | 0.081–22.5 | 28 | [43] |
| GCE/MnS | 0.02–2150 | 6.52 | [44] |
| Fe3O4-Au@Ag@(Au@Ag) | 0.0001–100 | 0.1 | [45] |
| MTO/Au/μ-Chip | 0.0001–1000 | 0.0027 | [46] |
| GCE/CS/MWCNTs-AuPtPd | 0.05–100 | 1.4 | This work |
| Sample | Added BPA (μM) | Found BPA (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 0 | 0 | / | / |
| 10 | 9.8 | 98.7 | 5.7 | |
| 25 | 25.9 | 103.6 | 4.8 | |
| Milk | 0 | 0 | / | / |
| 10 | 9.6 | 96.3 | 6.2 | |
| 25 | 23.6 | 94.4 | 3.9 | |
| Orange Juice | 0 | 0 | / | / |
| 10 | 9.5 | 95.4 | 4.8 | |
| 25 | 25.2 | 100.8 | 1.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, E.; Pan, Y.; Li, L.; Liu, Y.; Gu, Y.; Cai, J. Development of Sensitive Electrochemical Sensor Based on Chitosan/MWCNTs-AuPtPd Nanocomposites for Detection of Bisphenol A. Chemosensors 2023, 11, 331. https://doi.org/10.3390/chemosensors11060331
Han E, Pan Y, Li L, Liu Y, Gu Y, Cai J. Development of Sensitive Electrochemical Sensor Based on Chitosan/MWCNTs-AuPtPd Nanocomposites for Detection of Bisphenol A. Chemosensors. 2023; 11(6):331. https://doi.org/10.3390/chemosensors11060331
Chicago/Turabian StyleHan, En, Yingying Pan, Lei Li, Yuan Liu, Yuan Gu, and Jianrong Cai. 2023. "Development of Sensitive Electrochemical Sensor Based on Chitosan/MWCNTs-AuPtPd Nanocomposites for Detection of Bisphenol A" Chemosensors 11, no. 6: 331. https://doi.org/10.3390/chemosensors11060331
APA StyleHan, E., Pan, Y., Li, L., Liu, Y., Gu, Y., & Cai, J. (2023). Development of Sensitive Electrochemical Sensor Based on Chitosan/MWCNTs-AuPtPd Nanocomposites for Detection of Bisphenol A. Chemosensors, 11(6), 331. https://doi.org/10.3390/chemosensors11060331






