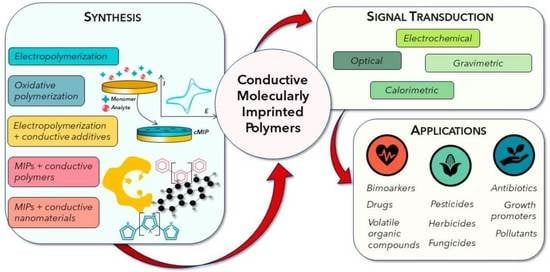
Conductive Molecularly Imprinted Polymers (cMIPs): Rising and Versatile Key Elements in Chemical Sensing
Abstract
1. Introduction
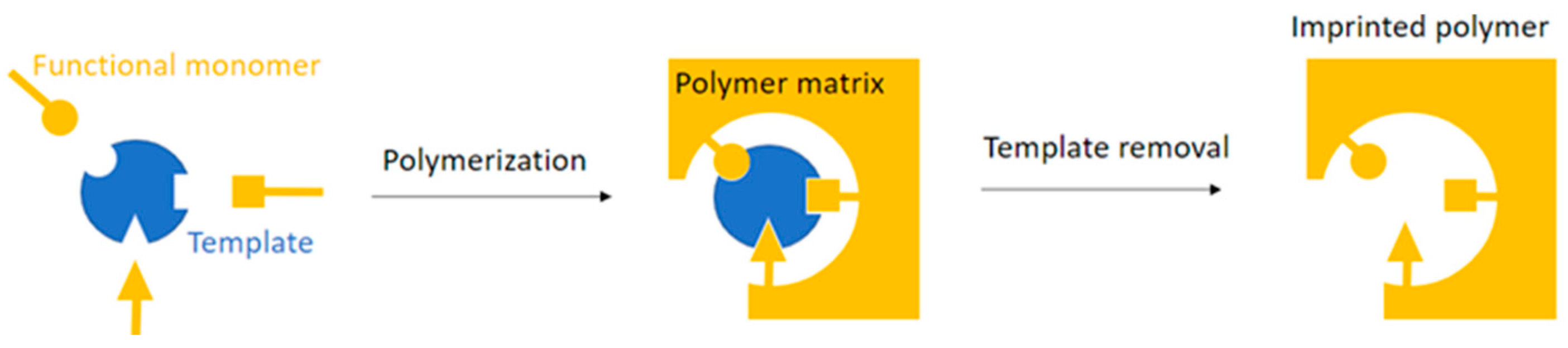
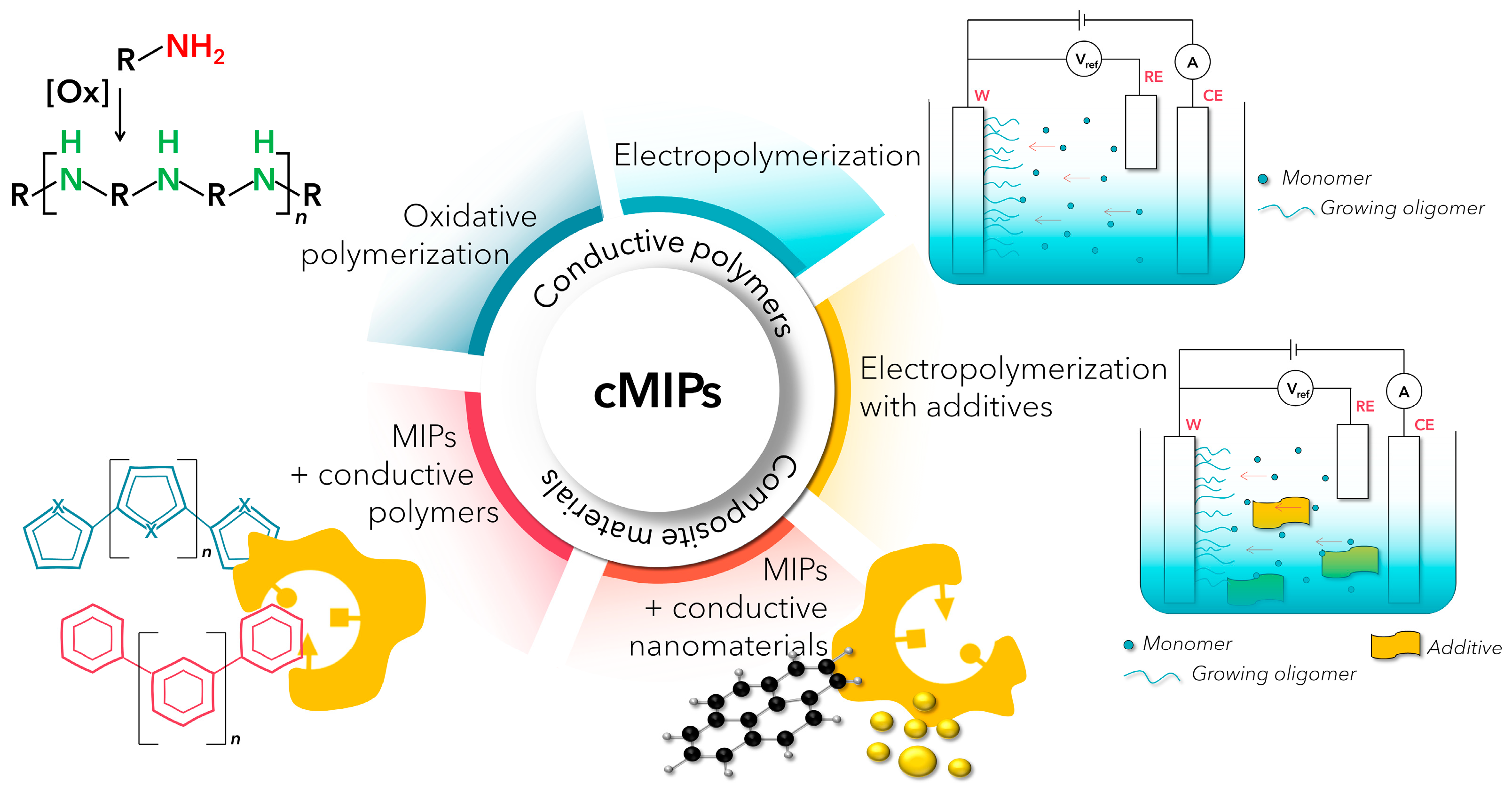
2. Conductive MIPs
2.1. Electropolymerization
2.1.1. Pyrrole
2.1.2. Aniline
2.1.3. Thiophene Derivatives
2.1.4. Alternative Monomers
2.2. Electropolymerization + Additives
2.2.1. Systems Based on Polypyrrole
2.2.2. Aniline-Based Systems
2.2.3. Thiophene-, Phenol-, and Benzoic-Acid-Based Systems
2.3. Oxidative Polymerization
2.4. MIPs + Conductive Nanomaterials
2.4.1. Acrylic Acid Derivatives as Monomers
2.4.2. Aliphatic/Non-Aromatic Monomers
2.4.3. Aromatic Monomers
2.5. Blending MIPs with Conductive Polymers
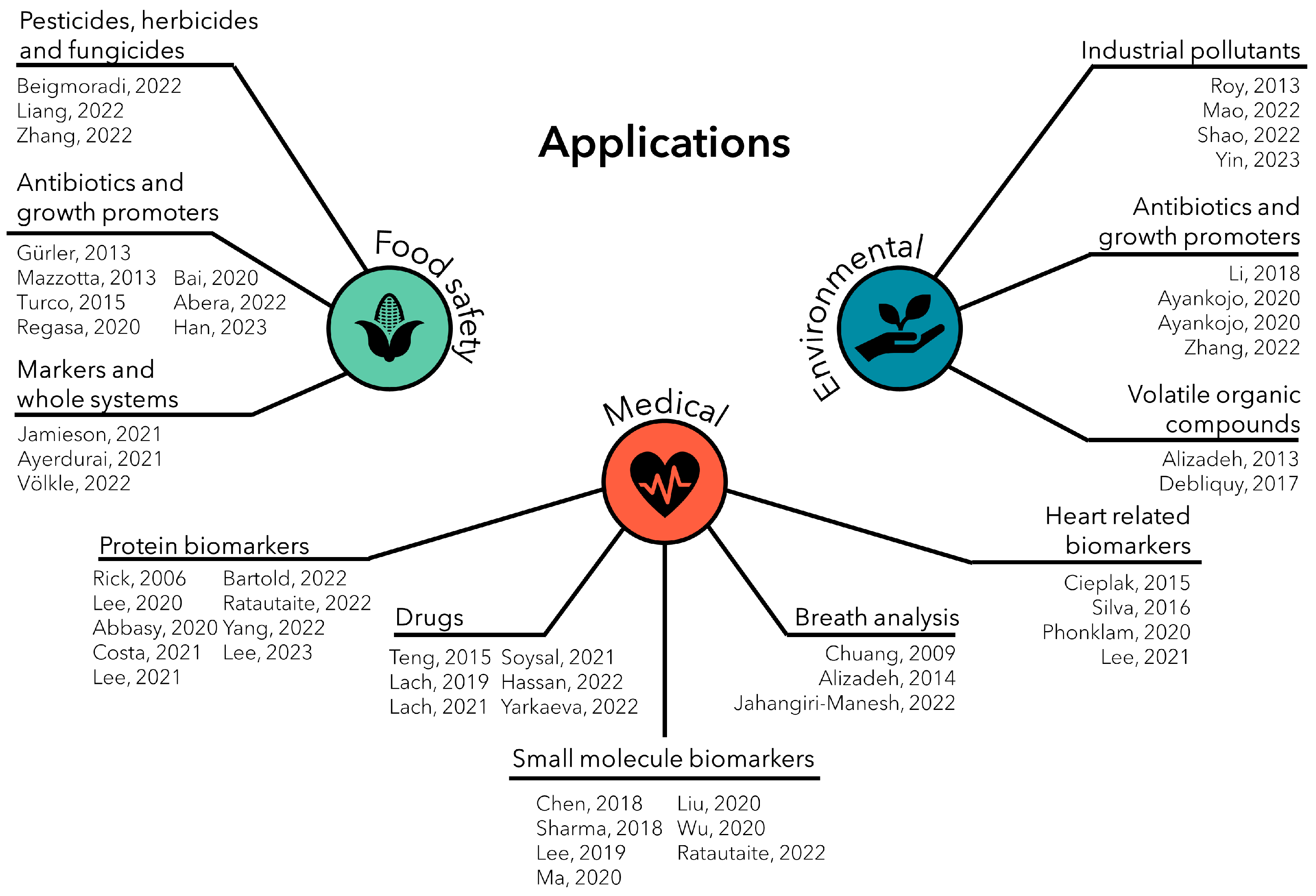
3. Applications in Chemical Sensing
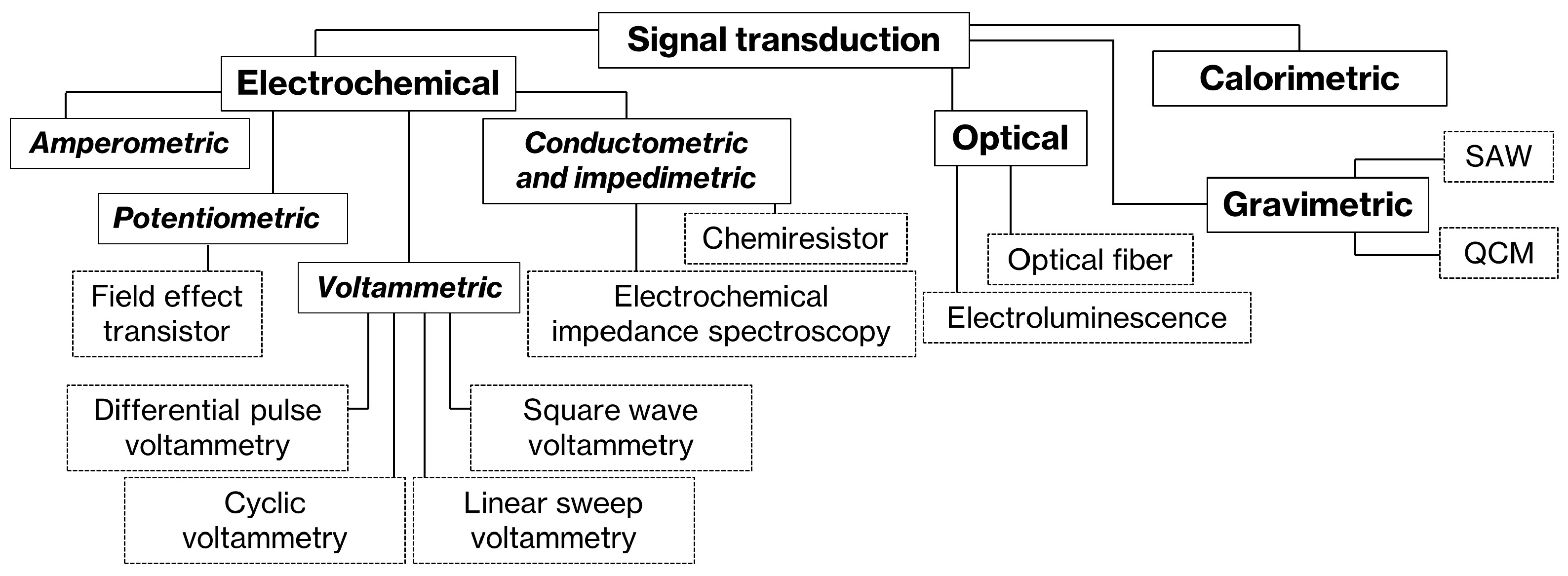
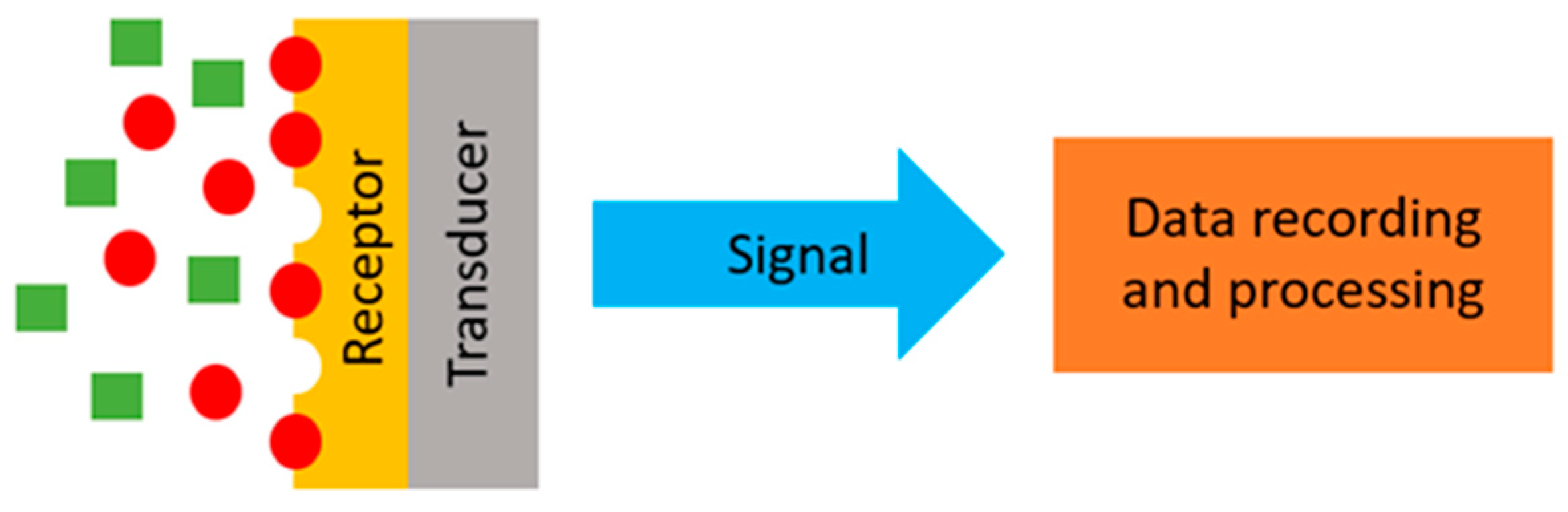
3.1. Transducers
3.2. Application Areas
3.2.1. Food Safety
3.2.2. Medical Applications
3.2.3. Environmental Applications
4. Summary and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wulff, G. Fourty years of molecular imprinting in synthetic polymers: Origin, features and perspectives. Microchim. Acta 2013, 180, 1359–1370. [Google Scholar] [CrossRef]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.V.; Allabush, F.; Bunka, D.; Tolley, A.; Mendes, P.M.; Tucker, J.H.R.; Turner, N.W. Hybrid aptamer-molecularly imprinted polymer (AptaMIP) nanoparticles selective for the antibiotic moxifloxacin. Polym. Chem. 2021, 12, 4394–4405. [Google Scholar] [CrossRef]
- Khan, M.A.R.; Aires Cardoso, A.R.; Sales, M.G.F.; Merino, S.; Tomás, J.M.; Rius, F.X.; Riu, J. Artificial receptors for the electrochemical detection of bacterial flagellar filaments from Proteus mirabilis. Sens. Actuators B Chem. 2017, 244, 732–741. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Gentile, G.J.; de Cortalezzi, M.M.F. Molecularly Imprinted Polymers (Mips) in Sensors for Environmental and Biomedical Applications: A Review. Molecules 2021, 26, 6233. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Wackerlig, J.; Lieberzeit, P.A. Molecularly Imprinted Polymer Nanoparticles in Chemical Sensing—Synthesis, Characterization and Application. Sens. Actuators B Chem. 2015, 207, 144–157. [Google Scholar] [CrossRef]
- Herrera-Chacón, A.; Cetó, X.; del Valle, M. Molecularly imprinted polymers—Towards electrochemical sensors and electronic tongues. Anal. Bioanal. Chem. 2021, 413, 6117–6140. [Google Scholar] [CrossRef]
- Han, Y.; Tao, J.; Ali, N.; Khan, A.; Malik, S.; Khan, H.; Yu, C.; Yang, Y.; Bilal, M.; Mohamed, A.A. Molecularly imprinted polymers as the epitome of excellence in multiple fields. Eur. Polym. J. 2022, 179, 111582. [Google Scholar] [CrossRef]
- Xu, S.; Wang, L.; Liu, Z. Molecularly Imprinted Polymer Nanoparticles: An Emerging Versatile Platform for Cancer Therapy. Angew. Chem. Int. Ed. 2021, 60, 3858–3869. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for commercial application in low-cost sensors and assays—An overview of the current status quo. Sens. Actuators B Chem. 2020, 325, 128973. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, M.V.; Kuleshina, L.; Neimark, I. On the Dependence of Silica Gel Adsorption Properties on the Character of Its Porosity. Zhurnal Fizieskoj Khimii/Akad. SSSR 1937, 10, 100–112. [Google Scholar]
- Dickey, F.H. The Preparation of Specific Adsorbents. Proc. Natl. Acad. Sci. USA 1949, 35, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Bolto, B.A.; Weiss, D. Electronic Conduction in Polymers. II. The Electrochemical Reduction of Polypyrrole at Controlled Potential. Aust. J. Chem. 1963, 16, 1076–1089. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Rasmussen, S.C. Early History of Polypyrrole: The First Conducting Organic Polymer. Bull. Hist. Chem. 2015, 40, 45–55. [Google Scholar]
- Gangopadhyay, R.; De, A. Conducting Polymer Nanocomposites: A Brief Overview. Chem. Mater. 2000, 12, 608–622. [Google Scholar] [CrossRef]
- Schadler, L.S.; Brinson, L.C.; Sawyer, W.G. Polymer nanocomposites: A small part of the story. JOM 2007, 59, 53–60. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Villa, C.C.; Sánchez, L.T.; Valencia, G.A.; Ahmed, S.; Gutiérrez, T.J. Molecularly imprinted polymers for food applications: A review. Trends Food Sci. Technol. 2021, 111, 642–669. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly imprinted polymers for sample preparation: A review. Anal. Chim. Acta 2010, 668, 87–99. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef] [PubMed]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Blanco-López, M.C.; Gutiérrez-Fernández, S.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical sensing with electrodes modified with molecularly imprinted polymer films. Anal. Bioanal. Chem. 2004, 378, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Fuchiwaki, Y.; Kubo, I. Electrochemical Sensor Based on Biomimetic Recognition Utilizing Molecularly Imprinted Polymer Receptor. In Biomimetics Learning from Nature; Vellore Institute of Technology University: Vellore, India, 2010. [Google Scholar] [CrossRef]
- Cowen, T.; Cheffena, M. Template Imprinting Versus Porogen Imprinting of Small Molecules: A Review of Molecularly Imprinted Polymers in Gas Sensing. Int. J. Mol. Sci. 2022, 23, 9642. [Google Scholar] [CrossRef]
- Sharma, P.S.; Pietrzyk-Le, A.; D’souza, F.; Kutner, W. Electrochemically synthesized polymers in molecular imprinting for chemical sensing. Anal. Bioanal. Chem. 2012, 402, 3177–3204. [Google Scholar] [CrossRef]
- Gürler, B.; Özkorucuklu, S.P.; Kır, E. Voltammetric behavior and determination of doxycycline in pharmaceuticals at molecularly imprinted and non-imprinted overoxidized polypyrrole electrodes. J. Pharm. Biomed. Anal. 2013, 84, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Malitesta, C.; Surdo, S.; Barillaro, G. Microstructuring conducting polymers and molecularly imprinted polymers by light-activated electropolymerization on micromachined silicon. Applications in electrochemical sensing. In Proceedings of the SENSORS, 2013 IEEE, Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Turco, A.; Corvaglia, S.; Mazzotta, E. Electrochemical sensor for sulfadimethoxine based on molecularly imprinted polypyrrole: Study of imprinting parameters. Biosens. Bioelectron. 2015, 63, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical sensors based on l-tryptophan molecularly imprinted polypyrrole and polyaniline. J. Electroanal. Chem. 2022, 917, 116389. [Google Scholar] [CrossRef]
- Choong, C.-L.; Milne, W.I. Dynamic modulation of detection window in conducting polymer based biosensors. Biosens. Bioelectron. 2010, 25, 2384–2388. [Google Scholar] [CrossRef]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowicz, P.; Noworyta, K.; D’souza, F.; Kutner, W. Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Ramanavicius, A. Molecularly imprinted polypyrrole-based synthetic receptor for direct detection of bovine leukemia virus glycoproteins. Biosens. Bioelectron. 2004, 20, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Costa, R.; Costa, J.; Moreira, P.; Brandão, A.T.S.C.; Mafra, I.; Silva, A.F.; Pereira, C.M. Molecularly imprinted polymer as a synthetic antibody for the biorecognition of hazelnut Cor a 14-allergen. Anal. Chim. Acta 2021, 1191, 339310. [Google Scholar] [CrossRef]
- Tokonami, S.; Nakadoi, Y.; Nakata, H.; Takami, S.; Kadoma, T.; Shiigi, H.; Nagaoka, T. Recognition of gram-negative and gram-positive bacteria with a functionalized conducting polymer film. Res. Chem. Intermed. 2014, 40, 2327–2335. [Google Scholar] [CrossRef]
- Jamieson, O.; Betlem, K.; Mansouri, N.; Crapnell, R.D.; Vieira, F.S.; Hudson, A.; Banks, C.E.; Liauw, C.M.; Gruber, J.; Zubko, M.; et al. Electropolymerised molecularly imprinted polymers for the heat-transfer based detection of microorganisms: A proof-of-concept study using yeast. Therm. Sci. Eng. Prog. 2021, 24, 100956. [Google Scholar] [CrossRef]
- Debliquy, M.; Lahem, D.; Tang, X.; Krumpmann, A.; Gonzalez Vila, A.; Raskin, J.-P.; Zhang, C. Molecularly Imprinted Polymers for VOC Sensing: Chemoresistive and Optical Sensors. In Proceedings of the 2017 4th International Conference on Machinery, Materials and Computer (MACMC 2017), Xi’an, China, 27–29 November 2017; p. 7. [Google Scholar]
- González-Vila, A.; Debliquy, M.; Lahem, D.; Zhang, C.; Mégret, P.; Caucheteur, C. Molecularly imprinted electropolymerization on a metal-coated optical fiber for gas sensing applications. Sens. Actuators B Chem. 2017, 244, 1145–1151. [Google Scholar] [CrossRef]
- Silva, B.V.; Rodríguez, B.A.; Sales, G.F.; Sotomayor, M.D.P.T.; Dutra, R.F. An ultrasensitive human cardiac troponin T graphene screen-printed electrode based on electropolymerized-molecularly imprinted conducting polymer. Biosens. Bioelectron. 2016, 77, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lee, U.-H.; Chang, S.-M.; Park, J.Y. Gravimetric detection of theophylline on pore-structured molecularly imprinted conducting polymer. Sens. Actuators B Chem. 2014, 200, 25–30. [Google Scholar] [CrossRef]
- Chen, L.; Liu, D.; Wang, C. A Novel Imprinted Electrochemical Sensor for Dopamine Determination Based on Electron Conductivity Enhanced by Ferrocenyl Chalcone Derivative Film. J. At. Mol. Sci. 2018, 9, 58–61. [Google Scholar] [CrossRef]
- Rick, J.; Chou, T.-C. Using protein templates to direct the formation of thin-film polymer surfaces. Biosens. Bioelectron. 2006, 22, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Iwersen-Bergmann, S.; Schmoldt, A. Acute intoxication with aniline: Detection of acetaminophen as aniline metabolite. Int. J. Leg. Med. 2000, 113, 171–174. [Google Scholar] [CrossRef]
- Khan, M.F.; Boor, P.J.; Gu, Y.; Alcock, N.W.; Ansari, G.A.S. Oxidative Stress in the Splenotoxicity of Aniline. Fundam. Appl. Toxicol. 1997, 35, 22–30. [Google Scholar] [CrossRef]
- Gandarilla, A.M.D.; Matos, R.S.; Barcelay, Y.R.; da Fonseca Filho, H.D.; Brito, W.R. Molecularly imprinted polymer on indium tin oxide substrate for bovine serum albumin determination. J. Polym. Res. 2022, 29, 166. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Liu, W.-C.; Zhang, Z.-X.; Liu, B.-D.; Yang, C.-H.; Lin, H.-Y. A multichannel system integrating molecularly imprinted conductive polymers for ultrasensitive voltammetric determination of four steroid hormones in urine. Microchim. Acta 2019, 186, 695. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.C.; Nisha, V.S.; Dhand, C.; Ali, M.A.; Malhotra, B.D. Molecularly imprinted polyaniline-polyvinyl sulphonic acid composite based sensor for para-nitrophenol detection. Anal. Chim. Acta 2013, 777, 63–71. [Google Scholar] [CrossRef]
- Regasa, M.B.; Refera Soreta, T.; Femi, O.E.; Ramamurthy, P.C. Development of Molecularly Imprinted Conducting Polymer Composite Film-Based Electrochemical Sensor for Melamine Detection in Infant Formula. ACS Omega 2020, 5, 4090–4099. [Google Scholar] [CrossRef] [PubMed]
- Lattach, Y.; Garnier, F.; Remita, S. Influence of Chemical and Structural Properties of Functionalized Polythiophene-Based Layers on Electrochemical Sensing of Atrazine. ChemPhysChem 2012, 13, 281–290. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-T.; Thomas, J.L.; Su, Z.-L.; O’hare, D.; Van Wuellen, T.; Chamarro, J.M.; Bolognin, S.; Luo, S.-C.; Schwamborn, J.C.; et al. Peptide-Imprinted Poly(hydroxymethyl 3,4-ethylenedioxythiophene) Nanotubes for Detection of α Synuclein in Human Brain Organoids. ACS Appl. Nano Mater. 2020, 3, 8027–8036. [Google Scholar] [CrossRef]
- Lach, P.; Cieplak, M.; Majewska, M.; Noworyta, K.R.; Sharma, P.S.; Kutner, W. “Gate Effect” in p-Synephrine Electrochemical Sensing with a Molecularly Imprinted Polymer and Redox Probes. Anal. Chem. 2019, 91, 7546–7553. [Google Scholar] [CrossRef] [PubMed]
- Ayerdurai, V.; Cieplak, M.; Noworyta, K.R.; Gajda, M.; Ziminska, A.; Sosnowska, M.; Piechowska, J.; Borowicz, P.; Lisowski, W.; Shao, S.; et al. Electrochemical sensor for selective tyramine determination, amplified by a molecularly imprinted polymer film. Bioelectrochemistry 2021, 138, 107695. [Google Scholar] [CrossRef]
- Yang, X.; Liu, H.; Ji, Y.; Xu, S.; Xia, C.; Zhang, R.; Zhang, C.; Miao, Z. A molecularly imprinted biosensor based on water-compatible and electroactive polymeric nanoparticles for lysozyme detection. Talanta 2022, 236, 122891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Iskierko, Z.; Noworyta, K.; Cieplak, M.; Borowicz, P.; Lisowski, W.; D’Souza, F.; Kutner, W. Synthesis and application of a “plastic antibody” in electrochemical microfluidic platform for oxytocin determination. Biosens. Bioelectron. 2018, 100, 251–258. [Google Scholar] [CrossRef]
- Soysal, M. An Electrochemical Sensor Based on Molecularly Imprinted Polymer for Methyl Paraben Recognition and Detection. J. Anal. Chem. 2021, 76, 381–389. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Ciocan, V.; Öpik, A.; Syritski, V. Molecularly imprinted polymer-based sensor for electrochemical detection of erythromycin. Talanta 2020, 209, 120502. [Google Scholar] [CrossRef]
- Ayankojo, A.G.; Reut, J.; Öpik, A.; Syritski, V. Sulfamethizole-imprinted polymer on screen-printed electrodes: Towards the design of a portable environmental sensor. Sens. Actuators B Chem. 2020, 320, 128600. [Google Scholar] [CrossRef]
- Bartold, K.; Iskierko, Z.; Borowicz, P.; Noworyta, K.; Lin, C.-Y.; Kalecki, J.; Sharma, P.S.; Lin, H.-Y.; Kutner, W. Molecularly imprinted polymer-based extended-gate field-effect transistor (EG-FET) chemosensor for selective determination of matrix metalloproteinase-1 (MMP-1) protein. Biosens. Bioelectron. 2022, 208, 114203. [Google Scholar] [CrossRef] [PubMed]
- Abbasy, L.; Mohammadzadeh, A.; Hasanzadeh, M.; Razmi, N. Development of a reliable bioanalytical method based on prostate specific antigen trapping on the cavity of molecular imprinted polymer towards sensing of PSA using binding affinity of PSA-MIP receptor: A novel biosensor. J. Pharm. Biomed. Anal. 2020, 188, 113447. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, H.F.; Olean-Oliveira, A.; Cardoso, C.X.; Teixeira, M.F.S. Development of a molecularly imprinted polymer for uric acid sensing based on a conductive azopolymer: Unusual approaches using electrochemical impedance/capacitance spectroscopy without a soluble redox probe. Sens. Actuators B Chem. 2021, 343, 130141. [Google Scholar] [CrossRef]
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736. [Google Scholar] [CrossRef]
- Saidman, S.B. The effect of pH on the electrochemical polymerisation of pyrrole on aluminium. J. Electroanal. Chem. 2002, 534, 39–45. [Google Scholar] [CrossRef]
- Liu, X.-X.; Zhang, L.; Li, Y.-B.; Bian, L.-J.; Su, Z.; Zhang, L.-J. Electropolymerization of aniline in aqueous solutions at pH 2 to 12. J. Mater. Sci. 2005, 40, 4511–4515. [Google Scholar] [CrossRef]
- Meteleva-Fischer, Y.V.; Von Hauff, E.; Parisi, J. Electrochemical synthesis of polypyrrole layers doped with glutamic ions. J. Appl. Polym. Sci. 2009, 114, 4051–4058. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Shiigi, H.; Yakabe, H.; Kishimoto, M.; Kijima, D.; Zhang, Y.; Sree, U.; Deore, B.A.; Nagaoka, T. Molecularly Imprinted Overoxidized Polypyrrole Colloids: Promising Materials for Molecular Recognition. Microchim. Acta 2003, 143, 155–162. [Google Scholar] [CrossRef]
- Kumpf, K.; Trattner, S.; Aspermair, P.; Bintinger, J.; Fruhmann, P. P3HT and PEDOT: PSS printed thin films on chemiresistors: An economic and versatile tool for ammonia and humidity monitoring applications. J. Appl. Polym. Sci. 2023, 140, 53733. [Google Scholar] [CrossRef]
- Bießmann, L.; Kreuzer, L.P.; Widmann, T.; Hohn, N.; Moulin, J.-F.; Müller-Buschbaum, P. Monitoring the Swelling Behavior of PEDOT:PSS Electrodes under High Humidity Conditions. ACS Appl. Mater. Interfaces 2018, 10, 9865–9872. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Mo, Y.; Liu, X.; Yang, H.; Zhou, D.; Cao, H.; Ye, T.; Xu, F. An ultra-sensitive and selective electrochemical sensor based on GOCS composite and ion imprinted polymer for the rapid detection of Cd2+ in food samples. Food Chem. 2023, 410, 135293. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, B.; Liu, M.; Hu, X.; Fang, G.; Wang, S. Molecularly imprinted electrochemical sensor based on polypyrrole/dopamine@graphene incorporated with surface molecularly imprinted polymers thin film for recognition of olaquindox. Bioelectrochemistry 2020, 132, 107398. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, F.; Dai, R.; Liu, G.; Zhang, Y.; Lu, L.; Yu, Y. Novel electrochemical sensing platform based on a molecularly imprinted polymer-decorated 3D-multi-walled carbon nanotube intercalated graphene aerogel for selective and sensitive detection of dopamine. Anal. Methods 2020, 12, 1845–1851. [Google Scholar] [CrossRef]
- Cai, D.; Ren, L.; Zhao, H.; Xu, C.; Zhang, L.; Yu, Y.; Wang, H.; Lan, Y.; Roberts, M.F.; Chuang, J.H.; et al. A molecular-imprint nanosensor for ultrasensitive detection of proteins. Nat. Nanotechnol. 2010, 5, 597–601. [Google Scholar] [CrossRef]
- Ma, X.; Tu, X.; Gao, F.; Xie, Y.; Huang, X.; Fernandez, C.; Qu, F.; Liu, G.; Lu, L.; Yu, Y. Hierarchical porous MXene/amino carbon nanotubes-based molecular imprinting sensor for highly sensitive and selective sensing of fisetin. Sens. Actuators B Chem. 2020, 309, 127815. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Chen, J.; Xue, Z.; Wu, B.; Lu, X. Acetylsalicylic acid electrochemical sensor based on PATP–AuNPs modified molecularly imprinted polymer film. Talanta 2011, 85, 1672–1679. [Google Scholar] [CrossRef]
- Essousi, H.; Barhoumi, H.; Bibani, M.; Ktari, N.; Wendler, F.; Al-Hamry, A.; Kanoun, O. Ion-Imprinted Electrochemical Sensor Based on Copper Nanoparticles-Polyaniline Matrix for Nitrate Detection. J. Sensors 2019, 2019, 4257125. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; El-Wekil, M.M.; Mahnashi, M.H.; Ali, M.F.B.; Alkahtani, S.A. Modification of N,S co-doped graphene quantum dots with p-aminothiophenol-functionalized gold nanoparticles for molecular imprint-based voltammetric determination of the antiviral drug sofosbuvir. Microchim. Acta 2019, 186, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
- Rezaei, B.; Khalili Boroujeni, M.; Ensafi, A.A. Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole, sol-gel, and gold nanoparticles hybrid nanocomposite modified pencil graphite electrode. Biosens. Bioelectron. 2014, 60, 77–83. [Google Scholar] [CrossRef]
- Komarova, E.; Aldissi, M.; Bogomolova, A. Design of molecularly imprinted conducting polymer protein-sensing films via substrate–dopant binding. Anal. 2015, 140, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Saumya, V.; Prathish, K.P.; Rao, T.P. In situ copper oxide modified molecularly imprinted polypyrrole film based voltammetric sensor for selective recognition of tyrosine. Talanta 2011, 85, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Hu, J.; Duan, P.; Qin, C.; Piao, Y. Ultrasensitive and highly reusable electrochemical sensor with ion imprinted nanobiochar. Sens. Actuators B Chem. 2022, 371, 132490. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Su, Z.-L.; Zhang, Z.-X.; Lin, C.-Y.; Huang, Y.-S.; Yang, C.-H.; Lin, H.-Y. Doping of transition metal dichalcogenides in molecularly imprinted conductive polymers for the ultrasensitive determination of 17β-estradiol in eel serum. Biosens. Bioelectron. 2020, 150, 111901. [Google Scholar] [CrossRef]
- Pandey, I.; Kant, R. Electrochemical impedance based chiral analysis of anti-ascorbutic drug: L-Ascorbic acid and d -ascorbic acid using C-dots decorated conductive polymer nano-composite electrode. Biosens. Bioelectron. 2016, 77, 715–724. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-H.; Thomas, J.L.; Chen, C.-Y.; Chen, C.-Y.; Yang, C.-H.; Lin, H.-Y. Doping of MXenes enhances the electrochemical response of peptide-imprinted conductive polymers for the recognition of C-Reactive protein. Biosens. Bioelectron. 2021, 200, 113930. [Google Scholar] [CrossRef]
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highly-sensitive cardiac troponin T detection. Sens. Actuators B Chem. 2020, 308, 127630. [Google Scholar] [CrossRef]
- Yarkaeva, Y.; Maistrenko, V.; Dymova, D.; Zagitova, L.; Nazyrov, M. Polyaniline and poly(2-methoxyaniline) based molecular imprinted polymer sensors for amoxicillin voltammetric determination. Electrochim. Acta 2022, 433, 141222. [Google Scholar] [CrossRef]
- Truta, L.A.A.N.A.; Sales, M.G.F. Carcinoembryonic antigen imprinting by electropolymerization on a common conductive glass support and its determination in serum samples. Sens. Actuators B Chem. 2019, 287, 53–63. [Google Scholar] [CrossRef]
- Zhang, R.-R.; Gan, X.-T.; Xu, J.-J.; Pan, Q.-F.; Liu, H.; Sun, A.-L.; Shi, X.-Z.; Zhang, Z.-M. Ultrasensitive electrochemiluminescence sensor based on perovskite quantum dots coated with molecularly imprinted polymer for prometryn determination. Food Chem. 2022, 370, 131353. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Fan, L.; Dai, Y.; Zhong, M.; Lu, X.; Kan, X. Electrochemical sensor for paracetamol recognition and detection based on catalytic and imprinted composite film. Biosens. Bioelectron. 2015, 71, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Abera, B.D.; Ortiz-Gómez, I.; Shkodra, B.; Romero, F.J.; Cantarella, G.; Petti, L.; Salinas-Castillo, A.; Lugli, P.; Rivadeneyra, A. Laser-Induced Graphene Electrodes Modified with a Molecularly Imprinted Polymer for Detection of Tetracycline in Milk and Meat. Sensors 2022, 22, 269. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Hua, Q.; Wang, X.; Luan, F.; Wang, L.; Li, Y.; Zhuang, X.; Tian, C. A novel electrochemiluminescence sensor based on a molecular imprinting technique and UCNPs@ZIF-8 nanocomposites for sensitive determination of imidacloprid. Analyst 2022, 147, 3917–3923. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, S.; Liang, Y.; Wang, X.; Zhuang, X.; Tian, C.; Luan, F.; Chen, L. Selective detection of enrofloxacin in biological and environmental samples using a molecularly imprinted electrochemiluminescence sensor based on functionalized copper nanoclusters. Talanta 2022, 236, 122835. [Google Scholar] [CrossRef]
- Xie, C.; Li, H.; Li, S.; Wu, J.; Zhang, Z. Surface Molecular Self-Assembly for Organophosphate Pesticide Imprinting in Electropolymerized Poly(p-aminothiophenol) Membranes on a Gold Nanoparticle Modified Glassy Carbon Electrode. Anal. Chem. 2010, 82, 241–249. [Google Scholar] [CrossRef]
- Lee, M.-H.; Thomas, J.L.; Su, Z.-L.; Yeh, W.-K.; Monzel, A.S.; Bolognin, S.; Schwamborn, J.C.; Yang, C.-H.; Lin, H.-Y. Transition metal dichalcogenides to optimize the performance of peptide-imprinted conductive polymers as electrochemical sensors. Microchim. Acta 2021, 188, 203. [Google Scholar] [CrossRef]
- Moreira, L.F.P.P.; Buffon, E.; de Sá, A.C.; Stradiotto, N.R. Fructose determination in fruit juices using an electrosynthesized molecularly imprinted polymer on reduced graphene oxide modified electrode. Food Chem. 2021, 352, 129430. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Xu, L. An electrochemical adenine sensor employing enhanced three-dimensional conductivity and molecularly imprinted sites of Au NPs bridged poly(3-thiophene acetic acid). Sens. Actuators B Chem. 2018, 255, 2952–2958. [Google Scholar] [CrossRef]
- Liu, F.; Kan, X. Conductive imprinted electrochemical sensor for epinephrine sensitive detection and double recognition. J. Electroanal. Chem. 2019, 836, 182–189. [Google Scholar] [CrossRef]
- Lach, P.; Cieplak, M.; Noworyta, K.R.; Pieta, P.; Lisowski, W.; Kalecki, J.; Chitta, R.; D’souza, F.; Kutner, W.; Sharma, P.S. Self-reporting molecularly imprinted polymer with the covalently immobilized ferrocene redox probe for selective electrochemical sensing of p-synephrine. Sens. Actuators B Chem. 2021, 344, 130276. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Yang, J.; Yu, Y.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L. MXene/carbon nanohorns decorated with conductive molecularly imprinted poly(hydroxymethyl-3,4-ethylenedioxythiophene) for voltammetric detection of adrenaline. Microchim. Acta 2021, 188, 420. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Lin, C.-C.; Kutner, W.; Thomas, J.L.; Lin, C.-Y.; Iskierko, Z.; Ku, Y.-S.; Lin, C.-Y.; Borowicz, P.; Sharma, P.S.; et al. Peptide-imprinted conductive polymer on continuous monolayer molybdenum disulfide transferred electrodes for electrochemical sensing of Matrix Metalloproteinase-1 in lung cancer culture medium. Biosens. Bioelectron. X 2023, 13, 100258. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Waterhouse, G.I.N.; Wang, M.; Qiao, X.; Xu, Z. Novel three-dimensional electrochemical sensor with dual signal amplification based on MoS2 nanosheets and high-conductive NH2-MWCNT@COF for sulfamerazine determination. Sens. Actuators B Chem. 2019, 281, 107–114. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Sleegers, N.; Florea, A.; Cowen, T.; Moretto, L.M.; Piletsky, S.; De Wael, K. Conductive imprinted polymers for the direct electrochemical detection of Β-lactam antibiotics: The case of cefquinome. Sens. Actuators B Chem. 2019, 297, 126786. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Ming, H.; Ai, S. Sensing of glycoprotein via a biomimetic sensor based on molecularly imprinted polymers and graphene–Au nanoparticles. Anal. 2013, 138, 1219–1225. [Google Scholar] [CrossRef]
- Martins, G.V.; Marques, A.C.; Fortunato, E.; Sales, M.G.F. Paper-based (bio)sensor for label-free detection of 3-nitrotyrosine in human urine samples using molecular imprinted polymer. Sens. Bio-Sensing Res. 2020, 28, 100333. [Google Scholar] [CrossRef]
- Blinova, N.V.; Stejskal, J.; Trchová, M.; Prokeš, J.; Omastová, M. Polyaniline and polypyrrole: A comparative study of the preparation. Eur. Polym. J. 2007, 43, 2331–2341. [Google Scholar] [CrossRef]
- Boeva, Z.A.; Sergeyev, V.G. Polyaniline: Synthesis, properties, and application. Polym. Sci. Ser. C 2014, 56, 144–153. [Google Scholar] [CrossRef]
- Chen, Z.; Wright, C.; Dincel, O.; Chi, T.-Y.; Kameoka, J. A Low-Cost Paper Glucose Sensor with Molecularly Imprinted Polyaniline Electrode. Sensors 2020, 20, 1098. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Lakshmi, G.B.V.S.; Fernandes, M.; Sarkar, T.; Gulati, P.; Singh, R.P.; Solanki, P.R. A simple detection platform based on molecularly imprinted polymer for AFB1 and FuB1 mycotoxins. Microchem. J. 2021, 171, 106730. [Google Scholar] [CrossRef]
- Liu, K.-H.; O’hare, D.; Thomas, J.L.; Guo, H.-Z.; Yang, C.-H.; Lee, M.-H. Self-assembly Synthesis of Molecularly Imprinted Polymers for the Ultrasensitive Electrochemical Determination of Testosterone. Biosensors 2020, 10, 16. [Google Scholar] [CrossRef]
- Mohseni, E.; Yaftian, M.R.; Shayani-Jam, H.; Zamani, A.; Piri, F. Molecularly imprinted poly (4,4′-methylenedianiline) as electrochemical sensor for determination of 1-benzothiophene. Synth. Met. 2020, 259, 116252. [Google Scholar] [CrossRef]
- Chuang, S.-W.; Rick, J.; Chou, T.-C. Electrochemical characterisation of a conductive polymer molecularly imprinted with an Amadori compound. Biosens. Bioelectron. 2009, 24, 3170–3173. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M. Fabrication of chemiresistive nanosensor using molecularly imprinted polymers for acetone detection in gaseous state. Iran. Polym. J. 2022, 31, 883–891. [Google Scholar] [CrossRef]
- Jahangiri-Manesh, A.; Mousazadeh, M.; Nikkhah, M.; Abbasian, S.; Moshaii, A.; Masroor, M.J.; Norouzi, P. Molecularly imprinted polymer-based chemiresistive sensor for detection of nonanal as a cancer related biomarker. Microchem. J. 2022, 173, 106988. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rezaloo, F. Toluene chemiresistor sensor based on nano-porous toluene-imprinted polymer. Int. J. Environ. Anal. Chem. 2013, 93, 919–934. [Google Scholar] [CrossRef]
- Alizadeh, T.; Rezaloo, F. A new chemiresistor sensor based on a blend of carbon nanotube, nano-sized molecularly imprinted polymer and poly methyl methacrylate for the selective and sensitive determination of ethanol vapor. Sens. Actuators B Chem. 2013, 176, 28–37. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamedsoltani, L. Graphene/graphite/molecularly imprinted polymer nanocomposite as the highly selective gas sensor for nitrobenzene vapor recognition. J. Environ. Chem. Eng. 2014, 2, 1514–1526. [Google Scholar] [CrossRef]
- Janfaza, S.; Banan Nojavani, M.; Nikkhah, M.; Alizadeh, T.; Esfandiar, A.; Ganjali, M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Microchim. Acta 2019, 186, 137. [Google Scholar] [CrossRef] [PubMed]
- Halim, F.A.N.; Musa, N.H.; Zakaria, Z.; Kamarudin, S.F.; Noor, A.Y.M.; Derman, M.N.; Shakaff, A.Y.M. Highly Sensitive Reduce Graphene Oxide—Molecular Imprinted Polymer Organic Thin Film Transistor for Serine Detection; IEEE: Piscataway, NJ, USA, 2016; ISBN 9781509030286. [Google Scholar]
- Halim, N.F.A.; Musa, N.H.; Zakaria, Z.; Von Schleusingen, M.; Ahmad, M.N.; Derman, N.; Shakaff, A.Y.M. Reduced graphene oxide/molecular imprinted polymer-organic thin film transistor for amino acid detection. In Proceedings of the 11th Asian Conference on Chemical Sensors: (ACCS2015), Penang, Malaysia, 16–18 November 2015; American Institute of Physics Inc.: College Park, MD, USA, 2015; Volume 1808. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, Y.; Kuang, X.; Ma, H.; Wei, Q. Directly assembled electrochemical sensor by combining self-supported CoN nanoarray platform grown on carbon cloth with molecularly imprinted polymers for the detection of Tylosin. J. Hazard. Mater. 2020, 398, 122778. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, W.; Zhao, X.; Huang, Y.; Kang, J.; Qi, Q.; Zhong, C. Electrochemical sensors based on molecularly imprinted polymers on Fe3O4/graphene modified by gold nanoparticles for highly selective and sensitive detection of trace ractopamine in water. Analyst 2018, 143, 5094–5102. [Google Scholar] [CrossRef]
- Beigmoradi, F.; Rohani Moghadam, M.; Bazmandegan-Shamili, A.; Masoodi, H.R. Electrochemical sensor based on molecularly imprinted polymer coating on metal–organic frameworks for the selective and sensitive determination of carbendazim. Microchem. J. 2022, 179, 107633. [Google Scholar] [CrossRef]
- Ge, L.; Chen, B.; Kawano, H.; Sassa, F.; Hayashi, K. Inkjet-printed Gas Sensor Matrix with Molecularly Imprinted Gas Selective Materials. In Proceedings of the IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; IEEE Sensors: Montreal, QC, Canada, 2019; Volume 2019, pp. 1–3. [Google Scholar] [CrossRef]
- Shinohara, S.; Sassa, F.; Hayashi, K. Gas Selective Chemiresistor Composed of Molecularly Imprinted Polymer Composit Ink. In Proceedings of the 2016 IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017. [Google Scholar]
- Prasad, B.B.; Prasad, A.; Tiwari, M.P. Multiwalled carbon nanotubes-ceramic electrode modified with substrate-selective imprinted polymer for ultra-trace detection of bovine serum albumin. Biosens. Bioelectron. 2013, 39, 236–243. [Google Scholar] [CrossRef]
- Wardani, N.I.; Kangkamano, T.; Wannapob, R.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Electrochemical sensor based on molecularly imprinted polymer cryogel and multiwalled carbon nanotubes for direct insulin detection. Talanta 2023, 254, 124137. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, Y.; Zheng, R.; Wang, P.; Zhao, Z.; An, J. Highly sensitive and selective surface molecularly imprinted polymer electrochemical sensor prepared by Au and MXene modified glassy carbon electrode for efficient detection of tetrabromobisphenol A in water. Adv. Compos. Hybrid Mater. 2022, 5, 3104–3116. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Ding, Z.; Li, G.; Liu, J.; Zuberi, Z.; He, Q. Rapid recognition and determination of tryptophan by carbon nanotubes and molecularly imprinted polymer-modified glassy carbon electrode. Bioelectrochemistry 2020, 131, 107393. [Google Scholar] [CrossRef]
- Antwi-Boampong, S.; Mani, K.S.; Carlan, J.; BelBruno, J.J. A selective molecularly imprinted polymer-carbon nanotube sensor for cotinine sensing. J. Mol. Recognit. 2013, 27, 57–63. [Google Scholar] [CrossRef]
- Han, B.; Li, W.; Shen, Y.; Li, R.; Wang, M.; Zhuang, Z.; Zhou, Y.; Jing, T. Improving the sensitivity and selectivity of sulfonamides electrochemical detection with double-system imprinted polymers. Sci. Total. Environ. 2023, 864, 161173. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P. Terpene Sensor Array with Bridge-Type Resistors by CMOS Technology. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing, Bangalore, India, 16 July 2015; Volume 87. [Google Scholar]
- Völkle, J.; Kumpf, K.; Feldner, A.; Lieberzeit, P.; Fruhmann, P. Development of conductive molecularly imprinted polymers (cMIPs) for limonene to improve and interconnect QCM and chemiresistor sensing. Sens. Actuators B Chem. 2022, 356, 131293. [Google Scholar] [CrossRef]
- Koudehi, M.F.; Pourmortazavi, S.M. Polyvinyl Alcohol/Polypyrrole/Molecularly Imprinted Polymer Nanocomposite as Highly Selective Chemiresistor Sensor for 2,4-DNT Vapor Recognition. Electroanalysis 2018, 30, 2302–2310. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Xu, Y.; Pan, H.; Guo, K.; Zhang, Y.; Chen, Y.; Liu, D.; Zhang, Y.; Yao, C.; et al. A novel molecularly imprinted polymer composite based on polyaniline nanoparticles as sensitive sensors for parathion detection in the field. Food Control 2022, 133, 108638. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Fathy, M.A. A novel screen-printed potentiometric electrode with carbon nanotubes/polyaniline transducer and molecularly imprinted polymer for the determination of nalbuphine in pharmaceuticals and biological fluids. Anal. Chim. Acta 2022, 1227, 340239. [Google Scholar] [CrossRef]
- Harvey, D. Modren Analytical Chemistry; McGraw-Hill Higher Education: New York, NY, USA, 2000; Volume 816. [Google Scholar]
- Antuña-Jiménez, D.; Díaz-Díaz, G.; Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Molecularly Imprinted Electrochemical Sensors: Past Present and Future. In Molecularly Imprinted Sensors; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–34. ISBN 9780444563316. [Google Scholar]
- Hua, Y.; Ahmadi, Y.; Kim, K.-H. Molecularly imprinted polymers for sensing gaseous volatile organic compounds: Opportunities and challenges. Environ. Pollut. 2022, 311, 119931. [Google Scholar] [CrossRef]
- Bräuer, B.; Unger, C.; Werner, M.; Lieberzeit, P.A. Biomimetic Sensors to Detect Bioanalytes in Real-Life Samples Using Molecularly Imprinted Polymers: A Review. Sensors 2021, 21, 5550. [Google Scholar] [CrossRef]
- De Carvalho Silva, J.; Rodrigues, J.J.P.C.; Alberti, A.M.; Solic, P.; Aquino, A.L.L. LoRaWAN—A Low Power WAN Protocol for Internet of Things: A Review and Opportunities. In Proceedings of the 2017 2nd International Multidisciplinary Conference on Computer and Energy Science (SpliTech), Split, Croatia, 12–14 July 2017; Volume 2017, pp. 3–8. [Google Scholar]

| Monomer | Analyte | Transducer | LOD [mol/L] | Ref. |
|---|---|---|---|---|
| Pyrrole | Doxycycline | DPV | 44 × 10−6 | [32] |
| Pyrrole | Sulfadimethoxine | Amperometric | 0.5 × 10−3 | [33] |
| Pyrrole | Sulfadimethoxine | Amperometric | 70 × 10−6 | [34] |
| Pyrrole | L-tryptophan | DPV | 17 × 10−6 | [35] |
| Pyrrole | Caffeine | Pulsed potential | 10 × 10−6 | [36] |
| Pyrrole | HSA | DPV EIS | 0.25 × 10−9 12.1 × 10−6 | [37] |
| Pyrrole | Glycoprotein (gp51) (bovine leukemia virus) | Pulsed amperometry | ~20 × 10−6 B | [38] |
| Pyrrole | SARS-CoV-2 spike glycoprotein | Pulsed amperometry | 12 × 10−9 B | [39] |
| Pyrrole | Hazelnut Cor a 14-allergen | SWV SPR | ~1.6 × 10−15 ~1.2 × 10−15 | [40] |
| Pyrrole | Bacteria | QCM | 1 × 10 9 CFU/mL A | [41] |
| Pyrrole | Yeast | Thermal resistance | 10 1.25 ± 0.09 CFU/mL | [42] |
| Pyrrole, pyrrole-3-carboxylic acid | Formaldehyde | Resistive and optical fiber | ~7 ppm (res.) 4.25 ppm (opt.) B | [43,44] |
| Pyrrole, pyrrole-3-carboxylic acid | Cardiac troponin T | DPV | 0.25 × 10−12 | [45] |
| Pyrrole, pyrrole-3-carboxylic acid | Theophylline | QCM | -- | [46] |
| OTPylFc, pyrrole | Dopamine | DPV | 1.7 × 10−6 D | [47] |
| Aminophenylboronic acid | Lysozyme/cytochrome c | CV | 7 × 10−9 (lys.) B | [48] |
| Aniline | BSA | DPV | 0.59 × 10−6 | [53] |
| Aniline, metanilic acid | Cortisol, progesterone, testosterone, 17β-estradiol | CV | C: 5.52 × 10−18 T: 34.67 × 10−18 P: 7.95 × 10−18 E: 33.03 × 10−18 | [54] |
| Aniline, polyvinylsulphonic acid | p-nitrophenol | DPV | 1 × 10−6 | [55] |
| Aniline, acrylic acid | Melamine | DPV | 17.2 × 10−3 | [56] |
| 3-acetic acid thiophene, EDOT | Atrazine | CV | 1.0 × 10−9 | [57] |
| Poly(hydroxymethyl 3,4-ethylenedioxythiophene) | α-synuclein | CV | 6.5 × 10−15 | [58] |
| 2,2′-bithiophene-5-carboxylic acid | p-synephrine | DPV EIS | 12.2 × 10−9 5.7 × 10−9 | [59] |
| 2,20-bithiophene-5-carboxylic acid and p-bis(2,20-bithien-5-yl)-methylbenzo-18-crown-6 | Tyramine | DPV | 159 × 10−6 | [60] |
| 3-aminothiophene, ATh-γ-PGA | Lysozyme | DPV | 0.1 × 10−9 B | [61] |
| 4-bis(2,2′-bithien-5-yl)methyl-benzoic acid glycol ester | Oxytocin nonapeptide | EIS | 60 × 10−6 | [62] |
| p-phenylenediamine | Methyl paraben | DPV | 10 × 10−6 | [63] |
| m-phenylenediamine | Erythromycin | DPV | 0.1 × 10−9 | [64] |
| m-phenylenediamine | Sulfamethizole | SAW/DPV | 0.9 × 10−9 (DPV) | [65] |
| Triphenylamine rhodanine- 3-acetic acid | Metalloproteinase-1 (MMP-1) | EG-FET | 20 × 10−9 (epitope 1) 60 × 10−9 (epitope 2) | [66] |
| Toluidine blue | Prostate specific antigen (PSA) | DPV | 29.4 × 10−15 C | [67] |
| Bismarck Brown Y | Uric acid | EIS/ECS | 0.160 × 10−6 | [68] |
| Monomer | Additive | Analyte | Transducer | LOD [mol/L] | Ref. |
|---|---|---|---|---|---|
| Pyrrole | CS2-functionalized GO | Cadmium | DPV | 2 × 10−9 | [77] |
| Pyrrole | Dopamine@graphene | Olaquindox | DPV | 7.5 × 10−9 | [78] |
| Pyrrole | MWCNT/GAs | Dopamine | DPV | 1.67 × 10−9 | [79] |
| Pyrrole | MXene/NH2-CNTs | Fisetin | DPV | 1.0 × 10−9 | [81] |
| Pyrrole | Prussian-Blue-porous carbon-CNT hybrids | Cysteine | DPV | 6 × 10−15 | [85] |
| Pyrrole | Au-NPs | Caffeine | DPV | 0.9 × 10−9 | [86] |
| Pyrrole | Coomassie BB | Ricin (chain A) | EIS | 3.13 × 10−12 | [87] |
| Pyrrole | Copper oxide | Tyrosine | DPV | 4.0 × 10−9 | [88] |
| Cysteine | Biochar | Pb2+, Cd2+ | Differential pulse anodic stripping voltammetry | 5.86 × 10−15 (Pb2+) 0.883 × 10−18 (Cd2+) | [89] |
| Aniline | Copper nanoparticles | Nitrate | LSV, EIS | 31 × 10−6 (EIS) 5 × 10−6 (LSV) | [83] |
| Aniline, metanilic acid | WS2 | 17β estradiol | CV | 0.2 × 10−18 | [90] |
| Aniline | C-dots | L-ascorbic acid, D-ascorbic acid | DPV | 0.00016 × 10−9 (D) 0.00073 × 10−9 (L) | [91] |
| Aniline, m-aminobenzenesulfonic acid | MXene (e.g., Ti2C) | C-reactive protein | CV | 1.67 × 10−21 | [92] |
| Aniline | PMB/MWCNTs | Cardiac troponin | DPV | 1.7 × 10−15 | [93] |
| Aniline or 2-methoxyaniline | GO | Amoxicillin | SWV | 2.6 × 10−6 6.1 × 10−7 | [94] |
| Aminophenol | Carbon ink | Carcinoembryonic antigen | EIS | 16.7 × 10−12 | [95] |
| Aminophenol | Perovskite quantum dots | Prometryn | Electroluminescence | 0.2 × 10−6 0.010 µg/kg (fish) | [96] |
| o-phenylenediamine | poly(p-aminobenzene sulfonic acid) | Paracetamol | DPV | 4.3 × 10−8 | [97] |
| o-phenylenediamine | Au-NPs | Tetracycline | DPV | 0.32 × 10−9 | [98] |
| o-phenylenediamine | UCNPs@ZIF-8 | Imidacloprid | Electroluminescence | 39.1 × 10−15 | [99] |
| o-phenylenediamine | MPA-Cu NCs | Enrofloxacin | Electroluminescence | 27 × 10−12 | [100] |
| p-ATP | Au-NPs | Aspirin | DPV | 0.3 × 10−9 | [82] |
| p-ATP | N,S co-doped GQDs, Au-NPs | Sofosbuvir | DPV | 0.36 × 10−9 | [84] |
| p-ATP | Au-NPs | Chlorpyrifos | CV | 0.33 × 10−6 | [101] |
| Aniline, m-aminobenzenesulfonic acid | WS2 | α-synuclein | CV | 0.04 × 10−15 | [102] |
| Phenylboronic acid | RGO | Fructose | DPV | 3.2 × 10−15 | [103] |
| 3-thiopheneacetic acid | Au-NPs | Adenine | DPV | 0.99 × 10−9 | [104] |
| 3-thiopheneboronic acid | Au NPs | Epinephrine | DPV | 76 × 10−9 | [105] |
| 2,2′-bithio-phene-5-carboxylic acid | bis-(2,2′-bithienyl)-4-ferrocenylphenyl methane | p-synephrine | DPV | 0.57 × 10−9 | [106] |
| Hydroxymethyl-3,4-ethylenedioxythiophene | MXene/carbon nanohorn | Adrenaline | DPV | 0.3 × 10−9 | [107] |
| Triphenylamine rhodanine-3-acetic acid, EDOT | MoS2 | Matrix metalloproteinase-1 | CV | 18.52 × 10−18 | [108] |
| para-aminobenzoic acid | MoS2/NH2-MWCNT@COF | Sulfamerazine | DPV | 0.11 × 10−6 | [109] |
| 4-aminobenzoic acid | MWCNTs | Cefquinome | SWV | 50 × 10−9 A | [110] |
| o-phenylenediamine, 3-aminophenylboronic acid monohydrate | graphene-Au NPs | BSA | Electrochem. oxidation of grafted 6-ferrocenyl-hexanthiol | 0.1 × 10−12 | [111] |
| Phenol | carbon ink | 3-nitrotyrosine | DPV | 22.3 × 10−9 | [112] |
| Phenol | CNTs | Human ferritin, human papillomavirus derived E7 protein | DPV | ~0.21 × 10−18 (hFtn) <0.91 × 10−18 (E7) | [80] |
| Monomer | Analyte | Transducer | LOD [mol/L] | Ref. |
|---|---|---|---|---|
| Aniline | Glucose | Resistive | 1.0048 × 10−3 | [115] |
| Aniline | Aflatoxin B1 Fumonisin B1 | DPV | 1.00 × 10−12 (AFB1) 44.61 × 10−12 (FuB1) | [116] |
| Aniline, metanilic acid | Testosterone | CV | ~3 × 10−6 | [117] |
| 4,4′-methylenedianiline | 1-benzothiophene | CV | 67.06 × 10−6 | [118] |
| 3-aminophenylboronic acid | N-(1-desoxy-ß-D-fructopyranose-1-yl)-L-valine | OCP | 10 × 10−3 A | [119] |
| Monomer | Additive | Analyte | Transducer | LOD [ppm] | Ref. |
|---|---|---|---|---|---|
| MAA | AuNPs | Acetone | Resistive | 66 | [120] |
| MAA | AuNPs | Nonanal | Resistive | 4.5 | [121] |
| MAA | Carbon black | Toluene | Resistive | 0.8 | [122] |
| MAA | MWCNTs | Ethanol | Resistive | 0.5 | [123] |
| MAA, vinyl benzene | Graphene | Nitrobenzene | Resistive | 0.2 | [124] |
| MAA | MWCNTs | Hexanal | Resistive | 10 | [125] |
| Polyacrylic acid | Carbon black | Acid gases A | Resistive | -- | [131] |
| Polyacrylic acid | Carbon black | Hexanoic acid | Resistive | 100 B | [132] |
| Monomer | Additive | Analyte | Transducer | LOD [mol/L] | Ref. |
| MAA | GO | L-serine | Thin film transistor | 0.19 × 10−3 | [126,127] |
| MAA | CoN nanowires | Tylosin | DPV | 5.5 × 10−12 | [128] |
| MAA | Au@Fe3O4@RGO-MIPs | Ractopamine | DPV | 0.02 × 10−9 | [129] |
| MAA | Cu-MOF | Carbendazim | DPV | 2 × 10−9 | [130] |
| Tetraethylene Glycol 3-morpholin propionate acrylate | MWCNTs | BSA | DPV | 0.36 × 10−9 | [133] |
| Acrylamide (AA) | MWCNTs | Insulin | SWV | 33 × 10−15 | [134] |
| 4-vinyl pyridine | MXene, AuNPs | Tetrabromobisphenol A (TBBPA) | DPV | 14.4 × 10−12 | [135] |
| Chitosan | MWCNTs | Tryptophan | Second-order derivative linear sweep voltammetry | 1.0 × 10−9 | [136] |
| Polyvinylphenol | SWCNTs | Cotinine | Resistive | 0.28 × 10−6 | [137] |
| Sodium p-styrenesulfonate, dopamine | MWCNTs, AgNPs | Sulfonamides | DPV | 4 × 10−9 | [138] |
| Monomer | Cond. Polymer | Analyte | Transducer | LOD [ppm] | Ref. |
|---|---|---|---|---|---|
| MAA | PANI | Terpenes A | Resistive | ~50 B | [139] |
| Styrene | P3HT | Limonene | QCM Resistive | 50 B | [140] |
| PVA | PPy | 2,4-DNT | Resistive | 0.1 | [141] |
| Monomer | Additive | Analyte | Transducer | LOD [mol/L] | Ref. |
| AA | FUN-PANI | Parathion | DPV | 1.13 × 10−8 | [142] |
| AA | MWCNT/PANI | Nalbuphine | Potentiometric | 1.1 × 10−7 | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldner, A.; Völkle, J.; Lieberzeit, P.; Fruhmann, P. Conductive Molecularly Imprinted Polymers (cMIPs): Rising and Versatile Key Elements in Chemical Sensing. Chemosensors 2023, 11, 299. https://doi.org/10.3390/chemosensors11050299
Feldner A, Völkle J, Lieberzeit P, Fruhmann P. Conductive Molecularly Imprinted Polymers (cMIPs): Rising and Versatile Key Elements in Chemical Sensing. Chemosensors. 2023; 11(5):299. https://doi.org/10.3390/chemosensors11050299
Chicago/Turabian StyleFeldner, Adriana, Julia Völkle, Peter Lieberzeit, and Philipp Fruhmann. 2023. "Conductive Molecularly Imprinted Polymers (cMIPs): Rising and Versatile Key Elements in Chemical Sensing" Chemosensors 11, no. 5: 299. https://doi.org/10.3390/chemosensors11050299
APA StyleFeldner, A., Völkle, J., Lieberzeit, P., & Fruhmann, P. (2023). Conductive Molecularly Imprinted Polymers (cMIPs): Rising and Versatile Key Elements in Chemical Sensing. Chemosensors, 11(5), 299. https://doi.org/10.3390/chemosensors11050299