Abstract
As a volatile organic compound, toluene is extremely harmful to the environment and human health. In this work, through a simple one-step solvothermal method, Ni-doped ZnO sensitive materials (0.5, 1, and 2 at% Ni-doped ZnO) with a core-shell morphology were synthesized for the first time for toluene gas detection. The sensing test results showed that the sensor based on 1 at% Ni-doped ZnO exhibited the best toluene sensing performance. The response was up to 210 to 100 ppm toluene at 325 °C. The sensor exhibited high selectivity, fast response/recovery characteristics (2/77 s), and low detection limit (500 ppb, 3.5). Furthermore, we carried out molecular-level research on the sensitive material prepared in this experiment by various characterization methods. The SEM characterization results showed that ZnO and Ni-doped ZnO possessed the core-shell morphology, and the average grain size decreased with the increase in the Ni doping content. The UV–Vis test showed that the band gap of ZnO became smaller with the increase in the Ni doping amount. The enhanced toluene sensing performance of 1 at% Ni-doped ZnO could be ascribed to the structural sensitization and Ni doping sensitization, which are discussed in detail in the sensing mechanism section.
1. Introduction
With the development of modern industry, various kinds of poisonous and harmful gases have been produced, which will cause harm to human health and the environment. In particular, volatile organic compounds (VOCs) such as ethanol, acetone, formaldehyde, and toluene, etc., which widely exist in our surroundings. Among them, toluene as a typical aromatic compound is a kind of colorless gas with a special fragrance, and is widely used in many aspects of production and life such as raw materials in the chemical industry, the production of dyes, pharmaceuticals, pesticides, explosives, and other fine chemicals. In addition, it can be used as a food preservative in major food production factories [1]. Meanwhile, toluene is a flammable and harmful gas and can produce an anesthetic effect on the central nervous system, causing great and irreparable harm to the human body. Therefore, toluene has been identified as a highly toxic carcinogen by the International Center for Research on Cancer [2]. Due to the harmfulness of toluene, it is very necessary and urgent to develop a high performance toluene gas sensor to monitor toluene in real-time to ensure human health and environmental safety.
Gas sensors based on semiconductor metal oxides have attracted extensive attention in recent years due to their advantages of high sensitivity, easy fabrication, low cost, and small size. As the most promising sensing material, gas sensors based on semiconductor metal oxides have become a research hotspot. Up to now, many metal oxide semiconductors have been developed and used as gas sensing materials, for instance, ZnO [3,4], In2O3 [5,6,7], SnO2 [8,9,10], WO3 [11,12,13], NiO [14,15], CuO [16,17], etc. Among the n-type and p-type single semiconductor oxides, ZnO is the most widely studied gas sensing material as ZnO is an n-type semiconductor oxide with a wide band gap energy (3.37 eV) and a large excite binding energy (60 MeV) [18,19]. The physical and chemical properties of ZnO are stable, and the electrical property of ZnO is tunable with transition metal doping [20]. Currently, different morphologies of ZnO have been reported to detect different gases [21,22,23,24,25,26,27] including nanoparticles [28], one-dimensional nanowires [29], nanorods and nanofibers, two-dimensional nanosheets [30], nanoribbons and nanorings as well as three-dimensional flower-like structures [31], core-shell structures, and hollow spheres. Studies have shown that the hierarchical structure of ZnO enables better gas sensing properties due to its larger specific surface area, porosity, and permeability.
In addition to constructing the hierarchical structure of ZnO, it is also an effective way to improve the gas sensitive performance by doping. There are many types of doping substances including metal oxides, precious metals, transition metals, carbon-based materials, and polymers [32,33,34,35,36]. Since transition metal ions can be doped into the semiconductor lattice, the lattice distortion of sensitive materials generates a large number of defects and vacancies. The increase in the defect concentration on the surface can improve the adsorption of oxygen anions on the surface of the material, thereby improving the gas-sensing response value of the sensing material. Thus far, many studies have reported that through transition metal doping, improved gas sensing performance can be achieved. Transition metal doping elements including Co, Sn, Fe, and Ni [37,38,39,40] etc. have been reported to tune the sensing performance of ZnO. The catalytic properties, surface properties, and charge carriers of pristine semiconductor materials can be improved by doping transition metal elements. Guo [41] et al. successfully synthesized Fe-doped ZnO/rGO nanocomposites via a simple hydrothermal process. The sensor exhibited a response of 12.7 to 5 ppm formaldehyde at 120 °C. Compared with pure ZnO, the response of the doped sensing material was about 3 times higher. The reason for the enhanced sensing performance is that the doping of Fe changes the band gap, making the band gap of ZnO smaller and generating a large number of oxygen vacancies.
Based on that demonstrated above, the gas sensing performance of semiconductor metal oxides can be improved by constructing a hierarchical structure to adjust the surface area and doping transition metal elements to tune the electrical and catalytic properties [42,43,44]. To our best knowledge, fabricating a core-shell Ni doped ZnO (Ni–ZnO) to achieve an enhanced sensing performance of toluene has rarely been reported.
Here, materials with different ratios of 0, 0.5, 1, and 2 at% Ni–ZnO core-shell morphologies were successfully prepared by a simple solvothermal method and used for toluene gas detection. Gas sensing measurements showed that out of all the four gas sensors, the sensor based on 1 at% Ni–ZnO had the highest toluene response of 210 (100 ppm, 325 °C), which was almost seven times higher than pure ZnO (33.6, 375 °C). Moreover, the sensor also had high selectivity, low detection limit, and fast response/recovery properties. The sensing mechanism of the enhanced sensing properties was analyzed in detail.
2. Materials and Methods
2.1. Synthesis of the Pure ZnO and Ni–ZnO Core-Shell Spheres
First, 20 mL of deionized water and 1 mmol Ni (CH3COO)2·4H2O were added into a 50 mL beaker and stirred for 20 min. Then, 7 mL of glycerol and 30 mL of isopropanol were added into a 50 mL beaker with vigorous stirring, and 0.45 mmol Zn (CH3COO)2·2H2O was dissolved into the above mixed solution with stirring for 15 min, named as solution A. Then 0, 45, 90, and 180 μL of the above prepared Ni (CH3COO)2·4H2O solution were added into solution A, respectively, and stirring was continued for another 20 min. Then, the four solutions were placed in an oven and kept at 180 °C for 24 h. After the reaction was completed, the temperature was naturally cooled to room temperature, and the product was separated by centrifugation, alternately washed with anhydrous ethanol and deionized water, and then dried at 80 °C for 10 h. The dried product was calcined at 500 °C for 2 h (heating rate, 2 °C·min−1). Finally, pure ZnO and 0.5, 1, and 2 at% Ni–ZnO core-shell products were obtained.
2.2. Characterization
In this paper, an X-ray powder diffractometer (XRD; Bruker D8 Discover, Billerica, MA, USA) was used to investigate the composition and crystallinity of pure ZnO and different proportions of the Ni–ZnO samples. Diffraction analysis of sensitive materials was carried out using Cu Kα1 rays (λ = 1.5406 Å) in the range of 2θ from 20 to 80°, and the scanning step was 10°·min−1. The microscopic topography of sensitive materials can be analyzed and studied using scanning electron microscopy (SEM; ZEISS Sigma 500, Jena, Germany). More information about the microscopic morphology and the lattice size of sensitive materials can be obtained by transmission electron microscopy and high-resolution transmission electron microscopy. In addition, energy dispersive X-ray spectrometry (EDS) enables the detection of element mapping distributions. The chemical element analysis was obtained from the X-ray photoelectron spectroscopy (XPS; Escalab 250 XI, Waltham, MA, USA) measurements, and the surface area and pore size distribution were calculated by the Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods using the nitrogen adsorption–desorption isotherm test (Micromeritics ASAP2000 system, USA). The absorbance and band gap of the sample were measured by using a UV–Visible spectrophotometer (UV–Vis; Lambda 1050+, Waltham, MA, USA) between 250 and 800 nm.
2.3. Fabrication and Measurement Process of the Gas Sensors
As schematically shown in Figure S1a,b, first, the as-synthesized sample (3–5 mg) and an appropriate amount (0.3–0.5 mL) of deionized water were mixed to form a slurry. Then, the slurry was evenly coated on the surface of the ceramic tube (outer diameter 1.2 mm, inner diameter 0.8 mm, length 4 mm) using a small brush. After drying in air for 15 min, the ceramic tube was placed in a Muffle furnace at 400 °C for 120 min with a heating rate of 2 °C min−1 to enhance the stability during the test process. After that, a Ni–Cr alloy wire, which provides the operating temperature by tuning the constant current, is inserted in the sintered ceramic tube. Finally, the sensor was welded on a six-legged sensor base, and the gas sensing performance was tested after aging for 24 h with 100 mA. Figure S1c is schematically shown as the static gas sensing test system. A constant-current power (Gwinstek GPD-3303S, New Taipei City, Taiwan) was used to supply a constant current for the Ni–Cr alloy wire to control the operating temperature. Two glass cavities with a volume of 1 L were used to hold air and test gas, respectively. A digital multimeter (Fluke 8846a, Everett, WA, USA) and a computer was used to record and monitor the resistance of the gas sensor. During the sensing test process, the sensor was alternately placed in 1 L of fresh air in the glass cavity for a few minutes to obtain a stabilized resistance value recorded as Ra. Then, the gas sensor device was transferred to another glass cavity with 1 L, which was filled with a mixture of fresh air and target gas, the stabilized resistance value in the target gas was recorded as Rg. The sensing test was carried out in a laboratory environment (25 °C, 30 RH%). The volatile organic gas used was an analytical grade liquid, and injected to the glass cavity by a microinjector. The gas preparation process is detailed in the Supplementary Materials. The gas sensing response was defined as S = Ra/Rg, and response/recovery time was the time taken by the sensor to reach 90% of the overall resistance change.
3. Results
The XRD pattern of the pure ZnO, 0.5, 1, and 2 at% Ni–ZnO core-shell spheres are shown in Figure 1a. The diffraction peaks appeared at 34.42°, 36.25°, 47.53°, 56.60°, 62.85°, 68.17°, 69.29°, which corresponded to the (100), (002), (101), (102), (110), (103), and (112) crystal planes of the hexagonal wurtzite phase ZnO (JCPDS No. 36-1451) [45]. However, no peak matched with Ni or NiO in the Ni–ZnO samples. No other diffraction peaks corresponding to the impurities were detected. Figure 1b is the high-resolution peak of the (101) plane of ZnO compared to pure ZnO, and the diffraction peak of Ni–ZnO shifted to a higher angle direction. The shift of 2 at% Ni–ZnO sample was the most obvious, which indicated that Ni2+ was incorporated in the ZnO crystal lattice due to the radius of Ni2+ being smaller than Zn2+.
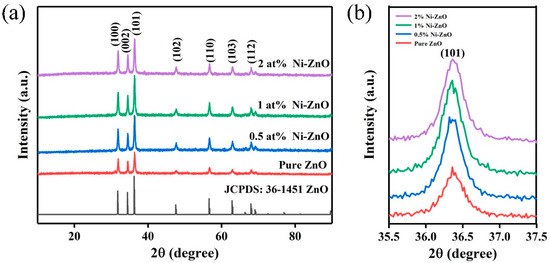
Figure 1.
(a) The XRD patterns of the pure and Ni–ZnO, (b) enlarged spectra of the XRD patterns of the Ni–ZnO.
Figure 2a–d shows the SEM images of the 0, 0.5, 1, and 2 at% Ni–ZnO sample, respectively. The pure ZnO, 0.5, and 1 at% Ni–ZnO samples showed a core-shell spherical hierarchical structure, and there was no obvious core-shell structure observed for 2 at% Ni–ZnO; the morphology was a simple spherical structure. It was found from the SEM images that with the increase in the Ni doping content, the surface of the ZnO microspheres became more and more coarse and the average particle size of the spheres decreased. The average particle size was 15.10, 9.42, 5.62, and 4.66 μm for pure ZnO, 0.5, 1 and 2 at% Ni–ZnO spheres, which suggests that the addition of Ni ions can inhibit the growth of ZnO particles in the hydrothermal reaction process. In addition, the inserted magnified SEM images showed that the core of the core-shell spheres was very large and next to the shell. Proper Ni doping, a rough surface, and loose shell are favorable for gas adsorption and diffusion, with a high gas sensing performance, which will be verified in later sections.
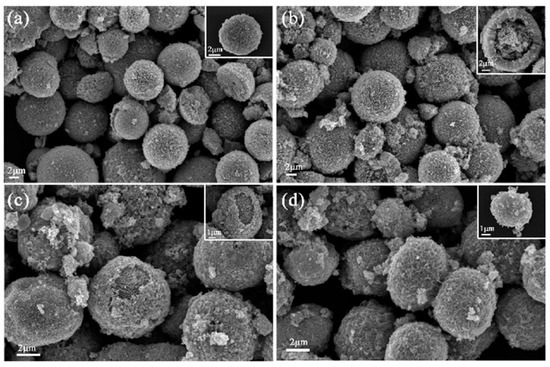
Figure 2.
The SEM images of the (a) pure (b) 0.5 at% (c) 1 at%, and (d) 2 at% Ni–ZnO.
Figure 3a,b are TEM images of different magnifications, whose shape and size are consistent with the SEM characterization results. The inset figure is the selected area electron diffraction (SAED) image and shows that the 1 at% Ni–ZnO sample had a polycrystalline structure. Figure 3c is the HRTEM images of the 1 at% Ni–ZnO sample. The lattice spacings were 0.286 and 0.249 nm, corresponding to the (100) and (101) crystal planes of ZnO [46,47]. Interplanar spacing corresponding to NiO was not found, which is consistent with the results of the XRD. From the element mapping test results in Figure 3d–g, the three elements (Ni, O, and Zn) were evenly distributed in the 1 at% Ni–ZnO sample.
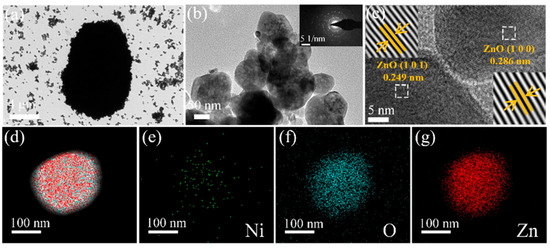
Figure 3.
The TEM image of 1 at% Ni–ZnO (a,b). (c) HRTEM images of 1 at% Ni–ZnO. (d) The overlapped elemental mapping of Ni, O, Zn in the 1 at% Ni–ZnO sample, and (e–g) elemental mapping of Ni, O, and Zn.
The specific surface area of the pure ZnO and Ni–ZnO samples was determined by the nitrogen adsorption/desorption isotherm. As illustrated in Figure 4a–e, the adsorption–desorption curve is an iv-type isotherm belonging to the H3 hysteresis loop, indicating that Ni–ZnO has a microporous structure [48].The specific surface area increased with the increase in the Ni doping amount, especially for 1 and 2 at% Ni–ZnO, the specific surface areas were 20.43 to 26.41, 37.54 and 40.14 m2·g−1 for the pure ZnO, 0.5, 1, and 2 at% Ni–ZnO. The results showed that with the proper amount of Ni doping, the specific area of ZnO could increase a lot; when the doping amount reached a certain value, the specific area did not increase too much. A large specific area could supply more surface-active sites for surface adsorbed oxygen species, thus improving the recognition function of the sensing material and contributing to the high gas sensing performance.
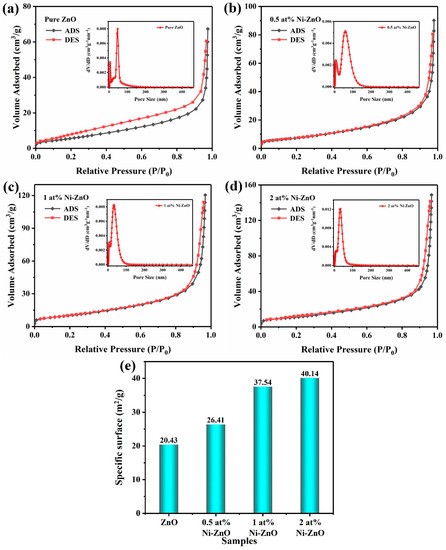
Figure 4.
The nitrogen absorption–desorption isotherms and pore size distribution curves (inset) of the (a) pure ZnO and (b) 0.5 at% (c) 1 at%, and (d) 2 at% Ni–ZnO. (e) Specific surface area of the samples.
Moreover, the inset of Figure 4 shows the nonlocal density functional theory (NLDFT) and grand canonical Monte Carlo (GCMC) pore size distribution of the Ni–ZnO, and through analysis, it was found that the pore sizes of the pure ZnO and 0.5 at%, 1 at%, and 2 at% Ni–ZnO were 46.8, 58.08, 32.72, and 32.71 nm, respectively. Appropriate Ni ion doping can increase the size of the pore size, and combined with the gas sensing data, it can be inferred that the pore size of the sensitive material in this experiment had little effect on the gas sensing performance. The increase in the specific surface area is one of the main factors affecting the gas sensing performance.
The XPS measurement was used to analyze the chemical composition and states of the elements in the pure ZnO and Ni–ZnO samples. Figure 5a exhibits the full spectrum of the ZnO and Ni–ZnO samples, where the full spectrum contained the peaks of Zn, O and Ni, proving the presence of Zn, O, and Ni in the sample. In Figure 5b, the binding energy around 1021.20 and 1044.28 eV corresponded to Zn 2p3/2 and Zn 2p1/2, respectively, proving that Zn was in the form of Zn2+ [49]. Figure 5c–e shows that the Ni 2p3/2 orbital can be separated into three peaks of about 854.1, 856.3, and 861.2 eV, corresponding to the Ni2+, Ni3+ and satellite peaks, respectively [50], indicating the existence of Ni ions in the ZnO crystals.
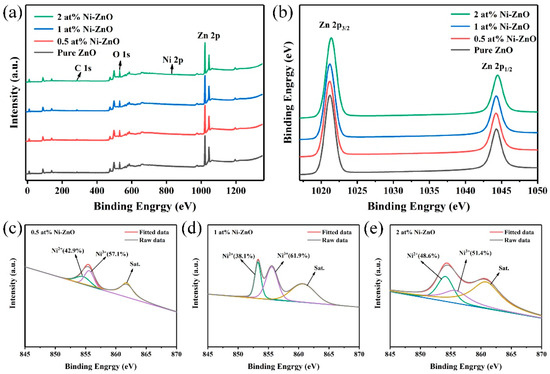
Figure 5.
The XPS spectra of the pure and Ni–ZnO for (a) the full scan, (b) Zn 2p, (c–e) Ni 2p3/2 of 0.5, 1, 2 at% Ni–ZnO.
Figure 6a–d is the XPS spectra of the O 1s of all samples, the peak shape is asymmetric, and can be allocated into three different oxygen components: chemisorbed gen (OC), oxygen vacancy (OV), and lattice oxygen (OL), which correspond to the peaks at around 532.2 ± 0.5 eV, 531.0 ± 0.5 eV, and 529.5 ± 0.5 eV [51,52], respectively. Table 1 shows the relative proportion of the three oxygen components of O 1s in all of the samples. The relative ratio of OC did not change a lot, but with Ni doping, the ratio of OL decreased, while relatively, the proportion of OV increased, and 1 at% Ni–ZnO showed the highest proportion of OV with 22.8%. Ni doping led to the formation of an oxygen vacancy in ZnO, and more OV concentration distributed to more surface-active sites, thus benefiting more surface negatively charged oxygen species.
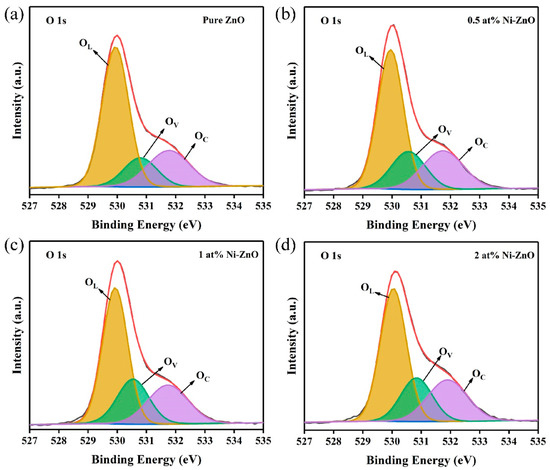
Figure 6.
The XPS spectra of O 1s for (a–d) pure, 0.5, 1, and 2 at% Ni–ZnO.

Table 1.
The relative percentages of O 1s in the XPS spectra ZnO, 0.5 at%, 1 at%, and 2 at% Ni–ZnO.
Gas Sensing Characteristics
The operating temperature can affect the carrier concentration and gas adsorption and desorption of the sensor resistance and gas response [53,54]. Response of the four sensors to 100 ppm toluene at different temperatures (Figure 7a) showed that the response values first increased and then decreased. The maximum response values of the four sensors reached at 375 °C (pure ZnO), 350 °C (0.5 at% Ni–ZnO), and 325 °C (1 and 2 at% Ni–ZnO), and the response values were 33.6, 111.4, 210.0 and 23.1, respectively. The 1 at% Ni–ZnO sample had the highest response of 210.0, which was seven times higher than the pure ZnO. With Ni doping, the operating temperature of 1 at% Ni–ZnO was significantly decreased, which could be explained by the change in the band gap, as illustrated in Figure S2. After Ni doping, the band gap reduced from 3.05 to 2.89 eV, and free electrons were easier to release from the conduction band. Figure 7b displays the responses of the four sensors to the six tested target gases (toluene, ethanol, acetone, methanol, formaldehyde, and xylene) with 100 ppm at their optimal operating temperatures (ZnO, 375 °C; 0.5 at% Ni–ZnO, 350 °C; 1 and 2 at% Ni–ZnO, 325 °C). Obviously, the four sensors had the highest response to toluene. Compared with the other tested gases, the 1 at% Ni–ZnO sensor showed the best toluene sensing performance, and the response to toluene and other gases was 210.0, 52.0, 3.4, 9.4, 3.1, and 1.5. The toluene response was 4–140 times higher than other measured gases, indicating that the sensor had excellent selectivity for toluene.
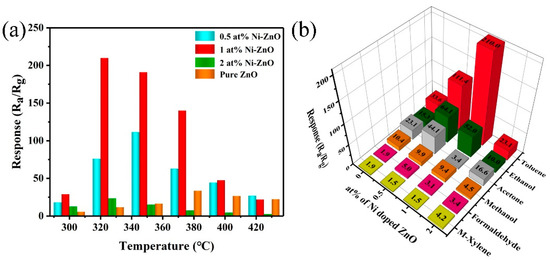
Figure 7.
(a) The response of the four gas sensors to 100 ppm toluene at different temperatures. (b) The selectivity of the four gas sensors to 100 ppm different VOC gases at their optimum operating temperature.
Figure 8a,b shows the real-time response curves of the four sensors at 375 °C (pure ZnO) and 325 °C (Ni–ZnO samples) with different concentrations of toluene. The response value increased with the increase in toluene concentration. The sensor response based on 1 at% Ni–ZnO increased significantly with increasing toluene concentration. The corresponding linear relationship between the toluene response and concentration is listed in Figure 8c,d. It can be observed that the linearity of the sensor was very good in both the low-concentration range and the high-concentration range. The response increased almost linearly with the toluene concentration, and 1 at% Ni–ZnO had the highest sensitivity. Furthermore, the sensor based on 1 at% Ni–ZnO had a low detection limit of 0.5 ppm with a response value of 3.5, which makes it promising in practical application.
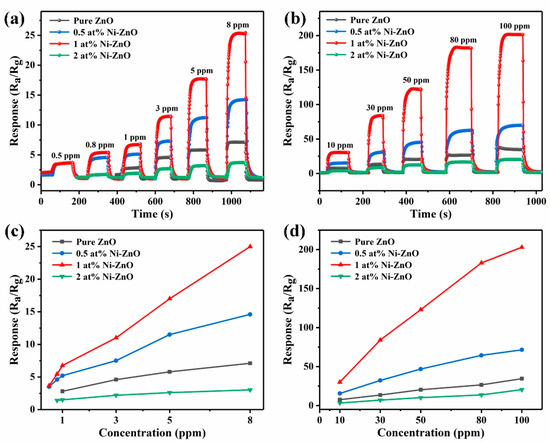
Figure 8.
(a,b) The dynamic response curves of the four sensors to toluene with different concentrations at 375 °C (pure ZnO) and 325 °C (Ni–ZnO samples). (c,d) The response of the pure, 0.5, 1, and 2 at% Ni–ZnO sensors to (0.5–100 ppm) toluene.
The response and recovery characteristics are also important parameters of gas sensors. Thus, the response and recovery transients of the sensor based on 1 at% Ni–ZnO to 100 ppm toluene at 325 °C are shown in Figure 9a. The response time and recovery time of the sensor based on 1 at% Ni–ZnO are 2 and 77 s, respectively, illustrating that the sensor had a relatively fast response and recovery property. Table 2 is a comparison of the toluene sensing performance of this work and the sensors reported in the literature [55,56,57,58,59]. The operating temperature was relatively higher than that in the literature, but the response and recovery time were fast and the toluene response was much higher than the reported literature.
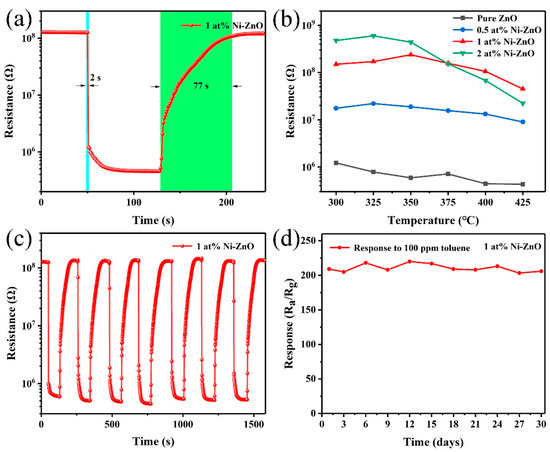
Figure 9.
(a) The response/recovery time of 1 at% Ni–ZnO gas sensor to 100 ppm toluene at 325 °C. (b) Resistance in the air of the four gas sensors at different operating temperatures. (c) Seven reversible cycles of 1 at% Ni–ZnO gas sensors. (d) The stability testing curves of 1 at% Ni–ZnO gas sensor to 100 ppm toluene in 30 days.

Table 2.
A comparison of the toluene gas sensing performance of this work and other sensing material in previously reported works.
Figure 9b is the resistance in air as a function of operating temperature. With the increase in Ni doping, the resistance of the sensor increased significantly. The resistance versus temperature plot of the sensors showed an abnormal PTCR (positive temperature coefficient of resistance) behavior during 300–350 °C. Based on the reported literature [60], this phenomenon was caused by Ni doping, which introduced defects in ZnO, thus forming oxygen vacancy-like defects in this temperature region.
Figure 9c is a seven-cycle test curve of the 1 at% Ni–ZnO sample to 100 ppm toluene at 325 °C. The resistance in air and toluene was within the allowable fluctuation range, proving that the sensor had good repeatability. The long-term stability was also studied in this work; the sensing response to 100 ppm toluene in the air for one month is shown in Figure 9d. During the test days, the response values varied and slightly decreased but did not show an obvious fluctuation. The results indicate that the 1 at% Ni–ZnO gas sensor has good long-term stability.
4. Discussion
The gas sensing mechanism can be explained by the interaction of multiple factors. In this work, toluene sensing mechanisms with exceptional response are discussed in detail. The gas sensing mechanism has been widely explored in many studies [61,62], and the extensively accepted theory is the chemical reaction between the target gas and chemically adsorbed oxygen species (O2− (T < 100 °C), O− (100 °C < T < 300 °C), and O2− (T > 300 °C)) [63,64]. When redox reactions take place on the surface of the semiconductors, charge transfer will occur during this process, which changes the resistance of the gas sensor.
As illustrated in Figure 10, when the Ni–ZnO gas sensor is exposed to air, O2 molecules will capture electrons in the conduction band of the material to form negatively charged oxygen species on the surface of the Ni–ZnO material, and a depletion layer will form on the surface at the same time, leading to a high resistance of the sensor in air. Then, when the sensor is exposed to a reducing gas (toluene) environment, the chemically adsorbed oxygen can react with the target gas molecules on the surface of the sensing material, the electron depletion layer becomes smaller. As a result, electrons that are released back into the conduction band induce lower resistance values in reducing gases. The relevant reactions are seen in Equations (1)–(3) (T > 300 °C) [65]:
O2 → O2(ads)
O2(ads) + 4e → 2O2−
C7H8 + 18O2− → 4H2O + 7CO2 + 36e−
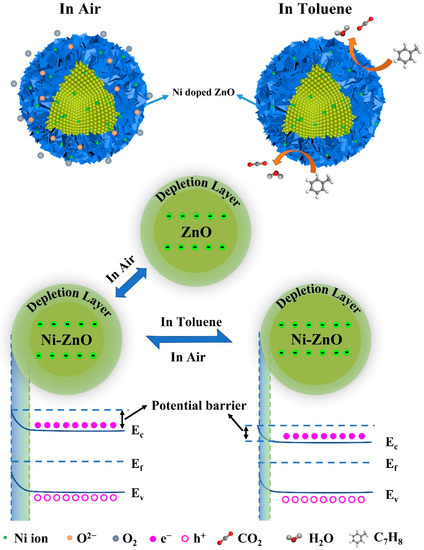
Figure 10.
A schematic diagram of the sensing mechanism.
The enhanced sensing performance of the 1 at% Ni–ZnO-based gas sensor can be attributed to the following two aspects including structural sensitization and modified sensitization. The first is structural sensitization. After Ni doping, the hierarchical microstructure of ZnO changed, and the surface of the Ni–ZnO core-shell spheres became rougher. This loose structure is very favorable for the diffusion of gas molecules, which can not only penetrate into the shell at the outer surface of the material, but may also further diffuse to the surface of the core. Therefore, the sensing material has a higher sensing performance [66]. Furthermore, the thickness of the shell of the sensing material becomes thinner with increasing Ni content. According to the literature [67,68], when the thickness of the shell layer is close to the Debye length, the gas sensing material has the best gas sensing performance. Finally, the size of the topography of the gas sensing material is also affected by Ni, resulting in a smaller morphology size. An appropriate reduction in the morphology size can lead to an increase in the specific surface area, which can adsorb more oxygen and toluene molecules and provide more active sites [69].
Modified sensitization includes the following points. First, the specific surface area increased with Ni doping, so the recognition function of the sensing material will be improved. Second, it can be seen from Table 2 that Ni doping increased the relative proportion of oxygen vacancy in ZnO. The oxygen vacancy improved from 15.6% to 22.8 for 1 at% Ni–ZnO, which means that more oxygen species will adsorb on the surface of 1 at% Ni–ZnO and the sensing performance will be enhanced. The third and most important point is that Ni2+ ions can be easily oxidized into Ni3+ with a higher oxidation state, which will facilitate the redox reaction, due to which Ni3+ usually plays the role of the catalyst during the redox process, as many studies have reported [70,71,72]. Therefore, a greater percentage of Ni3+ will obtain a good gas sensing property to a large extent. Figure 5c–e displays the fitted XPS results of Ni2+ and Ni3+ of the 0.5, 1, and 2 at% Ni–ZnO. The relative percentage of Ni2+ and Ni3+ varied in the three samples, and the ratio of Ni3+/Ni2+ was calculated to be 1.33, 1.62, and 1.05 for the 0.5, 1, and 2 at% Ni–ZnO samples. The 1 at% Ni–ZnO sample had the highest ratio of Ni3+/Ni2+ of 1.62, followed by the 0.5 at% Ni–ZnO and 2 at% Ni–ZnO. From the gas sensing tests, the highest sensing performance was obtained by the 1 at% Ni–ZnO, and then the 0.5 at% Ni–ZnO and 2 at% Ni–ZnO samples. The gas sensing results coincided with the inference of the Ni3+/Ni2+ ratio.
5. Conclusions
In summary, different Ni–ZnO core-shell spheres were successfully prepared by a one-step solvothermal method and gas sensors based on the prepared materials were prepared. The role of Ni doping in ZnO on the microstructure and gas sensing performance was studied in detail. The results showed that Ni doping changed the hierarchical microstructure of ZnO, and the BET specific area increased after Ni doping. Gas sensing measurements revealed that the operating temperature decreased due to the lower band gap after Ni doping. All of the sensing materials had the highest response to toluene, the best sensing performance was acquired by the 1 at% Ni–ZnO based gas sensor, with high response, excellent selectivity, fast response and recovery time, and relatively long-term stability. The enhanced sensing property can be mainly due to the increase in the specific surface area and relative percentage of oxygen vacancy after Ni doping, and more importantly, is the catalyst effect of Ni ions. This work provides a reasonable way to fabricate a high performance toluene sensing material.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors10080327/s1, Figure S1: The schematic figure of the (a) ceramic tube, (b) sensor device, and (c) the sensing test system. Figure S2: (a) The UV–Vis absorption spectrum and (b) energy band gap of the ZnO samples with different Ni doping amounts. Table S1: The parameter information for all of the gas samples.
Author Contributions
Conceptualization, X.Y. and Z.L.; Methodology, X.Y.; Formal analysis, Z.L.; Investigation, S.L., Z.S., Z.W., H.Z., C.S., H.L. and J.S.; Data curation, Z.L.; Writing—original draft preparation, Z.L.; Writing—review and editing, X.Y., G.P., Y.C. and L.G.; Supervision, X.Y., G.P., Y.C. and L.G.; Funding acquisition, X.Y. and G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Major National Science and Technology Special Projects (2016ZX02301003-004-007); the National Natural Science Foundation of China (62003123); the National Natural Science Foundation of Hebei Province (F2020202067); the Key Laboratory of Electronic Materials and Devices of Tianjin, China; and the National Demonstration Center for Experimental (Electronic and Communication Engineering) Education (Hebei University of Technology).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Xu, J.M.; Cheng, J.P. The advances of Co3O4 as gas sensing materials: A review. J. Alloys Compd. 2016, 686, 753–768. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Joshi, M.; Myung, J.H. Sputtered SnO2/ZnO heterostructures for improved NO2 gas sensing properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Hui, G.Z.; Zhu, M.Y.; Yang, X.L.; Liu, J.J.; Pan, G.F.; Wang, Z.Y. Highly sensitive ethanol gas sensor based on CeO2/ZnO binary heterojunction composite. Mater. Lett. 2020, 278, 128453. [Google Scholar] [CrossRef]
- Guo, L.L.; Zhang, B.; Yang, X.L.; Zhang, S.S.; Wang, Y.; Wang, G.D. Sensing platform of PdO-ZnO-In2O3 nanofibers using MOF templated catalysts for triethylamine detection. Sens. Actuators B 2021, 343, 130126. [Google Scholar] [CrossRef]
- Li, S.H.; Xie, L.L.; He, M.; Hu, X.B.; Luo, G.F.; Chen, C.; Zhu, Z. Metal-Organic frameworks-derived bamboo-like CuO/In2O3 Heterostructure for high-performance H2S gas sensor with Low operating temperature. Sens. Actuators B 2020, 310, 127828. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2 sensing properties of Au-loaded porous In2O3 gas sensors at low operating temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Yang, X.L.; Zhang, S.F.; Yu, Q.; Zhao, L.P.; Sun, P.; Wang, T.S.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Hiyoto, K.A.M.; Fisher, E.R. Utilizing plasma modified SnO2 paper gas sensors to better understand gas-surface interactions at low temperatures. J. Vac. Sci. Technol. A 2020, 38, 043202. [Google Scholar] [CrossRef]
- Zheng, L.; Bi, W.J.; Jin, Z.; Liu, S.T. Synthesis of hierarchical shell-core SnO2 microspheres and their gas sensing properties. Chin. Chem. Lett. 2020, 31, 2083–2086. [Google Scholar] [CrossRef]
- Ueda, T.; Maeda, T.; Huang, Z.D.; Higuchi, K.; Izawa, K.; Kamada, K.; Hyodo, T.; Shimizu, Y. Enhancement of methylmercaptan sensing response of WO3 semiconductor gas sensors by gas reactivity and gas diffusivity. Sens. Actuators B 2018, 273, 826–833. [Google Scholar] [CrossRef]
- Buyukkose, S. Highly selective and sensitive WO3 nanoflakes based ammonia sensor. Mater. Sci. Semicond. Process. 2020, 110, 104969. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, Y.H.; Qiu, P.P.; Duan, L.L.; Bi, W.; Chen, Y.; Guo, D.; Liu, Y.; Luo, W.; Deng, Y. Controllable synthesis of highly crystallized mesoporous TiO2/WO3 heterojunctions for acetone gas sensing. Chin. Chem. Lett. 2020, 31, 1119–1123. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Tshabalala, Z.P.; Hillie, K.T.; Swart, H.C.; Motaung, D.E. The blue luminescence of p-type NiO nanostructured material induced by defects: H2S gas sensing characteristics at a relatively low operating temperature. Appl. Surf. Sci. 2020, 525, 146002. [Google Scholar] [CrossRef]
- Simion, C.E.; Ghica, C.; Mihalcea, C.G.; Ghica, D.; Mercioniu, I.; Somacescu, S.; Florea, Q.G.; Stanoiu, A. Insights about CO gas-sensing mechanism with NiO-based gas sensors the influence of humidity. Chemosensors 2021, 9, 244. [Google Scholar] [CrossRef]
- Alev, O.; Sarica, N.; Ozdemir, O.; Arslan, L.C.; Buyukkose, S.; Oztur, Z.Z. Cu-doped ZnO nanorods based QCM sensor for hazardous gases. J. Alloys Compd. 2020, 826, 154177. [Google Scholar] [CrossRef]
- Umar, A.; Algadi, H.; Kumar, R.; Akhtar, M.S.; Ibrahim, A.A.; Albargi, H.; Alhamami, M.A.M.; Alsuwian, T.; Zeng, W. Ultrathin leaf-shaped CuO nanosheets based sensor device for enhanced hydrogen sulfide gas sensing application. Chemosensors 2021, 9, 221. [Google Scholar] [CrossRef]
- Reynolds, D.C.; Look, D.C.; Jogai, B.; Hoelscher, J.E.; Sherriff, R.E.; Harris, M.T.; Callahan, M.J. Time-resolved photoluminescence lifetime measurements of the Γ5 and Γ6 free excitons in ZnO. J. Appl. Phys. 2000, 88, 2152–2153. [Google Scholar] [CrossRef]
- Norton, D.P.; Heo, Y.W.; Ivill, M.P.; Ip, K.; Pearton, S.J.; Chisholm, M.F. ZnO: Growth, doping & processing. Mater. Today 2004, 7, 34–40. [Google Scholar]
- Modaberi, M.R.; Rooydell, R.; Brahma, S.; Akande, A.A.; Mwakikunga, B.W.; Liu, C.P. Enhanced response and selectivity of H2S sensing through controlled Ni doping into ZnO nanorods by using single metal organic precursors. Sens. Acuators B 2018, 273, 1278–1290. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liao, W.K.; Deng, X.S. Synthesis and substantially enhanced gas sensing sensitivity of homogeneously nanoscale Pd- and Au-particle decorated ZnO nanostructures. J. Alloys Compd. 2014, 599, 87–92. [Google Scholar] [CrossRef]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.Q.; Sang, S.B.; Li, P.W.; Deng, X.; Zhang, W.D.; Chen, Y.; Lian, K. Optimization of Pd content in ZnO microstructures for high-performance gas detection. J. Mater. Sci. 2015, 50, 1935–1942. [Google Scholar] [CrossRef]
- Wang, W.C.; Tian, Y.T.; Wang, X.C.; He, H.; Xu, Y.R.; He, C.; Li, H. Ethanol sensing properties of porous ZnO spheres via hydrothermal route. J. Mater. Sci. 2013, 48, 3232–3238. [Google Scholar] [CrossRef]
- Khoang, N.D.; Hong, H.S.; Trung, D.D.; van Duy, N.; Hoa, N.D.; Thinh, D.D.; Van Hieu, N. On-chip growth of wafer-scale planar-type ZnO nanorod sensors for effective detection of CO gas. Sens. Actuators B 2013, 181, 529–536. [Google Scholar] [CrossRef]
- Zhang, W.H.; Zhang, W.D.; Zhou, J.F. Solvent thermal synthesis and gas-sensing properties of Fe-doped ZnO. J. Mater. Sci. 2010, 45, 209–215. [Google Scholar] [CrossRef]
- Luo, J.; Ma, S.Y.; Li, F.M.; Li, X.B.; Li, W.Q.; Cheng, L.; Mao, Y.Z.; Gz, D.J. The mesoscopic structure of flower-like ZnO nanorods for acetone detection. Mater. Lett. 2014, 121, 137–140. [Google Scholar] [CrossRef]
- Xiong, H.M. ZnO nanoparticles applied to bioimaging and drug delivery. Adv. Mater. 2013, 25, 5329–5335. [Google Scholar] [CrossRef]
- Huang, J.R.; Xu, X.J.; Gu, C.P.; Yang, M.; Yang, M.; Liu, J.H. Large-scale synthesis of hydrated tungsten oxide 3D architectures by a simple chemical solution route and their gas-sensing properties. J. Mater. Chem. 2011, 21, 13283–13289. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, X. Three-dimensional architectures constructed using two-dimensional nanosheets. Sci. China Chem. 2015, 58, 1792–1799. [Google Scholar] [CrossRef]
- Sohila, S.; Rajendran, R.; Yaakob, Z.; Teridi, M.A.M.; Sopian, K. Photoelectrochemical water splitting performance of flower like ZnO nanostructures synthesized by a novel chemical method. J. Mater. Sci. Mater. Electron. 2016, 27, 2846–2851. [Google Scholar] [CrossRef]
- Akamatsu, T.; Itoh, T.; Tsuruta, A.; Masuda, Y. CH3SH and H2S Sensing Properties of V2O5/WO3/TiO2 Gas Sensor. Chemosensors 2021, 9, 113. [Google Scholar] [CrossRef]
- Li, Y.M.; Hu, H.J.; Zhang, W.F.; Tian, Z.Q.; Jiang, X.Q.; Wang, Y.H.; Zhang, S.; Zhang, Q.; Jian, J.; Zou, J. Theoretical Study on the Electrochemical Catalytic Activity of Au-Doped Pt Electrode for Nitrogen Monoxide. Chemosensors 2022, 10, 178. [Google Scholar] [CrossRef]
- Guo, W.W.; Jian, L.J.; Wang, X.M.; Zeng, W. Hydrothermal synthesis of Ni-doped hydrangea-like Bi2WO6 and the enhanced gas sensing property to n-butanol. Sens. Actuators B 2022, 357, 131396. [Google Scholar] [CrossRef]
- Sun, Q.H.; Wu, Z.F.; Cao, B.B.; Chen, X.; Zhang, C.C.; Shaymurat, T.; Duan, H.; Zhang, J.; Zhang, M. Gas sensing performance of biomass carbon materials promoted by nitrogen doping and p-n junction. Appl. Surf. Sci. 2022, 592, 153254. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.H.; Ma, B.S.; Shin, E.; Kim, Y.; Kim, T.S.; Kim, F.S.; Kim, I.D.; Kim, B.J. High-Performance, Flexible NO2 Chemiresistors Achieved by Design of Imine-Incorporated n-Type Conjugated Polymers. Adv. Sci. 2022, 9, 2200270. [Google Scholar] [CrossRef]
- Fan, Y.R.; Xu, Y.Y.; Wang, Y.X.; Sun, Y.Q. Fabrication and characterization of Co-doped ZnO nanodiscs for selective TEA sensor applications with high response, high selectivity and ppb-level detection limit. J. Alloys Compd. 2021, 876, 160170. [Google Scholar] [CrossRef]
- Lu, S.H.; Hu, X.F.; Zheng, H.; Qiu, J.W.; Tian, R.B.; Quan, W.J.; Min, X.; Ji, P.; Hu, Y.; Cheng, S.; et al. Highly selective, ppb-level xylene gas detection by Sn2+-doped NiO flower-like microspheres prepared by a one-step hydrothermal. Method Sens. 2019, 19, 2958. [Google Scholar] [CrossRef]
- Lee, C.S.; Li, H.Y.; Kim, B.Y.; Jo, Y.M.; Byun, H.G.; Hwang, I.S.; Abdel-Hady, F.; Wazzan, A.A.; Lee, J.-H. Discriminative detection of indoor volatile organic compounds using a sensor array based on pure and Fe-doped In2O3 nanofibers. Sens. Actuators B 2019, 285, 193–200. [Google Scholar] [CrossRef]
- Chacko, L.; Massera, E.; Aneesh, P.M. Enhancement in the selectivity and sensitivity of Ni and Pd functionalized MoS2 toxic gas sensors. J. Electrochem. Soc. 2020, 167, 106506. [Google Scholar] [CrossRef]
- Guo, W.W.; Zhao, B.Y.; Zhou, Q.L.; He, Y.Z.; Wang, Z.C.; Radacsi, N. Fe-doped ZnO/reduced graphene oxide nanocomposite with synergic enhanced gas sensing performance for the effective detection of formaldehyde. ACS Omega 2019, 4, 10252–10262. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Zhan, W.W.; He, Y.; Wang, Y.T.; Kong, X.J.; Kuang, Q.; Xie, Z.; Zheng, X. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl. Mater. Interfaces 2014, 6, 4186–4195. [Google Scholar] [CrossRef]
- Li, D.P.; Zhang, Y.; Xu, J.C.; Jin, H.X.; Jin, D.F.; Hong, B.; Peng, X.; Wang, P.; Ge, H.; Wang, X. Nanocasting synthesis and gas-sensing behavior of hematite nanowires. Phys. E Low-Dimens. Syst. Nanostruct. (Amst. Neth.) 2016, 84, 395–400. [Google Scholar] [CrossRef]
- Chen, H.D.; Jin, K.L.; Wang, P.F.; Xu, J.C.; Han, Y.B.; Jin, H.X.; Jin, D.F.; Peng, X.L.; Hong, B.; Li, J.; et al. Highly enhanced gas-sensing properties of indium-doped mesoporous hematite nanowires. J. Phys. Chem. Solids 2018, 120, 271–278. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.T.; Ren, C.; Li, B.L.; Wang, T.; Zeng, M.; Su, Y.; Hu, N.; Zhou, Z.; et al. Glucose-assisted synthesis of hierarchical NiO-ZnO heterostructure with enhanced glycol gas sensing performance. Sens. Actuators B 2021, 329, 129167. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.H.; Chen, H.D.; Lin, S.W. Low-operating-temperature NO2 sensor based on a CeO2/ZnO heterojunction. Sensors 2021, 21, 8269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Wang, S.; Zhai, X.; Shao, L.; Bai, X.J.; Liu, Y.L.; Wang, T.; Li, Y.; Zhang, L.; Fan, F.; et al. Construction of Zn/Ni bimetallic organic framework derived ZnO/NiO heterostructure with superior N-propanol sensing performance. ACS Appl. Mater. Interfaces 2021, 13, 9206–9215. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.H.; Zhao, Q.N.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.J.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B 2020, 317, 128204. [Google Scholar] [CrossRef]
- Chen, X.X.; Shen, Y.B.; Zhou, P.F.; Zhong, X.X.; Li, G.D.; Han, C.; Wei, D.; Li, S. Bimetallic Au/Pd nanoparticles decorated ZnO nanowires for NO2 detection. Sens. Actuators B 2019, 289, 160–168. [Google Scholar] [CrossRef]
- Zhou, C.G.; Meng, F.Q.; Chen, K.; Yang, X.L.; Wang, T.S.; Sun, P.; Liu, F.; Yan, X.; Shimanoe, K.; Lu, G. High sensitivity and low detection limit of acetone sensor based on NiO/Zn2SnO4 p-n heterojunction octahedrons. Sens. Actuators B 2021, 339, 129912. [Google Scholar] [CrossRef]
- Wang, C.; Cui, X.B.; Liu, J.Y.; Zhou, X.; Cheng, X.Y.; Sun, P.; Hu, X.; Li, X.; Zheng, J.; Lu, G. Design of superior ethanol gas sensor based on Al-doped NiO nanorod-flowers. ACS Sens. 2016, 1, 131–136. [Google Scholar] [CrossRef]
- Wang, S.; Huang, D.; Xu, S.S.; Jiang, W.K.; Wang, T.; Hu, J.; Hu, N.; Su, Y.; Zhang, Y.; Yang, Z. Two-dimensional NiO nanosheets with enhanced room temperature NO2 sensing performance via Al doping. Phys. Chem. Chem. Phys. 2017, 19, 19043–19049. [Google Scholar] [CrossRef]
- Wang, L.L.; Lou, Z.; Zhang, R.; Zhou, T.T.; Deng, J.N.; Zhang, T. Hybrid Co3O4/SnO2 core-shell nanospheres as real-time rapid-response sensors for ammonia gas. ACS Appl. Mater. Interfaces 2016, 8, 6539–6545. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches for the improvement of conductometric gas sensor parameters Part 1. Improvement of sensor sensitivity and selectivity (short survey). Sens. Actuators B 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.W.; Kim, S.S. Self-heating effects on the toluene sensing of Pt-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2017, 251, 781–794. [Google Scholar] [CrossRef]
- Chen, H.; Ao, S.R.; Li, G.D.; Gao, Q.; Zou, X.X.; Wei, C.D. Enhanced sensing performance to toluene and xylene by constructing NiGa2O4-NiO heterostructures. Sens. Actuators B 2019, 282, 331–338. [Google Scholar] [CrossRef]
- Nie, L.F.; Fan, G.J.; Wang, A.Q.; Zhang, L.; Guan, J.; Han, N.; Chen, Y. Finely dispersed and highly toluene sensitive NiO/NiGa2O4 heterostructures prepared from layered double hydroxides precursors. Sens. Actuators B 2021, 345, 130412. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Inada, M.; Yin, S. A facile method for preparation of uniformly decorated-spherical SnO2 by CuO nanoparticles for highly responsive toluene detection at high temperature. J. Mater. Sci. Technol. 2020, 51, 119–129. [Google Scholar] [CrossRef]
- Jamnik, D.J.S.S.I. Investigation of the PTCR effect in ZnO–NiO two-phase ceramics. Solid State Ion. 1997, 99, 125–135. [Google Scholar]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Jun, J.H.; Yun, J.; Cho, K.; Hwang, I.S.; Lee, J.H.; Kim, S. Necked ZnO nanoparticle-based NO2 sensors with high and fast response. Sens. Actuators B 2009, 140, 412–417. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Bai, J.L.; Zhao, H.; Yang, Z.Y.; Gu, X.Y.; Huang, B.Y.; Zhao, C.H.; Cairang, L.; Sun, G.Z.; Zhang, Z.X.; et al. Gas sensing enhancing mechanism via doping-induced oxygen vacancies for gas sensors based on indium tin oxide nanotubes. Sens. Actuators B 2018, 265, 273–284. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Patil, P.S.; Kim, J.H. Fabrication of nanostructured ZnO thin films based NO2 gas sensor via SILAR technique. Sens. Actuators B 2017, 239, 1185–1193. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, S.; Zhou, T.T.; Tu, J.C.; Zhang, T. Facile preparation of hierarchical structure based on p-type Co3O4 as toluene detecting sensor. Appl. Surf. Sci. 2020, 503, 144167. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Z.W.; Zhou, T.T.; Fei, T.; Wang, R.; Zhang, T. Improvement of gas sensing performance for tin dioxide sensor through construction of nanostructures. JCIS 2019, 557, 673–682. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Variation of shell thickness in ZnO-SnO2 core-shell nanowires for optimizing sensing behaviors to CO, C6H6, and C7H8 gases. Sens. Actuators B 2020, 302, 127150. [Google Scholar] [CrossRef]
- Bai, J.L.; Zhao, C.H.; Gong, H.M.; Wang, Q.; Huang, B.Y.; Sun, G.Z.; Wang, Y.; Zhou, J.; Xie, E.; Wang, F. Debye-length controlled gas sensing performances in NiO@ZnO p-n junctional core-shell nanotubes. J. Phys. D Appl. Phys. 2019, 52, 285103. [Google Scholar] [CrossRef]
- Zhou, T.T.; Zhang, T.; Zeng, Y.; Zhang, R.; Lou, Z.; Deng, J.N.; Wang, L. Structure-driven efficient NiFe2O4 materials for ultra-fast response electronic sensing platform. Sens. Actuators B 2018, 255, 1436–1444. [Google Scholar] [CrossRef]
- Kim, H.R.; Choi, K.I.; Kim, K.M.; Kim, I.D.; Cao, G.Z.; Lee, J.H. Ultra-fast responding and recovering C2H5OH sensors using SnO2 hollow spheres prepared and activated by Ni templates. Chem. Commun. 2010, 46, 5061–5063. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.Y.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.B.; Yang, J.; Sun, Y.; Lu, G. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuators B 2020, 313, 127965. [Google Scholar] [CrossRef]
- Luo, Y.B.; An, B.X.; Bai, J.L.; Wang, Y.R.; Cheng, X.; Wang, Q.; Li, J.; Yang, Y.; Wu, Z.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).