Abstract
Immunoassays are analytical tools that attract growing research attention in the field of sensors. Among the different analytical methods, the immunoassays based on optical readout have an important role due to the high sensitivity reached in past years by the instrumentation as well as by the preparation of new labels. This review aims to give an overview in term of basic concepts and practical examples of the most used optical immunoassays techniques, in order to help readers to choose the most useful techniques for their analyses. Particular emphasis is dedicated to the application of the presented immunoassays on the detection of the SARS-CoV-2 virus.
Keywords:
immunoassay; optical detection; sensor; antibody; antigen; analysis; fluorescence; electrochemiluminescence; SERS; ELISA; COVID-19 1. Introduction
Immunoassays are analytical tools used to quantify analytes of biological interest, such as bacteria [1] and proteins, by employing the specific binding between antibodies and antigens [2]. Due to the high selectivity of antibodies towards their corresponding antigens, the use of antibody–antigen recognition can be successfully performed even in complex matrixes containing other compounds. The biochemical interaction between antibodies and antigens can be observed by monitoring the variation of the properties of a specific compound (“tracer”) that interacts selectively either with the antibody or with the antigen. The tracer is generally an analyte or an antibody bearing a covalently bound label, which generates a signal upon a specific stimulus, such as light, electrical pulse, enzymatic action, and others. By recording the signal in different conditions, it is possible to generate a calibration curve, which represents the measured readout as a function of the concentration of the unlabelled analyte in the sample. Afterwards, by combining data and the calibration curve, the concentration of the investigated compound can be determined.
Additionally, the progress of different analytical methods, various immunoassays have been established depending on the nature of the signal recorded during the measurements. It is worth noting that the accuracy of the detection of the signal released by the label is critical for the different application fields in which immunoassays are used, such as disease diagnosis, food safety and environmental protection. Furthermore, even if many techniques are refined and established, significant improvement on the effectiveness of the detection process was achieved by coupling together different methods of analysis. Indeed, the high sensitivity and the low detection limit are parameters playing a pivotal role on the effectiveness of every immunoassay, together with the possibility to explore complex biological matrices (e.g., blood or urines).
Herein, we illustrate the basic principles of some of the common techniques employed in immunoassays, with particular emphasis on techniques based on the detection of optical readout generated by different stimuli, such as irradiation, chemical and electrochemical reactions. However, before describing the analytical methods, it is better to remind briefly some general concepts about immunoassays, such as their categorization and the important characteristics necessary to carry out good immunoassay experiments.
2. Classification of Immunoassays
Many immunoassays can be classified as either a homogeneous or heterogeneous assay [3]. The difference between the two assays refers to the experimental procedure used to prepare the assay (see below), which can avoid (homogeneous) or include (heterogeneous) a separation step. Moreover, both approaches can be performed in a competitive or non-competitive way [4]. In homogeneous immunoassays, the analyses are performed in the absence of solid support and the adopted operating procedures do not require the separation of the unbound tracer from the investigated sample. In fact, within this strategy, the labelled compound is able to generate the desired signal only when it binds to the analyte (immunometric immunoassay) or to the antibody (competitive assay). On the contrary, heterogeneous assays involve washing steps, allowing the separation of the bound form from the free-labelled antibody. In this case, the presence of solid support is important to perform automatic processes. This feature, together with the higher versatility, higher specificity and higher sensitivity allow the heterogeneous immunoassays to turn out as the most popular assay format.
Immunometric immunoassays, also known as sandwich immunoassays [5], are the simplest example to understand how the assay works. Figure 1 depicts the procedure of heterogeneous sandwich immunoassays. The antibodies immobilized onto a solid support bind the selected analyte from the sample, and a second labelled antibody able to recognize another part of the analyte is used for the signal generation. In fact, the latter antibody is bearing a tracer covalently bound, which is able to produce a specific readout, such as radioactivity or the emission of light. Sandwich immunoassays are commonly used for analytes with high molecular mass, thus with sufficient surface area able to locate two antibodies.
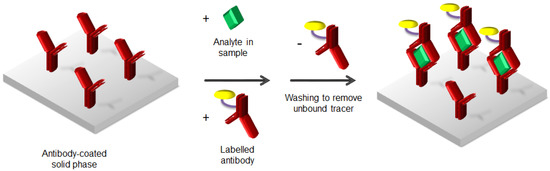
Figure 1.
Heterogeneous immunometric or sandwich immunoassay.
On the contrary, competitive immunoassays are instead employed if analytes are small molecules, by adding a labelled analyte to the sample solution, as depicted in Figure 2.
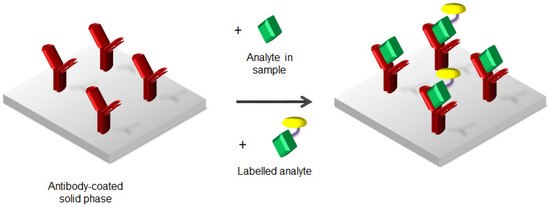
Figure 2.
Competitive heterogeneous assay.
3. How to Perform Good Experiments in Immunoassay
To define the performance of an immunoassay method, four fundamental characteristics must be considered:
- (1)
- Sensitivity;
- (2)
- Specificity;
- (3)
- Accuracy;
- (4)
- Precision.
“Sensitivity” is the quantitative measurement of a tiny concentration of analyte, while “specificity” corresponds to the discrimination between analytes with closely analogous molecular structures. This means that these first two features are strictly connected with the nature and the amount of analyte in the sample. In contrast, the other two features are more related to the comparison of recorded data. Indeed, “accuracy” refers to a quantification achieved through the comparison with reference standards and the use of calibration curves, and “precision” arises from the minimal variation between repeated measurements.
By considering the four above-mentioned characteristics of immunoassays, it appears clear how the chemical design and the properties of the tracer dramatically affect the effectiveness of the employed analytical technique.
4. Basic Principles of Methodologies Used in Protein Analysis
The various detection methods can be distinguished one to the other depending on the nature of the signal recorded during the measurements, which can be, for example, optical or electrical. Obviously, the chemical and physical properties of the label linked to the tracer determine the type of the immunoassay employed. In fact, radioisotopes or enzymes, as well as fluorescent or electrochemical markers can be used as labels for the detection of specific analytes.
4.1. -) Radioimmunoassay (RIA)
Radioimmunoassay (RIA) is one of the first methods employed for the detection of biological targets. Yalow and Berson introduced the use of radio-label for the recognition of insulin, thus developing RIA as analytical technique [6]. The importance of this research was recognized by assigning Yalow the Nobel prize in Medicine in 1977, the second woman of the history to receive the prestigious recognition [7].
The principle of the RIA is the competition between unlabelled and labelled antigens (Ag and Ag*, respectively) for a specific antibody (Ab). Starting from a constant concentration of Ab and Ag*, in which the labelled antigen is in relative excess over the antibody, unlabelled Ag is added, resulting in a competition binding process for the formation of the antigen–antibody complex. The increase in the concentration of Ag corresponds to a decrease in the radioactivity of the Ag*Ab complex. By calculating the percentage of the Ag*Ab, it is possible to determine the concentration of Ag, owing to the inverse proportionality between the concentration of Ag*Ab and the amount of unlabelled antigen added to the solution. As a consequence, either the decrease in the labelled antigen–antibody complex, or the increase of the free Ag* can be used for the determination of the unlabelled antigen Ag added to the medium, originating from the unknown to be measured, or from the standard used in the assay [8].
Standard employed radioactive labels are iodine, which exists in two different forms (125I, most abundant, with a half-life of 2 months and 131I, with a half-life of 8 days and higher specificity); tritium (3H); and carbon (14C).
The advantages of RIA lie mainly in its very high sensitivity, high specificity and the possibility to perform a large number of determinations simultaneously. However, this method also shows some drawbacks, mostly due to the presence of radioactivity. Indeed, this induces health and environment hazards, special attention for handling reagents, specific training of the operating staff, and expensive instruments to count radioactivity. In Table 1 are listed some examples of analytes investigated by RIA.

Table 1.
List of some compounds analysed in biological fluids by RIA.
4.2. -) Enzyme-Linked Immunosorbent Assay (ELISA)
As an alternative tracer to radioisotopes, enzymes chemically bound to antibodies can act as a label in immunoassay. The replacement of radiolabels with enzymes made immunoassay much simpler and more popular, and today the enzyme-linked immunosorbent assay (ELISA) is the most widely used immunoassay method [20,21]. This immunoassay method is based on an antibody- or antigen-coated solid surface with an enzyme involved in the signal generation process. The ELISA procedure implicates different steps: plate coating, blocking of the target, washing, signal generation and measurement. Generally, in ELISA, the antibodies (or antigens, if the goal is the detection of antibodies in the sample) are first bound to a polystyrene plate, then put in contact with an inert protein such as bovine serum albumin (BSA) to prevent nonspecific binding in the uncoated area of the plates. Afterwards, samples are added to the plates and incubated for a suitable time, allowing the binding between the analyte and the coated antibodies. The subsequent washing removes the unbound materials, allowing the measurement of specific analytes in crude preparation. Finally, the reaction is quantified by adding a specific enzyme-linked antibody, which induces a proportional change in a coloured reaction [22]. The generated signal appears highly amplified, due to the fact that a single enzyme can activate many molecules of the substrate. The sequence of steps described above builds a multilayer structure, which is commonly called a “sandwich assay” (Figure 3).
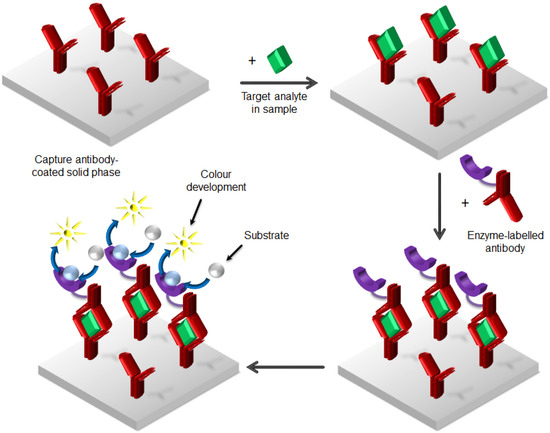
Figure 3.
Enzyme-linked immunosorbent assay (ELISA) method as “sandwich assay”. Washing steps are not indicated.
ELISA immunoassays can be also performed with alternative formats, called competitive binding assay and indirect ELISA (or antigen-down assay) (Figure 4).
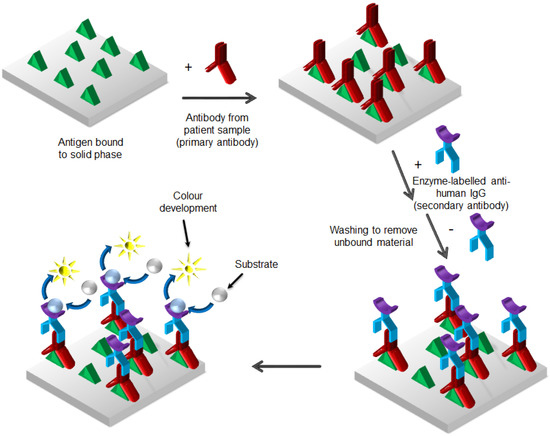
Figure 4.
Enzyme-linked immunosorbent assay (ELISA) method as “antigen-down immunoassay” or “indirect assay”.
The immobilization process of the reagents to a solid surface, followed by the binding of the analyte from the solution, makes ELISAs relatively easy to perform. Moreover, the use of 96-well microwell plates as solid support allows the ELISA assay to transform into a semi-automatic method, due to the high number of tests performed simultaneously (every single well acts as a separated reaction tube) and to the simplicity of the washing process of the wells [23,24]. Starting from this basic sequence, many researchers often develop their home-made ELISA methods, which gives an idea about the powerfulness of this assay.
4.3. -) Fluoroimmunoassay (FIA)
If the signal generated by the chemically bound label is fluorescent, the assay is called fluoroimmunoassay (FIA) [25]. In this particular case, the optical output is recorded upon irradiation of the sample with a lamp at a specific wavelength. To explain the concept of fluorescence, it is better to refer to the diagram depicted in Figure 5 (Jablonski diagram) [26], which schematically describes the electronic transitions involved in the absorption and emission phenomena at the molecular scale.
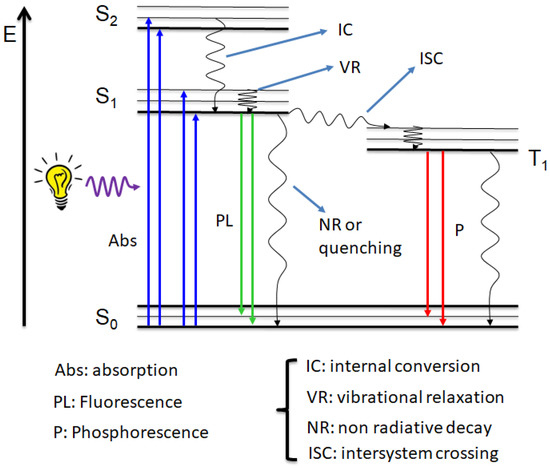
Figure 5.
Jablonski diagram. Sn: singlet states. T1: triplet state.
Different electronic states are reported in the diagram, depending on their energy and on their spin multiplicity. In particular, S (singlet) indicates states with a total spin number equal to zero, while T (triplet) indicates states with a total spin number equal to 1.
By irradiating a molecular compound with a light source, an electron from the ground state (S0) is promoted to an excited state (Sn) upon the absorption of a photon. Two types of molecular orbitals are involved in the absorption and fluorescence transitions, called HOMO (Highest Occupied Molecular Orbitals) and LUMO (Lower Unoccupied Molecular Orbitals), which refer to the ground state and the first excited state of the molecule. For this reason, we often refer to singlet–singlet and/or HOMO-LUMO transitions.
It appears evident from the Jablonski diagram that part of the energy absorbed is lost through non-radiative processes, such as vibrational relaxation and internal conversion. As a consequence, the radiative decay (i.e., electronic transition with emission of light) is shifted at a lower energy compared to the absorption. Because the energy is inversely proportional to the wavelength, the light emitted always falls at a longer wavelength than the absorption. Radiative decays are divided in two types depending on the nature of the excited state from which the photon is emitted: fluorescence (also called photoluminescence, PL) from a singlet state and phosphorescence from a triplet state.
By working with fluorescent compounds, it is important to be familiar with quantities usually employed to characterize the emission properties of different substances. The difference between the lowest absorption band and the first emission peak is called Stokes shift, while the number of emitted photons is defined as fluorescence quantum yield. The importance of these two quantities is reflected in the accuracy of the FIA: high quantum yield corresponds to a strong signal, and then to a higher sensitivity of the assay; while large Stokes shift avoids interferences from the excitation light in the emission measurements, resulting in a better precision. Another parameter often used in FIA is the radiative lifetime, which indicates the life of the populated excited state. To learn more details about the electronic transitions and physical quantities depicted in the Jablonski diagram, readers are invited to look at references [26,27].
There are two kinds of fluoroimmunoassays: those employing a fluorophore labelled directly, and enzyme immunoassays (EIAs) that use substrates that are converted in fluorescent end-products. Fluorophores (called also chromophores) are often organic compounds that emit at different wavelengths depending on their chemical structure. The presence of heterocycles as well as different conjugation lengths can dramatically change the emission properties of a class of molecules. Many chromophores are actually commercially available, such as derivatives of rhodamine, fluorescein, cyanine and BODIPY, but they suffer of some drawbacks, e.g., small Stokes shift. For this reason, the research activity devoted to design and investigate novel emitters with larger Stokes shift and high quantum yield is highly attractive and still in progress [28].
Quantum dots (QDs) represent a class of emitters employed in FIAs alternative to organic molecules [29]. They are inorganic semiconductor nanoparticles characterized by a narrow and intense emission that can be tuned by modifying their size. In fact, small quantum dots (1–4 nm) emit in the blue region, while bigger nanoparticles (10–12 nm) are red emitters [30]. Common quantum dots have a core-shell structure that ensures high stability and the confinement of the exciton, which generates the emission. The use of specific ligands as an external shell of the nanoparticles allows for the change in solubility of these colloids: for example, from organic solvents to water and, at the same time, can be used to link the quantum dots to enzymes or antibodies for FIAs. For instance, by using linkers bearing thiol on one side and biotin on the other, it is possible to stabilize the quantum dots and use the biotin/streptavidin complex formation to bind biological targets. However, even though their emissive properties are very promising, the application of quantum dots is still limited to in vitro tests due to the presence of cadmium in many of these semiconductors.
Generally, the use of fluorescent labels appears more convenient compared to radioisotopes, due to healthy reasons, easier design of the system, higher sensitivity and a faster readout process. A heterogeneous FIA approach was developed for the measurements of fluorescent signal on the solid microwell plates, appearing competitive with other heterogeneous immunoassay methods [31]. On the other hand, homogeneous FIA avoids the separation step of the free analyte before measuring the fluorescence, resulting in a faster process. Therefore, the analyte concentration in a sample can be directly determined in the reaction mixture. In these assays, the antibody-bound analyte induces a modification in the emission properties of the labelled compound. Examples of these variations are the change in the intensity of the emission (i.e., enhancement or quenching of the fluorescence, and/or shift of the emission wavelength) or in the polarization of the emitted light. In this case, the polarized emission of a fluorescent label generated by excitation with polarized light depends on the lifetime of the emitting label and on its rotational motion. When the labelled analyte is bound to the antibody, the rotational degrees decrease, resulting in an enhancement of polarization [32,33,34,35].
The detection limits of FIAs for conventional fluorescent labels can be limited by background interference, such as emission from some components in complex samples. A strategy employed to avoid this interference involves using a time delay between excitation and a recorded emission of the fluorophore, especially if the chromophore has a long lifetime decay. This approach is called time-resolved fluorescence and employs lanthanide complexes as emitters [36].
Lanthanide ions cannot be excited directly due to the forbidden f-f transitions. Then, they are usually coordinated by organic ligands that absorb light and transfer the energy to the lanthanide. The mechanism first involves the population of the triplet state of the ligand (called also “antenna”) through the intersystem crossing process (ISC, see Figure 5), which is enhanced by the spin–orbit coupling induced by the presence of the heavy metal ion [37]. The emission colour depends on the f-f transition; thus, every lanthanide emits at a well-defined wavelength. Europium (Eu) and terbium (Tb) are the most used lanthanide ions for immunoassay and imaging due to their high fluorescence in the visible region (red and green for europium and terbium, respectively) [38]. Lanthanide complexes show large Stokes shift and long lifetime decay, together with high quantum yield, even if the quantum yield is connected to the energy transfer efficiency between the antenna and metal ion. Although many lanthanide chelates have been reported, the research focused on the design of novel ligands to prepare more efficient complexes is still an active field [39,40,41,42,43,44,45,46,47,48,49,50].
In FIAs, two approaches were developed by using europium complexes (Figure 6). The first strategy employs a non-fluorescent lanthanide complex covalently bound to an antibody, which is converted in a fluorescent complex after immunological reaction with another coordinating ligand (enhancer), usually a fluorinated β-diketonate (dissociation-enhanced lanthanide fluoroimmunoassay) (Figure 6a). This assay was commercially distributed by Delfia (Perkin Elmer, MA, USA) and Pharmacia systems. The main drawbacks are the possible contamination of the europium complex and the enhanced background signal due to the high concentration of free ligand in the solution. The second approach involves a highly emitting europium complex covalently bound to proteins, which can bind the antibody through the interaction between biotin and streptavidin (Figure 6b). The use of lanthanide complex as labels allowed reaching very low detection limits, i.e., in the range of 10−3 pg/mL [51,52].
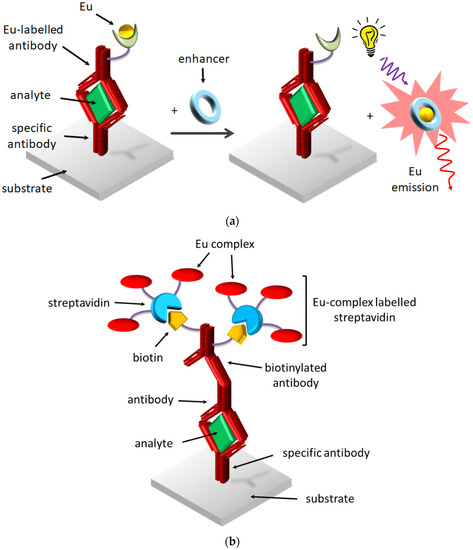
Figure 6.
Two strategies employing Eu complexes: (a) enhancement of Eu emission by DELFIA, (b) use of Eu complex-labelled proteins.
4.4. -) Surface-Enhanced Raman Scattering (SERS)
Another kind of optical immunoassays is based on the detection of the surface-enhanced Raman scattering (SERS) as a signal readout [53,54]. Raman scattering is the inelastic scattering of a photon after its interaction with the matter, a phenomenon discovered by Raman in 1928 [55] that allows for the gaining of structural and quantitative information on molecules and materials. However, the intensity of the signal measured in Raman spectroscopy is generally weak, which restricts its application in immunoassays. In the 1970s, the discovery of the phenomenon of enhancement of the Raman signal, which was observed by using pyridine adsorbed at the silver electrode surface [56], permitted the development of biochemical assays based on SERS.
Commonly, a SERS-based immunoassay is composed of two parts, i.e., the substrate modified with targeting molecules that bind specific analyte from sample solution, and the SERS probes to quantify the concentration of the analyte. The SERS probes are obtained by combining the Raman reporter, usually small organic molecules, such as 4-mercaptobenzoic acid [57], 4-nitrothiophenol [58] and rhodamine 6G [59], with metal nanoparticles, mainly silver and gold, which act as signal amplifiers. Ag and Au nanosystems are prepared with different shapes in order to enhance the Raman signal. Indeed, it was observed that both isotropic (nanospheres [60,61]) and anisotropic structures, such as nanoplates [62], nanorods [63] and nanostars [64,65,66,67] show a remarkable SERS effect in different spectral regions, depending on the form of the metal substrate employed. It is also worth noting the important role of the ligand coordinating the metal particles, which increases the solubility of the systems and can also be used to bind specific targets [68,69].
SERS immunoassays can be performed in different conditions, in vitro [70] and ex or in vivo [71,72] allowing the raise of SERS as technique for bioanalytical measurements [73,74,75,76,77,78,79,80,81,82,83,84], which was firstly commercialized in 2015 [85].
As well as other techniques reported in this review, the immunoassays based on SERS can be classified as heterogeneous or homogenous (Figure 7) and competitive or non-competitive, depending on the procedure followed to perform the measurements.
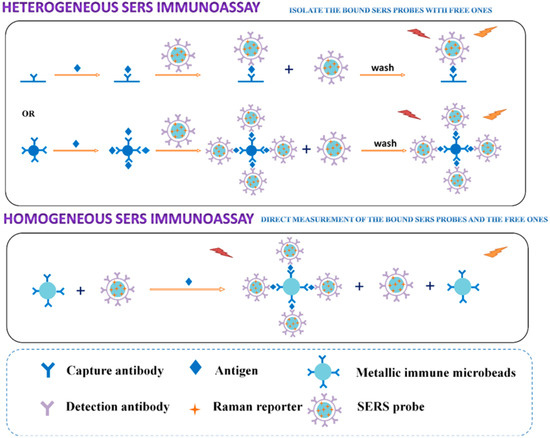
Figure 7.
Principle of the heterogeneous and homogeneous SERS immunoassay. Reprinted with permission from ref. [53]. Copyright 2017 American Chemical Society.
4.5. -) Surface Plasmon Resonanace (SPR) Analysis
It is worth mentioning the occurrence of other methods based on optical readout in combination with metal substrates, such as the surface plasmon resonance (SPR) analysis [86]. This measurement is based on the interaction of light with thin metal films typically made of gold onto a glass substrate. The analysis surface consists of a capture biomolecule, which has an affinity for the analyte of interest, covalently bound to the gold surface. The analysis substrate is optically coupled to a hemispherical or hemicylindrical prism by an index matching fluid. Light impinges on the gold film through the prism, which is called the Kretschmann configuration. The SPR sensor based on the Kretschmann prism structure can also be applied in biological detection. In the satisfaction of the phase matching condition, a partial incident optical coupling plasmon exciton-reduces the light reflection at a specific angle. This method can be measured with a high sensitivity of refractive index in the order of 10−6 to 10−7 RIU [87,88]. SPR have been recently studied in combination with optical fiber-gratings [89].
However, this method can be performed only under heterogeneous configuration due to the grafted bioreceptors on the metal surface (film or nanoparticles).
4.6. -) Chemiluminescence Immunoassay (CLIA)
Another strategy to generate an optical readout alternative to fluorescence is the chemiluminescence (CL), which arises from chemical reactions producing an optical signal. By using labels showing chemiluminescence, it is possible to apply this phenomenon to immunoassays [90]. In details, the product of the chemical reaction is formed in the excited state, which subsequently relaxes to the ground state with emission of a photon. It is also possible that the excited intermediate transfers the energy to a suitable fluorophore, which can then exhibit its characteristic emission. The intensity of the emitted light depends on the rate of the chemical reaction, the number of generated excited species and the efficiency of the radiative decay. It then appears clear that most chemiluminescent reactions have quantum yields lower than those obtained through photoluminescence. However, the decrease in background signal, due to the lack of a light source, the simple instrumentation required to detect the signal and the high sensitivity due to the selectivity of the chemical reaction, is an important advantage that allows a large diffusion of CL immunoassay (CLIA) [91]. The energy able to generate electronically excited species usually arises from bond cleavage or electron transfer processes. In the first case, chemiluminescent systems, such as luminol or peroxyoxalates, can be used only once, while systems involving electron transfer, such as rubrene and tris(2,2′-bipyridil)ruthenium(II), generate light without bond cleavage or rearrangement and can be recycled. An alternative to the above-mentioned chemiluminescent reactions, chemiluminescence, can be also generated by a chemical reaction in the presence of an enzyme, and this approach is the most employed in CLIA. The label for CL can be divided in direct chemical or enzyme-based, depending on if an enzyme must be used to activate the chemical reaction.
Among the compounds used as label, luminol (5-amino-2,3-dihydro-1,4-phthalazinedione), acridinium ester (typically aromatic ester of the 10-methylacridinium-9-carboxylic acid), 4-methoxy-4-(3-phosphatephenyl)-spiro-(1,2-dioxoetane-3,2-adamantane, AMPPD) and their derivatives are the most common organic molecules (Figure 8).

Figure 8.
Molecular structure of common organic molecules for chemiluminescence.
Luminol is oxidized in alkaline solution to yield the excited state 3-aminophtalate, which decays by emitting light in the blue region with maximum peak around 425 nm (Figure 9).
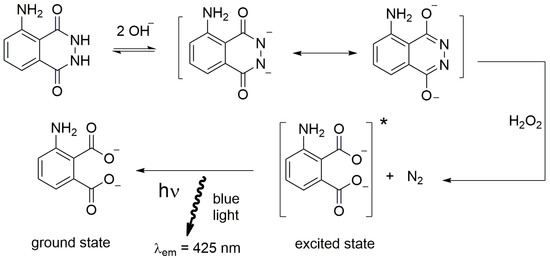
Figure 9.
Schematic reaction of luminol. * indicates the excited state.
Among the oxidants employed in tandem with luminol, which also include iodine, potassium permanganate and sodium hypochlorite, hydrogen peroxide (H2O2) is the most commonly used. The oxidation reaction between luminol and H2O2 proceeds in the presence of a catalyst. The latter can have a different origin, such as transition metal cations and some metal complexes, and the most frequently employed catalysts are the peroxidases [92]. Table 2 summarizes the main features of the most used reagents for CL in solution, i.e., the environmental condition typical for the reagent, the analytes activating the chemiluminescent reaction and the corresponding wavelength of the CL emission.

Table 2.
Examples of liquid-phase chemiluminescence reactions.
Due to the direct proportionality between the intensity of the chemiluminescence and the concentration of luminol, catalyst and hydrogen peroxide, this chemical reaction represents a useful method to establish the amount of these species inside a sample. In fact, the measurement of hydrogen peroxide produced by reactions of oxidase enzymes is employed to detect the presence of different analytes, for example antioxidants, such as ascorbate, urate or vitamin E [93], uric acid [94] and glucose [95]. Peroxidases are enzymes commonly used as label for analytes in immunoassays and, in particular, horseradish peroxidase (HRP) is the most frequently used enzyme label in binding assay due to its high stability. However, the generated CL suffers from low intensity and short lifetime, so the further addition of an enhancer (e.g., certain substituted phenols and naphthols) to the substrate mixture is a useful strategy to increase the efficiency of CLIA.
As well as luminol, acridinium derivatives can also be used in combination with HRP to generate CL, and substituted phenols act as enhancers too (Figure 10). Although the emitted signal is similar to that obtained by luminol, it is worth noting that an acridan derivative shows CL emission even with a pH level as low as 2.6, while luminol does not emit at a pH level lower than 5.6 [96].
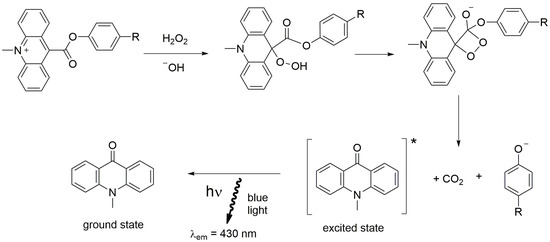
Figure 10.
Schematic reaction of acridinium ester. * indicates the excited state.
The third class of organic molecules suitable for CL is made by the AMPPD derivatives. The adamantyl 1,2-dioxoetane arylphosphate is sensitive to alkaline phosphatase, which produces an unstable anion that decomposes with the emission of light by cleaving the phosphate group (Figure 11). The emission colour can be tuned by introducing different substituents with electron withdrawing characteristics on the phenyl ring [97].
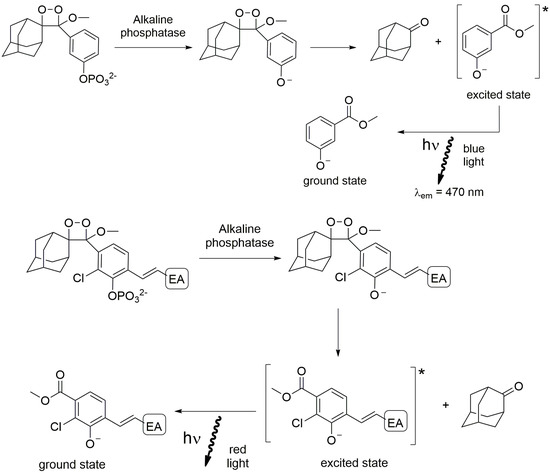
Figure 11.
Schematic reaction of adamantyl derivatives. * indicates the excited state.
In addition to fluoroimmunoassay, inorganic nanoparticles, such as quantum dots and colloidal gold nanoparticles, have found application as labels in CLIA, even if their diffusion is still limited [98].
The simplicity of the instrumentation and the accuracy of the measurements make CLIA an important method for the development of portable biosensors by using common smartphones [99], with applications in many different fields, spanning from medical diagnosis to cultural analysis, passing through assays to monitor the health of astronauts in space [100,101,102].
4.7. -) Electrochemiluminescence Immunoassay (ECLIA)
Electrochemiluminescence (ECL), or electrogenerated chemiluminescence, was first investigated by Hercules [103], Visco [104] and Bard [105] in the 1960s by studying aromatic compounds. Afterwards, the description of the ECL properties of the ruthenium complex tris(2,2′-bipiridine)Ru(II) dichloride [Ru(bpy)32+] in acetonitrile in 1972 [106] opened new possibilities for immunoassays, owing to the high electrochemical stability, the easy detection of the ECL emission and the possibility to operate at a low potential offered by this metal complex. Furthermore, the lack of background signal, relatively low-cost instrumentation, high sensitivity, direct proportionality of the intensity of the signal emitted with the concentration of the species involved, and the possibility to perform experiments in aqueous solution rapidly established ECL as an important analytical tool.
As suggested by the name, the chemiluminescence produced in this process occurs as result of the reaction between species (radical ions) electrochemically generated. This reaction leads to the formation of the excited state of the ECL active chromophore, which decays to its ground state by emitting a photon [107]. Usually, the ECL is classified in two categories: annihilation and coreactant (Figure 12).
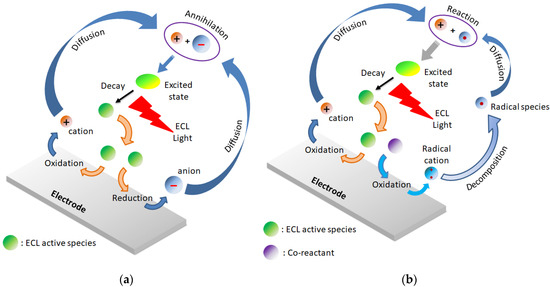
Figure 12.
ECL mechanisms: (a) annihilation, (b) coreactant.
The annihilation involves the electron transfer reaction between radical cations and radical anions generated through oxidation, and reduction processes of an ECL luminophore. In this case, the emitter is recycled and not consumed during the process; thus, it can be used in small concentrations. It is useful to underline three general parameters necessary to observe an efficient ion annihilation ECL: (i) stable radical ions of the molecules in the electrolyte of interest, (ii) good fluorescence efficiency of the emitting excited state, and (iii) sufficient energy in the electron-transfer process to generate the excited state.
In the coreactant ECL, an additional species (called coreactant or coreagent) added to the reaction mixture is involved in the formation of the excited state of the ECL dye, operating with one directional scanning (either positive or negative). The coreactant is a consuming agent because it forms a strongly oxidative (or reductive) species upon decomposition of its oxidized (or reduced) form, depending on its nature and on the polarity of the applied potential. The emission of light occurs after the reaction between the powerful reducing (or oxidizing) species with the oxidized (or reduced) luminophore that produces the excited state. The corresponding ECL reactions are referred as “cathodic or reductive oxidation” [108] and “anodic or oxidative reduction” [109], respectively. Table 3 are summarized the most commonly used coreactants in combination with the Ru(bpy)32+ complex. Generally, the goal of the presence of the coreactant is to overcome either the limited potential window of the solvent or the poor stability of the generated radical anions or cations.

Table 3.
List of common co-reactants used in combination with Ru(bpy)32+.
Several criteria must be considered for a good reactant compound. First of all, the coreactant should be appropriately soluble in the reaction media, owing to the proportional relationship between the ECL intensity and the concentration of the coreactants (solubility). Moreover, the intermediate species generated electrochemically and chemically should be sufficiently stable to react with the ECL precursor (stability). The coreactant should be easily oxidized or reduced with the ECL active compound at or near the electrode surface and should undergo a rapid chemical reaction to form the intermediate species with sufficient reducing or oxidizing energy to react with the oxidized or reduced dye to form the excited state (electrochemical properties). Additionally, the reaction rate between the reactive intermediate and the oxidized, or reduced luminophore species, must be rapid (kinetics). It is also important that the coreactant and its redox products should not be good quenchers of the emission generated by ECL compounds (quenching effect). Finally, the coreactant itself should not give any ECL signal over the potential range scanned (ECL background).
Among the ECL luminophores, 9,10-diphenylanthracene (DPA) and Ru(bpy)32+ are used as references to compare the efficiency of the new systems, even if compounds with higher efficiency have been reported [110,111,112]. Due to their peculiar properties, Ru(bpy)32+ and closely related analogues are mainly employed as emitting species in analytical applications. In fact, the photophysics and the electrochemistry of Ru complexes are extensively investigated, showing emission in the orange-red visible region with a maximum around 620 nm, a relative long lifetime (between 0.5 and 1 µs) and a fully reversible behaviour in both oxidation and reduction within the useful potential window of several solvents (+1.3 V and −1.3 V vs. Ag/AgCl) [113,114].
Moreover, Ru(bpy)32+ can be efficiently combined with different coreagents in both “reductive oxidation” (e.g., with peroxydisulfate and benzoyl peroxide) and “oxidative reduction” (e.g., with oxalate and amine) ECL pathways and its solubility in water favours the application in immunoassays [115]. In particular, tri-n-propylamine (TrPA) is the coreactant mainly involved in the majority of ECL applications in combination with Ru complexes, due to the highest ECL efficiency showed by the Ru(bpy)32+/TrPA system. The list of some interesting ECL applications by using the Ru(bpy)32+/TrPA system is reported in Table 4, and readers who are interested in particular aspects are encouraged to consult the corresponding references cited.

Table 4.
Example of analytes detect by using the Ru(bpy)32+/TPrA, system.
ECL detections in immunosensors are performed with ECL emitter onto solid phase, thus in heterogeneous formats, including direct, competitive and sandwich immunoassays (Figure 13) [122].
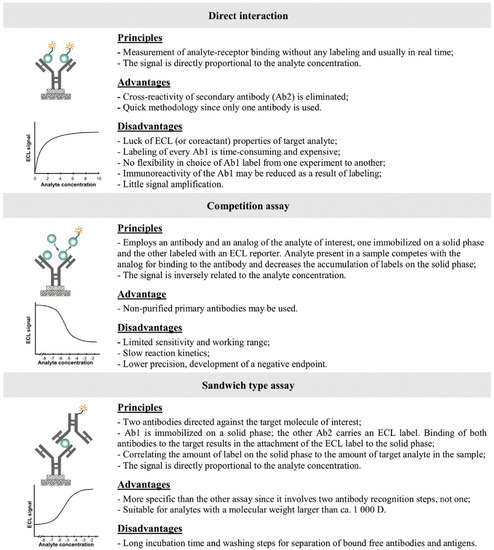
Figure 13.
Types of ECLIA immunoassays. Reprinted with permission from ref. [122], Copyright 2014, Elsevier.
The immobilization of ECL luminophores onto a solid substrate allows for the amplifying of the ECL signal, and thus enhances the efficiency of immunosensors [123]. Although Ru complexes are the only emitters used in commercially available devices for ECLIA immunoassays based on functionalized solid support (Elecsys® technology) [124,125], the development of novel functionalized surfaces for ECL purposes is an up-to-date research activity [126,127].
Besides DPA and Ru complexes, it is worth noting that the research focuses on obtaining higher ECL efficiency and/or different emission colours to be applied in multiassays analysis is in continuous expansion. Many other compounds are actually under investigation, such as metal transition complexes (mainly iridium [128,129,130] and platinum [131,132]), quantum dots [133,134], nanomaterials [135] and polyaromatic molecules (luminol [122,136], spirobifluorene derivatives [110,111,126] and many others [137,138,139]).
Other electrochemical techniques have been employed in immunoassay, depending on the monitored quantities that change in the presence of the analyte, such as potential, current, resistance, and capacitance [140,141]. Owing to the lack of optical readout, these methods will be not analysed in this review, thus interested readers are invited to check the corresponding literature.
Table 5 summarizes the characteristics of the reported immunoassays in terms of advantages and disadvantages for each, in order to help the readers better compare the different methods.

Table 5.
Comparison of the immunoassay methods reported in the paragraph.
5. SARS-CoV-2 Detection: A Recent Example of Application of Immunoassays
Since December 2019, we have been in the battlefield with a new threat to humanity that the World Health Organization (WHO) named “COVID-19”. The International Committee on Taxonomy of Viruses (ICTV) called the virus “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) [142]. The early and accurate detection of the virus is pivotal to prevent the rapid spread of the infection and to isolate contacts. Currently, the reverse transcription polymerase chain reaction (RT-PCR) test is the standard method for the detection of COVID-19, which consists of analysing the RNA of the virus presents in respiratory samples [143,144]. This ongoing global pandemic motivated researchers to investigate new alternatives for the detection, diagnosis, treatment and vaccinations of this virus. Many reviews about these developments have been recently published [145,146,147,148]; thus, this paragraph will report only a short overview focused on immunoassays with optical readout to show how the immunoassays are strictly connected to the “real world” and play a pivotal role on the development of point-of-care devices.
Traditional ELISA assays were successfully used with qualitative or quantitative outcomes typically in 1 to 5 h. The diffusion and the sensitivity of the technique allowed the commercialization of ELISA-based tests by different organizations or manufacturers approved by the Food and Drug Administration (FDA) [149].
Chemiluminescence immunoassays [150,151] were also approved by the FDA as a test for COVID-19. The CLIA-based tests appear to be faster than the ELISA immunoassays (only 30 min instead of hours) and sometimes even more sensitive depending on the target antigens [152]; but, they can suffer from the presence of contaminants.
Fluorescence immunoassays confirm to be a simple and fast method to detect antibodies of COVID-19 [153,154,155], showing efficiency comparable with chemiluminescence immunoassays [156].
Although, to the best of our knowledge, electrochemiluminescence immunoassays ECLIA for COVID-19 are not approved by the FDA yet, they are very promising due to the high sensitivity reported in literature [157,158,159].
Other methods are employed to enhance the performance of the lateral flow immunoassay LFIA, which usually can give more qualitative results. For example, by combining LFIA with SERS, it was possible to obtain quantitative analyses comparable with ELISA’s outcomes [160,161]. This approach aims to improve the application of LFIA-based systems, which are cheap and could be more easily accessible by single patients.
For more complete reviews about the comparison among the different detection methods of SARS-CoV-2, interested readers are invited to check additional recent literature.
6. Outlook and Perspectives
This review is devoted to giving a general overview about the basic principles of several immunoassay methods, by focusing mainly on techniques with an optical readout. Besides a short reminder about the classification of immunoassays and the important characteristics to perform good measurements, the basic theoretical description of the phenomena involves in the analysed techniques were reported. At the same time, advantages and drawbacks of methods were also discussed, in order to inform about the effectiveness of each techniques. The comparison of the different immunoassays shows that the reported techniques are efficient and very sensitive, with the exception of FIA, which shows slightly lower sensitivity. The presence of dedicated (and sometimes very expensive) instrumentation is a major drawback of several immunoassays. In order to overcome this limit, research groups are devoted to developing cheaper and portable instruments, which can also be combined with smartphone devices. The main goal is the diffusion of more accessible medical analyses to the citizens, but also to simplify the analytical procedures in other application fields of immunoassays, such as food safety and environmental analysis and preservation of cultural heritage. The research on portable instruments attracts a lot of interest and is very promising, thanks to the increasing miniaturization of the optical detectors.
In addition to discussing each technique, inputs about the actual research activity in the field of emitting labels are also presented in the review, with the aim of updating the readers regarding the latest developments of several methods, and in which direction science is moving in the field of immunoassays. From this point of view, it appears clear how dynamic the scientific progress accompanying the application of different kind of immunoassays is in many fields, indicating the great potential of the immunoassay approaches and their importance in the development of our society. To additionally emphasize the impact of immunoassays on the society and on the “everyday life”, examples of immunoassays with optical readout employed to detect the SARS-CoV-2 virus are discussed in the last section of the review. Although this field is continuously under evolution due to the worldwide pandemic situation, the discussed data underline the versatility of the immunoassays, which were rapidly and successfully applied to monitor the diffusion of the virus.
In addition to the development of new efficient emitting systems, in the next future enhancement of the sensitivity and thus earlier detection of illness or pollution could be reached by combining different methods, for example by employing SERS-emitters in ELISA immunoassays. In addition, the use of multiassays with different readouts is expected to have a tremendous impact in the field of immunoassays, thus it is attracting a great interest. However, it remains a challenging task, which could be overcome with the further development of miniaturized devices. In these terms, the role of the multidisciplinary is fundamental in the field of immunoassays, where chemistry, physics, medicine and engineering can work together aiming to improve our society and preserve our planet.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG) grant number RI 2635/6-1, project number: 464509280.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Zourob, M.; Elwary, S.; Turner, A. (Eds.) Principles of Bacteria Detection: Biosensors, Recognition Receptors and Microsystems; Springer: New York, NY, USA, 2008. [Google Scholar]
- Gosling, J.P. A decade of development in Immunoassay methodology. Clin. Chem. 1990, 36, 1406–1427. [Google Scholar] [CrossRef]
- Dobosz, P.; Puchades, R.; Morais, S.; Maquieira, A. Highly sensitive homogeneous-heterogeneous nanogold-based microimmunoassays for multi-residue screening of pesticides in drinking water. Case Stud. Chem. Environ. Eng. 2022, 5, 100199. [Google Scholar] [CrossRef]
- Darwish, I.A. Immunoassay methods and their application in pharmaceutical analysis: Basic methodology and recent advances. Int. J. Biomed. Sci. 2006, 2, 217–235. [Google Scholar] [PubMed]
- Zhang, C.; Liu, Z.; Bai, M.; Wang, Y.; Liao, X.; Zhang, Y.; Wang, P.; Wei, J.; Zhang, H.; Wang, J.; et al. An ultrasensitive sandwich chemiluminescent enzyme immunoassay based on phage-mediated double-nanobody for detection of Salmonella Typhimurium in food. Sens. Actuators B 2022, 352, 131058. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma Insulin in human subjects by immunological methods. Nature 1959, 184, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.nobelprize.org (accessed on 1 June 2022).
- Felber, J.P. Radioimmunoassay of Polypeptide Hormones and Enzymes. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons: New York, NY, USA, 1974; Volume 22, pp. 1–94. [Google Scholar]
- Ikeda, Y.; Fujii, M.; Yamazaki, M.; Fujii, Y. Measurement of serum digitoxin in patients by radioimmunoassay using specific antiserum. Clin. Chim. Acta 2001, 314, 245–247. [Google Scholar] [CrossRef]
- Vakkuri, O.; Arnason, S.S.; Pouta, A.; Vuolteenaho, O.; Leppäluoto, J. Radioimmunoassay of plasma ouabain in healthy and pregnant individuals. J. Endocrinol. 2000, 165, 669–677. [Google Scholar] [CrossRef][Green Version]
- Mei, J.V.; Hannon, W.H.; Dobbs, T.L.; Bell, C.J.; Spruill, C.; Gwinn, M. Radioimmunoassay for monitoring zidovudine in dried blood spot specimens. Clin. Chem. 1998, 44, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Clive, D.R.; Sudhaker, D.; Giacherio, D.; Gupta, M.; Schreiber, M.J.; Sackrison, J.L.; MacFarlane, G.D. Analytical and clinical validation of a radioimmunoassay for the measurement of 1,25 dihydroxy vitamin D. Clin. Biochem. 2002, 35, 517–521. [Google Scholar] [CrossRef]
- De Clerck, I.; Daenens, P. Development of a Radioimmunoassay for the Determination of Zolpidem in Biological Samples. Analyst 1997, 122, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Tagliaro, F.; Camilot, M.; Valentini, R.; Mengarda, F.; Antoniazzi, F.; Tatò, L. Determination of thyroxine in the hair of newborns by radioimmunoassay with high-performance liquid chromatographic confirmation. J. Chromatogr. B Biomed. Sci. Appl. 1998, 716, 77–82. [Google Scholar] [CrossRef]
- Deberg, M.; Houssa, P.; Frank, B.H.; Sodoyez-Goffaux, F.; Sodoyez, J.-C. Highly specific radioimmunoassay for human insulin based on immune exclusion of all insulin precursors. Clin. Chem. 1998, 44, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Chatterton, R.T., Jr.; Vogelsong, K.M.; May, L.K. Direct Radioimmunoassay of Progesterone in Saliva. J. Immunoass. 1997, 18, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Giton, F.; Valleix, A.; Boudou, P.; Villette, J.M.; Bélanger, A.; Galons, H.; Fiet, J. Specific radioimmunoassay of estrone sulfate: Application to measurement in male plasma. J. Steroid. Biochem. Mol. Biol. 2002, 81, 85–94. [Google Scholar] [CrossRef]
- Tagliaro, F.; Valentini, R.; Manetto, G.; Crivellente, F.; Carli, G.; Marigo, M. Hair analysis by using radioimmunoassay, high-performance liquid chromatography and capillary electrophoresis to investigate chronic exposure to heroin, cocaine and/or ecstasy in applicants for driving licences. Forensic Sci. Int. 2000, 107, 121–128. [Google Scholar] [CrossRef]
- Mannaert, E.; Tytgat, J.; Daenens, P. Development of a stereospecific radioimmunoassay for the analysis of zopiclone and metabolites in urine. Clin. Chim. Acta 1996, 253, 103–115. [Google Scholar] [CrossRef]
- Engvall, E.; Perlmann, P. Enzyme-linked immunosorbent assay (ELISA) Quantitative assay of immunoglobin G. Immunochemistry 1971, 8, 871–874. [Google Scholar] [CrossRef]
- Li, J.; Ding, Y.; Chen, H.; Sun, W.; Huang, Y.; Liu, F.; Wang, M.; Hua, X. Development of an in-direct competitive enzyme-linked immunosorbent assay for propiconazole based on monoclonal antibody. Food Control 2022, 134, 108751. [Google Scholar] [CrossRef]
- Wild, D.G. (Ed.) The Immunoassay Handbook, 4th ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lavery, P.; Brown, M.J.; Pope, A.J. Simple absorbance-based assay for ultra-high throughput screening. J. Biomol. Screen. 2001, 6, 3–9. [Google Scholar] [CrossRef][Green Version]
- Asthagiri, A.R.; Horwitz, A.F.; Lauffenburger, D.A. A rapid and sensitive quantitative kinase activity using a convenient 96-well format. Anal. Biochem. 1999, 269, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hemmilä, I. Fluoroimmunoassays and immunofluorometric assays. Clin. Chem. 1985, 31, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley-VCH GmbH: Weinheim, Germany, 2001. [Google Scholar]
- Schlüter, F.; Riehemann, K.; Kehr, N.S.; Quici, S.; Daniliuc, C.G.; Rizzo, F. A highly fluorescent water soluble spirobifluorene dye with a large Stokes shift: Synthesis, characterization and bio-applications. Chem. Commun. 2018, 54, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Yemets, A.; Plokhovska, S.; Pushkarova, N.; Blume, Y. Quantum dot-antibody conjugates for immunofluorescence studies of biomolecules and subcellular structures. J. Fluoresc. 2022. [Google Scholar] [CrossRef]
- Pietryga, J.M.; Park, Y.-S.; Lim, J.; Fidler, A.F.; Bae, W.K.; Brovelli, S.; Klimov, V.I. Spectroscopic and device aspects of nanocrystal quantum dots. Chem. Rev. 2016, 116, 10513–10622. [Google Scholar] [CrossRef]
- Kakabakos, S.E.; Georgiou, S.; Petrou, P.S.; Christofidis, I. Heterogeneous fluoroimmunoassay using fluorescein as label with measurement of the fluorescence signal directly onto the solid-phase. J. Immunol. Methods 1999, 222, 183–187. [Google Scholar] [CrossRef]
- Dandliker, W.B.; de Saussure, V.A. Fluorescence polarization in immunochemistry. Immunochemistry 1970, 7, 799–828. [Google Scholar] [CrossRef]
- Dandliker, W.B.; Kelly, R.J.; Dandliker, J.; Farquhar, J.; Levin, J. Fluorescence polarization immunoassay. Theory and experimental method. Immunochemistry 1973, 10, 219–227. [Google Scholar]
- Wang, Y.; Adeoye, D.I.; Wang, Y.J.; Roper, M.G. Increasing insulin measurement throughput by fluorescence anisotropy imaging immunoassays. Anal. Chim. Acta 2022, 1212, 339942. [Google Scholar] [CrossRef]
- Fukuyama, M.; Nakamura, A.; Nishiyama, K.; Imai, A.; Tokeshi, M.; Shigemura, K.; Hibara, A. Noncompetitive Fluorescence Polarization Immunoassay for Protein Determination. Anal. Chem. 2020, 92, 14393–14397. [Google Scholar] [CrossRef]
- Lövgren, T.; Meriö, L.; Mitrunen, K.; Mäkinen, M.-L.; Mäkelä, M.; Blomberg, K.; Palenius, T.; Petterson, K. One-step all-in-one dry reagent immunoassays with fluorescent europium chelate label and time-resolved fluorometry. Clin. Chem. 1996, 42, 1196–1201. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Lanthanide-containing molecular and supramolecular polymetallic functional assemblies. Chem. Rev. 2002, 102, 1897–1928. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanide: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Barry, D.E.; Caffrey, D.F.; Gunnlaugsson, T. Lanthanide-directed synthesis of luminescent self-assembly supramolecular structures and mechanically bonded systems from acyclic coordinating organic ligands. Chem. Soc. Rev. 2016, 45, 3244–3274. [Google Scholar] [CrossRef]
- Bottaro, G.; Rizzo, F.; Cavazzini, M.; Armelao, L.; Quici, S. Efficient luminescence from fluorene- and spirobifluorene-based lanthanide complexes upon near-visible irradiation. Chem. Eur. J. 2014, 20, 4598–4607. [Google Scholar] [CrossRef]
- Rizzo, F.; Meinardi, F.; Tubino, R.; Pagliarin, R.; Dellepiane, G.; Papagni, A. Synthesis of 8-hydroxyquinoline functionalised DO3A ligand and Eu(III) and Er(III) complexes: Luminescence properties. Synth. Metals 2009, 159, 356–360. [Google Scholar] [CrossRef]
- Ottonelli, M.; Musso, G.F.; Rizzo, F.; Dellepiane, G.; Porzio, W.; Destri, S. Quantum chemical prediction of antennae structures in lanthanide complexes. Mater. Sci. Eng. B 2008, 146, 50–53. [Google Scholar] [CrossRef]
- Destri, S.; Pasini, M.; Porzio, W.; Rizzo, F.; Dellepiane, G.; Ottonelli, M.; Musso, G.F.; Meinardi, F.; Veltri, L. New erbium complexes emitting in infrared region based on oligothiophene and thiophenefluorene carboxylate. J. Lumin. 2007, 127, 601–610. [Google Scholar] [CrossRef]
- Lama, M.; Mamula, O.; Kottas, G.S.; Rizzo, F.; De Cola, L.; Nakamura, A.; Kuroda, R.; Stoeckli-Evans, H. Lanthanide class of trinuclear enantiopure helical architecture containing chiral ligands: Synthesis, structure, and properties. Chem. Eur. J. 2007, 13, 7358–7373. [Google Scholar] [CrossRef]
- Gagliardo, M.; Rizzo, F.; Lutz, M.; Speck, A.L.; van Klink, G.P.M.; Merbach, A.E.; De Cola, L.; van Koten, G. A PCP-pincer RuII-terpyridine building block as a potential “antenna unit” for intramolecular sensitization. Eur. J. Inorg. Chem. 2007, 2007, 2853–2861. [Google Scholar] [CrossRef]
- De Paoli, G.; Džolic, Z.; Rizzo, F.; De Cola, L.; Vögtle, F.; Müller, W.M.; Richardt, G.; Žinic, M. Reversible luminescent gels containing metal complexes. Adv. Funct. Mater. 2007, 17, 821–828. [Google Scholar] [CrossRef]
- Ottonelli, M.; Izzo, G.M.M.; Rizzo, F.; Musso, G.F.; Dellepiane, G.; Tubino, R. Semiempirical study of the electronic and optical properties of the Er(8-hydroxyquinolinate)3 complex. J. Phys. Chem. B 2005, 109, 19249–19256. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Papagni, A.; Meinardi, F.; Tubino, R.; Ottonelli, M.; Musso, G.F.; Dellepiane, G. Novel lanthanide complexes for visible and IR emission. Synth. Metals 2004, 147, 143–147. [Google Scholar] [CrossRef]
- Parker, D.; Dickins, R.S.; Puschmann, H.; Crossland, C.; Howard, J.A.K. Being excited by lanthanide coordination complexes: Aqua species, chirality, excited-state chemistry, and exchange dynamics. Chem. Rev. 2002, 102, 1977–2010. [Google Scholar] [CrossRef]
- Hewitt, S.H.; Butler, S.J. Application of lanthanide luminescence in probing enzyme activity. Chem. Commun. 2018, 54, 6635–6647. [Google Scholar] [CrossRef]
- Yuan, J.; Matsumoto, K.; Kimura, H. A new tetradentate β-diketonate-europium chelate that can be covalently bound to proteins for time-resolved fluoroimmunoassay. Anal. Chem. 1998, 70, 596–601. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-activated platforms for immunoassay: Probes, encoding methods, and applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Cheng, L.; Ding, S.; Wang, G.; Choo, J.; Chen, L. SERS-based test strips: Principles, designs and applications. Biosens. Bioelectron. 2021, 189, 113360. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Michota, A.; Bukowska, J. Suface-enhanced Raman scattering (SERS) of 4-mercaptobenzoic acid on silver and gold substrates. J. Raman Spectrosc. 2003, 34, 21–25. [Google Scholar] [CrossRef]
- Li, M.; Kang, J.W.; Sukumar, S.; Dasari, R.R.; Barman, I. Multiplexed detection of serological cancer markers with plasmon-enhanced Raman spectro-immunoassay. Chem. Sci. 2015, 6, 3906–3914. [Google Scholar] [CrossRef]
- Hong, W.; Seo, H.K.; Jung, Y.M. SERS immunoassay using microcontact printing for application of sensitive biosensors. Bull. Korean Chem. Soc. 2011, 32, 4281–4285. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, S.; Khan, A.; Ruttner, G.; Leigh, S.Y.; Murray, M.; Abeytunge, S.; Peterson, G.; Rajadhyaksha, M.; Dintzis, S.; et al. Quantitative molecular phenotyping with topically applied SERS nanoparticles for intraoperative guidance of breast cancer lumpectomy. Sci. Rep. 2016, 6, 21242. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Jana, D.; Mandal, A.; De, G. High Raman enhancing shape-tunable Ag nanoplates in alumina: A reliable and efficient SERS technique. ACS Appl. Mater. Interfaces 2012, 4, 3330–3334. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, Z.; Zong, S.; Huang, Z.; Zhang, P.; Cui, Y. A SERS-based immunoassay with highly increased sensitivity using gold/silver core–shell nanorods. Biosens. Bioelectron. 2012, 38, 94–99. [Google Scholar] [CrossRef]
- Kohout, C.; Santi, C.; Polito, L. Anisotropic gold nanoparticles in biomedical applications. Int. J. Mol. Sci. 2018, 19, 3385. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, A.; Lay, L.; Psaro, R.; Polito, L.; Evangelisti, C. Fluidic manufacture of star-shape gold nanoparticles. Chem. Eur. J. 2017, 23, 9732–9735. [Google Scholar] [CrossRef]
- Harmsen, S.; Huang, R.; Wall, M.A.; Karabeber, H.; Samii, J.M.; Spaliviero, M.; White, J.R.; Monette, S.; O’Connor, R.; Pitter, K.L.; et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl. Med. 2015, 7, 271ra277. [Google Scholar] [CrossRef]
- Zeng, L.; Pan, Y.; Wang, S.; Wang, X.; Zhao, X.; Ren, W.; Lu, G.; Wu, A. Raman reporter-coupled Agcore@Aushell nanostars for in vivo improved surface enhanced Raman scattering imaging and near-infrared-triggered photothermal therapy in breast cancers. ACS Appl. Mater. Interfaces 2015, 7, 16781–16791. [Google Scholar] [CrossRef]
- Compostella, F.; Pitirollo, O.; Silvestri, A.; Polito, L. Glyco-gold nanoparticles: Synthesis and applications. Beilstein J. Org. Chem. 2017, 13, 1008–1021. [Google Scholar] [CrossRef]
- Potara, M.; Baia, M.; Farcau, C.; Astilean, S. Chitosan-coated anisotropic silver nanoparticles as a SERS substrate for single-molecule detection. Nanotechnology 2012, 23, 055501. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, L.E.; Asiala, S.M.; Gracie, K.; Faulds, K.; Graham, D. Bioanalytical measurements enabled by surface-enhanced Raman scattering (SERS) probes. Annu. Rev. Anal. Chem. 2017, 10, 415–437. [Google Scholar] [CrossRef]
- McQueenie, R.; Stevenson, R.; Benson, R.; MacRitchie, N.; MacInnes, I.; Maffia, P.; Faulds, K.; Graham, D.; Brewer, J.; Garside, P. Detection of inflammation in vivo by surface-enhanced Raman scattering provides higher sensitivity than conventional fluorescence imaging. Anal. Chem. 2012, 84, 5968–5975. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Jamieson, L.E.; Faulds, K.; Graham, D. Surface-enhanced Raman spectroscopy for in vivo biosensing. Nat. Rev. Chem. 2017, 1, 0060. [Google Scholar] [CrossRef]
- Rohr, T.E.; Cotton, T.; Fan, N.; Tarcha, P.J. Immunoassay employing surface-enhanced Raman spectroscopy. Anal. Biochem. 1989, 182, 388–398. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Houck, K.; Stokes, D.L. Surface-enhanced Raman gene probes. Anal. Chem. 1994, 66, 3379–3383. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.; Mallinder, B.J.; Smith, W.E. Surface-enhanced resonance Raman scattering as a novel method of DNA discrimination. Angew. Chem. Int. Ed. 2000, 39, 1061–1063. [Google Scholar] [CrossRef]
- Stuart, D.A.; Yuen, J.M.; Shah, N.; Lyandres, O.; Yonzon, C.R.; Glucksberg, M.R.; Walsh, J.T.; van Duyne, R.P. In vivo glucose measurement by surface-enhanced Raman spectroscopy. Anal. Chem. 2006, 78, 7211–7215. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Peng, X.-H.; Ansari, D.O.; Yin-Goen, Q.; Chen, G.Z.; Shin, D.M.; Yang, L.; Young, A.N.; Wang, M.D.; Nie, S. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol. 2008, 26, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Keren, S.; Zavaleta, C.; Cheng, Z.; de la Zerda, A.; Gheysens, O.; Gambhir, S.S. Noninvasive molecular imaging of small living subjects using Raman spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Centrone, A.; Park, J.-H.; Ramanathan, R.; Sailor, M.J.; Hatton, T.A.; Bhatia, S.N. SERS-coded gold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv. Mater. 2009, 21, 3175–3180. [Google Scholar] [CrossRef]
- Samanta, A.; Maiti, K.K.; Soh, K.-S.; Liao, X.; Vendrell, M.; Dinish, U.S.; Yun, S.-W.; Bhuvaneswari, R.; Kim, H.; Rautela, S.; et al. Ultrasensitive near-infrared Raman reporters for SERS-based in vivo cancer detection. Angew. Chem. Int. Ed. 2011, 50, 6089–6092. [Google Scholar] [CrossRef] [PubMed]
- Gracie, K.; Correa, E.; Mabbott, S.; Dougan, J.A.; Graham, D.; Goodacre, R.; Faulds, K. Simultaneous detection and quantification of three bacterial meningitis pathogens by SERS. Chem. Sci. 2014, 5, 1030–1040. [Google Scholar] [CrossRef]
- Indrasekara, A.S.D.S.; Meyers, S.; Shubeita, S.; Feldman, L.C.; Gustafsson, T.; Fabris, L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale 2014, 6, 8891–8899. [Google Scholar] [CrossRef]
- Garai, E.; Sensarn, S.; Zavaleta, C.L.; Loewke, N.O.; Rogalla, S.; Mandella, M.J.; Felt, S.A.; Friedland, S.; Liu, J.T.C.; Gambhir, S.S.; et al. A real-time clinical endoscopic system for intraluminal, multiplexed imaging of surface-enhanced Raman scattering nanoparticles. PLoS ONE 2015, 10, e0123185. [Google Scholar] [CrossRef]
- Harmsen, S.; Bedics, M.A.; Wall, M.A.; Huang, R.; Detty, M.R.; Kircher, M.F. Rational design of a chalcogenopyrylium-based surface-enhanced resonance Raman scattering nanoprobe with attomolar sensitivity. Nat. Commun. 2015, 6, 6570. [Google Scholar] [CrossRef] [PubMed]
- Renishaw Launches RenDx® Multiplex Assay System and Fungiplex Assay. Available online: https://www.technologynetworks.com/diagnostics/product-news/renishaw-launches-rendx-multiplex-assay-system-and-fungiplex-assay-222450 (accessed on 1 June 2022).
- Lakowicz, J.R. Radiative decay engineering 3. Surface plasmon-coupled directional emission. Anal. Biochem. 2004, 324, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Kretschmann, E.; Raether, H. Radiative Decay of Nonradiative Surface Plasmons Excited by Light. Z. Naturforsch. A 1968, 23, 2135–2136. [Google Scholar] [CrossRef]
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; González-Villa, A.; Loyez, M.; Caucheteur, C. Plasmonic Optical Fiber-Grating Immunosensing: A Review. Sensors 2017, 17, 2732. [Google Scholar] [CrossRef] [PubMed]
- Barnett, N.W.; Francis, P.S. Chemiluminescence-Overview. In Encyclopedia of Analytical Science, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 506–511. [Google Scholar]
- Roda, A.; Mirasoli, M.; Michelini, E.; di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lai, J.; Wu, K.; Huang, X.; Guo, S.; Zhang, L.; Liu, J. Peroxidase-catalyzed chemiluminescence system and its application in immunoassay. Talanta 2018, 180, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Thorpe, G.H.G.; Maxwell, S.R.J. Enhanced chemiluminescence assay for antioxidant capacity in biological fluids. Anal. Chim. Acta 1992, 266, 265–277. [Google Scholar] [CrossRef]
- Jansen, E.H.J.M.; van den Berg, R.H.; Bergman, J.J. Effect of iron chelates on luminol chemiluminescence in the presence of xanthine oxidase. Anal. Chim. Acta 1989, 227, 57–63. [Google Scholar] [CrossRef]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. Adding biomolecular recognition capability to 3D printed objects. Anal. Chem. 2016, 88, 10767–10772. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.M.; Zomer, G.; Laane, C.; Hilhorst, R. Comparative studies of the chemiluminescent horseradish peroxidase-catalyzed peroxidation of acridan (GZ-11) and luminol reactions: Effect of pH and scavengers of reactive oxygen species on the light intensity of these systems. Luminescence 2000, 15, 189–197. [Google Scholar] [CrossRef]
- Bruemmer, K.J.; Green, O.; Su, T.A.; Shabat, D.; Chang, C.J. Chemiluminescent Probes for Activity-Based Sensing of Formaldehyde Released from Folate Degradation in Living Mice. Angew. Chem. Int. Ed. 2018, 57, 7508–7512. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, C.-C.; Zhang, C.-Y. Development of quantum dot-based biosensors: Principles and applications. J. Mater. Chem. B 2018, 6, 6173–6190. [Google Scholar] [CrossRef]
- Roda, A.; Michelini, E.; Zangheri, M.; di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Chu, W.; Chen, Y.; Liu, W.; Zhao, M.; Li, H. Paper-based chemiluminescence immunodevice with temporal controls of reagent transport technique. Sens. Actuators B 2017, 250, 324–332. [Google Scholar] [CrossRef]
- Sciutto, G.; Zangheri, M.; Anfossi, L.; Guardigli, M.; Prati, S.; Mirasoli, M.; di Nardo, F.; Baggiani, C.; Mazzeo, R.; Roda, A. Miniaturized biosensors to preserve and monitor cultural heritage: From medical to conservation diagnosis. Angew. Chem. Int. Ed. 2018, 57, 7385–7389. [Google Scholar] [CrossRef]
- Roda, A.; Mirasoli, M.; Guardigli, M.; Zangheri, M.; Caliceti, C.; Calabria, D.; Simoni, P. Advanced biosensors for monitoring astronauts’ health during long duration space missions. Biosens. Bioelectron. 2018, 111, 18–26. [Google Scholar] [CrossRef]
- Hercules, D.M. Chemiluminescence resulting from electrochemically generated species. Science 1964, 145, 808–809. [Google Scholar] [CrossRef]
- Visco, R.E.; Chandross, E.A. Electroluminescence in solutions of aromatic hydrocarbons. J. Am. Chem. Soc. 1964, 86, 5350–5351. [Google Scholar] [CrossRef]
- Santhanam, K.S.V.; Bard, A.J. Chemiluminescence of electrogenerated 9,10-diphenylanthracene anion radical. J. Am. Chem. Soc. 1965, 87, 139–140. [Google Scholar] [CrossRef]
- Tokel, N.E.; Bard, A.J. Electrogenerated chemiluminescence. IX. Electrochemistry and emission from systems containing tris(2,2’-bipiridine)ruthenium(II) dichloride. J. Am. Chem. Soc. 1972, 94, 2862–2863. [Google Scholar] [CrossRef]
- Bard, A.J. (Ed.) Electrogenerated Chemiluminescence; Marcel Dekker Inc.: New York, NY, USA, 2004. [Google Scholar]
- White, H.S.; Bard, A.J. Electrogenerated chemiluminescence. 41. Electrogenerated chemiluminescence and chemiluminescence of the Ru(2,2’-bpy)32+-S2O82− system in acetonitrile-water solutions. J. Am. Chem. Soc. 1982, 104, 6891–6895. [Google Scholar] [CrossRef]
- Rubinstein, I.; Bard, A.J. Electrogenerated chemiluminescence. 37. Aqueous ECL systems based on Ru(2,2’-bipydine)32+ and oxalate or organic acids. J. Am. Chem. Soc. 1981, 103, 512–516. [Google Scholar] [CrossRef]
- Rizzo, F.; Polo, F.; Bottaro, G.; Fantacci, S.; Antonello, S.; Armelao, L.; Quici, S.; Maran, F. From blue to green: Fine-tuning of photoluminescence and electrochemiluminescence in bifunctional organic dyes. J. Am. Chem. Soc. 2017, 139, 2060–2069. [Google Scholar] [CrossRef]
- Polo, F.; Rizzo, F.; Veiga-Gutierrez, M.; De Cola, L.; Quici, S. Efficient greenish blue electrochemiluminescence from fluorene and spirobifluorene derivatives. J. Am. Chem. Soc. 2012, 134, 15402–15409. [Google Scholar] [CrossRef]
- Shen, M.; Rodriguez-Lopez, J.; Huang, J.; Liu, Q.; Zhu, X.-H.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of dithienylbenzothiadiazole derivative. Differential reactivity of donor and acceptor groups and simulations of radical cation-anion and dication-radical anion annihilation. J. Am. Chem. Soc. 2010, 132, 13453–13461. [Google Scholar] [CrossRef]
- Campagna, S.; Puntoriero, F.; Nastasi, F.; Bergamini, G.; Balzani, V. Photochemistry and photophysics of coordination compound: Ruthenium. Top. Curr. Chem. 2007, 280, 117–214. [Google Scholar]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Usami, M.; Suzuki, O. Highly Sensitive Electrochemiluminescence Immunoassay Using the Ruthenium Chelate-Labeled Antibody Bound on the Magnetic Micro Beads. Anal. Sci. 1999, 15, 1087–1093. [Google Scholar] [CrossRef]
- Yilmaz, N.; Erbagci, A.B.; Aynacioglu, A.S. Cytochrome P4502C9 genotype in Southeast Anatolia and possible relation with some serum tumour markers and cytokines. Acta Biochim. Pol. 2001, 48, 775–782. [Google Scholar] [CrossRef]
- Khorkova, O.E.; Pate, K.; Heroux, J.; Sahasrabudhe, S. Modulation of amyloid precursor protein processing by compounds with various mechanisms of action: Detection by liquid phase electrochemiluminescent system. J. Neurosci. Methods 1998, 82, 159–166. [Google Scholar] [CrossRef]
- Sapin, R.; le Galudec, V.; Gasser, F.; Pinget, M.; Grucker, D. Elecsys Insulin Assay: Free Insulin Determination and the Absence of Cross-Reactivity with Insulin Lispro. Clin. Chem. 2001, 47, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Juzgado, A.; Soldà, A.; Ostric, A.; Criado, A.; Valenti, G.; Rapino, S.; Conti, G.; Fracasso, G.; Paolucci, F.; Prato, M. Highly sensitive electrochemiluminescence detection of a prostate cancer biomarker. J. Mater. Chem. B 2017, 5, 6681–6687. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Carbayo, M.; Mauri, M.; Alfayate, R.; Miralles, C.; Soria, F. Analytical and clinical evaluation of TSH and thyroid hormones by electrochemiluminescent immunoassays. Clin. Biochem. 1999, 32, 395–403. [Google Scholar] [CrossRef]
- Muzyka, K. Current trends in the development of the electrochemiluminescent immunosensors. Biosens. Bioelectron. 2014, 54, 393–407. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Electro-chemiluminescent biosensing. Anal. Bioanal. Chem. 2008, 390, 155–168. [Google Scholar] [CrossRef] [PubMed]
- First instrument distributed by Igen International Inc., now part of Roche Diagnostics Corporation. Available online: www.roche.com (accessed on 1 June 2022).
- Meyers, M.B. Elecsys® immunoassay system, Ch. 7.17. In The Immunoassay Handbook, 4th ed.; Wild, D.G., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kudruk, S.; Villani, E.; Polo, F.; Lamping, S.; Körsgen, M.; Arlinghaus, H.F.; Paolucci, F.; Ravoo, B.J.; Valenti, G.; Rizzo, F. Solid state electrochemiluminescence from homogenous and patterned monolayers of bifunctional spirobifluorene. Chem. Commun. 2018, 54, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Stringer, B.D.; Shokouhi, A.; Ramkissoon, P.; Agugiaro, J.; Wilson, D.J.D.; Barnard, P.J.; Hogan, C.F. Unusually strong electrochemiluminescence from iridium-based redox polymer immobilized as thin layers or polymer nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 37251–37257. [Google Scholar] [CrossRef] [PubMed]
- Kapturkiewicz, A. Cyclometalated iridium(III) chelates-a new exceptional class of the electrochemilunescent luminophores. Anal. Bioanal. Chem. 2016, 408, 7013–7033. [Google Scholar] [CrossRef]
- Haghighatbin, M.A.; Laird, S.E.; Hogan, C.F. Electrochemiluminescence of cyclometalated iridium (III) complexes. Curr. Opin. Electrochem. 2018, 7, 216–223. [Google Scholar] [CrossRef]
- Kerr, E.; Doeven, E.H.; Barbante, G.J.; Hogan, C.F.; Bower, D.J.; Donnelly, P.S.; Connell, T.U.; Francis, P.S. Annihilation electrogenerated chemiluminescence of mixed metal chelates in solution: Modulating emission colour by manipulating the energetic. Chem. Sci. 2015, 6, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Carrara, S.; Alilprandi, A.; Hogan, C.F.; De Cola, L. Aggregation-induced electrochemiluminescence of platinum(II) complexes. J. Am. Chem. Soc. 2017, 139, 14605–14610. [Google Scholar] [CrossRef] [PubMed]
- Reid, E.F.; Cook, V.C.; Wilson, D.J.D.; Hogan, C.F. Facile tuning of luminescent platinum(II) Schiff base complexes from yellow to near-infrared: Photophysics, electrochemistry, electrochemiluminescence and theoretical calculations. Chem. Eur. J. 2013, 19, 15907–15917. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ugo, P. Recent advances in electrochemiluminescence with quantum dots and arrays of nanoelectrodes. ChemElectroChem 2017, 4, 1663–1676. [Google Scholar] [CrossRef]
- Yao, J.; Li, L.; Li, P.; Yang, M. Quantum dots: From fluorescence to chemiluminescence, bioluminescence, electrochemiluminescence, and electrochemistry. Nanoscale 2017, 9, 13364–13383. [Google Scholar] [CrossRef] [PubMed]
- Kesakar, S.; Rampazzo, E.; Zanut, A.; Palomba, F.; Marcaccio, M.; Valenti, G.; Prodi, L.; Paolucci, F. Dye-doped nanomaterials: Strategic design and role in electrochemiluminescence. Curr. Opin. Electrochem. 2018, 7, 130–137. [Google Scholar] [CrossRef]
- Marquette, C.A.; Blum, L.J. Applications of the luminol chemiluminescent reaction in analytical chemistry. Anal. Bioanal. Chem. 2006, 385, 546–554. [Google Scholar] [CrossRef]
- Li, H.; Voci, S.; Wallabregue, A.; Adam, C.; Labrador, G.M.; Duwald, R.; Delgado, I.H.; Pascal, S.; Bosson, J.; Lacour, J.; et al. Efficient annihilation electrochemiluminescence of cationic helicene luminophores. ChemElectroChem. 2017, 4, 1750–1756. [Google Scholar] [CrossRef]
- Li, H.; Daniel, J.; Verlhac, J.-B.; Blanchard-Desce, M.; Sojic, N. Bright electrogenerated chemiluminescence of a bis-donor quadrupolar spirofluorene dye and its nanoparticles. Chem. Eur. J. 2016, 22, 12702–12714. [Google Scholar] [CrossRef]
- Fiorani, A.; Difonzo, M.; Rizzo, F.; Valenti, G. Versatile electrochemiluminescent organic emitters. Curr. Opin. Electrochem. 2022, 34, 10098. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.B.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef]
- Wen, W.; Yan, X.; Zhu, C.; Du, D.; Lin, Y. Recent advances in electrochemical immunosensors. Anal. Chem. 2017, 89, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Tena, N.; Asuero, A.G. Current state of diagnostic, screening and surveillance testing methods for COVID-19 from an analytical chemistry point of view. Microchem. J. 2021, 167, 106305. [Google Scholar] [CrossRef]
- Lino, A.; Cardoso, M.A.; Gonçalves, H.M.R.; Martins-Lopes, P. SARS-CoV-2 Detection methods. Sensors 2022, 10, 221. [Google Scholar] [CrossRef]
- Kontou, P.I.; Braliou, G.G.; Dimou, N.L.; Nikolopoulos, G.; Bagos, P.G. Antibody Tests in Detecting SARS-CoV-2 Infection: A Meta-Analysis. Diagnostics 2020, 10, 319. [Google Scholar] [CrossRef]
- Mattiolli, I.A.; Hassan, A.; Crespilho, O.N.O.J.F.N. On the Challenges for the Diagnosis of SARS-CoV-2 Based on a Review of Current Methodologies. ACS Sens. 2020, 5, 3655–3677. [Google Scholar] [CrossRef]
- Mohit, E.; Rostami, Z.; Vahidi, H. A comparative review of immunoassays for COVID-19 detection. Expert. Rev. Clin. Immunol. 2021, 17, 573–599. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/medical-devices/emergency-use-authorizations-medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices (accessed on 1 June 2022).
- Lin, D.; Liu, L.; Zhang, M.; Hu, Y.; Yang, Q.; Guo, J.; Dai, Y.; Xu, Y.; Cai, Y.; Chen, X.; et al. Evaluations of the serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2271–2277. [Google Scholar] [CrossRef]
- Cai, X.-F.; Chen, J.; Hu, J.-L.; Long, Q.-X.; Deng, H.-J.; Liu, P.; Fan, K.; Liao, P.; Liu, B.-Z.; Wu, G.-C.; et al. A Peptide-Based Magnetic Chemiluminescence Enzyme Immunoassay for Serological Diagnosis of Coronavirus Disease 2019. J. Infec. Dis. 2020, 222, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Schnurra, C.; Reiners, N.; Biemann, R.; Kaiser, T.; Trawinski, H.; Jassoy, C. Comparison of the diagnostic sensitivity of SARS-CoV-2 nucleoprotein and glycoprotein-based antibody tests. J. Clin. Virol. 2020, 129, 104544. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, P.; Tian, Y.; Wang, J.; Zeng, H.; Wang, J.; Liu, J.; Chen, Z.; Zhang, L.; He, H.; et al. Clinical Significance of an IgM and IgG Test for Diagnosis of Highly Suspected COVID-19. Front. Med. 2021, 8, 569266. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; An, T.; Situ, B.; Hu, Y.; Ou, Z.; Li, Q.; He, X.; Zhang, Y.; Tian, P.; Sun, D.; et al. Heat inactivation of serum interferes with the immunoanalysis of antibodies to SARS-CoV-2. J. Clin. Lab. Anal. 2020, 34, e23411. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Song, K.; Cai, K.; Liu, L.; Tang, D.; Long, W.; Zhai, B.; Chen, J.; Tao, Y.; Zhao, Y.; et al. B-Cell-Epitope-Based Fluorescent Quantum Dot Biosensors for SARS-CoV-2 Enable Highly Sensitive COVID-19 Antibody Detection. Viruses 2022, 14, 1031. [Google Scholar] [CrossRef]
- Meschi, S.; Colavita, F.; Bordi, L.; Matusali, G.; Lapa, D.; Amendola, A.; Vairo, F.; Ippolito, G.; Capobianchi, M.R.; Castilletti, C. Performance evaluation of Abbott ARCHITECT SARS-CoV-2 IgG immunoassay in comparison with indirect immunofluorescence and virus microneutralization test. J. Clin. Virol. 2020, 129, 104539. [Google Scholar] [CrossRef]
- Jiang, C.; Mu, X.; Liu, S.; Liu, Z.; Du, B.; Wang, J.; Xu, J. A Study of the Detection of SARS-CoV-2 ORF1ab Gene by the Use of Electrochemiluminescent Biosensor Based on Dual-Probe Hybridization. Sensors 2022, 22, 2402. [Google Scholar] [CrossRef]
- Gutiérrez-Gálvez, L.; del Caño, R.; Menéndez-Luque, I.; García-Nieto, D.; Rodríguez-Peña, M.; Luna, M.; Pineda, T.; Pariente, F.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescent nanostructured DNA biosensor for SARS-CoV-2 detection. Talanta 2022, 240, 123203. [Google Scholar] [CrossRef]
- Favresse, J.; Eucher, C.; Elsen, M.; Tré-Hardy, M.; Dogné, J.-M.; Douxfils, J. Clinical Performance of the Elecsys Electrochemiluminescent Immunoassay for the Detection of SARS-CoV-2 Total Antibodies. Clin. Chem. 2020, 66, 1104–1106. [Google Scholar] [CrossRef]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.-Y.; Tian, Z.; Shang, Y.; Xing, J.; Jiang, Y.; Zeng, J. Ag Nanoparticles with Ultrathin Au Shell-Based Lateral Flow Immunoassay for Colorimetric and SERS Dual-Mode Detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94, 8466–8473. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).