Abstract
The application of ion-selective electrodes (ISEs) in the detection and determination of environmental pollutants has become a very important mission in the last few years. Two selective and sensitive membrane electrodes were fabricated in the laboratory and intended to evaluate the electrochemical response of bromazepam (BRZ) using phosphotungstic acid (PTA) and sodium tetraphenylborate (TPB) as ion pairing agents. The linearity range of the fabricated electrodes was between 1 × 10−6 M to 1 × 10−3 M. Nernstian slopes of 54 mV/decade and 57 mV/decade were obtained for the BRZ-PTA and BRZ-TPB membrane electrodes, respectively. The performance of the fabricated membranes was optimum in the pH range of 3–6. Optimum electrochemical response was attained through the careful adjustment of all assay settings. The cited method was successfully applied for the selective determination of BRZ in either its pure form or real wastewater samples obtained from a pharmaceutical industrial plant. The main core of novelty in the suggested method lies in the application of the membranes for the sensitive, selective, and economic determination of BRZ in real wastewater effluents without the tedious sample pretreatment procedures. This can make the suggested method considered an eco-friendly method, as it minimizes the use of organic solvents and chemicals used in the pretreatment process.
1. Introduction
Bromazepam (BRZ) is a classical benzodiazepine that is medically applied for the effective handling of insomnia, control of panic attacks, or anxiety. It gives its action by acting as a positive modulator that binds to gamma amino buteric acid (GABA)A receptors, performing a conformational alteration and increasing the inhibitory effects of GABA [1]. Regarding the International Narcotics Control Board (INCB), BRZ is a widespread used drug worldwide [2]. This widespread use and the increase in its production may lead to its being present commonly in the wastewater effluents coming from pharmaceutical factories, which constitute an enormous risk on either the ecosystem or human health [3,4]. Using BRZ for a long period has many adverse effects, including neurotoxicity, as well as cognitive decline and hip fracture [5]. Many studies attempted to determine the levels of active pharmaceutical ingredients (APIs) in the environment, particularly in wastewater effluents [6,7,8]. Because the presence of APIs in the environment poses a significant risk to humans, there is no permissible minimum limit for their presence in either natural water or soil [9].
BRZ is chemically known as 7-bromo- 1,3-dihydro-5-(2-pyridyl)-2H-1,4-benzodiazepine-2-one (Figure 1). It is pharmacologically classified as benzodiazepine, applied for the handling of anxiety and panic attacks [1].
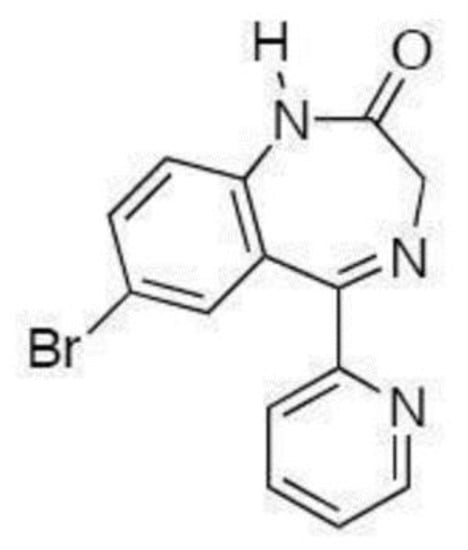
Figure 1.
Chemical structure of bromazepam (BRZ).
According to the literature survey concerning the analytical techniques applied for the quantification of BRZ, there are many papers dealing with its determination in different samples. These methods comprise spectrophotometric and spectrofluorimetric methods [10,11,12]. Also, high performance liquid chromatography (HPLC) [13,14,15,16,17] and liquid chromatography coupled to tandem mass spectroscopy (LC-MS/MS) [18,19,20] were applied for the quantification of the studied drug in bulk, dosage forms, and biological fluids. At the same time, many electrochemical methods were applied for the determination of BRZ, which itself was determined by the application of different polarographic and voltammetric techniques [21,22,23,24]. Also, different kinds of electrodes were applied for the determination of BRZ, including solid contact ion-selective electrodes (SC-ISEs) [25], carbon paste electrodes [26], and boron-doped diamond electrodes [27].
BRZ was not investigated enough in surface water. This fact is coupled with the huge elevation in its production and occurrence in biological treatment systems [28]. Therefore, there is an urgent need to develop new sensitive, selective, and accurate analytical methods that can be applied for sensing and determining such pollutants in the surrounding environment.
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) after sample preparation by solid-phase extraction (SPE) is the most commonly used strategy for quantifying BRZ in surface water [29]. LC-MS/MS suffers from the drawback of being a complicated technique that comprises many sample pretreatment steps for analyte extraction and matrix effect removal [30].
Electrochemical analysis via fabrication of membrane sensitive electrodes is a technique applied for the quantification of different organic and inorganic analytes due to its simplicity, being an economic and non-destructive technique [31].
BRZ was analyzed using equilibrium potentiometry through the in-laboratory synthesis of ISEs for its sensing and determination in dosage form [32]. The mentioned method suffers from low sensitivity, as it cannot determine the studied drug at concentrations below 1 × 10−4 M. Therefore, in this work, efforts were exerted to propose an analytical method applying equilibrium potentiometry to determine BRZ with better sensitivity and selectivity in real wastewater effluents without the tedious sample pretreatment procedure, which is considered the main core of the work novelty.
After an extensive literature survey, there were no articles concerned with the BRZ determination in industrial wastewater effluents through the fabrication of membrane sensitive electrodes. Therefore, the main task of this work is to develop, validate, and apply a sensitive, accurate, and simple technique for the determination of BRZ in diverse samples, particularly in industrial wastewater effluents and complex matrices, without any sample pretreatment.
2. Materials and Methods
2.1. Instrumentation
A digital mV/pH meter (Model 3510; Jenway, UK) was used for electrochemical measurements. It was connected with a bi-junctional reference electrode of the Ag/AgCl type. The pH measurement was performed with a pH glass electrode (Jenway, UK).
2.2. Chemicals and Reagents
BRZ bulk powder was kindly supplied by F. Hoffmann-La Roche AG, Grenzacherstrasse, Basel, Switzerland. Its percentage purity was labeled as 100.78 ± 0.57. Sodium tetraphenylborate (TPB), phosphotungstic acid (PTA), hydrochloric acid, NaOH, KCl, silver nitrate, and tetrahydrofuran (THF) were supplied by Prolabo, France. Dioctyl phthalate (DOP), nitrophenyl octyl ether (NPOE), and dibutyl sebacate (DBS) were purchased from Sigma, Germany. Potassium chloride and polyvinyl chloride (PVC) (HMW grade) were purchased from Fluka Chemie, Germany. Deionized H2O was supplied from the water still of Aquatron Automotive, Bibby Sterillin Ltd., (Staffordshire, UK).
2.3. Collection, Preparation, and Storage of the Samples
Collection of the real wastewater samples was carried out at a pharmaceutical industrial plant producing BRZ in tablet dosage form. The samples’ pH was adjusted to 4.0 by the addition of either 10−2 M HCl or 10−2 M NaOH. Filtration of the collected samples was done using nylon membrane filters (0.45 µm) to remove fine particulate matter. To guard against any degradation of the sample, the filtrate was stored in amber glass bottles at 4 °C.
2.4. Standard Solutions
BRZ Stock standard solution (2 × 10−2 M) was prepared by dissolving the appropriate amount of BRZ in 2 × 10−2 M HCl and the volume was completed to 100 mL using deionized water. Working standard solutions in the concentration range (1 × 10−3 to 1 × 10−7 M) were prepared by accurate dilution with deionized water. Also, standard solutions of PTA and TPB (2 × 10−2 M) were prepared by dissolving appropriate weights of PTA and TPB in deionized water.
2.5. Preparation of Ion Pair Complexes
Twenty mL each of standard aqueous solutions of BRZ (2 × 10−2 M) and PTA (2 × 10−2 M) or TPB (2 × 10−2 M) were mixed and vortexed for about 5 min. The obtained precipitates of BRZ-PTA and BRZ-TPB ion pair complexes were processed and washed with cold deionized water. The ion pair complex was completely dried at room temperature and then ground to produce a fine powder.
2.6. Sensors’ Fabrication, Calibration, and Optimization
Ten mg of the ion pair complex (BRZ-PTA or BRZ-TPB) was mixed with 190 mg PVC and 400 mg NPOE in two separate 5 cm diameter petri dishes. Addition of five mL THF was done to perform the task of the previous mixture dissolution. Removal of the solvent was done by leaving the petri dishes at room temperature for 24 h. A membrane of 8 mm diameter and 0.1 thickness was taken out and applied to a tip of PVC with the aid of THF, which was joined to the end of the electrode’s body. Mixing equal volumes of BRZ standard solution and KCl of the same molarity (1 × 10−2 M) was performed to prepare the internal reference solution. A wire of Ag/AgCl of 1 mm diameter was made as internal reference electrode, and was then dipped in the internal reference solution.
The fabricated membranes were conditioned by soaking in 1 × 10−2 M BRZ standard solution for 24 h. The sensors were then calibrated using BRZ standard solutions in the range of 1 × 10−8–1 × 10−2 M. The standard graphs were plotted and used for the subsequent determination of unknown concentrations of BRZ.
The electrodes’ competence was examined, optimized, and validated according to the IUPAC recommendation [33]. The impact of the solvent mediator was assessed on using various plasticizers of different polarities. The investigated plasticizers were DBP, DOP, and NPOE to get optimum electrode performance. The pH effect was studied using BRZ standard solution of concentration 10−4 M via the careful drop wise addition of 1 × 10−2 M NaOH or HCl. Potential stability and repeatability of BRZ sensors were traced for 30 days. Calculation of the slope (mV/decade) was performed for each fabricated membrane and compared with the slopes obtained during the first-time calibration. Thermal stability of the fabricated sensors was investigated at three temperature levels (25 °C, 30 °C, and 40 °C). The fabricated membranes selectivity was effectively examined via the matched potential method (MPM) by calculating the potentiometric selectivity coefficients (PSCs) for the examined interferents. The PSC can be defined as the ratio of the primary ion concentration to the interfering ion concentration which gives the same potential change in a reference solution [34,35].
2.7. Application of the Suggested Method in Spiked Water Samples and Real Wastewater Effluent
The proposed method was applied to the determination of BRZ in distilled and tap water samples spiked with known BRZ concentrations and adjusted to pH 4. Also, six real wastewater samples were analyzed using fabricated sensors. The standard curves were utilized to compute the unknown samples’ concentrations. The results obtained for the real wastewater samples were compared to those obtained on applying a reference method [13] after solid-phase extraction of the studied drug from the samples.
3. Results
The three main components of measuring an ISE are an internal reference solution and an outer sample solution separated by an ion-selective membrane. The potential developed at the membrane is the result of an ion exchange process occurring at each interface between the membrane and the solution. At each solution-membrane interface, BRZ molecules are selectively bonded with specific sites in the membrane (PTA or TPB) until an ion-exchange equilibrium is established. The partitioning of BRZ between the aqueous solution phase and the membrane phase depends on its activity or concentration. The resulting charge separation at each interface results in a phase-boundary potential. If BRZ concentration on each side of the membrane is equal, the potential difference across the membrane will be zero. However, if the concentrations are not equal, a membrane potential will develop [36,37,38].
3.1. Performance Characteristics of the Fabricated Membranes
The performance criteria of the fabricated membranes were studied and presented in Table 1. The fabricated sensors showed nearly a Nearnstian response in a range of concentration 1 × 10−6 to 1 × 10−3 M. Slopes of 54 mV and 57 mV per decade for the BRZ-PTA membrane electrode and BRZ-TPB membrane electrode, respectively (Table 1). The nearly Nearnstian slopes can be considered as strong evidence for the 1:1 ratio between the studied drug and PTA or TPB in the ion pair complex. The standard plots are constructed and given in Figure 2.

Table 1.
Electrochemical response characteristics of the fabricated electrodes.
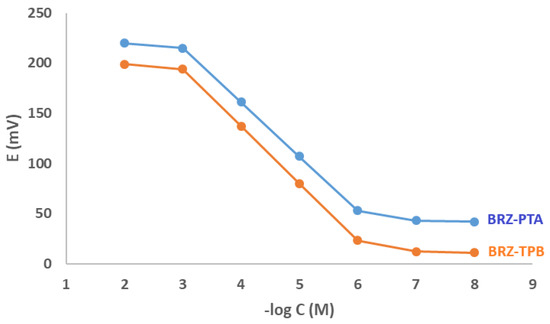
Figure 2.
Profile of the potential (in mV) versus −log concentration (in M) for bromazepam-phosphotungstate (BRZ-PTA) and bromazepam-tetraphenyl borate (BRZ-TPB membrane sensors at pH 4.
The fabricated membranes’ performance was examined according to the IUPAC guidelines [33]. The screened factors included the effect of solvent mediators, response time, effect of pH, linearity ranges, detection limits, lifetime, and selectivity.
The impact of the type of plasticizer was examined by trying three different plasticizers with variable polarity. The examined plasticizers were DOP, NPOE, and DBS. The plasticizer that gave the optimum performance was NPOE, as its fabricated sensors gave slopes close to the Nernstian behavior.
Detection limits were computed at the intersection point of the extrapolated segments coming from the two linear parts of the calibration curves [33]. The detection limits extended down to 0.25 µg/mL which revealed excellent sensitivity (Table 1).
The fabricated sensors’ performance was affected by the pH of the medium. It was examined in the range of 1–10 pH units. They were obtained through the action of 1 × 10−2 M HCl and 1 × 10−2 M NaOH on a standard solution of 1 × 10−4 M BRZ. It was noticed that the optimum electrochemical response was attained in the pH range of 3–6 (Figure 3).
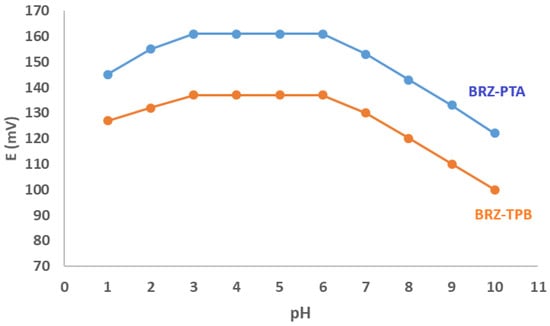
Figure 3.
Effect of pH on the potential response bromazepam-phosphotungstate (BRZ-PTA) and bromazepam-tetraphenyl borate (BRZ-TPB membrane sensors.
The fabricated sensors’ lifetime was 21 days for both electrodes. The acquired potential was almost stable in this period, as declared in Figure 4. The response time was 10–20 S for prepared sensors when used for BRZ standard solutions in the concentration range of 10−3–10−6 M. On the other hand, the response time was 20–30 S when the sensors were used for potential measurement at concentrations below 10−6 M. After three weeks, the response time was increased to 1.5 min., which reflected a marked decrease in electrode stability.
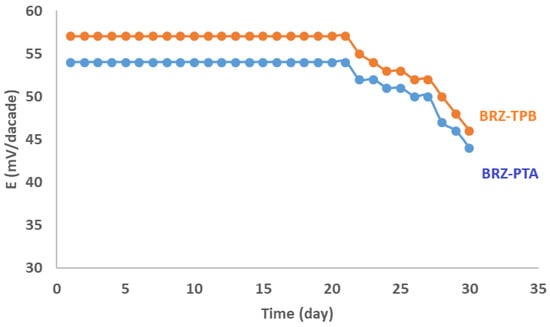
Figure 4.
Stability of the bromazepam-phosphotungstate (BRZ-PTA) and bromazepam-tetraphenyl borate (BRZ-TPB membrane sensors.
The influence of temperature on the fabricated sensors’ performance was examined at 3 temperatures (25 °C, 30 °C, and 40 °C) by plotting the calibration graphs at the three studied temperatures. No significant variations in the calibration plots were noticed, indicating the excellent temperature stability of the fabricated electrodes. The potential slopes and the detection limits exhibited no significant variations, confirming the thermal stability of the studied electrodes up to 40 °C.
To examine the method accuracy, fabricated membranes were applied to analyze different BRZ concentrations. Method robustness was examined by checking the capability of the method to tolerate deliberate changes in experimental conditions. In addition, the ruggedness was checked to measure the method repeatability (Table 1).
Sensors’ selectivity can be considered as a determining factor in the application of the fabricated electrodes, as it points out the usefulness and scope in handling real samples. The membranes’ selectivity was examined by checking the membrane performance in the presence of different interfering materials and calculating the PSCs [35]. Many salts and structurally related benzodiazepines were tested as interferents. Table 2 presents the calculated PSCs, which were of the order of 10−3 or smaller, showing excellent sensors’ selectivity.

Table 2.
Potentiometric selectivity coefficients of the proposed bromazepam-selective sensors by matched potential method.
3.2. Quantification of BRZ in Spiked Water Samples
The fabricated membranes were successfully applied for the reliable determination of BRZ in distilled and tap water samples spiked with BRZ. The results are declared in Table 3, showing excellent method accuracy.

Table 3.
Determination of BRZ in spiked water samples using the fabricated membrane sensors.
3.3. Quantification of BRZ in Real Wastewater Samples
The working conditions were optimized to get the optimum membrane performance. The fabricated sensors were then applied for the careful analysis of different real wastewater samples directly without any sample pretreatment. The lack of sample pretreatment can be considered as excellent merit for the proposed analytical method. Comparison of the obtained results was done against those obtained by applying a reference method [13] to the same real wastewater samples after pretreatment via solid-phase extraction (Table 4).

Table 4.
Determination of BRZ in actual wastewater samples from pharmaceutical industrial plant.
4. Discussion
There is no doubt that the monitoring of environmental pollutants has been considered a crucial task in the last few years. In this work, two membrane-sensitive electrodes were fabricated to be effectively applied for the selective and sensitive determination of BRZ in real wastewater samples without any specimen pretreatment. First, the working parameters were optimized to ensure optimum membrane behavior. The examined parameters were the working pH of the medium, detection limits, response time, lifetime, and selectivity. The optimization procedures were followed according to the IUPAC recommendations [33].
Regarding the effect of plasticizers on the membrane performance, three different plasticizers were tried (NPOE, DBS, and DOP), which were of variable polarity. NPOE was the plasticizer that gave sensors their optimum electrochemical performance. The potential slope exhibited by the NPOE membranes showed the nearest response to the Nernstian behavior, which can be attributed to the good match in polarity between the studied drug and NPOE. This matching enabled the optimum ion exchange across the membranes.
The electrochemical response of the fabricated membranes was evaluated and compared with that of a membrane in a cited method, which depends on the same equilibrium potentiometric technique [32] (Table 1). The BRZ-PTA and BRZ-TPB membranes showed slopes of 54 and 57 mv/decade, respectively, which are closer to the ideal Nermstian behavior than the case in the published method (52 mv/decade). This may reflect the superior performance of the fabricated membranes. Also, the proposed membranes showed a faster response time (10–20 S) than the situation in the published method (>20 S). The fabricated membranes showed steady response over a pH range of 3–6, which is a wider range than the membrane in the published method (pH 3). A marked deviation from linearity was noticed in the calibration curves at pH levels below three. This behavior was due to the elevated hydrogen ion concentrations. The loss of calibration curve linearity over pH six may be attributed to the BRZ formation of its base form that is characterized by its lower solubility, leading to a marked decrease in electrode potentials [39]. Also, the fabricated membranes were characterized by superior sensitivity, as evidenced by their lower detection limits (0.25 µg/mL), if compared with the membrane in the published method (9.4 µg/mL). The sensitivity issue is a determining factor in the successful application of fabricated sensors for the detection and quantification of BRZ in real wastewater samples.
Regarding the long-term stability, the fabricated sensors showed high potential stability up to 21 days, which indicates the suitability of PTA and TPB as ion pairing materials capable of sensing and determining the studied drug. The fabricated sensors showed a marked reduction in their stability after 21 days. This may be attributed to ion-pair leaching out of the fabricated sensors.
BRZ fabricated sensors exhibited excellent thermal stability as declared by the in-variation in the potential slopes and lower detection limits up to 40 °C.
Accuracy, ruggedness, and robustness are important items in the validation scheme. Different BRZ samples were analyzed using fabricated electrodes to evaluate the method’s accuracy. At the same time, robustness and ruggedness were carefully evaluated to show that the proposed method could be applied even in the case of slight variation in the experimental conditions and declare method repeatability. All these parameters are presented in Table 1.
Method selectivity is an important parameter that should be carefully evaluated to be confident enough in the applicability of the proposed method in the presence of a wide range of interfering materials. The membrane selectivity is dependent on the stereo specificity, which means the extent of fitting between the lipophilicity sites of the two competing species in the test solution side and the ion exchanger receptor [40]. It is defined according to the MPM. It is a theory for the determination of the PSCs of ISEs for two ions with any charge. This MPM theory is based on electrical diffuse layers on both the membrane and aqueous sides of the interface, avoiding disadvantages encountered by the Nicolsky–Eisenman equation which include the inability to apply in the case of non-Nernstian response of the investigated interferent and dissimilar charges of the sample and the interfering ions [34,35]. Different interfering materials, commonly present in the wastewater effluent of pharmaceutical industrial plants, were tried. The tried and tested interferents included different inorganic salts with variable charges. Also, benzodiazepines with similar chemical structures to BRZ (diazepam and clonazepam) were examined. The fabricated membranes showed excellent selectivity, as evidenced by the calculated PSCs as presented in Table 2.
Application of the fabricated membranes for the selective and accurate analysis of distilled and tap water samples spiked with BRZ was carried out. The obtained Rec.% values, which are not deviating than the 100% by more than 2%, indicate excellent membranes’ performance. The results were introduced in Table 3. Meanwhile, real wastewater samples were successfully analyzed for BRZ using the fabricated sensors without applying any sample pretreatment procedure, indicating the suitability of the proposed method for the simple, selective, and accurate environmental monitoring of BRZ in industrial wastewater effluents, which is the main core of this work. The results for determination of BRZ in real wastewater samples were validated by analyzing the same samples with reference analytical method [13], showing excellent matching between the results (Table 4). The lack of sample pretreatment procedure is considered to be a major advantage for the proposed method over other analytical procedures applied for determination of BRZ in aquatic samples such as LC-MS/MS. The lack of sample pretreatment confirms that the proposed method is simple and economic. Also, the cited method can be considered an eco-friendly one because it minimizes the use of organic solvents and chemicals.
5. Conclusions
The novelty of this work originates from the absence of any published work dealing with the electrochemical determination of BRZ in real wastewater effluents. The aspect of superiority of this work over other techniques applied for the quantification of the studied drug in environmental samples is the lack of a sample pretreatment protocol that ensures the simplicity of the proposed method. Also, the proposed method is economical enough that it has a low sample analysis cost when compared with LC-MS/MS and UPLC.
Constant, fast potential responses were obtained over a broad pH and concentration range, indicating the ease of applicability of the proposed method for the sensing and determination of BRZ in real wastewater samples.
Author Contributions
Conceptualization, S.A.A.-G. and H.H.A.; Data curation, S.A.A.-G. and H.H.A.; Formal analysis, S.A.A.-G.; Investigation, S.A.A.-G. and H.H.A.; Methodology, S.A.A.-G., and H.H.A.; Resources, S.A.A.-G. and H.H.A.; Software, S.A.A.-G. and H.H.A.; Supervision, S.A.A.-G.; Validation, S.A.A.-G.; Visualization, S.A.A.-G. and H.H.A.; Writing-Original draft, S.A.A.-G.; Writing-Review & editing, S.A.A.-G. and H.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The current work was supported by Taif University Researchers Supporting Project number (TURSP-2020/29), Taif University, Taif, Saudi Arabia (to Hany H. Arab).
Conflicts of Interest
The authors declare that they have no competing interest in this manuscript.
References
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- International Narcotics Control Board (INCB). Psychotropic Substances—Statistics; International Narcotics Control Board (INCB): New York, NY, USA, 2015. [Google Scholar]
- Brodin, T.; Nordling, J.; Lagesson, A.; Klaminder, J.; Hellström, G.; Christensen, B.; Fick, J. Environmental relevant levels of a benzodiazepine (oxazepam) alters important behavioral traits in a common planktivorous fish, (Rutilus rutilus). J. Toxicol. Environ. Health A 2017, 80, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Olfson, M.; Kroenke, K. Balancing the Risks and Benefits of Benzodiazepines. JAMA 2021, 32, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Streltzer, J. Risks Associated with Long-Term Benzodiazepine Use. Am. Fam. Physician 2013, 88, 224–225. [Google Scholar] [PubMed]
- Seifrtová, M.; Aufartová, J.; Vytlacilová, J.; Pena, A.; Solich, P.; Nováková, L. Determination of fluoroquinolone antibiotics in wastewater using ultra high-performance liquid chromatography with mass spectrometry and fluorescence detection. J. Sep. Sci. 2010, 33, 2094–2108. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; Afzal, O.; Arab, H.H.; Alabbas, A.B.; Alqarni, A.M. Fabrication of Membrane Sensitive Electrodes for the Validated Electrochemical Quantification of Anti-Osteoporotic Drug Residues in Pharmaceutical Industrial Wastewater. Molecules 2021, 26, 5093. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, S.A.; Arab, H.H.; Alabbas, A.B. Validated Simultaneous Gradient Ultra-Performance Liquid Chromatographic Quantification of Some Proton Pump Inhibitor Drug Residues in Saudi Pharmaceutical Industrial Wastewater. Molecules 2021, 26, 4358. [Google Scholar] [CrossRef]
- Boxall, A.B. The environmental side effects of medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- El-Brashy, A.M.; Aly, F.A.; Belal, F. Determination of 1,4-benzodiazepines in drug dosage forms by difference spectrophotometry. Microchim. Acta 1993, 110, 55–60. [Google Scholar] [CrossRef]
- Salem, A.A.; Barsoum, B.N.; Izake, E.L. Spectrophotometric and fluorimetric determination of diazepam, bromazepam and clonazepam in pharmaceutical and urine samples. Spectrochim. Acta A 2004, 60, 771–780. [Google Scholar] [CrossRef]
- Darwish, H.W.; Ali, N.A.; Naguib, I.A.; El Ghobashy, M.R.; Al-Hossaini, A.M.; Abdelrahman, M.M. Stability indicating spectrophotometric methods for quantitative determination of bromazepam and its degradation product. Spectrochim. Acta A 2020, 238, 118433. [Google Scholar] [CrossRef] [PubMed]
- Le Solleu, H.; Demotes-Mainard, F.; Vinçon, G.; Bannwarth, B. The determination of bromazepam in plasma by reversed-phase high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1993, 11, 771–775. [Google Scholar] [CrossRef]
- Capella-Peiró, M.E.; Bose, D.; Martinavarro-Domínguez, A.; Gil-Agustí, M.; Esteve-Romero, J. Direct injection micellar liquid chromatographic determination of benzodiazepines in serum. J. Chromatogr. B 2002, 780, 241–249. [Google Scholar] [CrossRef]
- Podilsky, G.; Berger-Gryllaki, M.; Testa, B.; Pannatier, A. Development and validation of an HPLC method for the simultaneous monitoring of bromazepam and omeprazole. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 878–890. [Google Scholar] [CrossRef]
- Darwish, H.W.; Ali, N.A.; Naguib, I.A.; El Ghobashy, M.R.; Al-Hossaini, A.M.; Abdelrahman, M.M. Development and validation of a stability indicating RP-HPLC-DAD method for the determination of bromazepam. PLoS ONE 2021, 16, e0244951. [Google Scholar] [CrossRef]
- Al-Hawasli, H.; Al-Khayat, M.A.; Al-Mardini, M.A. Development of a validated HPLC method for the separation and analysis of a Bromazepam, Medazepam and Midazolam mixture. J. Pharm. Anal. 2012, 2, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.C.S.; Monteiro, T.M.; de Miranda Neves, C.S.; da Silva Gram, K.R.; Volpato, N.M.; Silva, V.A.; Caminha, R.; Gonçalves Mdo, R.; Santos, F.M.; Silveira, G.E.; et al. On-line solid-phase extraction coupled with high-performance liquid chromatography and tandem mass spectrometry (SPE-HPLC-MS-MS) for quantification of bromazepam in human plasma: An automated method for bioequivalence studies. Ther. Drug Monit. 2005, 27, 601–607. [Google Scholar] [CrossRef]
- Laurito, T.L.; Mendes, G.D.; Santagada, V.; Caliendo, G.; de Moraes, M.E.A.; De Nucci, G. Bromazepam determination in human plasma by high-performance liquid chromatography coupled to tandem mass spectrometry: A highly sensitive and specific tool for bioequivalence studies. J. Mass Spectrum. 2004, 39, 168–176. [Google Scholar] [CrossRef]
- Chèze, M.; Villain, M.; Pépin, G. Determination of bromazepam, clonazepam and metabolites after a single intake in urine and hair by LC-MS/MS: Application to forensic cases of drug facilitated crimes. Forensic Sci. Int. 2004, 145, 123–130. [Google Scholar] [CrossRef]
- Brooks, M.A.; Hackman, M.R. Trace Level Determination of 1,4-benzodiazepines in blood by differential pulse polarography. Anal. Chem. 1975, 47, 2059–2062. [Google Scholar] [CrossRef]
- Dos Santos, M.M.C.; Famila, V.; Simões Gonçalves, M.L. Square-wave voltammetric techniques for determination of psychoactive 1,4-benzodiazepine drugs. Anal. Bioanal. Chem. 2002, 374, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Hernández, L.; Zapardiel, A.; López, J.A.P.; Bermejo, E. Determination of camazepam and bromazepam in human serum by adsorptive stripping voltammetry. Analyst 1987, 112, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Valdeón, J.L.; Escribano, M.T.S.; Hernandez, L.H. Determination of bromazepam and its urinary metabolites, with a previous hydrolysis reaction, by voltammetric and spectrophotometric techniques. Analyst 1987, 112, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; Barsoum, B.N.; Izake, E.L. Potentiometric determination of diazepam, bromazepam and clonazepam using solid contact ion-selective electrodes. Anal. Chim. Acta 2003, 498, 79–91. [Google Scholar] [CrossRef]
- Samiec, P.; Navrátilová, Z. Electrochemical behavior of bromazepam and alprazolam and their determination in the pharmaceutical tablets Lexaurin and Xanax on carbon paste electrode. Monatsh. Chem. 2016, 148, 449–455. [Google Scholar] [CrossRef]
- Samiec, P.; Švorc, Ľ.; Stanković, D.M.; Vojs, M.; Marton, M.; Navrátilová, Z. Mercury-free and modification-free electroanalytical approach towards bromazepam and alprazolam sensing: A facile and efficient assay for their quantification in pharmaceuticals using boron-doped diamond electrodes. Sens. Actuators B-Chem. 2017, 245, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Cunha, D.L.; de Araujo, F.G.; Marques, M. Psychoactive drugs: Occurrence in aquatic environment, analytical methods, and ecotoxicity—A review. Environ. Sci. Pollut. Res. 2017, 24, 24076–24091. [Google Scholar] [CrossRef]
- de Araujo, F.G.; Bauerfeldt, G.F.; Marques, M.; Martins, E.M. Development and Validation of an Analytical Method for the Detection and Quantification of Bromazepam, Clonazepam and Diazepam by UPLC-MS/MS in Surface Water. Bull. Environ. Contam. Toxicol. 2019, 103, 362–366. [Google Scholar] [CrossRef]
- Seger, C. Usage and limitations of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in clinical routine laboratories. Wien. Med. Wochenschr. 2012, 162, 499–504. [Google Scholar] [CrossRef]
- Coşofre, V.V.; Buck, R.P. Recent advances in pharmaceutical analysis with potentiometric membrane sensors. Crit. Rev. Anal. Chem. 1993, 24, 1–58. [Google Scholar] [CrossRef]
- Al Attas, A.S. Construction and analytical application of ion selective bromazepam sensor. Int. J. Electrochem. Sci. 2009, 4, 20–29. [Google Scholar]
- Lindner, E.; Umezawa, Y. Performance evaluation criteria for preparation and measurement of macro-and microfabricated ion-selective electrodes (IUPAC Technical Report). Pure Appl. Chem. 2008, 80, 85–104. [Google Scholar] [CrossRef]
- Tohda, K.; Dragoe, D.; Shibata, M.; Umezawa, Y. Studies on the matched potential method for determining the selectivity coefficients of ion-selective electrodes based on neutral ionophores: Experimental and theoretical verification. Anal. Sci. 2001, 17, 733–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.K.; Mehtab, S.; Jain, A.K. Selective electrochemical sensor for copper (II) ion based on chelating ionophores. Anal. Chim. Acta. 2006, 575, 25–31. [Google Scholar] [CrossRef]
- Bakker, E.; Buhlmann, P.; Pretsch, E. The phase-boundary potential model. Talanta 2004, 63, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Meyerholf, M.E.; Opdycke, W.N. In Advances in Clinical Chemistry; Spiegel, H.E., Ed.; Academic Press Inc.: Orlando, FL, USA, 2013; Volume 25, pp. 1–47. [Google Scholar]
- Riad, S.M.; Mostafa, N.M. Ion selective electrodes for potentiometric determination of baclofen in pharmaceutical preparations. Anal. Bioanal. Electrochem. 2013, 5, 494–505. [Google Scholar]
- Nie, L.; Liu, D.; Yao, S. Potentiometric determination of diazepam with a diazepam ion-selective electrode. J. Pharm. Biomed. Anal. 1990, 8, 379–383. [Google Scholar] [CrossRef]
- Abdel Ghani, N.T.; Rizk, M.S.; El-Nashar, R.M. Salbutamol plastic membrane electrodes based on individual and mixed ion-exchangers of salbutamolium phosphotungstate and phosphomolybdate. Analyst 2000, 125, 1129–1133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).