1. Introduction
Heavy metals are pollutants that significantly affect the environment and their toxicity has been a problem of increasing significance for ecological, evolutionary, nutritional and environmental reasons [
1]. Due to human activity and consequent environmental pollution, heavy metals enter the biosphere, where, due to their toxicity, they lead to certain changes in specific metabolic pathways in living organisms. In addition to the organisms, heavy metals reach the soil. Technological operations that are a part of everyday industrial workflows are the main cause of soil contamination by deposition of harmful substances. Further, agricultural processes and waste disposal sites, as well as air pollution and pollutant deposition by wastewater treatment, account for a large portion of soil contamination causative factors [
2]. Interestingly, growing plants on soils that contained increased levels of heavy metals led to the discovery of the metal-accumulating properties of many species known today. However, not all heavy metals have a toxic effect on plants. In other words, normal plant growth requires the presence of heavy metals, which is why it up to the type of metal, its chemical form, pH, the composition of soil or sole plant species whether a metal will have deleterious effects on a particular plant or not [
1].
The majority of metals cannot be eliminated from the environment by chemical or biological transformation. They cause a state of oxidative stress in the plant and it is necessary to better understand the mechanisms of their harmful effects, as well as the tolerance of certain species and the specific reaction of their varieties to the increased availability of heavy metals in the environment.
Lead, as a non-essential trace metal, is a strong environmental pollutant that is toxic in very low concentrations and accumulates in various parts of the plant. Copper is a micronutrient highly important for plants because of its ability to maintain vital plant activity. Any deficiency of a nutrient affects plant growth and decreases crop yields [
3]. There are different sources of lead and copper in the environment, such as natural sources (geologic parent material or sedimentary rock, volcanoes, marine sources) and industrial sources (energy supplying power stations, metallurgy and electroplating, chemical industry, pharmaceuticals). Other sources of heavy metals include waste incineration, landfills and transportation (cars, diesel-powered vehicles and aircraft) [
1]. For copper to be biologically available, the pH should optimally be between 4.5 and 6, while the mobility of lead does not depend on the pH value of the soil and is quite small due to its tendency to bind to the organic matter [
4].
Crop plants have been used to extract heavy metals from soil and sediments, followed by translocation of contaminants to the harvestable stalks and leaves of the plants [
2]. As many metals that are being stockpiled by plants are essential nutrients, there is great potential for their use in food fortification and phytoremediation. Thus, analysis of metal accumulation capacity represents a promising aspect of plant use [
5]. Over 700 metal-hyperaccumulating and tolerant plant species are known, particularly in the context of affinity towards Nickel (over 500) [
6]. Species associated with storing cobalt, copper or zinc in higher amounts are second in rank, although much smaller in number, with those hyperaccumulating arsenic, cadmium, gold, lead, manganese and thallium being in the third place [
7].
According to Baker and Brooks [
8], the largest numbers of hyperaccumulating species belong to the Brassicaceae family. Likewise, Sarma emphasizes in his paper [
9] that metal hyperaccumulation is a property widespread among the representatives of the Brassicaceae family. Ni and Zn hyperaccumulation, the former being first discovered in 1948 in Alyssum bertolonii/Brassicaceae, and later in 1865 in Noccaea caerulescens (formerly, Thlaspi caerulescens)/Brassicaceae, began to attract increasing attention in the early 1990s as incidences of the alternative metal accumulating strategies [
10].
A hyperaccumulator has been defined as a plant that can accumulate cadmium (>100 mg/kg), copper and lead (>1000 mg/kg), zinc (>10.000 mg/kg) in its shoot dry matter. Further, in these plants, metal concentrations in shoots are greater than in roots, showing a peculiar ability of a plant to absorb and transport metals, and store them in their above-ground parts [
8]. A plethora of factors affect hyperaccumulation, and some of them are described in the work of Peng et al. [
6].
Brassicaceae plants often feature regularly in diets as raw or preserved vegetables and vegetable oil [
11].
Lunaria annua L. is a biennial cruciferous oilseed crop. The biennial character of
Lunaria is the main constraint for economically feasible cultivation. The seeds contain 30–35% oil, which contains 67% of long-chain fatty acids (44% erucic acid, C22:1, and 23% nervonic acid, C24:1). The oil is suitable as a lubricant [
12,
13]. In representatives of the genus, the boiled root of a plant is edible, while unripe fruits can be chopped and used as a spice.
Determinations of heavy metals in environmental samples, whether qualitative or quantitative, can be performed using different spectroscopic and electroanalytical methods. These include atomic absorption spectrometry (AAS), inductively coupled plasma atomic emission spectroscopy (ICP-AES) [
14], microwave-induced plasma optical emission spectroscopy (MIP-OES), inductively coupled plasma optical emission (ICP-OES) and stripping voltammetry [
15]. The latter is a widely used electrochemical technique for the detection of heavy metals in soil and water samples due to its ability to measure metal ions at trace concentrations. Stripping analysis consisted of two steps, a preconcentration and a stripping one. During the former one, the metal ions were reduced to a metal of interest and accumulated on the surface of a working electrode; during the latter, by applying a positive (anodic) or negative (cathodic) potential scan to the electrode, a metal of interest was oxidized back to its ion form and stripped out into the solution. In the meantime, the amount of the element was determined by measuring the generated current [
3].
In this work, we investigated the accumulation properties of Lunaria annua L. in the context of copper and lead-abundant soil. Metal concentrations of copper and lead were measured before planting and after the plants were harvested in soils, seeds and plants depending on the stage of growth. COMPO SANA, a commercially available organic mineral substrate and a soil sample collected from the Botanical Garden of the Faculty of Science, University of Split (30 cm-deep soil surface), were used in these experiments.
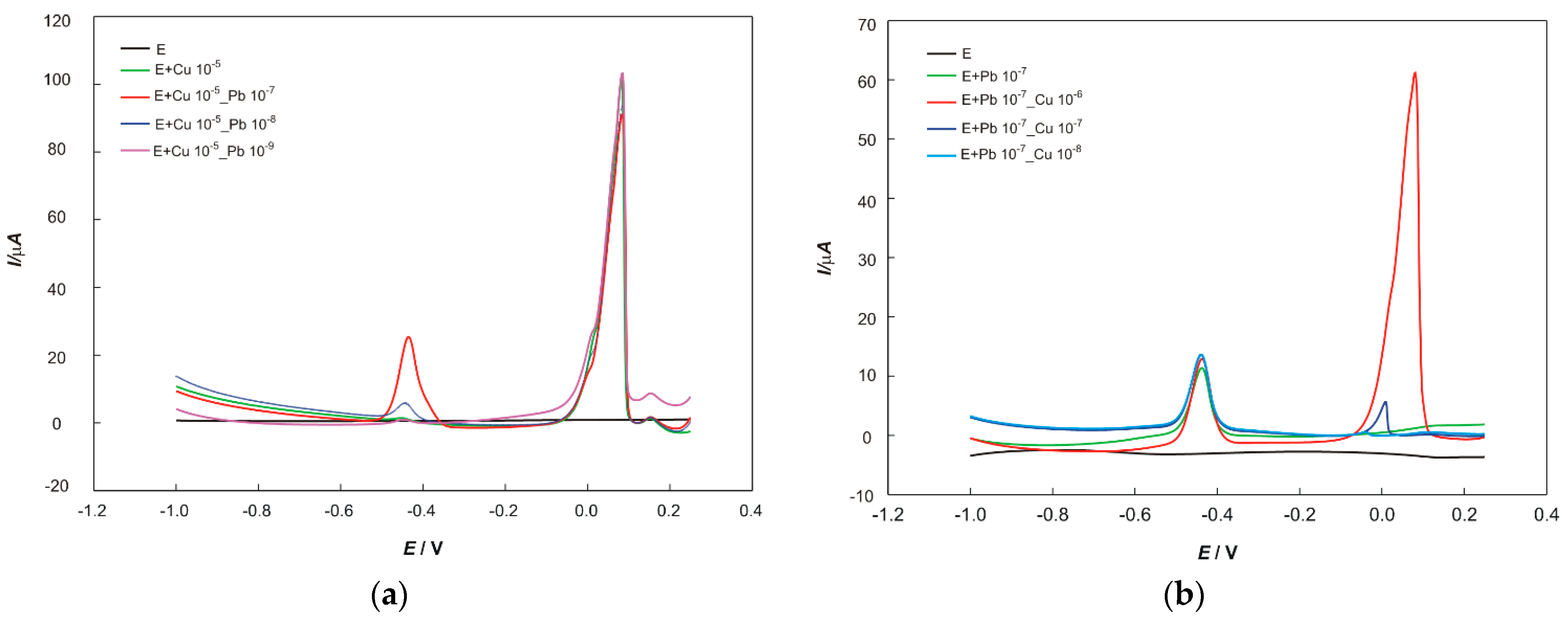
For the determination of Cu and Pb, square wave anodic stripping voltammetry (SWASV), using a glassy carbon electrode modified with mercury film, was applied. The standard addition method was used to determine and calculate the concentrations of copper and lead.
2. Materials and Methods
2.1. Chemicals and Materials
All chemicals and reagents were at least of analytical grade, and Milli-Q water was used throughout the experiment. Lead(II) nitrate, potassium nitrate and phosphoric acid were purchased from T.T.T. (Zagreb, Croatia); copper(II) nitrate, mercury(II) nitrate monohydrate and sulfuric acid were purchased from Kemika (Zagreb, Croatia). Nitric acid was purchased from Merck (Darmstadt, Germany). Hydrochloric acid was purchased from VWR Chemicals (Radnor, Pennsylvania, SAD). Ethanol was purchased from Gram-mol (Zagreb, Croatia). Standard solutions (1 × 10−5 M) of each metal were prepared by dissolving exact salt weight (Pb(NO3)2), Cu(NO3)2 × 3H2O) in 100 mL deionized water.
All experiments were carried out in a conventional three-electrode electrochemical cell at 25 °C. The glassy carbon (ϕ = 3 mm) served as a working electrode, Ag/AgCl/3 M KCl as a reference electrode and platinum as an auxiliary electrode. Electrochemical measurements were carried out using a potentiostat (Autolab PGSTAT 302N), connected to a PC and driven by the GPES4.9 software (Eco Chemie).
The supporting electrolyte required for the determination of Pb(II) and Cu(II) was a mixture of 0.01 M H3PO4 and 0.01 M HNO3 acids. To eliminate the influence of matrix in soil samples, measurements were performed with the addition of 100 µL aqua regia (2 mL of concentrated HNO3 and 6 mL of concentrated HCl into a 50 mL volumetric flask and filled with deionized water up to the volume). All experiments were carried out at room temperature (approximately 25 °C) without removing the dissolved gases.
2.2. Sample Preparation
2.2.1. Preparation of Soil Samples
The soil sample collected from the Botanical Garden (hereinafter S1) and a commercially available organic mineral substrate (COMPO SANA) (hereinafter S2) were air-dried for three weeks, mixed into a homogenous mixture and then sieved through a 2.5 mm, 1.0 mm, 0.5 mm and 0.25 mm mesh.
In total, 1.0 g of each soil sample was put on porcelain crucibles, placed in a cool muffle furnace and ashed at 550 °C for 3 h. Afterwards, the ashes were cooled and dissolved in 8 mL of aqua regia. The solution was filtered, the ashes were washed with diluted aqua regia and the filtrate was transferred into a 50 mL volumetric flask and diluted to the mark with deionized water.
For soil pH determination, two replicates of a 10.00 g portion of each soil sample were individually transferred to 50 mL glass beakers, and 25 mL of Milli-Q water (Ω = 18.2 MΩ/cm) was added. A premium hotplate stirrer model MSH-20A (Witeg Labortechnik, GmbH, Wertheim, Germany) was used for stirring the solutions. The pH of the samples was measured using a pH/conductivity combimeter (Orion Star Series Meter Thermo Fischer Scientifc Inc., Beverly, MA, USA).
2.2.2. Preparation of Seeds Samples
In total, 1.0 g of the plant was put on porcelain crucibles, placed in a cool muffle furnace and ashed at 550 °C for 3 h. Afterwards, the ashes were cooled and dissolved in 8 mL of aqua regia. The solution was filtered, the ashes were washed with diluted aqua regia and the filtrate was transferred into a 50 mL volumetric flask and diluted to the mark with deionized water.
2.2.3. Preparation of Plant Samples
Four flowerpots were prepared for each plant species; two of them were filled with S1, and two of them with S2. The seeds of Lunaria annua L. were planted in the flowerpots with the prepared soils. Two flower pots were watered with 15 mL of tap water of pH 7 (samples S1 + H2O and S2 + H2O) and the other two with 15 mL of a prepared solution which contained 8 mM of Pb(NO3)2 and 8 mM Cu(NO3)2 × 3H2O (samples S1 + Pb/Cu and S2 + Pb/Cu), for thirty days. Unseeded and untreated soils were used as controls.
After thirty days of cultivation, when plants had flowered, a plant from each flowerpot was harvested and left to dry at room temperature (ca. 25 °C) over a period of two days, and afterwards, in a laboratory drying oven at 75 °C to a constant mass, in order to obtain dry mass. Plant samples were cut to pieces and milled into a homogenous powder. In total, 1.0 g of a plant was put in a porcelain crucible, placed in a cool muffle furnace and ashed at 550 °C for 3 h. Afterwards, the ashes were cooled and dissolved in 8 mL of aqua regia. The solution was filtered, the ashes were washed with diluted aqua regia and the filtrate was transferred into a 50 mL volumetric flask and diluted to the mark with deionized water.
2.3. Electrode Preparation
A working electrode was polished with alumina powder to obtain a mirror-like surface, washed with deionized water, sonicated in ethanol solution for 2 min, washed with deionized water and dried. After, the electrode was electrochemically cleaned in 0.5 M sulfuric acid in the potential area of −1 V to 1 V, and the scan rate of 0.2 Vs−1.
Plating was carried out by immersion in a solution of 0.1 M KNO3, 0.01 M HNO3, and 2 × 10−4 M Hg(NO3)2 × H2O, while the electrode potential was held at −1.0 V for 2 min. After each experiment, the electrode was electrochemically re-prepared, and the mercury film was cleaned by wiping the electrode with a wet tissue, followed by plating a new mercury film for performing a new experiment.
2.4. Voltammetric Measurements
The concentrations of lead and copper were determined using the square wave anodic stripping voltammetry (SWASV) under optimized parameters, i.e., electrodeposition potential and final potential of −1.5 and +0.25 V, respectively; electrodeposition time of 60 s; step potential = 2 mV, step amplitude = 25 mV, and frequency = 10 Hz.
Before the measurements, to avoid any contamination, the electrochemical cell was rinsed with concentrated HNO3 and Milli-Q water.
2.5. Analysis of Samples
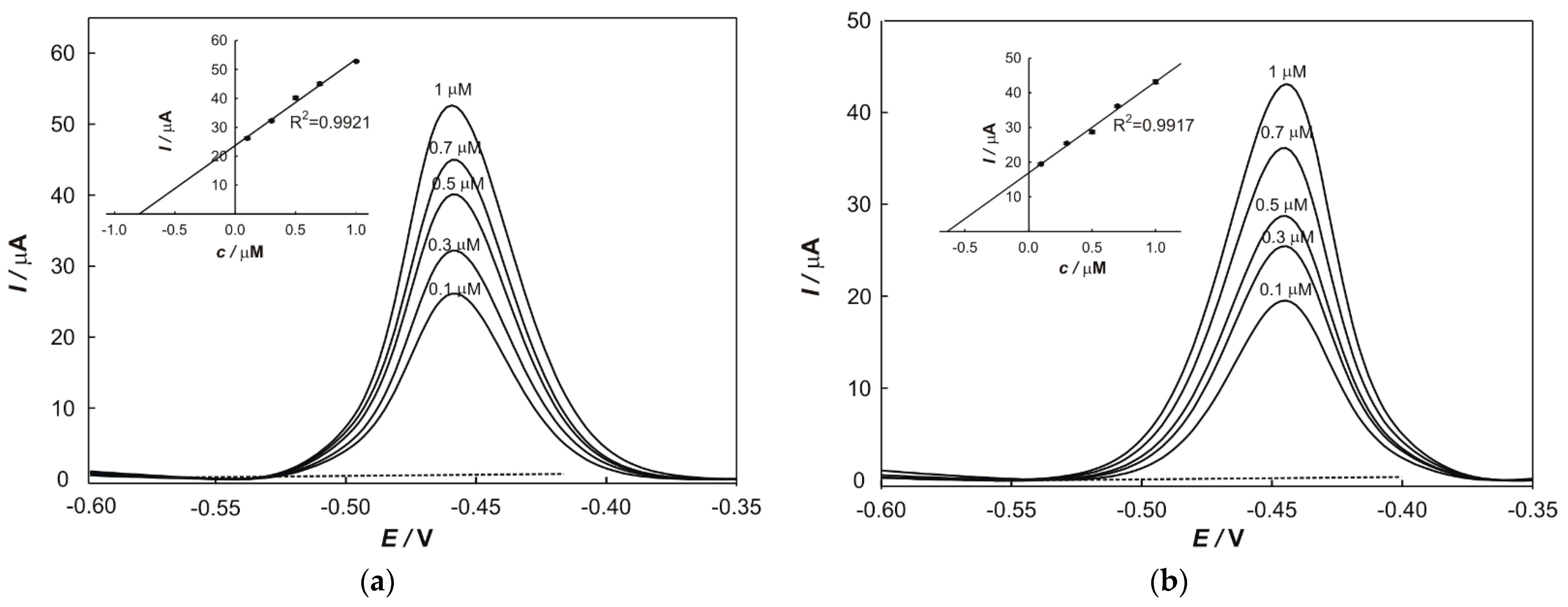
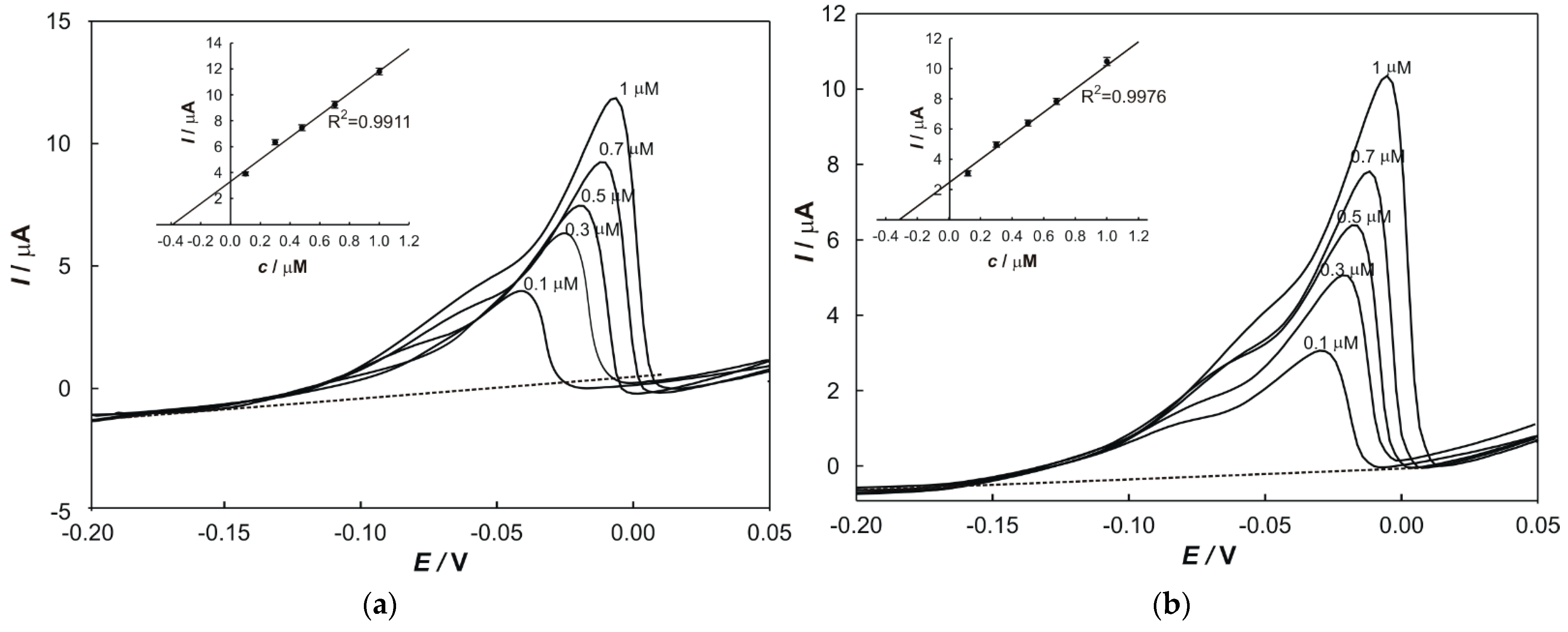
In total, 50 mL of supporting electrolyte was transferred into an electrochemical cell, 100 µL of each digested soil or plant sample and 100 µL of aqua regia was added. The solution was stirred thoroughly and the potential was scanned. The obtained values of the peak current are given in the results chapter and were used to determine the concentrations of Pb and Cu in samples, respectively.
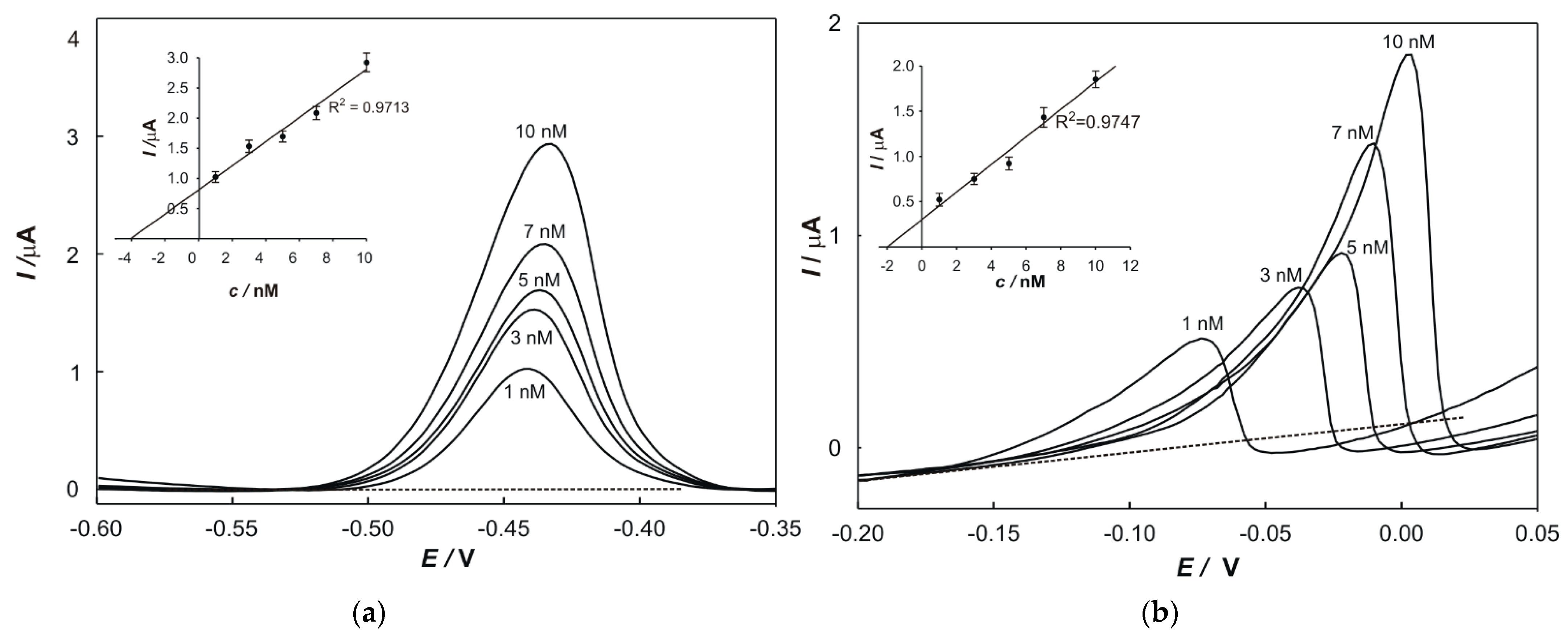
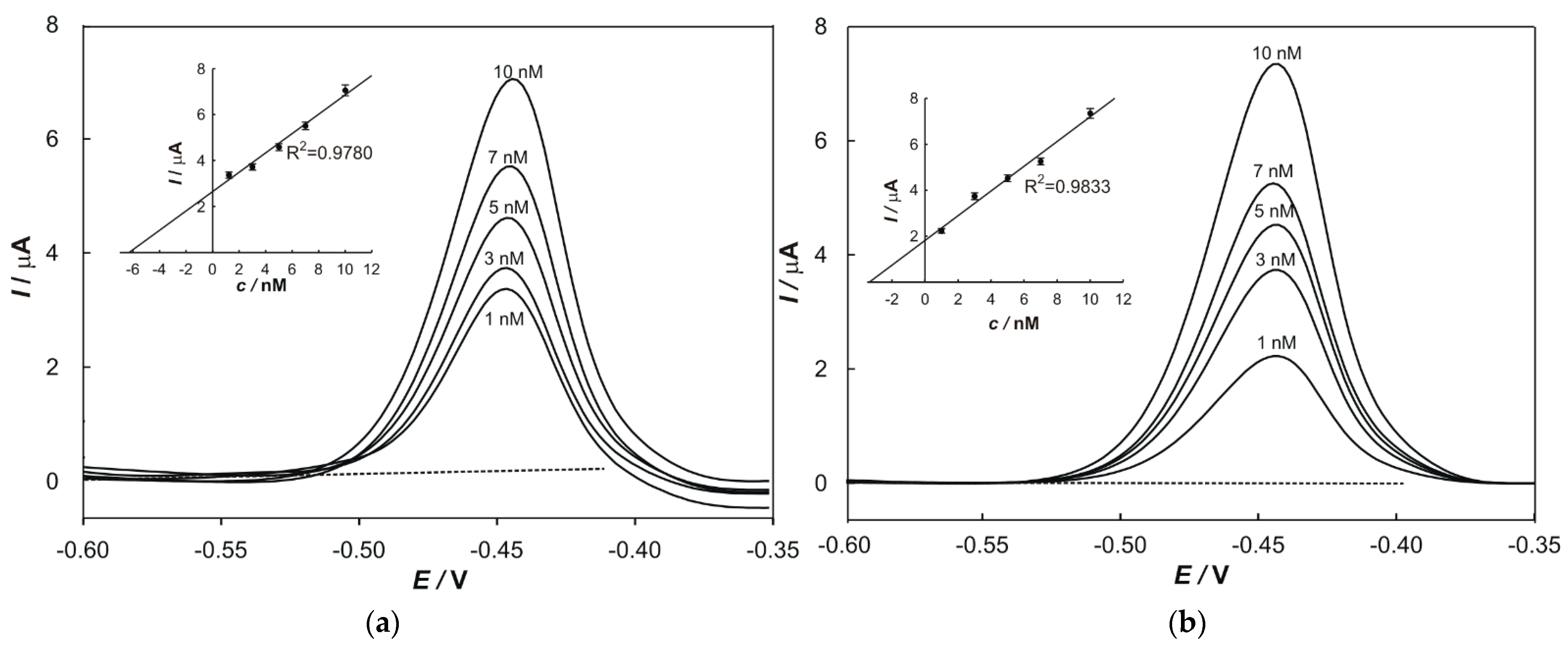
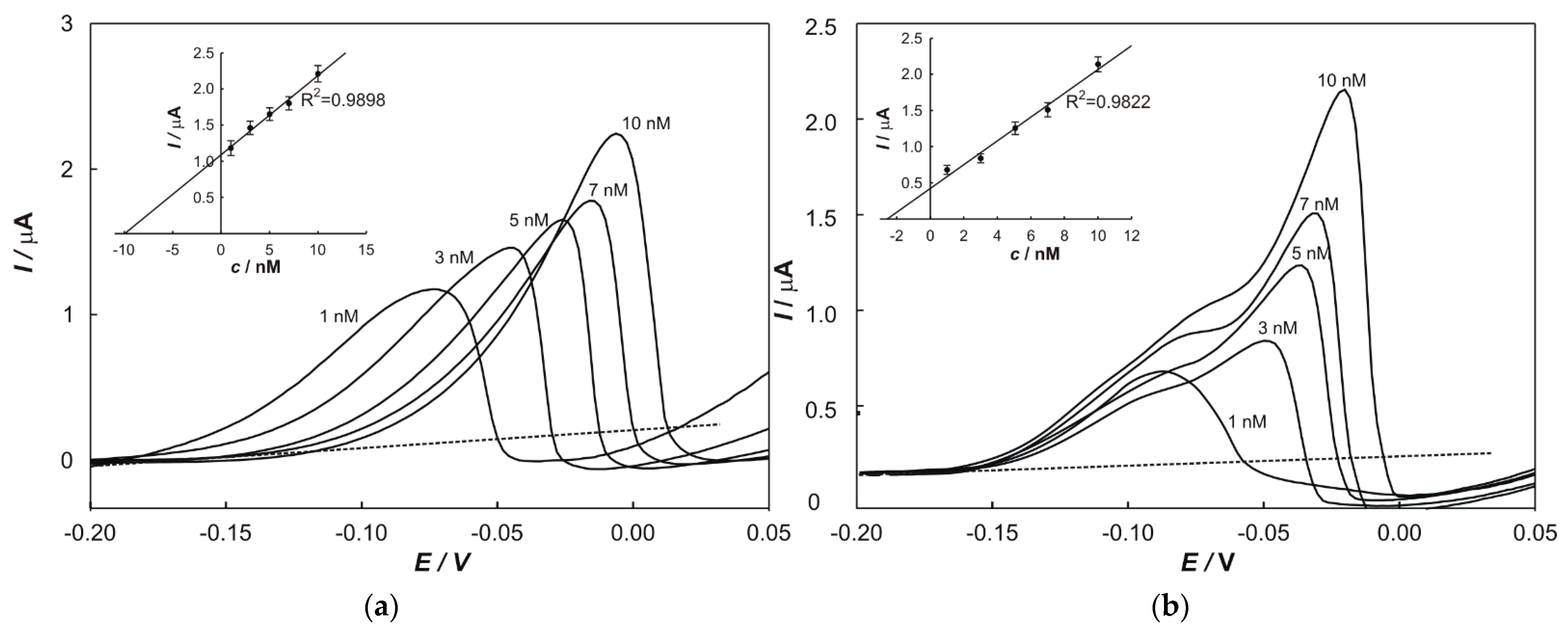
2.6. Standard Addition Method
The standard addition method was used to determine the concentrations of metals in samples and to eliminate the matrix effects. In total, 100 µL of a prepared sample and a particular volume of standard solutions (1 × 10
−5 M) of each metal were transferred into a 50 mL volumetric flask. The peak current value, relevant to each addition, was plotted on the
y-axis, while the
x-axis was graduated in terms of the concentration of a standard in the cell. The regression line was calculated and extrapolated back to the point on the
x-axis at which
y = 0. The content of the determined element in the sample (w) was calculated according to the following equation:
where 2.5 × 10
7 represents the correlation factor.
4. Conclusions
The experiments showed that SWASV could be used successfully to determine metals, particularly copper and lead, in contaminated soils and plant samples. Moreover, such a technique may be a good alternative to spectroscopy due to its simplicity and lower equipment costs.
Obtained concentrations for copper in plants from both types of soil watered with tap water are lower in comparison to the unseeded and untreated soil. Unlike copper, lead concertation in plants from both types of soil watered with tap water were much higher in comparison with unseeded and untreated soil. This confirms the well-known fact that for the bioavailability of copper, the optimal pH should be between 4.5 and 6, while the mobility of lead does not depend on pH value.
The obtained results showed that the higher the pH, the lower the possibility of metal accumulation.
Various species of the Brassicaceae family are known to hyperaccumulate metals. Our results suggest that Lunaria annua L. is capable of accumulating toxic amounts of copper and lead. None of the plant samples accumulated copper in concentrations above 1000 mg kg−1, meaning the criteria for a hyperaccumulator were not met. However, given the obtained concentration values, we can conclude that Lunaria annua L. is a potential copper accumulator and a heavy metal-tolerant species. In contrast to the obtained values of copper, the values for lead were greater than 1000 mg kg−1, indicating that Lunaria annua L. is a hyperaccumulator of lead. Hence, the results presented in this paper indicate a possible health risk if the plant material of Lunaria annua L. were used for human consumption.