Recent Advances in Surface Plasmon Resonance Microscopy
Abstract
:1. Introduction
2. Principle and Configurations of SPRM
2.1. Principle of SPRM
2.2. Research Progress of SPRM Technologies
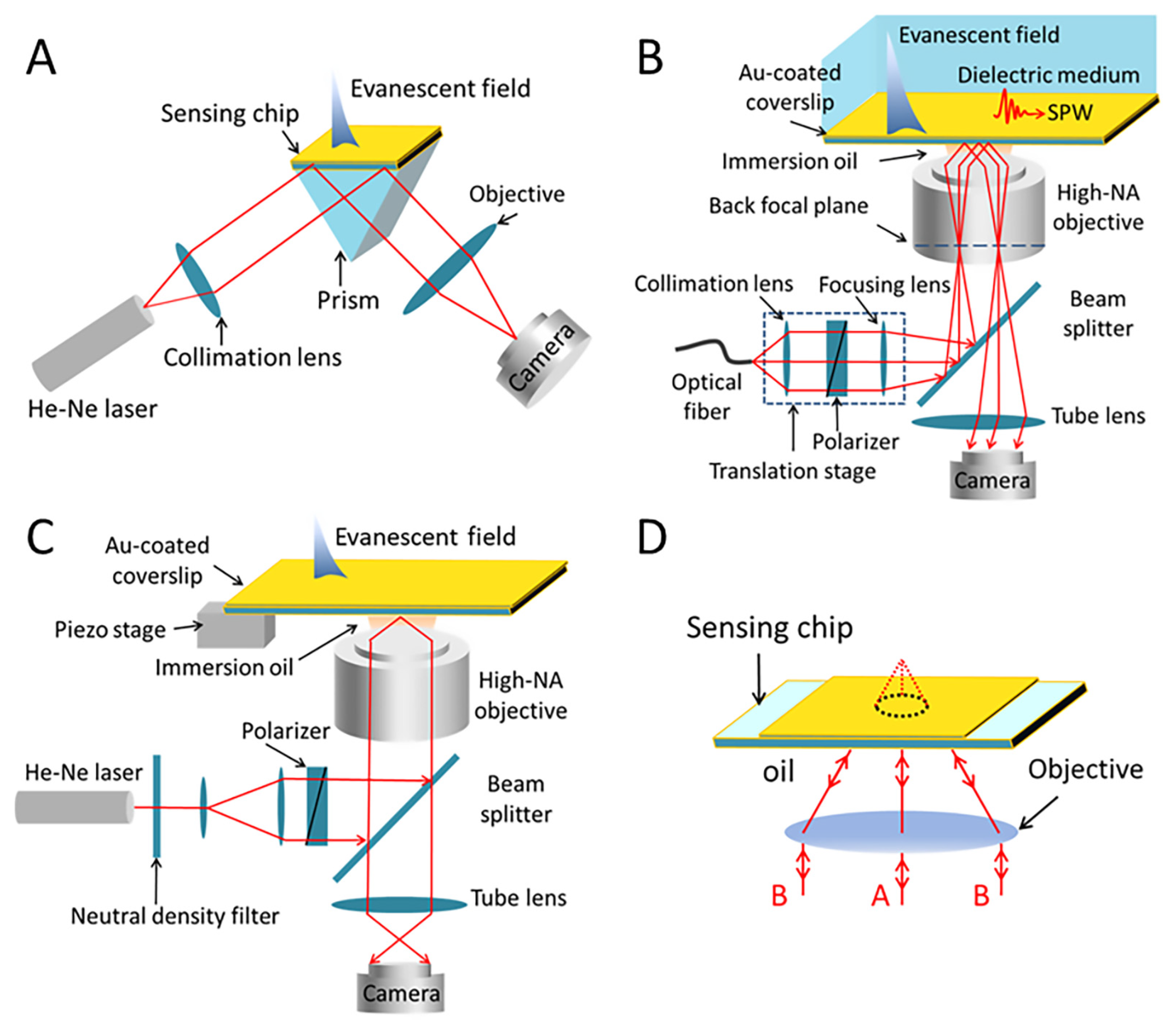
2.3. SPRM’s Resolution Enhancement Methods
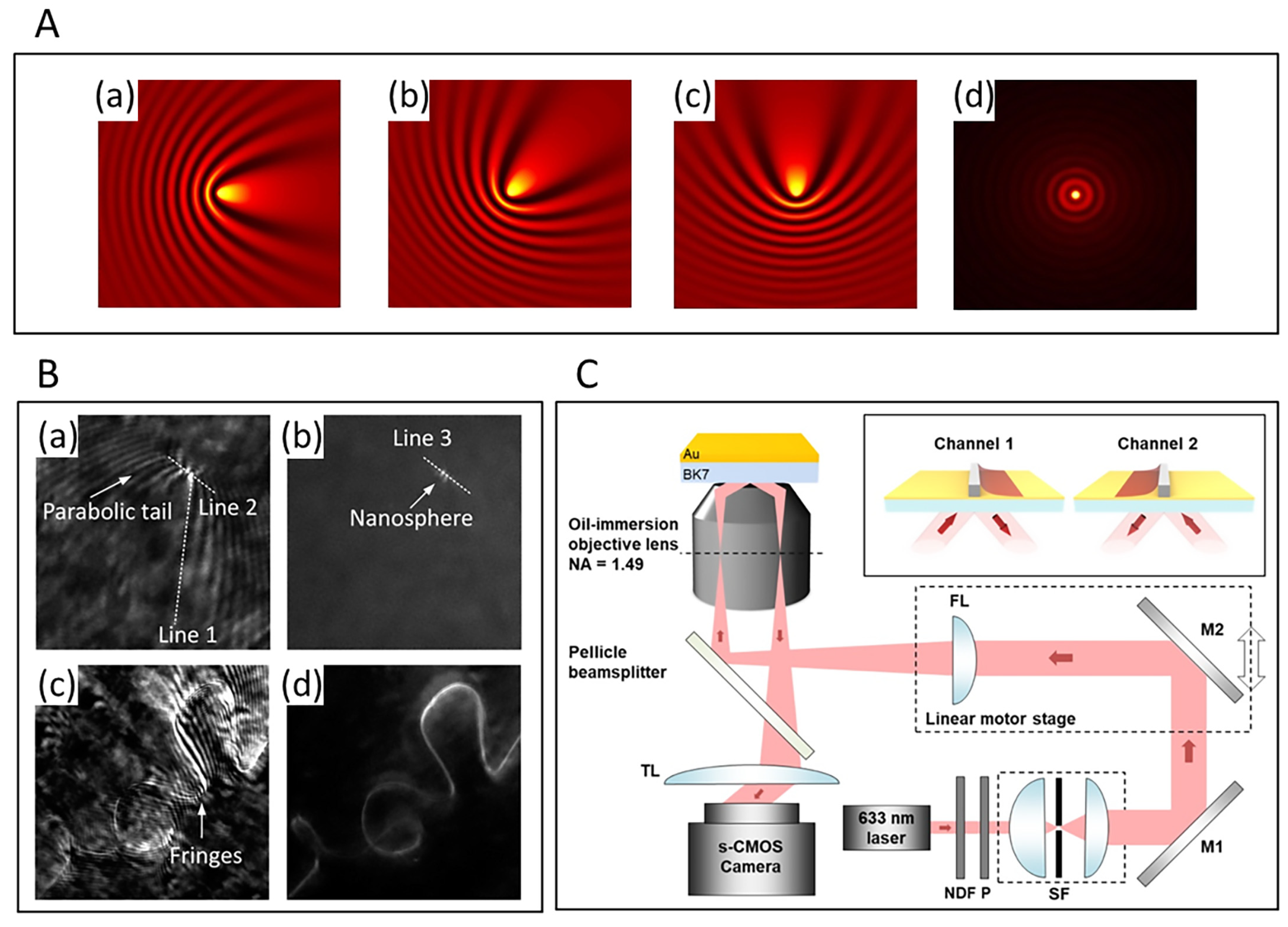
3. Advances in Biological Detection
3.1. Live Cell Studies
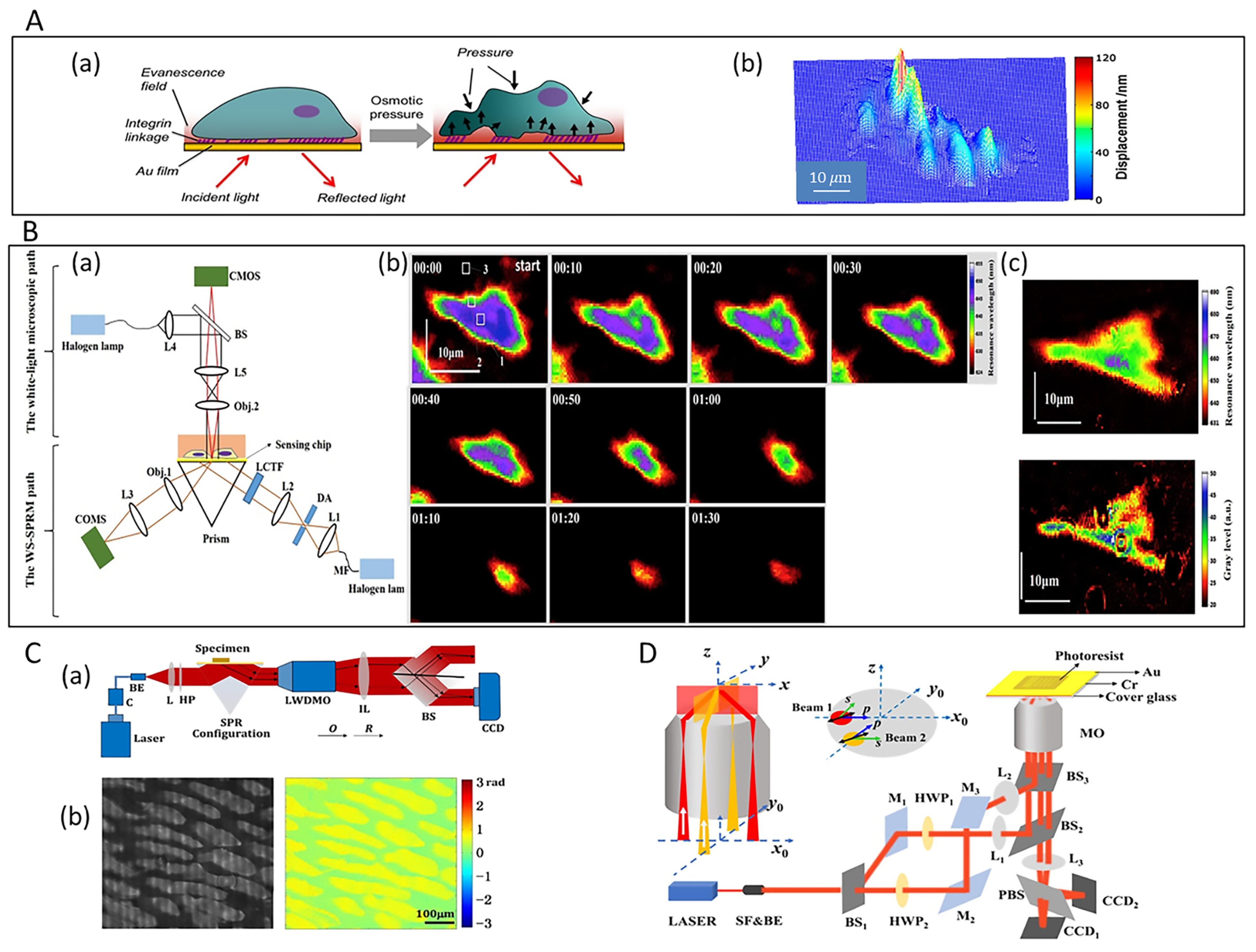
3.2. Single-Virus and Single-Bacteria Imaging
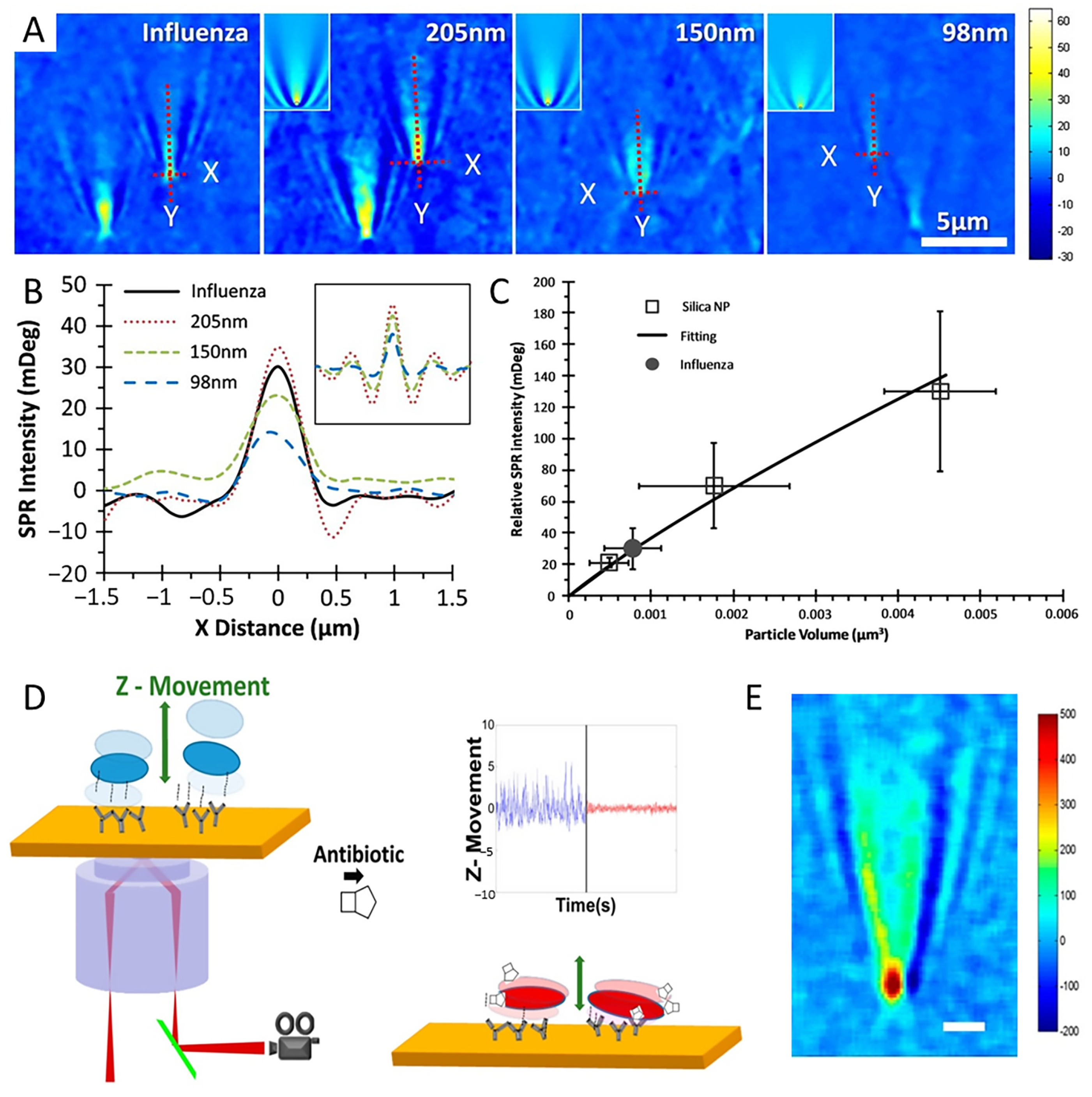
3.3. Study of Biomolecules
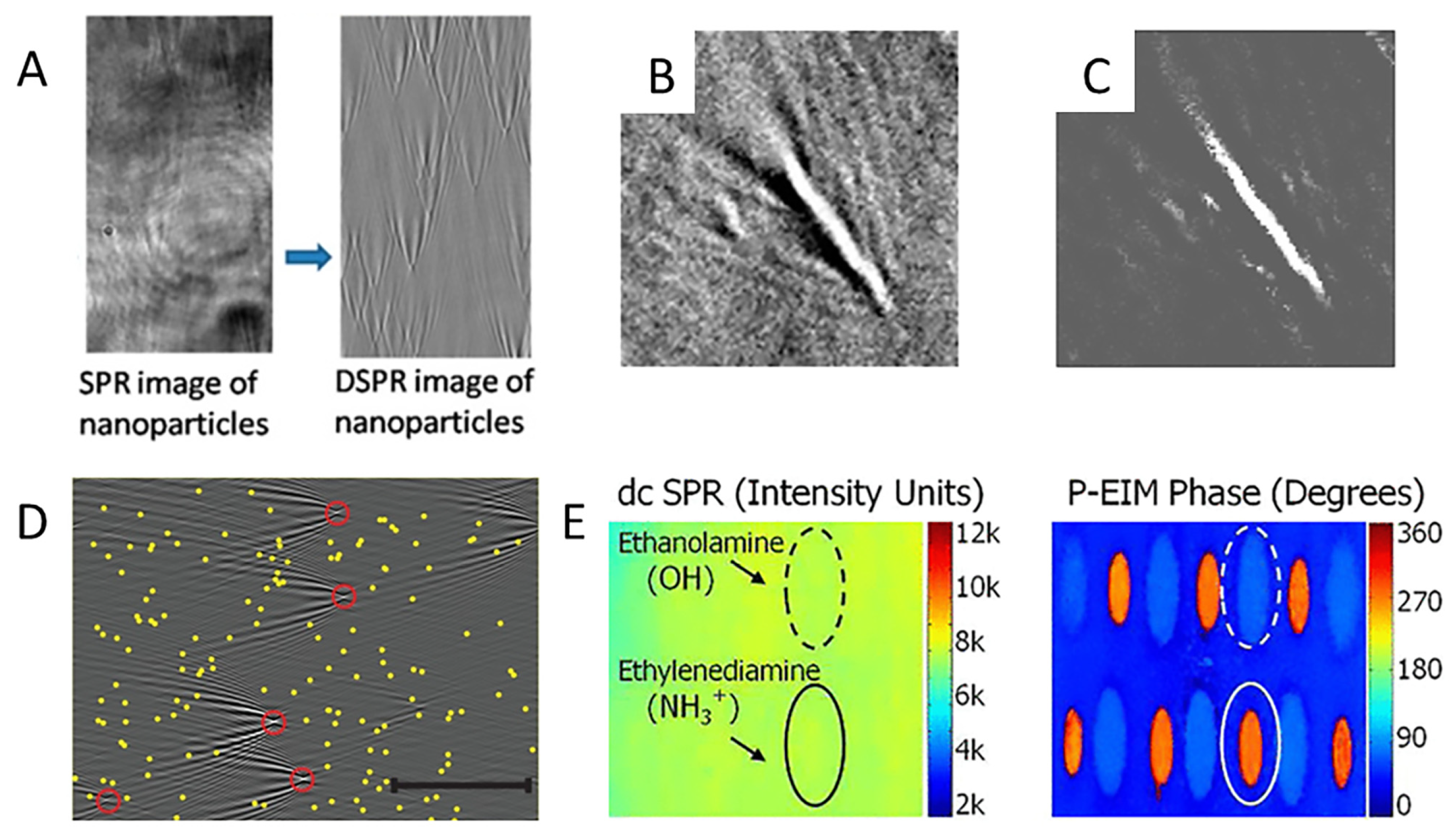
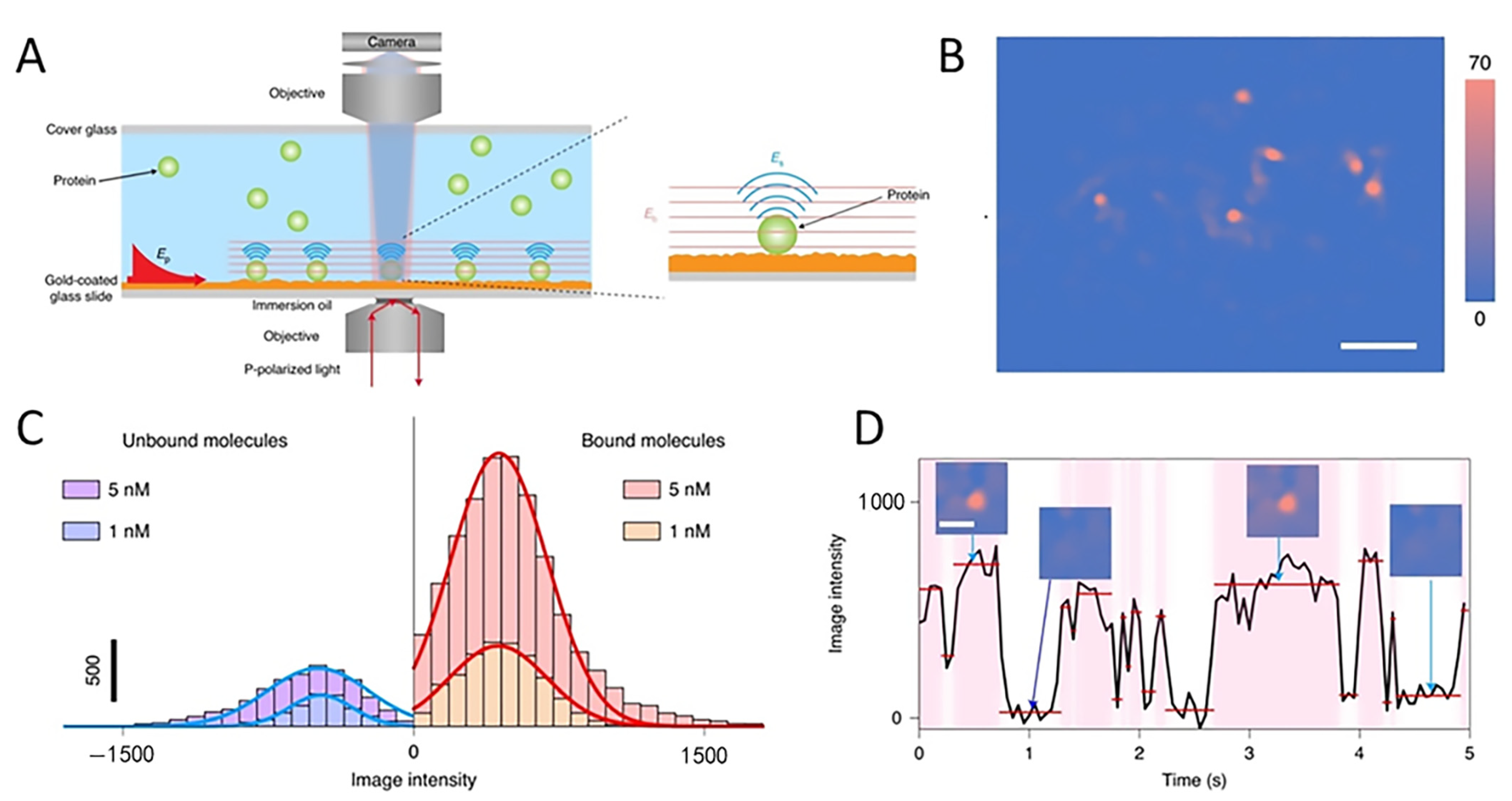
4. Novel Technologies for SPRM Enhancement
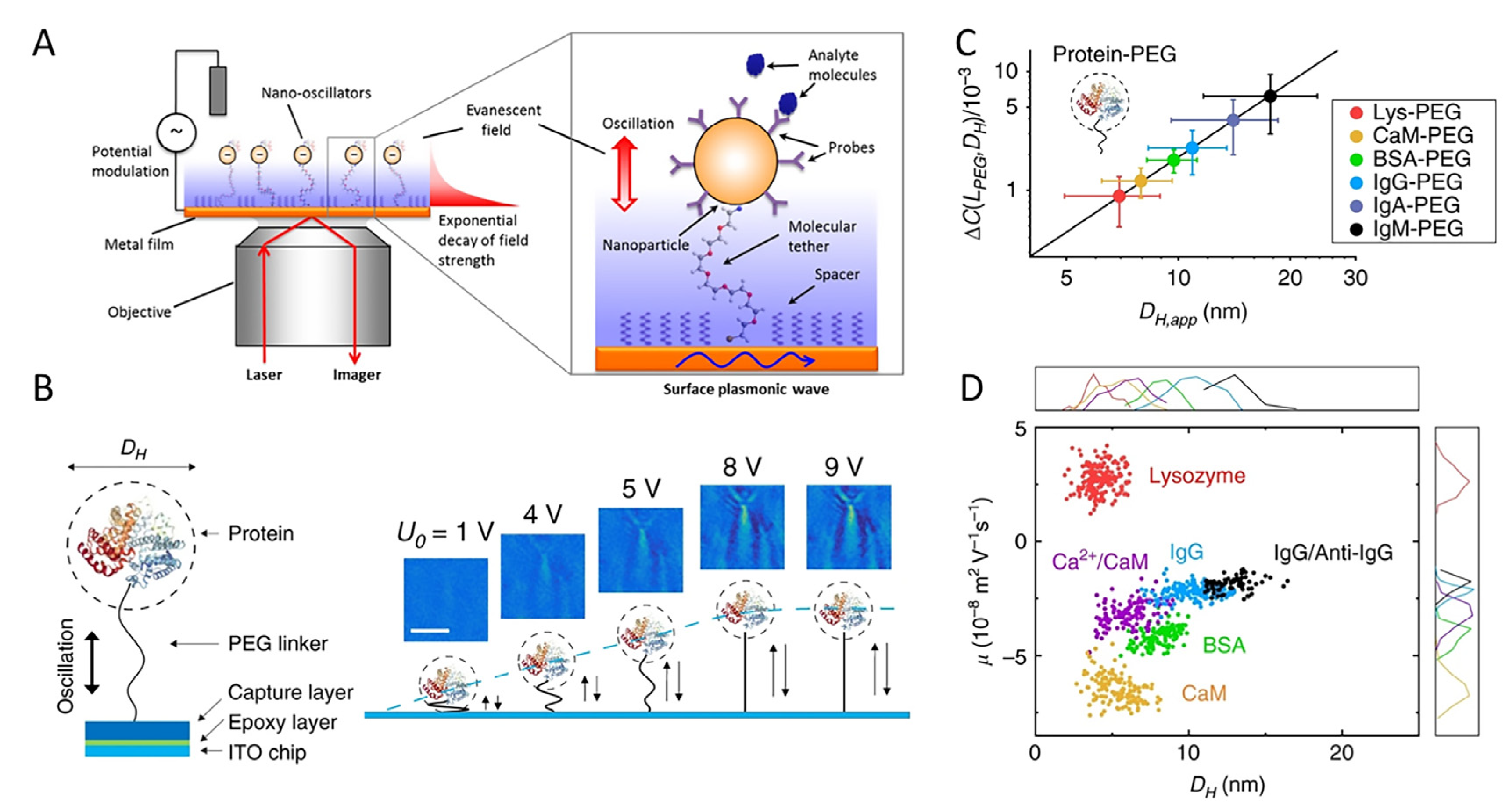
4.1. Development of Multifunctional Nano-Oscillators
4.2. Applications of Machine Learning Algorithms in SPRM
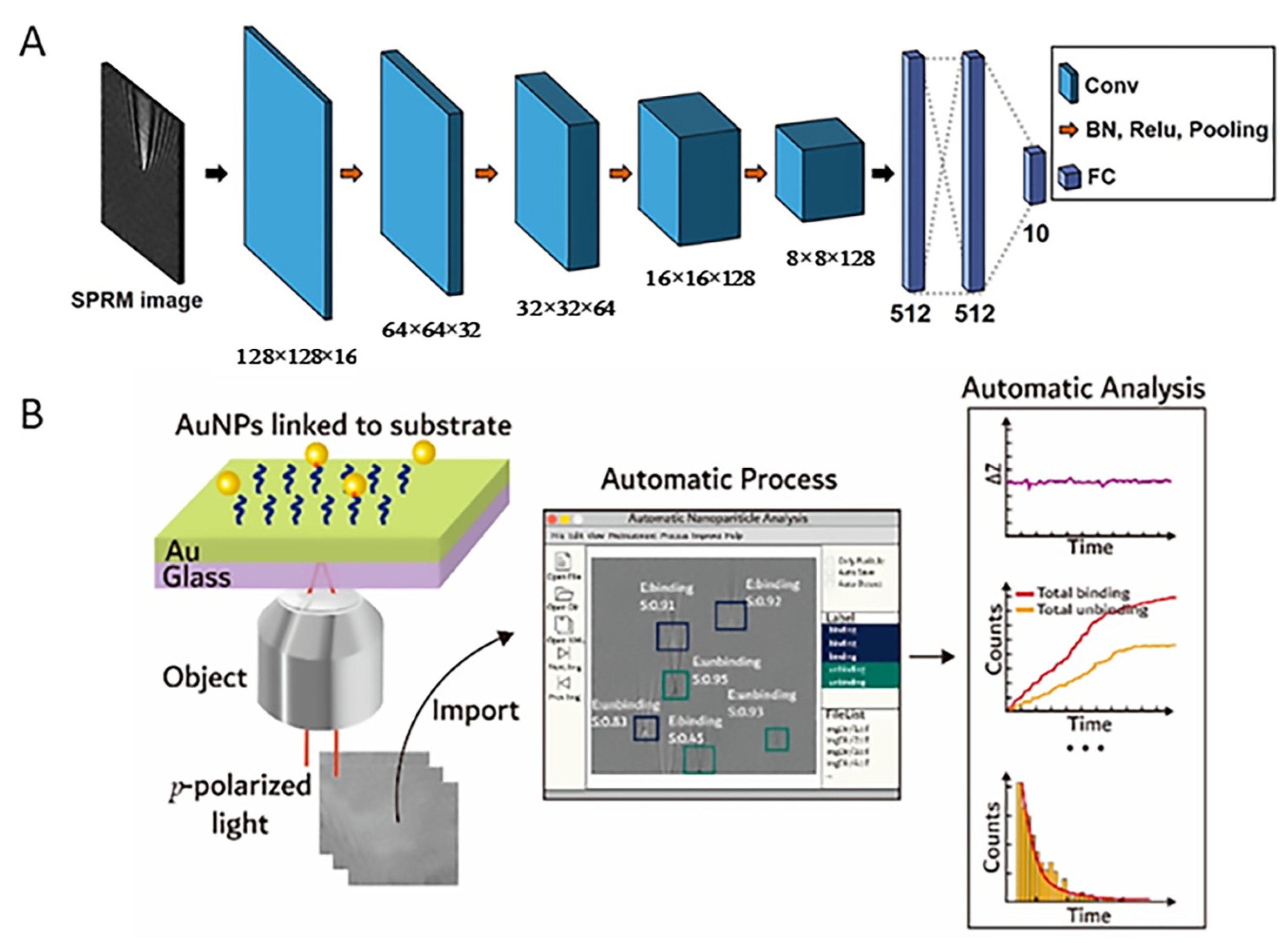
4.3. Integration of Optical Manipulation Technology
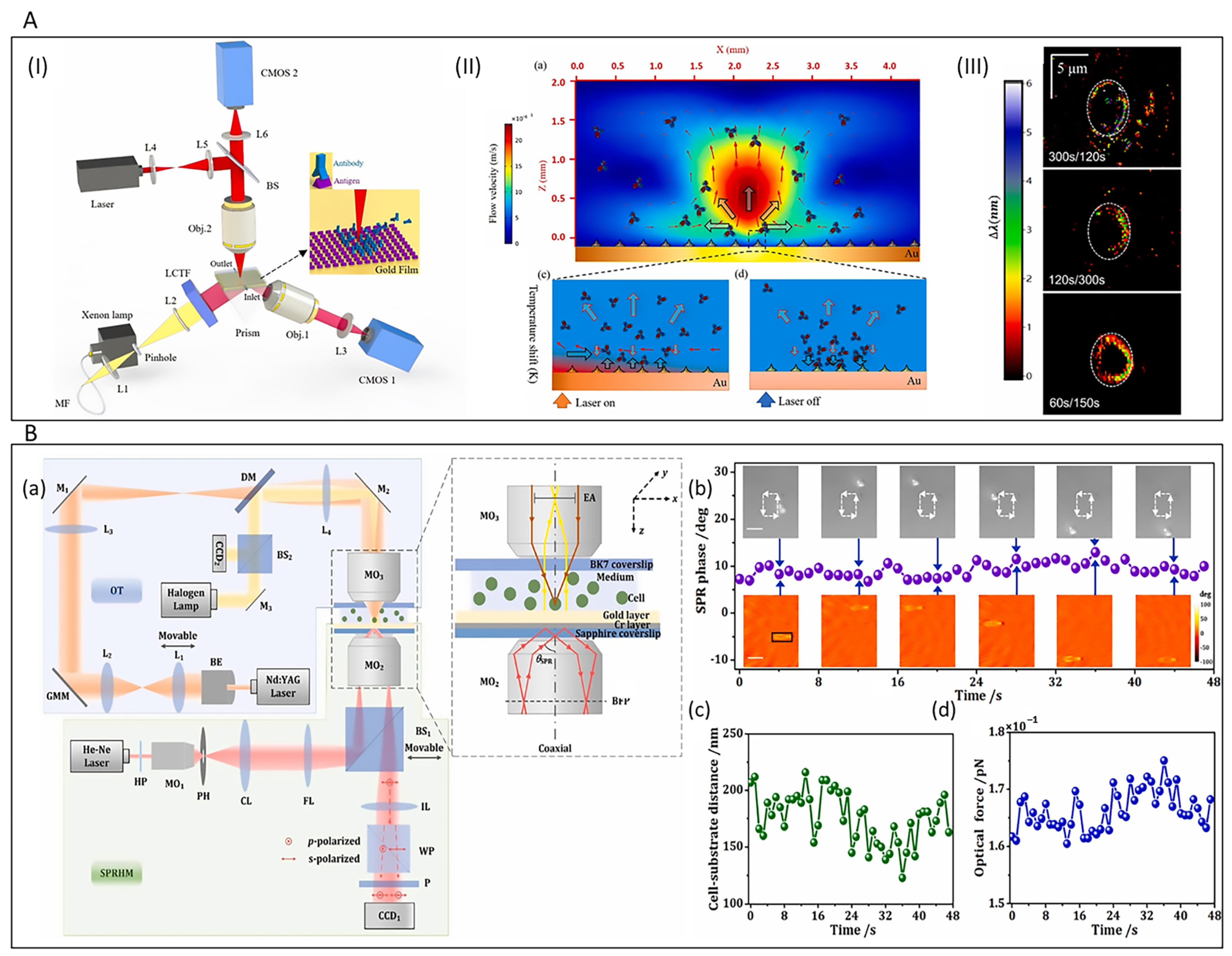
4.4. Applications of Optical Vortex
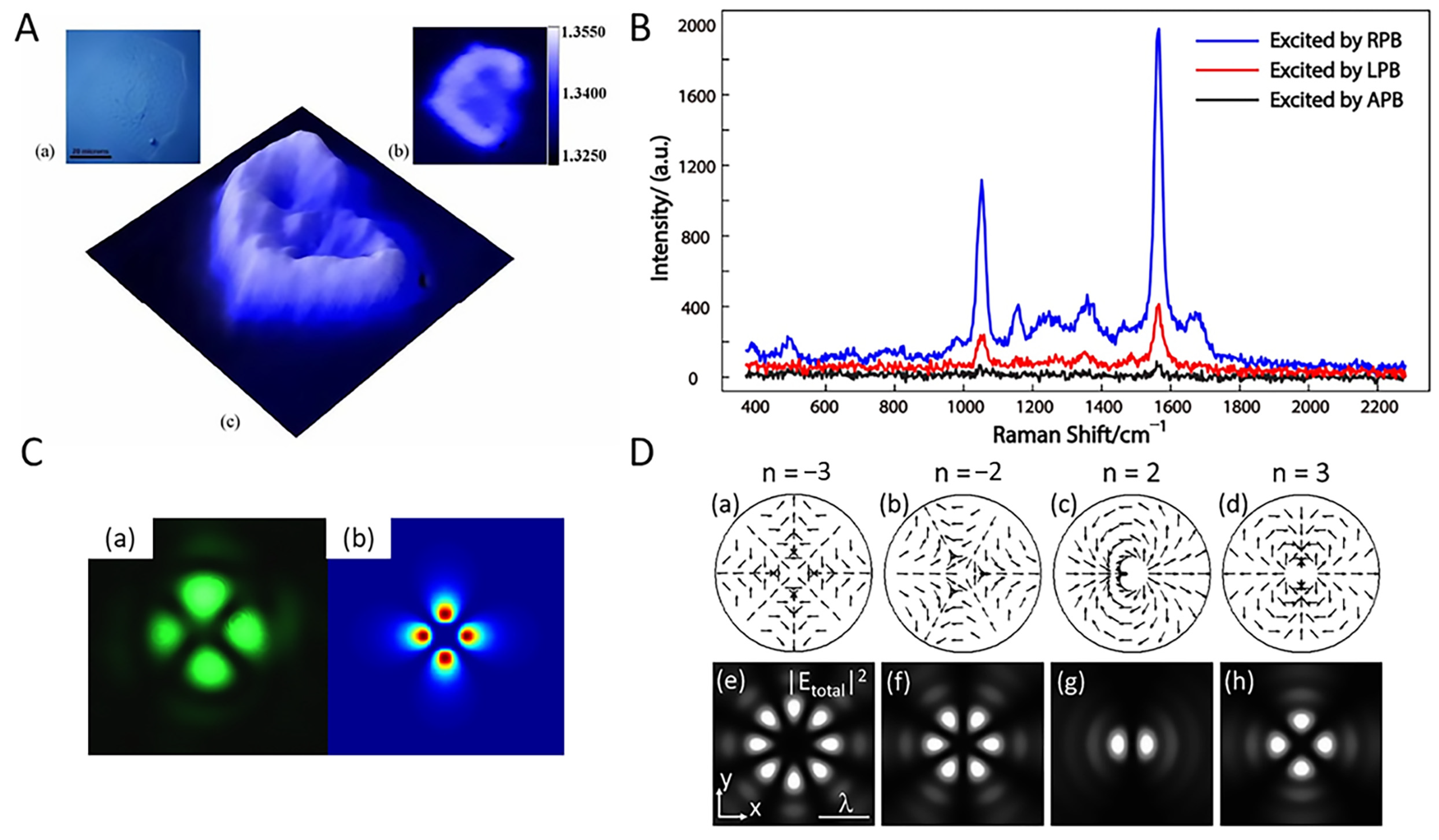
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Day, Y.S.; Baird, C.L.; Rich, R.L.; Myszka, D.G. Direct comparison of binding equilibrium, thermodynamic, and rate constants determined by surface- and solution-based biophysical methods. Protein Sci. 2002, 11, 1017–1025. [Google Scholar]
- Wood, R.W. On a remarkable case of uneven distribution of light in a diffraction grating spectrum. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2009, 4, 396–402. [Google Scholar]
- Johnston, S.K.; Mar, M.; Yee, S.S. Prototype of a multi-channel planar substrate spr probe. Sens. Actuators B Chem. 1999, 54, 57–65. [Google Scholar]
- Jansson, M.; Uhlen, M.; Nilsson, B. Structural changes in insulin-like growth factor (igf) i mutant proteins affecting binding kinetic rates to igf binding protein 1 and igf-i receptor. Biochemistry 1997, 36, 4108–4117. [Google Scholar]
- Carrascosa, L.G.; Sina, A.A.; Palanisamy, R.; Sepulveda, B.; Otte, M.A.; Rauf, S.; Shiddiky, M.J.; Trau, M. Molecular inversion probe-based spr biosensing for specific, label-free and real-time detection of regional DNA methylation. Chem. Commun. 2014, 50, 3585–3588. [Google Scholar]
- Pollet, J.; Delport, F.; Janssen, K.P.; Jans, K.; Maes, G.; Pfeiffer, H.; Wevers, M.; Lammertyn, J. Fiber optic spr biosensing of DNA hybridization and DNA-protein interactions. Biosens. Bioelectron. 2009, 25, 864–869. [Google Scholar]
- Zhang, F.; Wang, S.; Yin, L.; Yang, Y.; Guan, Y.; Wang, W.; Xu, H.; Tao, N. Quantification of epidermal growth factor receptor expression level and binding kinetics on cell surfaces by surface plasmon resonance imaging. Anal. Chem. 2015, 87, 9960–9965. [Google Scholar]
- Campbell, C.T.; Kim, G. Spr microscopy and its applications to high-throughput analyses of biomolecular binding events and their kinetics. Biomaterials 2007, 28, 2380–2392. [Google Scholar]
- Fasoli, J.B.; Corn, R.M. Surface enzyme chemistries for ultrasensitive microarray biosensing with spr imaging. Langmuir 2015, 31, 9527–9536. [Google Scholar]
- Hinman, S.S.; Ruiz, C.J.; Drakakaki, G.; Wilkop, T.E.; Cheng, Q. On-demand formation of supported lipid membrane arrays by trehalose-assisted vesicle delivery for spr imaging. ACS Appl Mater. Interfaces 2015, 7, 17122–17130. [Google Scholar]
- Manuel, G.; Luptak, A.; Corn, R.M. A microwell-printing fabrication strategy for the on-chip templated biosynthesis of protein microarrays for surface plasmon resonance imaging. J. Phys. Chem. C Nanomater. Interfaces 2016, 120, 20984–20990. [Google Scholar]
- Scarano, S.; Mascini, M.; Turner, A.P.; Minunni, M. Surface plasmon resonance imaging for affinity-based biosensors. Biosens. Bioelectron. 2010, 25, 957–966. [Google Scholar]
- Giebel, K.F.; Bechinger, C.; Herminghaus, S.; Riedel, M.; Leiderer, P.; Weiland, U.; Bastmeyer, M. Imaging of cell/substrate contacts of living cells with surface plasmon resonance microscopy. Biophys. J. 1999, 76, 509–516. [Google Scholar]
- Huang, B.; Yu, F.; Zare, R.N. Surface plasmon resonance imaging using a high numerical aperture microscope objective. Anal. Chem. 2007, 79, 2979–2983. [Google Scholar]
- Smith, E.A.; Corn, R.M. Surface plasmon resonance imaging as a tool to monitor biomolecular interactions in an array based format. Appl. Spectrosc. 2003, 57, 320A–332A. [Google Scholar]
- Wang, D.; Loo, J.F.C.; Chen, J.; Yam, Y.; Chen, S.C.; He, H.; Kong, S.K.; Ho, H.P. Recent advances in surface plasmon resonance imaging sensors. Sensors 2019, 19, 1266. [Google Scholar]
- Kretschmann, E.; Raether, H. Notizen: Radiative decay of non radiative surface plasmons excited by light. Z. Für. Nat. A 1968, 23, 2135–2136. [Google Scholar]
- Yu, H.; Shan, X.; Wang, S.; Chen, H.; Tao, N. Molecular scale origin of surface plasmon resonance biosensors. Anal. Chem. 2014, 86, 8992–8997. [Google Scholar]
- Kano, H.; Knoll, W. Locally excited surface-plasmon-polaritons for thickness measurement of lbk films. Opt. Commun. 1998, 153, 235–239. [Google Scholar]
- Somekh, M.G.; Liu, S.G.; Velinov, T.S.; See, C.W. Optical v(z) for high-resolution 2pi surface plasmon microscopy. Opt. Lett. 2000, 25, 823–825. [Google Scholar]
- Zhang, B.; Pechprasarn, S.; Zhang, J.; Somekh, M.G. Confocal surface plasmon microscopy with pupil function engineering. Opt. Express 2012, 20, 7388–7397. [Google Scholar]
- Watanabe, K.; Miyazaki, R.; Terakado, G.; Okazaki, T.; Morigaki, K.; Kano, H. High resolution imaging of patterned model biological membranes by localized surface plasmon microscopy. Appl. Opt. 2010, 49, 887–891. [Google Scholar]
- Kuai, Y.; Chen, J.; Tang, X.; Xiang, Y.; Lu, F.; Kuang, C.; Xu, L.; Shen, W.; Cheng, J.; Gui, H.; et al. Label-free surface-sensitive photonic microscopy with high spatial resolution using azimuthal rotation illumination. Sci. Adv. 2019, 5, eaav5335. [Google Scholar]
- Chen, Y.; Zhang, D.; Han, L.; Rui, G.; Wang, X.; Wang, P.; Ming, H. Surface-plasmon-coupled emission microscopy with a polarization converter. Opt. Lett. 2013, 38, 736–738. [Google Scholar]
- Son, T.; Lee, C.; Seo, J.; Choi, I.H.; Kim, D. Surface plasmon microscopy by spatial light switching for label-free imaging with enhanced resolution. Opt. Lett. 2018, 43, 959–962. [Google Scholar]
- Son, T.; Lee, C.; Moon, G.; Lee, D.; Cheong, E.; Kim, D. Enhanced surface plasmon microscopy based on multi-channel spatial light switching for label-free neuronal imaging. Biosens. Bioelectron. 2019, 146, 111738. [Google Scholar]
- Yu, H.; Shan, X.; Wang, S.; Tao, N. Achieving high spatial resolution surface plasmon resonance microscopy with image reconstruction. Anal. Chem. 2017, 89, 2704–2707. [Google Scholar]
- Yang, Y.; Shen, G.; Wang, H.; Li, H.; Zhang, T.; Tao, N.; Ding, X.; Yu, H. Interferometric plasmonic imaging and detection of single exosomes. Proc. Natl. Acad. Sci. USA 2018, 115, 10275–10280. [Google Scholar]
- Yang, Y.; Yu, H.; Shan, X.; Wang, W.; Liu, X.; Wang, S.; Tao, N. Label-free tracking of single organelle transportation in cells with nanometer precision using a plasmonic imaging technique. Small 2015, 11, 2878–2884. [Google Scholar]
- Shan, X.; Fang, Y.; Wang, S.; Guan, Y.; Chen, H.Y.; Tao, N. Detection of charges and molecules with self-assembled nano-oscillators. Nano Lett. 2014, 14, 4151–4157. [Google Scholar]
- Wang, W.; Wang, S.; Liu, Q.; Wu, J.; Tao, N. Mapping single-cell-substrate interactions by surface plasmon resonance microscopy. Langmuir 2012, 28, 13373–13379. [Google Scholar]
- Zeng, Y.; Zhou, J.; Wang, X.; Cai, Z.; Shao, Y. Wavelength-scanning surface plasmon resonance microscopy: A novel tool for real time sensing of cell-substrate interactions. Biosens. Bioelectron. 2019, 145, 111717. [Google Scholar]
- Zhang, J.; Dai, S.; Ma, C.; Di, J.; Zhao, J. Common-path digital holographic microscopy for near-field phase imaging based on surface plasmon resonance. Appl. Opt. 2017, 56, 3223–3228. [Google Scholar]
- Dou, J.; Dai, S.; Dong, C.; Zhang, J.; Di, J.; Zhao, J. Dual-channel illumination surface plasmon resonance holographic microscopy for resolution improvement. Opt. Lett. 2021, 46, 1604–1607. [Google Scholar]
- Yang, C.T.; Mejard, R.; Griesser, H.J.; Bagnaninchi, P.O.; Thierry, B. Cellular micromotion monitored by long-range surface plasmon resonance with optical fluctuation analysis. Anal. Chem. 2015, 87, 1456–1461. [Google Scholar]
- Wang, W.; Yang, Y.; Wang, S.; Nagaraj, V.J.; Liu, Q.; Wu, J.; Tao, N. Label-free measuring and mapping of binding kinetics of membrane proteins in single living cells. Nat. Chem. 2012, 4, 846–853. [Google Scholar]
- Wang, S.; Shan, X.; Patel, U.; Huang, X.; Lu, J.; Li, J.; Tao, N. Label-free imaging, detection, and mass measurement of single viruses by surface plasmon resonance. Proc. Natl. Acad. Sci. USA 2010, 107, 16028–16032. [Google Scholar]
- Syal, K.; Iriya, R.; Yang, Y.; Yu, H.; Wang, S.; Haydel, S.E.; Chen, H.Y.; Tao, N. Antimicrobial susceptibility test with plasmonic imaging and tracking of single bacterial motions on nanometer scale. ACS Nano 2016, 10, 845–852. [Google Scholar]
- Yu, H.; Shan, X.; Wang, S.; Chen, H.; Tao, N. Plasmonic imaging and detection of single DNA molecules. ACS Nano 2014, 8, 3427–3433. [Google Scholar]
- Tan, H.M.; Pechprasarn, S.; Zhang, J.; Pitter, M.C.; Somekh, M.G. High resolution quantitative angle-scanning widefield surface plasmon microscopy. Sci. Rep. 2016, 6, 20195. [Google Scholar]
- Halpern, A.R.; Wood, J.B.; Wang, Y.; Corn, R.M. Single-nanoparticle near-infrared surface plasmon resonance microscopy for real-time measurements of DNA hybridization adsorption. ACS Nano 2014, 8, 1022–1030. [Google Scholar]
- Foley, K.J.; Shan, X.; Tao, N.J. Surface impedance imaging technique. Anal. Chem. 2008, 80, 5146–5151. [Google Scholar]
- MacGriff, C.; Wang, S.; Wiktor, P.; Wang, W.; Shan, X.; Tao, N. Charge-based detection of small molecules by plasmonic-based electrochemical impedance microscopy. Anal. Chem. 2013, 85, 6682–6687. [Google Scholar]
- Zhang, P.; Ma, G.; Dong, W.; Wan, Z.; Wang, S.; Tao, N. Plasmonic scattering imaging of single proteins and binding kinetics. Nat. Methods 2020, 17, 1010–1017. [Google Scholar]
- Zhang, P.; Ma, G.; Wan, Z.; Wang, S. Quantification of single-molecule protein binding kinetics in complex media with prism-coupled plasmonic scattering imaging. ACS Sens. 2021, 6, 1357–1366. [Google Scholar]
- Chen, Z.; Peng, Y.; Cao, Y.; Wang, H.; Zhang, J.R.; Chen, H.Y.; Zhu, J.J. Light-driven nano-oscillators for label-free single-molecule monitoring of microrna. Nano Lett. 2018, 18, 3759–3765. [Google Scholar]
- Ma, G.; Wan, Z.; Yang, Y.; Zhang, P.; Wang, S.; Tao, N. Optical imaging of single-protein size, charge, mobility, and binding. Nat. Commun. 2020, 11, 4768. [Google Scholar]
- Moon, G.; Son, T.; Lee, H.; Kim, D. Deep learning approach for enhanced detection of surface plasmon scattering. Anal. Chem. 2019, 91, 9538–9545. [Google Scholar]
- Wang, X.; Zeng, Q.; Xie, F.; Wang, J.; Yang, Y.; Xu, Y.; Li, J.; Yu, H. Automated nanoparticle analysis in surface plasmon resonance microscopy. Anal. Chem. 2021, 93, 7399–7404. [Google Scholar]
- Chen, J.; Zeng, Y.; Zhou, J.; Wang, X.; Jia, B.; Miyan, R.; Zhang, T.; Sang, W.; Wang, Y.; Qiu, H.; et al. Optothermophoretic flipping method for biomolecule interaction enhancement. Biosens. Bioelectron. 2022, 204, 114084. [Google Scholar]
- Liu, S.; Lin, L.; Sun, H.B. Opto-thermophoretic manipulation. ACS Nano 2021, 15, 5925–5943. [Google Scholar]
- Chen, J.; Kang, Z.; Kong, S.K.; Ho, H.P. Plasmonic random nanostructures on fiber tip for trapping live cells and colloidal particles. Opt. Lett. 2015, 40, 3926–3929. [Google Scholar]
- Dai, S.; Mi, J.; Dou, J.; Lu, H.; Dong, C.; Ren, L.; Zhao, R.; Shi, W.; Zhang, N.; Zhou, Y.; et al. Optical tweezers integrated surface plasmon resonance holographic microscopy for characterizing cell-substrate interactions under noninvasive optical force stimuli. Biosens. Bioelectron. 2022, 206, 114131. [Google Scholar]
- Zhou, J.; Dai, X.; Jia, B.; Qu, J.; Ho, H.-P.; Gao, B.Z.; Shao, Y.; Chen, J. Nanorefrigerative tweezers for optofluidic manipulation. Appl. Phys. Lett. 2022, 120, 163701. [Google Scholar]
- Tan, P.S.; Yuan, X.C.; Lin, J.; Wang, Q.; Mei, T.; Burge, R.E.; Mu, G.G. Surface plasmon polaritons generated by optical vortex beams. Appl. Phys. Lett. 2008, 92, 111108. [Google Scholar]
- Moh, K.J.; Yuan, X.C.; Bu, J.; Zhu, S.W.; Gao, B.Z. Surface plasmon resonance imaging of cell-substrate contacts with radially polarized beams. Opt. Express 2008, 16, 20734–20741. [Google Scholar]
- Shen, J.; Wang, J.; Zhang, C.; Min, C.; Fang, H.; Du, L.; Zhu, S.; Yuan, X.C. Dynamic plasmonic tweezers enabled single-particle-film-system gap-mode surface-enhanced raman scattering. Appl. Phys. Lett. 2013, 103, 191119. [Google Scholar]
- Dou, X.; Yang, A.; Min, C.; Du, L.; Zhang, Y.; Weng, X.; Yuan, X. Polarization-controlled gap-mode surface-enhanced raman scattering with a single nanoparticle. J. Phys. D Appl. Phys. 2017, 50, 255302. [Google Scholar]
- Hu, Z.J.; Yuan, X.C.; Zhu, S.W.; Yuan, G.H.; Tan, P.S.; Lin, J.; Wang, Q. Dynamic surface plasmon patterns generated by reconfigurable “cogwheel-shaped” beams. Appl. Phys. Lett. 2008, 93, 181102. [Google Scholar]
- Man, Z.; Du, L.; Min, C.; Zhang, Y.; Zhang, C.; Zhu, S.; Paul Urbach, H.; Yuan, X.C. Dynamic plasmonic beam shaping by vector beams with arbitrary locally linear polarization states. Appl. Phys. Lett. 2014, 105, 011110. [Google Scholar]
- Jia, B.; Chen, J.; Zhou, J.; Zeng, Y.; Ho, H.-P.; Shao, Y. Passively and actively enhanced surface plasmon resonance sensing strategies towards single molecular detection. Nano Res. 2022, 15, 8367–8388. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Chen, J.; Zhang, T.; Dai, X.; Wang, X.; Zhou, J.; Kong, W.; Liu, Q.; Qu, J.; Shao, Y. Recent Advances in Surface Plasmon Resonance Microscopy. Chemosensors 2022, 10, 509. https://doi.org/10.3390/chemosensors10120509
Huang S, Chen J, Zhang T, Dai X, Wang X, Zhou J, Kong W, Liu Q, Qu J, Shao Y. Recent Advances in Surface Plasmon Resonance Microscopy. Chemosensors. 2022; 10(12):509. https://doi.org/10.3390/chemosensors10120509
Chicago/Turabian StyleHuang, Songfeng, Jiajie Chen, Teliang Zhang, Xiaoqi Dai, Xueliang Wang, Jianxing Zhou, Weifu Kong, Qian Liu, Junle Qu, and Yonghong Shao. 2022. "Recent Advances in Surface Plasmon Resonance Microscopy" Chemosensors 10, no. 12: 509. https://doi.org/10.3390/chemosensors10120509
APA StyleHuang, S., Chen, J., Zhang, T., Dai, X., Wang, X., Zhou, J., Kong, W., Liu, Q., Qu, J., & Shao, Y. (2022). Recent Advances in Surface Plasmon Resonance Microscopy. Chemosensors, 10(12), 509. https://doi.org/10.3390/chemosensors10120509







