What Can COVID-19 Teach Us about Using AI in Pandemics?
Abstract
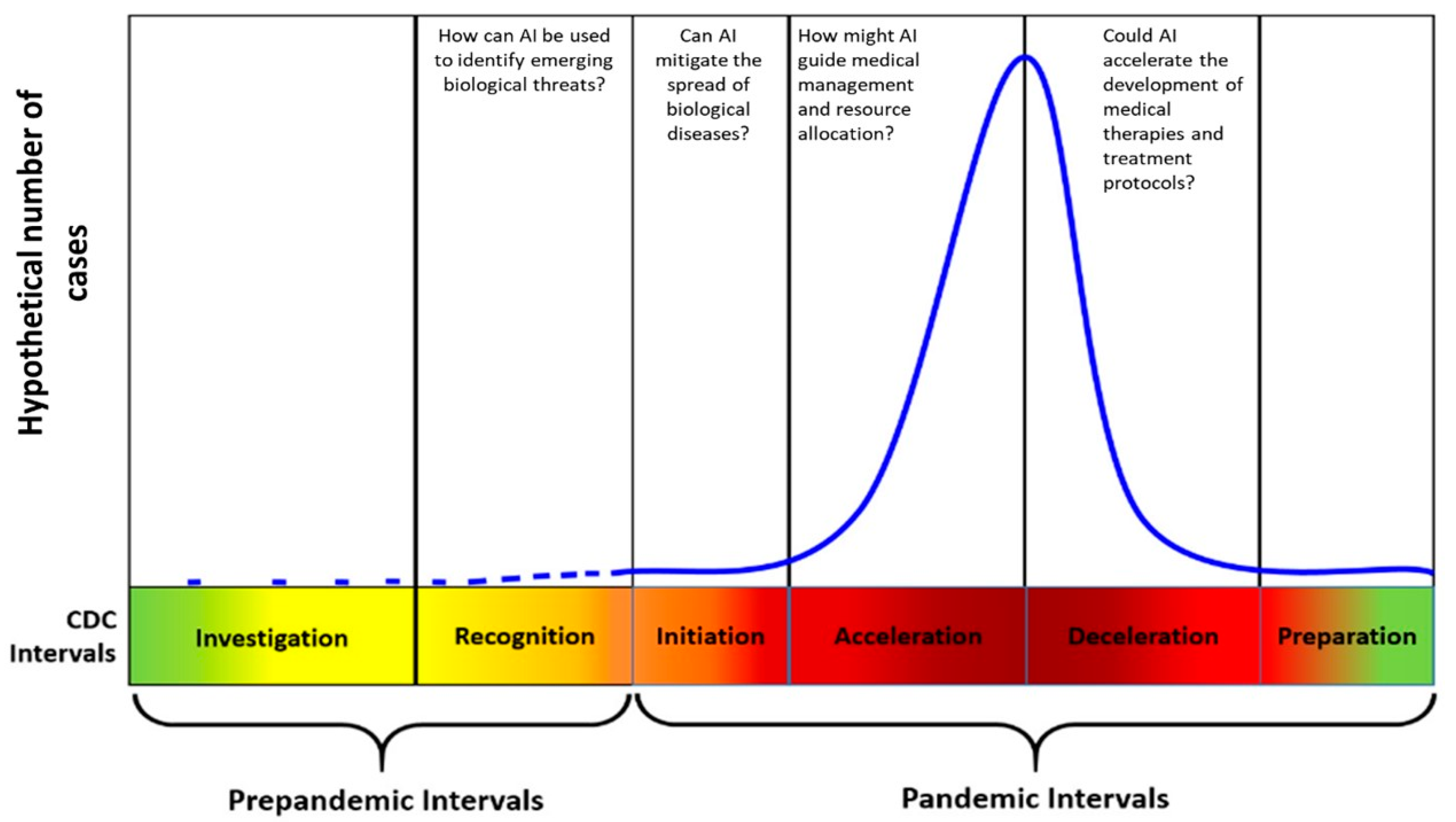
1. Background and Significance
2. Materials and Methods
3. Results
3.1. How Can AI Be Used to Identify Emerging Biological Threats during Investigation and Detection?
3.2. Can AI Forecast and Mitigate the Spread of Biological Diseases during the Initiation and Acceleration of a Pandemic?
3.3. How Might AI Guide Medical Management and Resource Allocation during Acceleration and Deceleration of a Pandemic?
3.4. How Might AI Accelerate the Development of Medical Therapies and Treatment Protocols?
3.5. Weaknesses of AI
4. Final Points
Author Contributions
Funding
Conflicts of Interest
References
- Bogoch, I.I.; Watts, A.; Thomas-Bachli, A.; Huber, C.; Kraemer, M.U.G.; Khan, K. Pneumonia of unknown aetiology in Wuhan, China: Potential for international spread via commercial air travel. J. Travel Med. 2020, 27, taaa008. [Google Scholar] [CrossRef]
- Contini, C.; Di Nuzzo, M.; Barp, N.; Bonazza, A.; De Giorgio, R.; Tognon, M.; Rubino, S. The novel zoonotic COVID-19 pandemic: An expected global health concern. J. Infect. Dev. Ctries. 2020, 14, 254–264. [Google Scholar] [CrossRef]
- Nicola, M.; O’Neill, N.; Sohrabi, C.; Khan, M.; Agha, M.; Agha, R. Evidence based management guideline for the COVID-19 pandemic—Review article. Int. J. Surg. 2020, 77, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Poston, J.T.; Patel, B.K.; Davis, A.M. Management of Critically Ill Adults with COVID-19. JAMA 2020, 323, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.L.; Zhang, W.J.; Lu, Y.; Guo, C.; Guo, Z.M.; Liao, C.H.; Zhang, X.; Zhang, Y.; Han, X.H.; Li, Q.L.; et al. From severe acute respiratory syndrome-associated coronavirus to 2019 novel coronavirus outbreak: Similarities in the early epidemics and prediction of future trends. Chin. Med. J. 2020, 133, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Jain, S.; Finelli, L.; Shaw, M.W.; Lindstrom, S.; Garten, R.J.; Gubareva, L.V.; Xu, X.; Bridges, C.B.; Uyeki, T.M. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009, 360, 2605–2615. [Google Scholar] [CrossRef] [PubMed]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.; Horovitz, D.D.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.; Neri, J.I.; Neto, J.M.; Wanderley, H.Y.; et al. Possible Association between Zika Virus Infection and Microcephaly—Brazil, 2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- Holloway, R.; Rasmussen, S.A.; Zaza, S.; Cox, N.J.; Jernigan, D.B. Updated preparedness and response framework for influenza pandemics. MMWR Recomm. Rep. 2014, 63, 1–18. [Google Scholar]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database J. Biol. Databases Curation 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Healthc. J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Gutierrez, G. Artificial Intelligence in the Intensive Care Unit. Crit. Care 2020, 24, 101. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Niel, O.; Bastard, P. Artificial Intelligence in Nephrology: Core Concepts, Clinical Applications, and Perspectives. Am. J. Kidney Dis. 2019, 74, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Carin, L.; Dzau, V.; Wong, T.Y. Digital technology and COVID-19. Nat. Med. 2020, 26, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.L. SARS and other coronaviruses in humans and animals. Adv. Exp. Med. Biol. 2006, 581, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.; Nunn, M.; Grossel, G.; Burgman, M. Comparison of web-based biosecurity intelligence systems: BioCaster, EpiSPIDER and HealthMap. Transbound. Emerg. Dis. 2012, 59, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Freifeld, C.C.; Mandl, K.D.; Reis, B.Y.; Brownstein, J.S. HealthMap: Global infectious disease monitoring through automated classification and visualization of Internet media reports. J. Am. Med. Inform. Assoc. 2008, 15, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hossain, N.; Househ, M. Using HealthMap to Analyse Middle East Respiratory Syndrome (MERS) Data. Stud. Health Technol. Inform. 2016, 226, 213–216. [Google Scholar] [PubMed]
- Rezaei, M.; Shahidi, M. Zero-Shot Learning and its Applications from Autonomous Vehicles to COVID-19 Diagnosis: A Review. Intell. Based Med. 2020, 3–4, 100005. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, A.H.; Jochmans, D.; Posthuma, C.C.; Zevenhoven-Dobbe, J.C.; van Nieuwkoop, S.; Bestebroer, T.M.; van den Hoogen, B.G.; Neyts, J.; Snijder, E.J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014, 58, 4875–4884. [Google Scholar] [CrossRef] [PubMed]
- BlueDot Protects People around the World from Infectious Diseases with Human and Artificial Intelligence. 2020. Available online: https://bluedot.global/about/ (accessed on 15 May 2020).
- Agbehadji, I.E.; Awuzie, B.O.; Ngowi, A.B.; Millham, R.C. Review of Big Data Analytics, Artificial Intelligence and Nature-Inspired Computing Models towards Accurate Detection of COVID-19 Pandemic Cases and Contact Tracing. Int. J. Environ. Res. Public Health 2020, 17, 5330. [Google Scholar] [CrossRef] [PubMed]
- Dataminr. Real-Time Event and Risk Detection. 2020. Available online: https://www.dataminr.com (accessed on 15 May 2020).
- An AI Epidemiologist Sent the First Warnings of the Wuhan Virus. Available online: https://utulsa.edu/an-ai-epidemiologist-sent-the-first-warnings-of-the-wuhan-virus/ (accessed on 15 May 2020).
- Wang, P.; Zheng, X.; Ai, G.; Liu, D.; Zhu, B. Time series prediction for the epidemic trends of COVID-19 using the improved LSTM deep learning method: Case studies in Russia, Peru and Iran. Chaos Solitons Fractals 2020, 140, 110214. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kelley, B.P.; Nasser, J.S.; Chung, K.C. Implementing Precision Medicine and Artificial Intelligence in Plastic Surgery: Concepts and Future Prospects. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2113. [Google Scholar] [CrossRef] [PubMed]
- Matheny, M.E.; Whicher, D.; Thadaney Israni, S. Artificial Intelligence in Health Care: A Report from the National Academy of Medicine. JAMA 2020, 323, 509–510. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Tsang, I.W.; Liu, C. Complementary Attributes: A New Clue to Zero-Shot Learning. IEEE Trans. Cybern. 2019. [Google Scholar] [CrossRef] [PubMed]
- Benkler, Y. Don’t let industry write the rules for AI. Nature 2019, 569, 161. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.; Nevejans, N.; Allen, C.; Blyth, A.; Leonard, S.; Pagallo, U.; Holzinger, K.; Holzinger, A.; Sajid, M.I.; Ashrafian, H. Legal, regulatory, and ethical frameworks for development of standards in artificial intelligence (AI) and autonomous robotic surgery. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2019, 15, e1968. [Google Scholar] [CrossRef]
- Kluge, E.W. Artificial intelligence in healthcare: Ethical considerations. Healthc. Manag. Forum 2020, 33, 47–49. [Google Scholar] [CrossRef]
- Chimmula, V.K.R.; Zhang, L. Time series forecasting of COVID-19 transmission in Canada using LSTM networks. Chaos Solitons Fractals 2020, 135, 109864. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Saba, A.I.; Elaziz, M.A.; Lu, S.; Shanmugan, S.; Muthuramalingam, T.; Kumar, R.; Mosleh, A.O.; Essa, F.A.; Shehabeldeen, T.A. Deep learning-based forecasting model for COVID-19 outbreak in Saudi Arabia. Process Saf. Environ. Prot. Trans. Inst. Chem. Eng. Part B 2020, 149, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Saba, A.I.; Elsheikh, A.H. Forecasting the prevalence of COVID-19 outbreak in Egypt using nonlinear autoregressive artificial neural networks. Process Saf. Environ. Prot. Trans. Inst. Chem. Eng. Part B 2020, 141, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hawas, M. Generated time-series prediction data of COVID-19’s daily infections in Brazil by using recurrent neural networks. Data Brief 2020, 32, 106175. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Jo, H.; Son, H.; Hwang, H.J. Real-World Implications of a Rapidly Responsive COVID-19 Spread Model with Time-Dependent Parameters via Deep Learning: Model Development and Validation. J. Med. Internet Res. 2020, 22, e19907. [Google Scholar] [CrossRef]
- Prasanth, S.; Singh, U.; Kumar, A.; Tikkiwal, V.A.; Chong, P.H.J. Forecasting spread of COVID-19 using Google Trends: A hybrid GWO-Deep learning approach. Chaos Solitons Fractals 2020, 110336. [Google Scholar] [CrossRef]
- Shastri, S.; Singh, K.; Kumar, S.; Kour, P.; Mansotra, V. Time series forecasting of Covid-19 using deep learning models: India-USA comparative case study. Chaos Solitons Fractals 2020, 140, 110227. [Google Scholar] [CrossRef] [PubMed]
- Pourghasemi, H.R.; Pouyan, S.; Farajzadeh, Z.; Sadhasivam, N.; Heidari, B.; Babaei, S.; Tiefenbacher, J.P. Assessment of the outbreak risk, mapping and infection behavior of COVID-19: Application of the autoregressive integrated-moving average (ARIMA) and polynomial models. PLoS ONE 2020, 15, e0236238. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Park, S.; Chong, M. Conversations and Medical News Frames on Twitter: Infodemiological Study on COVID-19 in South Korea. J. Med. Internet Res. 2020, 22, e18897. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.V.; Garchitorena, A.; Rakotonanahary, R.J.L.; Drake, J.M.; Andriamihaja, B.; Rajaonarifara, E.; Ngonghala, C.N.; Roche, B.; Bonds, M.H.; Rakotonirina, J. Reconciling model predictions with low reported cases of COVID-19 in Sub-Saharan Africa: Insights from Madagascar. Glob. Health Action 2020, 13, 1816044. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Srivastava, S.; Chaudhary, G.; Al-Turjman, F. A systematic approach for COVID-19 predictions and parameter estimation. Pers. Ubiquitous Comput. 2020, 1–13. [Google Scholar] [CrossRef]
- Ansumali, S.; Kaushal, S.; Kumar, A.; Prakash, M.K.; Vidyasagar, M. Modelling a pandemic with asymptomatic patients, impact of lockdown and herd immunity, with applications to SARS-CoV-2. Annu. Rev. Control 2020. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Das, K.; Parida, S.K. Inter nation social lockdown versus medical care against COVID-19, a mild environmental insight with special reference to India. Sci. Total Environ. 2020, 728, 138914. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Murali Sundram, B.; Rajendran, K.; Boon Law, K.; Aris, T.; Ibrahim, H.; Chandra Dass, S.; Singh Gill, B. Forecasting daily confirmed COVID-19 cases in Malaysia using ARIMA models. J. Infect. Dev. Ctri. 2020, 14, 971–976. [Google Scholar] [CrossRef] [PubMed]
- He, J.L.; Luo, L.; Luo, Z.D.; Lyu, J.X.; Ng, M.Y.; Shen, X.P.; Wen, Z. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir. Med. 2020, 168, 105980. [Google Scholar] [CrossRef] [PubMed]
- Lessmann, N.; Sánchez, C.I.; Beenen, L.; Boulogne, L.H.; Brink, M.; Calli, E.; Charbonnier, J.P.; Dofferhoff, T.; van Everdingen, W.M.; Gerke, P.K.; et al. Automated Assessment of CO-RADS and Chest CT Severity Scores in Patients with Suspected COVID-19 Using Artificial Intelligence. Radiology 2020, 202439. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Li, Y.; Zhang, T.; Gao, L.; Jin, D.; Sun, Y.; Ye, X.; Yu, L.; Hu, Z.; et al. From community-acquired pneumonia to COVID-19: A deep learning-based method for quantitative analysis of COVID-19 on thick-section CT scans. Eur. Radiol. 2020, 30, 6828–6837. [Google Scholar] [CrossRef]
- Neri, E.; Miele, V.; Coppola, F.; Grassi, R. Use of CT and artificial intelligence in suspected or COVID-19 positive patients: Statement of the Italian Society of Medical and Interventional Radiology. Radiol. Med. 2020, 125, 505–508. [Google Scholar] [CrossRef]
- ACR Issues Statement for Use of Chest Radiography, CT for Suspected COVID-19 Infection. 2020. Available online: https://appliedradiology.com/communities/CT-Imaging/acr-issues-statement-for-use-of-chest-radiography-ct-for-suspected-covid-19-infection (accessed on 15 May 2020).
- Rahimzadeh, M.; Attar, A. A modified deep convolutional neural network for detecting COVID-19 and pneumonia from chest X-ray images based on the concatenation of Xception and ResNet50V2. Inform. Med. Unlocked 2020, 19, 100360. [Google Scholar] [CrossRef]
- Chowdhury, N.K.; Rahman, M.M.; Kabir, M.A. PDCOVIDNet: A parallel-dilated convolutional neural network architecture for detecting COVID-19 from chest X-ray images. Health Inf. Sci. Syst. 2020, 8, 27. [Google Scholar] [CrossRef]
- Lee, K.S.; Kim, J.Y.; Jeon, E.T.; Choi, W.S.; Kim, N.H.; Lee, K.Y. Evaluation of Scalability and Degree of Fine-Tuning of Deep Convolutional Neural Networks for COVID-19 Screening on Chest X-ray Images Using Explainable Deep-Learning Algorithm. J. Pers. Med. 2020, 10, 213. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Z.Q.; Wong, A. COVID-Net: A tailored deep convolutional neural network design for detection of COVID-19 cases from chest X-ray images. Sci. Rep. 2020, 10, 19549. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, D.; Dong, J.; Wang, N.; Huang, H.; Xu, H.; Xia, C. False-Negative Results of Real-Time Reverse-Transcriptase Polymerase Chain Reaction for Severe Acute Respiratory Syndrome Coronavirus 2: Role of Deep-Learning-Based CT Diagnosis and Insights from Two Cases. Korean J. Radiol. 2020, 21, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 200642. [Google Scholar] [CrossRef] [PubMed]
- Schwyzer, M.; Martini, K.; Skawran, S.; Messerli, M.; Frauenfelder, T. Pneumonia Detection in Chest X-Ray Dose-Equivalent CT: Impact of Dose Reduction on Detectability by Artificial Intelligence. Acad. Radiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; Urraro, F.; Grassi, R.; Giacobbe, G.; Patelli, G.; Cappabianca, S.; Reginelli, A. Artificial intelligence to codify lung CT in Covid-19 patients. Radiol. Med. 2020, 125, 500–504. [Google Scholar] [CrossRef]
- Cath, C. Governing artificial intelligence: Ethical, legal and technical opportunities and challenges. Philos. Trans. Math Phys. Eng. Sci. 2018, 376. [Google Scholar] [CrossRef]
- Chen, M.; Decary, M. Artificial intelligence in healthcare: An essential guide for health leaders. Healthc. Manag. Forum 2020, 33, 10–18. [Google Scholar] [CrossRef]
- Martin, A.; Nateqi, J.; Gruarin, S.; Munsch, N.; Abdarahmane, I.; Zobel, M.; Knapp, B. An artificial intelligence-based first-line defence against COVID-19: Digitally screening citizens for risks via a chatbot. Sci. Rep. 2020, 10, 19012. [Google Scholar] [CrossRef]
- Badell-Grau, R.A.; Cuff, J.P.; Kelly, B.P.; Waller-Evans, H.; Lloyd-Evans, E. Investigating the Prevalence of Reactive Online Searching in the COVID-19 Pandemic: Infoveillance Study. J. Med. Internet Res. 2020, 22, e19791. [Google Scholar] [CrossRef]
- Knebel, A.R.; Sharpe, V.A.; Danis, M.; Toomey, L.M.; Knickerbocker, D.K. Informing the gestalt: An ethical framework for allocating scarce federal public health and medical resources to states during disasters. Disaster Med. Public Health Prep. 2014, 8, 79–88. [Google Scholar] [CrossRef]
- Wolf, L.; Hensel, W. Valuing lives: Allocating scarce medical resources during a public health emergency and the Americans with Disabilities Act (perspective). PLoS Curr. 2011, 3, RRN1271. [Google Scholar] [CrossRef] [PubMed]
- Daugherty Biddison, E.L.; Gwon, H.; Schoch-Spana, M.; Cavalier, R.; White, D.B.; Dawson, T.; Terry, P.B.; London, A.J.; Regenberg, A.; Faden, R.; et al. The community speaks: Understanding ethical values in allocation of scarce lifesaving resources during disasters. Ann. Am. Thorac. Soc. 2014, 11, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Vergano, M.; Bertolini, G.; Giannini, A.; Gristina, G.R.; Livigni, S.; Mistraletti, G.; Riccioni, L.; Petrini, F. Clinical ethics recommendations for the allocation of intensive care treatments in exceptional, resource-limited circumstances: The Italian perspective during the COVID-19 epidemic. Crit. Care 2020, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, S. Principles of scarce medical resource allocation in natural disaster relief: A simulation approach. Med. Decis. Mak. 2012, 32, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Persad, G.; Wertheimer, A.; Emanuel, E.J. Principles for allocation of scarce medical interventions. Lancet 2009, 373, 423–431. [Google Scholar] [CrossRef]
- Huesch, M.D. One and done? Equality of opportunity and repeated access to scarce, indivisible medical resources. BMC Med. Ethics 2012, 13, 11. [Google Scholar] [CrossRef]
- Guo, R.; Farnsworth, T.J.; Hermanson, P.M. Information Resources for Hospital Administrator Healthcare Management Decision-Making. J. Hosp. Librariansh. 2015, 15, 274–283. [Google Scholar] [CrossRef][Green Version]
- Lai, J.; Ma, S.; Wang, Y.; Cai, Z.; Hu, J.; Wei, N.; Wu, J.; Du, H.; Chen, T.; Li, R.; et al. Factors Associated With Mental Health Outcomes Among Health Care Workers Exposed to Coronavirus Disease 2019. JAMA Netw. Open 2020, 3, e203976. [Google Scholar] [CrossRef]
- Sherman, M.F.; Gershon, R.R.; Riley, H.E.M.; Zhi, Q.; Magda, L.A.; Peyrot, M. Emergency Preparedness Safety Climate and Other Factors Associated with Mental Health Outcomes among World Trade Center Disaster Evacuees. Disaster Med. Public Health Prep. 2017, 11, 326–336. [Google Scholar] [CrossRef]
- DeLucia, J.A.; Bitter, C.; Fitzgerald, J.; Greenberg, M.; Dalwari, P.; Buchanan, P. Prevalence of Post-Traumatic Stress Disorder in Emergency Physicians in the United States. West. J. Emerg. Med. 2019, 20, 740–746. [Google Scholar] [CrossRef]
- Di Castelnuovo, A.; Bonaccio, M.; Costanzo, S.; Gialluisi, A.; Antinori, A.; Berselli, N.; Blandi, L.; Bruno, R.; Cauda, R.; Guaraldi, G.; et al. Common cardiovascular risk factors and in-hospital mortality in 3894 patients with COVID-19: Survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1899–1913. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.J.; Xu, J.; Yin, J.M.; Li, L.; Hou, W.; Zhang, L.L.; Zhou, Z.; Yu, Y.Z.; Li, H.J.; Feng, Y.M.; et al. Lower Circulating Interferon-Gamma Is a Risk Factor for Lung Fibrosis in COVID-19 Patients. Front. Immunol. 2020, 11, 585647. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Wittbold, K.A.; Dadabhoy, F.Z.; Sato, R.; Landman, A.B.; Schwamm, L.H.; He, S.; Patel, R.; Wei, N.; Zuccotti, G.; et al. Digital triage: Novel strategies for population health management in response to the COVID-19 pandemic. Healthcare 2020, 8, 100493. [Google Scholar] [CrossRef] [PubMed]
- Chassagnon, G.; Vakalopoulou, M.; Battistella, E.; Christodoulidis, S.; Hoang-Thi, T.N.; Dangeard, S.; Deutsch, E.; Andre, F.; Guillo, E.; Halm, N.; et al. AI-driven quantification, staging and outcome prediction of COVID-19 pneumonia. Med. Image Anal. 2020, 67, 101860. [Google Scholar] [CrossRef]
- Suri, J.S.; Puvvula, A.; Biswas, M.; Majhail, M.; Saba, L.; Faa, G.; Singh, I.M.; Oberleitner, R.; Turk, M.; Chadha, P.S.; et al. COVID-19 pathways for brain and heart injury in comorbidity patients: A role of medical imaging and artificial intelligence-based COVID severity classification: A review. Comput. Biol. Med. 2020, 124, 103960. [Google Scholar] [CrossRef]
- Gao, Y.; Cai, G.Y.; Fang, W.; Li, H.Y.; Wang, S.Y.; Chen, L.; Yu, Y.; Liu, D.; Xu, S.; Cui, P.F.; et al. Machine learning based early warning system enables accurate mortality risk prediction for COVID-19. Nat. Commun. 2020, 11, 5033. [Google Scholar] [CrossRef]
- Neves, N.; Bitencourt, F.; Bitencourt, A.G.V. Ethical dilemmas in COVID-19 times: How to decide who lives and who dies? Rev. Assoc. Med. Bras. 2020, 66 (Suppl. S2), 106–111. [Google Scholar] [CrossRef]
- Suh, S.; Lee, H.; Lukowicz, P.; Lee, Y.O. CEGAN: Classification Enhancement Generative Adversarial Networks for unraveling data imbalance problems. Neural Netw. Off. J. Int. Neural Netw. Soc. 2020, 133, 69–86. [Google Scholar] [CrossRef]
- Blobel, B.; Ruotsalainen, P.; Brochhausen, M.; Oemig, F.; Uribe, G.A. Autonomous Systems and Artificial Intelligence in Healthcare Transformation to 5P Medicine-Ethical Challenges. Stud. Health Technol. Inform. 2020, 270, 1089–1093. [Google Scholar] [CrossRef]
- Lian, W.; Wen, L.; Zhou, Q.; Zhu, W.; Duan, W.; Xiao, X.; Mhungu, F.; Huang, W.; Li, C.; Cheng, W.; et al. Emergency response to the COVID-19 pandemic using digital health technologies: Practical experience of a tertiary hospital in China. J. Med. Internet Res. 2020. [Google Scholar] [CrossRef]
- Govindan, K.; Mina, H.; Alavi, B. A decision support system for demand management in healthcare supply chains considering the epidemic outbreak: A case study of coronavirus disease 2019 (COVID-19). Transp. Res. Part E Logist. Transp. Rev. 2020, 138, 101967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Coffee, M.; Bari, A.; Wang, J.; Jiang, X.; Huang, J.; Shi, J.; Dai, J.; Cai, J.; Zhang, T.; et al. Towards an artificial intelligence framework for data-driven prediction of coronavirus clinical severity. Comput. Mater. Contin. 2020, 63, 537–551. [Google Scholar] [CrossRef]
- Doctors Are Using AI to Triage COVID-19 Patients. the Tools May Be Here to Stay. Available online: https://axisimagingnews.com/imaging-news/doctors-are-using-ai-to-triage-covid-19-patients-the-tools-may-be-here-to-stay (accessed on 15 May 2020).
- Baden, L.R.; Rubin, E.J. Covid-19—The Search for Effective Therapy. N. Engl. J. Med. 2020, 382, 1851–1852. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.; Romm, T.; Gregg, A.; Hamburger, T. Scramble for Medical Equipment Descends into Chaos as U.S. States and Hospitals Compete for Rare Supplies. Available online: https://www.washingtonpost.com/business/2020/03/24/scramble-medical-equipment-descends-into-chaos-us-states-hospitals-compete-rare-supplies/ (accessed on 25 March 2020).
- Srinivasa Rao, A.S.R.; Vazquez, J.A. Identification of COVID-19 can be quicker through artificial intelligence framework using a mobile phone-based survey when cities and towns are under quarantine. Infect. Control Hosp. Epidemiol. 2020, 41, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Chakir, I.; El Khaili, M.; Mestari, M. Logistics Flow Optimization for Advanced Management of the Crisis Situation. Procedia Comput. Sci. 2020, 175, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rasul, G. A Framework for Improving Policy Priorities in Managing COVID-19 Challenges in Developing Countries. Front. Public Health 2020, 8, 589681. [Google Scholar] [CrossRef]
- Wang, M.; Xia, C.; Huang, L.; Xu, S.; Qin, C.; Liu, J.; Cao, Y.; Yu, P.; Zhu, T.; Zhu, H.; et al. Deep learning-based triage and analysis of lesion burden for COVID-19: A retrospective study with external validation. Lancet Digit. Health 2020, 2, e506–e515. [Google Scholar] [CrossRef]
- Bird, J.J.; Barnes, C.M.; Premebida, C.; Ekárt, A.; Faria, D.R. Country-level pandemic risk and preparedness classification based on COVID-19 data: A machine learning approach. PLoS ONE 2020, 15, e0241332. [Google Scholar] [CrossRef]
- Etzioni, E.; Decario, N. AI Can Help Scientists Find a Covid-19 Vaccine. Wired. 28 March 2020. Available online: https://www.wired.com/story/opinion-ai-can-help-find-scientists-find-a-covid-19-vaccine/ (accessed on 28 March 2020).
- LitCOVID. 2020. Available online: https://www.ncbi.nlm.nih.gov/research/coronavirus/ (accessed on 15 May 2020).
- Soam, S.S.; Bhasker, B.; Mishra, B.N. Improved prediction of MHC class I binders/non-binders peptides through artificial neural network using variable learning rate: SARS corona virus, a case study. Adv. Exp. Med. Biol. 2011, 696, 223–229. [Google Scholar] [CrossRef]
- Khan, A.; Ali, S.S.; Khan, M.T.; Saleem, S.; Ali, A.; Suleman, M.; Babar, Z.; Shafiq, A.; Khan, M.; Wei, D.Q. Combined drug repurposing and virtual screening strategies with molecular dynamics simulation identified potent inhibitors for SARS-CoV-2 main protease (3CLpro). J. Biomol. Struct. Dyn. 2020, 1–12. [Google Scholar] [CrossRef]
- Hage-Melim, L.; Federico, L.B.; de Oliveira, N.K.S.; Francisco, V.C.C.; Correia, L.C.; de Lima, H.B.; Gomes, S.Q.; Barcelos, M.P.; Francischini, I.A.G.; da Silva, C. Virtual screening, ADME/Tox predictions and the drug repurposing concept for future use of old drugs against the COVID-19. Life Sci. 2020, 256, 117963. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, O.V.; Rocha, G.B.; Paluch, A.S.; Costa, L.T. Repurposing approved drugs as inhibitors of SARS-CoV-2 S-protein from molecular modeling and virtual screening. J. Biomol. Struct. Dyn. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Krishnan, V.; de Bono, S.; Ottaviani, S.; Casalini, G.; Richardson, P.J.; Monteil, V.; Lauschke, V.M.; Mirazimi, A.; Youhanna, S.; et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol. Med. 2020, 12, e12697. [Google Scholar] [CrossRef] [PubMed]
- Metz, C. How AI streered doctors towards possible coronavirus treatment. The New York Times, 30 April 2020. [Google Scholar]
- NIH Clinical Trial of Investigational Vaccine for COVID-19 Begins NIH. 2020. Available online: https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (accessed on 15 May 2020).
- Yang, X.; Wang, Y.; Byrne, R.; Schneider, G.; Yang, S. Concepts of Artificial Intelligence for Computer-Assisted Drug Discovery. Chem. Rev. 2019, 119, 10520–10594. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Ozaki, M.; Otsuka, C.; Vecchione, A.; Arai, T.; Kitagawa, T.; Ofusa, K.; Yabumoto, M.; Hirotsu, T.; et al. COVID-19 Drug Discovery Using Intensive Approaches. Int. J. Mol. Sci. 2020, 21, 2839. [Google Scholar] [CrossRef] [PubMed]
- Yong, E. A Popular Algorithm Is No Better at Predicting Crimes Than Random People. The Atlantic, 17 January 2018. [Google Scholar]
- Souza Filho, E.M.; Fernandes, F.A.; Soares, C.L.A.; Seixas, F.L.; Santos, A.; Gismondi, R.A.; Mesquita, E.T.; Mesquita, C.T. Artificial Intelligence in Cardiology: Concepts, Tools and Challenges—“The Horse is the One Who Runs, You Must Be the Jockey”. Arq. Bras. Cardiol. 2019. [Google Scholar] [CrossRef]
- Gurupur, V.; Wan, T.T.H. Inherent Bias in Artificial Intelligence-Based Decision Support Systems for Healthcare. Medicina 2020, 56, 141. [Google Scholar] [CrossRef]
- Randhawa, G.K.; Jackson, M. The role of artificial intelligence in learning and professional development for healthcare professionals. Healthc. Manag. Forum 2020, 33, 19–24. [Google Scholar] [CrossRef]
- Asan, O.; Bayrak, A.E.; Choudhury, A. Artificial Intelligence and Human Trust in Healthcare: Focus on Clinicians. J. Med. Internet Res. 2020, 22, e15154. [Google Scholar] [CrossRef]
| Key Questions | The Promise of AI | The Peril of AI | References |
|---|---|---|---|
| How can AI be used to identify emerging biological threats? |
|
| [1,9,10,11,12,15,17,26,63,64,65,66,67] |
| Can AI mitigate the spread of biological diseases and guide early treatment? |
|
| [1,11,12,68] |
| How might AI guide medical management and resource allocation? |
|
| [13,14,15,16,17,18,20,25,26,37,69,70,71,72] |
| How might AI accelerate development of medical therapies and treatment protocols? |
|
| [41,42,43,45,46,48,73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laudanski, K.; Shea, G.; DiMeglio, M.; Restrepo, M.; Solomon, C. What Can COVID-19 Teach Us about Using AI in Pandemics? Healthcare 2020, 8, 527. https://doi.org/10.3390/healthcare8040527
Laudanski K, Shea G, DiMeglio M, Restrepo M, Solomon C. What Can COVID-19 Teach Us about Using AI in Pandemics? Healthcare. 2020; 8(4):527. https://doi.org/10.3390/healthcare8040527
Chicago/Turabian StyleLaudanski, Krzysztof, Gregory Shea, Matthew DiMeglio, Mariana Restrepo, and Cassie Solomon. 2020. "What Can COVID-19 Teach Us about Using AI in Pandemics?" Healthcare 8, no. 4: 527. https://doi.org/10.3390/healthcare8040527
APA StyleLaudanski, K., Shea, G., DiMeglio, M., Restrepo, M., & Solomon, C. (2020). What Can COVID-19 Teach Us about Using AI in Pandemics? Healthcare, 8(4), 527. https://doi.org/10.3390/healthcare8040527





