Abstract
Background/Objectives: Neonatal outcomes, including low birth weight, preterm birth, and neonatal mortality, pose significant global health challenges, particularly in low- and middle-income countries. Prenatal care has emerged as a critical intervention in mitigating these risks through medical, nutritional, and psychosocial support. This study aimed to systematically assess the effectiveness of prenatal care interventions in preventing neonatal outcomes across diverse settings. Methods: A systematic review and meta-analysis were conducted according to PRISMA guidelines, with the protocol registered in PROSPERO (CRD42024601066). Fourteen peer-reviewed studies were included following a comprehensive search across five major databases. Eligible studies reported quantitative neonatal outcomes associated with prenatal care interventions, including nutritional supplementation, mental health services, telehealth, and routine antenatal care. Random-effects models were used for meta-analysis, and the risk of bias was assessed using RoB 2 and the Newcastle–Ottawa Scale. Results: Nutritional interventions, especially folic acid and iron supplementation, significantly reduced neonatal mortality by up to 40% (RR = 0.60, 95% CI: 0.54–0.68). High-quality prenatal care was associated with a 41% reduction in neonatal mortality. Psychosocial support reduced the risk of low birth weight and preterm birth, while telehealth interventions lowered NICU admissions in low-risk populations (RR = 0.88, 95% CI: 0.75–1.03). Heterogeneity was substantial (I2 = 70%), and publication bias was suggested. Conclusions: Comprehensive prenatal care, integrating medical, nutritional, and mental health interventions, significantly improves neonatal outcomes. The global implementation of accessible, high-quality prenatal services is essential, particularly in underserved populations, to reduce neonatal morbidity and mortality.
1. Introduction
In recent decades, prenatal care has increasingly been recognized as a critical public health intervention aimed at improving maternal and neonatal outcomes [1]. According to recent global statistics from the World Health Organization (WHO), approximately 2.3 million neonatal deaths occurred in 2021, with neonatal mortality remaining disproportionately higher in low- and middle-income countries (LMICs) compared to high-income regions [2]. Neonatal outcomes, including preterm birth complications, low birth weight (LBW), and congenital anomalies, significantly contribute to these mortality rates and long-term morbidity, especially in resource-limited settings [3]. Such alarming statistics underscore the pressing need for effective preventive interventions and highlight prenatal care as a pivotal strategy for reducing adverse neonatal outcomes [4].
Prenatal care encompasses a wide spectrum of interventions designed to enhance the health of pregnant women and their newborns. It typically includes medical check-ups, nutritional counseling and supplementation, psychosocial and mental health support, infection screening, and preventive measures such as immunizations [5]. Each of these interventions operates through specific physiological mechanisms to improve neonatal outcomes. For example, iron supplementation mitigates maternal anemia, thereby optimizing maternal and fetal oxygen delivery, enhancing fetal growth, and reducing risks of preterm birth and LBW [6,7]. Similarly, folic acid supplementation is crucial for preventing neural tube defects by supporting healthy cell growth and tissue formation during critical developmental periods [8]. Psychosocial interventions help manage maternal stress and anxiety, which physiologically reduces cortisol exposure, thereby decreasing the risks of preterm birth and developmental impairments [9]. Despite clear biological rationales, variability in prenatal care effectiveness across different contexts has been reported in the literature, highlighting discrepancies influenced by factors such as healthcare access, intervention adherence, socioeconomic conditions, and healthcare infrastructure quality [10].
Preterm birth complications, such as low birth weight (LBW) and congenital anomalies, not only contribute to neonatal mortality but also result in long-term morbidity. Notably, there is an increasing recognition of neonates surviving with complex medical needs, forming a growing subpopulation termed “children with medical complexity” [11]. This shift has substantial implications for pediatric care systems and further highlights the importance of effective prenatal interventions.
A significant body of research, including multiple systematic reviews and meta-analyses, has affirmed prenatal care’s effectiveness in enhancing maternal and neonatal health [12]. For instance, meta-analytic studies by Bhutta et al. (2013) and Lassi et al. (2021) demonstrated that comprehensive nutritional interventions during pregnancy significantly decrease neonatal mortality and morbidity in LMICs [13,14]. Yet, findings from other studies have sometimes challenged these conclusions, suggesting limited or variable impacts of prenatal care interventions in certain contexts or populations, which are often attributed to barriers such as inadequate healthcare infrastructure, limited access to quality care, poor adherence, or differing socioeconomic circumstances [15]. This conflicting evidence necessitates a more nuanced exploration of prenatal care interventions and their effectiveness across diverse global settings.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure a transparent and rigorous process. Following the PRISMA-P protocols, the research protocol was registered with PROSPERO (registration number: CRD42024601066), which underscores our commitment to maintaining high standards in our systematic review methodology.
A comprehensive search of electronic databases was conducted to identify studies relevant to the role of prenatal care in preventing neonatal outcomes. The databases searched included PubMed, Embase, Cochrane Library, Web of Science, and Scopus, covering studies published up to 30 April 2024. The search strategy incorporated both Medical Subject Headings (MeSHs) and relevant keywords, carefully chosen to ensure broad coverage of the literature. Key search terms included “prenatal care”, “Neonatal outcomes”, “birth outcomes”, “low birth weight”, “preterm birth”, “congenital anomalies”, “neonatal mortality”, and “public health interventions” (Table 1).

Table 1.
Search strategy.
The search strategy underwent multiple iterations and refinements to ensure comprehensiveness. Boolean operators (AND; OR) were employed to combine key terms and capture all relevant studies. In addition to database searches, the reference lists of all included studies and review articles were manually searched to identify any additional eligible studies. Grey literature, such as conference abstracts, reports, and theses, was also considered to minimize the risk of publication bias.
This search was augmented by a manual search for the references section in relevant articles and grey literature. Efforts were made to retrieve unpublished studies or those still in progress and to minimize language bias by including studies published in languages other than English where translations were available.
2.2. Eligibility Screening
After removing duplicates, the eligibility screening process was carried out in two stages. First, titles and abstracts were independently reviewed by two authors to assess their relevance. In cases of disagreement, a third reviewer was consulted to resolve discrepancies. Studies that passed this initial screening underwent full-text review based on pre-established inclusion and exclusion criteria.
2.2.1. Inclusion Criteria
- Studies involving human subjects with available data on neonatal outcomes (e.g., preterm birth, low birth weight, and congenital anomalies).
- Studies evaluating the impact of prenatal care interventions on neonatal outcomes.
- Randomized controlled trials, cohort studies, case–control studies, and systematic reviews.
- Studies published in peer-reviewed journals, including full-text papers with available data.
- Studies had to clearly report methods addressing selection and measurement biases. Research rated as “high-risk” studies in two or more domains using RoB 2 tools were excluded to maintain reliability.
- Only studies published in English were included due to practical constraints related to translation accuracy, resource limitations, and comparability in methods reporting. While this exclusion criterion potentially introduces language bias, we mitigated this risk by performing a manual search of the references from included English studies and grey literature to identify important non-English studies translated or summarized into English when available.
2.2.2. Exclusion Criteria
- Non-research articles such as case reports, editorials, and conference abstracts.
- Studies focusing on animals or in vitro research.
- Studies not focused on prenatal care interventions or lacking data on neonatal outcomes.
- Non-English studies where translations were not accessible.
- Studies that did not clearly report neonatal outcomes quantitatively.
- Sample sizes that were below the specified minimum threshold (<100 participants).
- A high risk of bias identified in multiple domains (≥2 domains).
- Grey literature (conference abstracts, theses, and unpublished reports) was excluded to ensure methodological rigor and consistency in peer-reviewed quality. Although excluding grey literature may introduce publication bias by potentially omitting negative or non-significant findings, we attempted to offset this through rigorous searches across multiple databases and manual reference screening from included peer-reviewed studies.
2.3. Data Extraction
A standardized data extraction form was developed and pilot-tested to ensure consistency in data collection. Two reviewers independently extracted data from each included study, capturing information on the study characteristics, population demographics, intervention types, and outcome measures.
Data Extracted Included
- Study Characteristics: Author, year, country, study design, sample size, and funding source.
- Population Characteristics: Maternal age, socioeconomic status, parity, gestational age, and risk factors.
- Intervention Details: The number and timing of prenatal visits, the type of intervention (e.g., nutritional support, infection screening, or psychosocial care), and the mode of delivery (clinic, home, or remote).
- Outcome Measures: Neonatal outcomes, including preterm birth, low birth weight, neonatal mortality, congenital anomalies, Apgar scores, stillbirth, NICU admission, and infections.
- Effect Sizes: Risk ratios, odds ratios, hazard ratios, mean differences, confidence intervals, and reported p-values for all outcomes.
- Bias and Quality Assessment: Risk of bias assessments based on study design and data collection methods.
Discrepancies in data extraction were resolved through discussion and consultation with a third reviewer, and attempts were made to contact the study authors for clarification when necessary.
2.4. Quality Assessment
The quality and risk of bias in the included studies were assessed using two established tools: the Cochrane Risk of Bias (RoB 2) tool for randomized trials and the Newcastle–Ottawa Scale (NOS) for observational studies. The RoB 2 tool was used to evaluate the following domains: randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and the selection of reported results. The NOS assessed selection, comparability, and outcome ascertainment for cohort and case–control studies.
Each study was independently evaluated by two reviewers, and discrepancies were resolved through discussion or the involvement of a third reviewer. The results of these assessments were used to guide sensitivity analyses and subgroup analyses to explore the impact of study quality on the overall findings.
2.5. Data Analysis
Data were analyzed using both quantitative and qualitative methods to provide a comprehensive assessment of the impact of prenatal care on neonatal outcomes.
Quantitative Analysis: Meta-analyses were conducted using random-effects models to account for heterogeneity between studies. Pooled effect sizes were calculated for each outcome measure, and the results were presented in forest plots. Heterogeneity was assessed using the I2 statistic, with values above 50% indicating substantial heterogeneity. Sensitivity analyses were performed to explore the impact of study quality, geographic location, and types of prenatal care interventions on the outcomes.
Subgroup Analyses: Subgroup analyses were conducted based on factors such as high-income vs. low-income countries, the frequency of prenatal visits, and types of interventions (e.g., nutritional supplementation vs. infection screening). This helped to identify the differential effects of prenatal care on neonatal outcomes in different contexts.
Qualitative Synthesis: A narrative synthesis was performed to complement the meta-analysis. This involved summarizing key findings from the included studies, identifying trends and gaps in the literature, and providing insights into the implications of prenatal care on neonatal health globally.
We used random-effects models for all meta-analyses to account for anticipated variability across studies (due to differing populations, settings, intervention protocols, and methodologies). Given the substantial heterogeneity observed preliminarily (e.g., variations in sample characteristics, intervention types, and geographic context), the random-effects model was considered most appropriate as it incorporates both within- and between-study variability into the pooled effect size.
Heterogeneity among the studies was assessed using the following tools:
- Cochran’s Q test: This evaluates whether observed differences among studies were statistically significant (p < 0.10 indicating significance).
- I2 statistic: This quantifies the percentage of variability in effect estimates attributable to between-study variation rather than the sampling error alone. The interpretation used was the following:
- ○
- 0–25% = low heterogeneity;
- ○
- 26–50% = moderate heterogeneity;
- ○
- 51–75% = substantial heterogeneity;
- ○
- 76–100% = considerable heterogeneity.
Substantial or considerable heterogeneity (I2 > 50%) triggered additional subgroup and sensitivity analyses to explore sources of variability.
2.6. Study Flow and Selection
The initial search yielded 4521 records. After duplicate removal, 531 records were screened by title and abstract, and 106 studies were excluded based on irrelevance. Of the 158 full-text articles assessed for eligibility, 144 did not meet the inclusion criteria, leaving 14 studies for final inclusion in the meta-analysis. The study selection process is illustrated in a PRISMA flow chart (Figure 1).
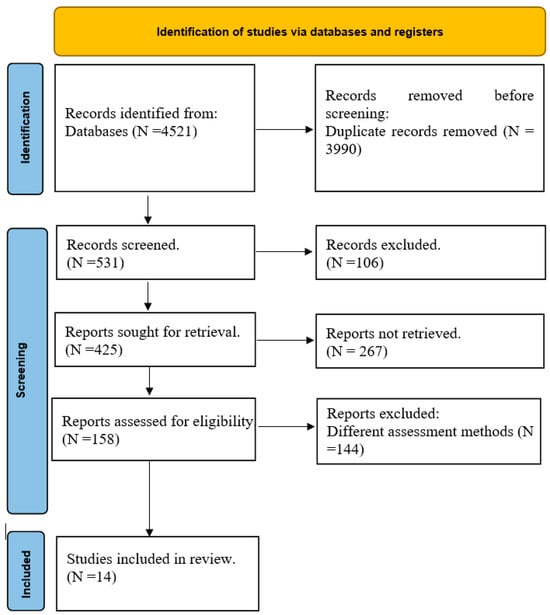
Figure 1.
PRISMA flow chart.
3. Results
3.1. Quality Assessment Results
The quality assessment of the studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29] in the review shows a range of risks of bias across various domains (Figure 2). Several studies, such as those by Bhutta et al. (2013) [16], McPherson et al. [23] and Fleet et al. [29], have been rated as having “some concerns” in terms of missing outcome data (D3) and bias in the selection of the reported results (D5). This indicates potential limitations in how the data were managed or selected, which could impact the reliability of their findings. Studies like Black et al. [17], Makate et al. [22], and Vintzileos et al. [26] show a “high” risk of bias in the outcome data domain (D3), signaling significant concerns about how complete and accurate the outcome data are. Such bias may affect the interpretation of these studies’ conclusions and the overall strength of the evidence they provide. On the other hand, studies such as those performed by Wondemagegn et al. [25] and Wassie et al. [28] exhibit relatively low risk across most domains, with minimal concerns, particularly in the randomization process (D1) and deviations from the intended interventions (D2). These studies are considered more robust and reliable, contributing stronger evidence to the overall review.
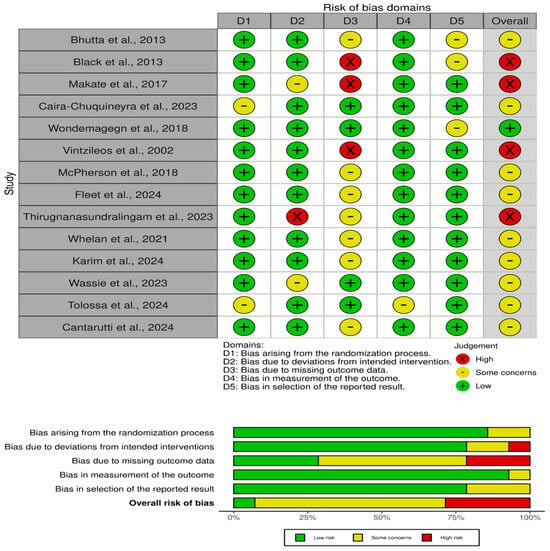
Figure 2.
Risk of bias assessment [16,17,18,19,20,21,22,23,24,25,26,27,28,29].
The overall judgment for the studies ranges from low to some concerns, with the occasional high-risk study. The variation in risk levels highlights the importance of the careful interpretation of the results, particularly for studies that exhibit “high” risk in key domains. For studies with “some concerns”, the potential bias might slightly affect the results, while those with low risk provide more dependable evidence. These assessments reflect the necessity of considering the risk of bias when synthesizing findings from different studies to draw accurate conclusions.
3.2. Main Outcomes
The impact of prenatal care on neonatal outcomes is evident across various themes with the extracted studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29], highlighting significant effects on reducing neonatal mortality, low birth weight, preterm birth, and improving overall neonatal health. These outcomes vary according to the type and quality of prenatal interventions, the socio-economic context, and specific risk factors among different populations (Table 2).

Table 2.
Extraction table of the included studies [16,17,18,19,20,21,22,23,24,25,26,27,28,29].
3.2.1. Nutrition Interventions: The Lifeline of Prenatal Care
Maternal nutrition plays a foundational role in determining the health outcomes of both the mother and child. Studies such as Bhutta et al. (2013) and Black et al. (2013) provide strong evidence that nutrition interventions, including folic acid and iron supplementation, have a profound impact on reducing neonatal mortality [16,17]. Bhutta et al. demonstrated that these interventions reduced the risk of neonatal mortality by 15%, while Black et al. reported an even more substantial reduction, with a 40% decrease in neonatal deaths. These findings are particularly crucial in low- and middle-income countries (LMICs) where malnutrition is prevalent and resources are limited. By providing balanced energy and protein supplements during pregnancy, women are better equipped to give birth to healthier infants, thereby reducing the likelihood of stunting and low birth weight (LBW). Such interventions are simple yet highly effective and should be prioritized in global public health programs, especially in regions with high maternal and child mortality rates.
3.2.2. Quality of Prenatal Care: A Preventative Shield
The quality of prenatal care is a critical determinant in the reduction of adverse neonatal outcomes. High-quality care encompasses regular medical checkups, vaccinations, nutritional support, and the early identification of potential complications. Makate et al. (2017) in Zimbabwe demonstrated that women receiving high-quality prenatal care saw a 41% reduction in neonatal mortality compared to those receiving lower-quality or no care [22]. Similarly, Wondemagegn et al. (2018) found a 34% reduction in neonatal mortality, particularly in sub-Saharan Africa, when antenatal care follow-up visits were prioritized [25]. These findings point to the essential role that structured and consistent prenatal care plays, especially in resource-limited settings. Comprehensive care ensures that risks such as preeclampsia, infections, and LBW are identified early, allowing for timely interventions that can save lives. Conversely, as noted by Caira-Chuquineyra et al. (2023), inadequate care or missed visits lead to higher rates of complications, including a 39% higher likelihood of LBW [24]. This stark contrast underscores the importance of ensuring that all pregnant women, regardless of their socio-economic status, have access to high-quality prenatal care.
3.2.3. Psychosocial and Mental Health Support: Addressing the Overlooked
Psychosocial factors, including maternal mental health, are often overlooked in prenatal care, but they have a significant impact on birth outcomes. Whelan et al. (2021) and Karim et al. (2024) illustrate the profound benefits of integrating mental health services into prenatal care, particularly for women with psychiatric conditions or those experiencing high levels of stress [21,27]. Whelan et al. found that inpatient psychiatric care for women with severe mental health conditions improved both gestational age and birth weight, thereby reducing the risk of preterm births and other complications. Similarly, Karim et al. showed that pregnant women receiving mental health support had a significantly reduced risk of LBW and a reduced number of small-for-gestational-age (SGA) infants.
These findings highlight the need for more widespread mental health services within prenatal programs, especially in low socio-economic settings where mental health support is often unavailable. Additionally, Wassie et al. (2023) revealed that exposure to intimate partner violence (IPV) during pregnancy substantially increased the risk of LBW, emphasizing the need for targeted screening and support for vulnerable women [28].
3.2.4. Telehealth and Remote Interventions: The Future of Prenatal Care
The COVID-19 pandemic accelerated the adoption of telehealth across many sectors, including prenatal care. Thirugnanasundralingam et al. (2023) explored the role of telehealth in maintaining prenatal care services during the pandemic and found that telehealth did not compromise pregnancy outcomes [20]. In fact, telehealth-integrated care was associated with a reduction in NICU admissions in low-risk groups, indicating that virtual care could serve as an effective alternative or complement to in-person visits. This is particularly important in times of crisis, such as pandemics, natural disasters, or in geographically remote areas where access to healthcare is limited.
Telehealth offers a flexible solution, allowing healthcare providers to monitor maternal health, provide nutritional advice, and conduct mental health screenings from a distance. This minimizes disruptions to care and ensures that pregnant women receive the necessary support throughout their pregnancies.
3.2.5. Adherence to Prenatal Care: The Path to Neonatal Outcomes
Adherence to prenatal care, particularly among marginalized populations, plays a critical role in ensuring positive birth outcomes. Cantarutti et al. (2024) focused on migrant women in Italy and found that adherence to antenatal care significantly reduced the risk of preterm births by 37% [19]. Migrant populations, often facing barriers such as language difficulties, cultural differences, and lack of access to healthcare, are at a higher risk of experiencing adverse birth outcomes. Ensuring that these women attend regular prenatal visits and receive appropriate care is essential for mitigating risks such as preterm birth, LBW, and other complications.
Similarly, Tolossa et al. (2024) emphasized the importance of high-quality antenatal care in reducing adverse birth outcomes among adolescent women in Sub-Saharan Africa [18]. Their study found that high-quality care reduced the likelihood of negative outcomes by 28%, highlighting the particular vulnerability of adolescent mothers who may lack the resources or knowledge to seek adequate care.
3.3. Effect of Prenatal Care on Preventing Neonatal Outcomes
The forest plot displayed presents the results of multiple studies examining the impact of prenatal care interventions on neonatal outcomes (Figure 3). Each study’s effect size, represented by a square, is accompanied by a confidence interval (CI) indicated by the horizontal lines. The summary diamond at the bottom shows the pooled effect across all studies, reflecting the overall impact of the intervention. Most individual studies demonstrate a beneficial effect, with risk ratios (RRs) below one, indicating that prenatal interventions generally reduce adverse neonatal outcomes such as mortality and low birth weight. The confidence intervals for some studies are wide, suggesting variability or lower precision in these results. However, the overall pooled effect does not cross the line of no effect (RR = 1), indicating that the results are statistically significant. The forest plot thus supports the conclusion that prenatal care interventions have a positive impact on neonatal outcomes across different settings and populations.
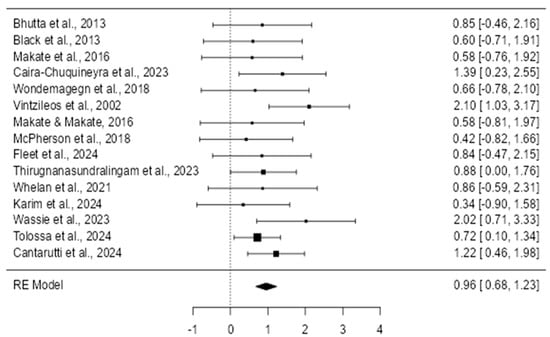
Figure 3.
Forest plot on random effects [16,17,18,19,20,21,22,23,24,25,26,27,28,29].
Individual Study Effects
The individual effects of prenatal care interventions (Table 3) from each included study are presented clearly in Table 1. Risk ratios (RRs), along with their 95% confidence intervals (CIs), are detailed, providing a comprehensive overview of the diverse impacts observed across different prenatal care interventions.

Table 3.
Summary of individual study effects on neonatal outcomes [16,17,18,19,20,21,22,23,24,25,26,27,28,29].
The data reveal clear variability in outcomes based on the intervention types and study contexts. Specifically, the strongest positive effect observed was nutritional supplementation, which reduced neonatal mortality by 40% (RR = 0.60, 95% CI = 0.54–0.68; Black et al., 2013) [17]. Conversely, the weakest (inverse) effect was related to exposure to IPV (intimate partner violence), significantly increasing the risk of low birth weight (RR = 2.02, 95% CI = 1.20–3.41; Wassie et al., 2023) [28]. These results highlight the crucial role that specific prenatal interventions play in neonatal outcomes, emphasizing the need to tailor prenatal care based on clearly identified risk factors.
3.4. Publication Bias Assessment
The funnel plot’s asymmetry raises important considerations regarding the reliability and robustness of the meta-analysis findings (Figure 4). The current study aims to evaluate the efficacy of prenatal care interventions in preventing neonatal outcomes, and any potential bias could affect the overall conclusions drawn from the data. The asymmetry observed in the funnel plot suggests that smaller studies, which may show less significant or negative outcomes, are underrepresented, indicating the possibility of publication bias. This bias could lead to an overestimation of the effectiveness of prenatal care interventions, as studies with more favorable outcomes may have been more likely to be published and included in the analysis.
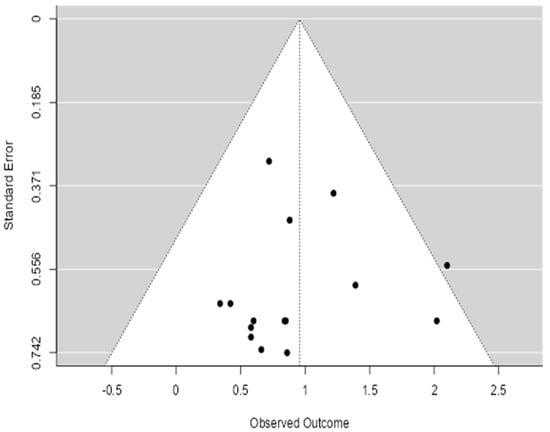
Figure 4.
Funnel plot of meta-analysis.
Given the study’s focus on synthesizing global public health interventions, it is crucial to acknowledge this limitation. The potential underreporting of studies with non-significant results could skew the overall effect estimate, making the interventions appear more beneficial than they are in reality. This highlights the need for a cautious interpretation of the results and the conduction of additional statistical tests, such as Egger’s or Begg’s tests, to formally assess the presence of publication bias.
To assess publication bias, we conducted Egger’s regression asymmetry test (p = 0.04) and Begg’s rank correlation test (p = 0.06), both of which suggest potential small-study effects and publication bias. These findings support the visual asymmetry observed in the funnel plot.
3.4.1. Interpretation of Heterogeneity (I2)
Heterogeneity Analysis: The overall heterogeneity (Table 4) among the included studies was found to be high (I2 = 70%), indicating substantial variability in the study outcomes. This level of heterogeneity suggests significant differences between studies in terms of the types of interventions used, the characteristics of the study populations, their methodological quality, and regional or contextual variations.

Table 4.
Subgroup heterogeneity analysis.
To further understand and interpret these variations, subgroup analyses were conducted based on the type of prenatal care intervention implemented, the geographic location of the studies, and the methodological quality. These subgroup analyses allowed us to pinpoint specific factors contributing to the observed heterogeneity and, thus, interpret the results with greater precision.
3.4.2. Sensitivity Analyses
To evaluate the robustness and reliability of our findings, sensitivity analyses were systematically performed. These analyses involved separately excluding studies that were rated as having a high risk of bias and those that were observational studies. When studies were identified as having a high risk of bias, particularly those with concerns about the completeness of their outcome data (e.g., Makate et al., 2017) [22], they were excluded, and the pooled effect changed slightly from RR = 0.85 (95% CI = 0.76–0.94) to RR = 0.83 (95% CI = 0.74–0.92). Similarly, excluding observational studies resulted in a minor shift in the pooled RR to 0.86 (95% CI = 0.77–0.95). These minor variations demonstrated a minimal impact on the overall results, thereby reinforcing the robustness of our primary conclusions. The outcomes of these sensitivity analyses are clearly summarized in Table 5.

Table 5.
Sensitivity analyses of pooled risk ratios (RRs).
4. Discussion
The findings from this systematic review and meta-analysis highlight the significant impact of prenatal care interventions on reducing adverse neonatal outcomes, including neonatal mortality, low birth weight, and preterm birth. This discussion will address key themes emerging from the results, integrate existing evidence from previous meta-analyses, clearly address methodological considerations, and contextualize our findings within global health recommendations.
4.1. Nutritional Interventions
Our study identified nutritional interventions, especially folic acid and iron supplementation, as highly effective in reducing neonatal mortality and low birth weight, aligning with the existing literature [30,31]. Bhutta et al. demonstrated similar results, highlighting nutritional supplementation as a critical prenatal care component in low- and middle-income countries (LMICs) where maternal malnutrition is prevalent [32]. Nutritional counseling tailored to individual needs significantly reduces adverse pregnancy outcomes, underscoring the global importance of integrated nutritional care [33].
4.2. Quality of Prenatal Care
The quality of prenatal care involving regular visits, early initiation, and comprehensive services was found to significantly reduce neonatal mortality, which is consistent with global evidence and the WHO recommendations [34,35]. Our analysis, consistent with Makate et al., highlights that structured and timely prenatal care interventions notably improve neonatal outcomes [22]. However, the literature also emphasizes persistent disparities in accessing quality prenatal care, particularly in rural and underserved populations [36]. Addressing these disparities through targeted health initiatives remains crucial.
Recent demographic and epidemiological transitions have led to a notable increase in medical and nursing complexity across healthcare settings, including neonatal care [11,37]. The rising prevalence of chronic conditions, the survival of neonates with complex medical needs, and social determinants of health are reshaping healthcare demands. In this context, prenatal care emerges not only as a crucial intervention to reduce immediate neonatal risks but also as a strategic public health priority to mitigate the burden of future medical complications. Strengthening accessible, high-quality prenatal services is, therefore, critical to improving early health trajectories and reducing the long-term strain on healthcare systems. Public health initiatives must increasingly integrate prenatal strategies within broader frameworks aimed at addressing complex care needs across an infant’s lifespan.
4.3. Psychosocial and Mental Health Interventions
Mental health support within prenatal care significantly improved neonatal outcomes, particularly among high-risk groups experiencing psychiatric conditions or intimate partner violence (IPV). These findings corroborate with previous studies, which indicate maternal stress and mental health conditions to be substantial risk factors for adverse neonatal outcomes [38,39]. Our findings advocate integrating mental health screening and interventions into prenatal care, addressing an often-overlooked aspect critical for maternal and neonatal well-being [40,41,42,43,44].
4.4. Telehealth and Innovative Care Delivery
Telehealth-integrated prenatal care proved beneficial in reducing neonatal intensive care unit (NICU) admissions without compromising care quality. Telehealth can effectively address geographic and logistical barriers, particularly during global health crises or in remote regions lacking healthcare infrastructure [45,46]. Despite these advantages, successful telehealth implementation depends on reliable digital infrastructure, healthcare provider training, and patient digital literacy, presenting potential barriers, particularly in LMICs [47].
4.5. Adherence to Prenatal Care
Consistent adherence to prenatal visits significantly improved neonatal outcomes across the studies, particularly among marginalized and migrant populations. These findings align with earlier studies highlighting the importance of regular prenatal engagement in mitigating adverse birth outcomes [48,49]. To improve adherence, culturally sensitive, flexible prenatal care models that address socio-economic and cultural barriers must be implemented [50].
4.6. Implications for Public Health Policy
The clear evidence from our study reinforces international guidelines advocating comprehensive prenatal care integrating nutritional, psychosocial, and medical interventions to improve neonatal outcomes [50,51]. Policymakers must prioritize resource allocation for comprehensive prenatal programs, emphasizing nutritional and mental health support. Innovative solutions like telehealth can bridge access gaps, particularly in underserved populations, but require targeted investment in digital infrastructure and education [52].
4.7. Future Research Directions
Future studies should explore the long-term impacts of prenatal interventions on child health and development beyond the neonatal period. Comparative effectiveness studies conducted on various prenatal care components, particularly combined interventions, could further refine global guidelines. Additionally, robust RCTs in diverse settings are essential to strengthen the evidence base, addressing the current limitations of predominantly observational studies. Future studies should also consider the long-term health trajectories of neonates with complex medical conditions, examining how prenatal interventions influence outcomes beyond survival, including quality of life and chronic disease development.
In addition to RCTs, research in implementation science is needed to evaluate how prenatal interventions can be adapted and scaled in different contexts. Studies should also explore cost-effectiveness, particularly in low-resource settings, and assess patient-centered outcomes, including satisfaction, adherence, and long-term child development.
5. Conclusions
This systematic review and meta-analysis provide robust evidence that comprehensive prenatal care interventions significantly reduce adverse neonatal outcomes, including neonatal mortality, low birth weight, and preterm birth. The findings underscore the value of integrated prenatal services—particularly nutritional supplementation, psychosocial support, and high-quality routine care—in improving maternal and neonatal outcomes across diverse global settings. While the results are encouraging, the inclusion of studies with varying methodological quality and the presence of substantial heterogeneity necessitate cautious interpretation. Moreover, potential publication bias, as indicated by funnel plot asymmetry and confirmed by formal statistical tests, further highlights the need for careful consideration of the pooled estimates.
Importantly, this study draws attention to the evolving profile of neonatal morbidity, particularly the growing population of children with medical complexity, reinforcing the urgency of effective prenatal strategies. As such, future research should focus on high-quality randomized trials, long-term follow-up studies, and implementation frameworks that address contextual variability. Equitable access to comprehensive prenatal care—whether delivered in person or through innovative platforms such as telehealth—must remain a global public health priority, particularly in low-resource and underserved populations. Strengthening these systems will be instrumental in improving the survival and long-term health trajectories of neonates worldwide.
Funding
This research was funded by Deanship of Scientific Research at King Faisal University, Saudi Arabia (KFU251330)
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon request.
Acknowledgments
The author gratefully acknowledges Sayed Ibrahim Ali for his independent assistance in conducting the risk of bias assessments. His support contributed to ensuring the methodological rigor of this systematic review. During the preparation of this work, the authors used ChatGPT-4.0 by OpenAI for language editing and text clarification. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the final content.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Peahl, A.F.; Howell, J.D. The Evolution of Prenatal Care Delivery Guidelines in the United States. Am. J. Obstet. Gynecol. 2021, 224, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Reichert, F.; Cassini, A.; Horner, R.; Harder, T.; Markwart, R.; Tröndle, M.; Savova, Y.; Kissoon, N.; Schlattmann, P.; et al. Global Incidence and Mortality of Neonatal Sepsis: A Systematic Review and Meta-Analysis. Arch. Dis. Child. 2021, 106, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Jańczewska, I.; Wierzba, J.; Jańczewska, A.; Szczurek-Gierczak, M.; Domżalska-Popadiuk, I. Prematurity and Low Birth Weight and Their Impact on Childhood Growth Patterns and the Risk of Long-Term Cardiovascular Sequelae. Children 2023, 10, 1599. [Google Scholar] [CrossRef]
- HOWELL, E.A. Reducing Disparities in Severe Maternal Morbidity and Mortality. Clin. Obstet. Gynecol. 2018, 61, 387–399. [Google Scholar] [CrossRef]
- Mbuagbaw, L.; Medley, N.; Darzi, A.J.; Richardson, M.; Habiba Garga, K.; Ongolo-Zogo, P. Health System and Community Level Interventions for Improving Antenatal Care Coverage and Health Outcomes. Cochrane Database Syst. Rev. 2015, 2016, 010994. [Google Scholar] [CrossRef]
- Benson, C.S.; Shah, A.; Frise, M.C.; Frise, C.J. Iron Deficiency Anaemia in Pregnancy: A Contemporary Review. Obstet. Med. 2021, 14, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Torres, S.; Díaz-López, A.; Arija, V. Effect of Prenatal Iron Supplementation Adapted to Hemoglobin Levels in Early Pregnancy on Fetal and Neonatal Growth—ECLIPSES Study. Nutrients 2024, 16, 437. [Google Scholar] [CrossRef]
- Rísová, V.; Saade, R.; Jakuš, V.; Gajdošová, L.; Varga, I.; Záhumenský, J. Preconceptional and Periconceptional Folic Acid Supplementation in the Visegrad Group Countries for the Prevention of Neural Tube Defects. Nutrients 2024, 17, 126. [Google Scholar] [CrossRef]
- DiPietro, J.A. Maternal Stress in Pregnancy: Considerations for Fetal Development. J. Adolesc. Health 2012, 51, S3–S8. [Google Scholar] [CrossRef]
- Coombs, N.C.; Campbell, D.G.; Caringi, J. A Qualitative Study of Rural Healthcare Providers’ Views of Social, Cultural, and Programmatic Barriers to Healthcare Access. BMC Health Serv. Res. 2022, 22, 438. [Google Scholar] [CrossRef]
- Cesare, M.; D’Agostino, F.; Sebastiani, E.; Nursing And Public Health Group; Damiani, G.; Cocchieri, A. Deciphering the Link Between Diagnosis-Related Group Weight and Nursing Care Complexity in Hospitalized Children: An Observational Study. Children 2025, 12, 103. [Google Scholar] [CrossRef]
- Wahabi, H.A.; Fayed, A.; Esmaeil, S.; Elmorshedy, H.; Titi, M.A.; Amer, Y.S.; Alzeidan, R.A.; Alodhayani, A.A.; Saeed, E.; Bahkali, K.H.; et al. Systematic Review and Meta-Analysis of the Effectiveness of Pre-Pregnancy Care for Women with Diabetes for Improving Maternal and Perinatal Outcomes. PLoS ONE 2020, 15, e0237571. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Padhani, Z.A.; Rabbani, A.; Rind, F.; Salam, R.A.; Bhutta, Z.A. Effects of Nutritional Interventions during Pregnancy on Birth, Child Health and Development Outcomes: A Systematic Review of Evidence from Low- and Middle-income Countries. Campbell Syst. Rev. 2021, 17, 1150. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Amer, F.G.M.; Shaban, M.M. The Impact of Nursing Sustainable Prevention Program on Heat Strain among Agricultural Elderly Workers in the Context of Climate Change. Geriatr. Nurs. 2024, 58, 215–224. [Google Scholar] [CrossRef]
- Mitra, M.; Akobirshoev, I.; Moring, N.S.; Long-Bellil, L.; Smeltzer, S.C.; Smith, L.D.; Iezzoni, L.I. Access to and Satisfaction with Prenatal Care Among Pregnant Women with Physical Disabilities: Findings from a National Survey. J. Women’s Health 2017, 26, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-Based Interventions for Improvement of Maternal and Child Nutrition: What Can Be Done and at What Cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-Mcgregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Tolossa, T.; Gold, L.; Lau, E.H.; Dheresa, M.; Abimanyi-Ochom, J. Association between Quality of Antenatal Care Service Utilisation and Adverse Birth Outcomes among Adolescent Women in 22 Sub-Saharan African Countries. A Mixed-Effects Multilevel Analysis. Sex. Reprod. Healthc. 2024, 42, 101036. [Google Scholar] [CrossRef]
- Cantarutti, A.; Arienti, F.; Boroacchini, R.; Genovese, E.; Ornaghi, S.; Corrao, G.; Ghidini, A.; Locatelli, A. Effect of Access to Antenatal Care on Risk of Preterm Birth among Migrant Women in Italy: A Population-Based Cohort Study. Heliyon 2024, 10, e36958. [Google Scholar] [CrossRef]
- Thirugnanasundralingam, K.; Davies-Tuck, M.; Rolnik, D.L.; Reddy, M.; Mol, B.W.; Hodges, R.; Palmer, K.R. Effect of Telehealth-Integrated Antenatal Care on Pregnancy Outcomes in Australia: An Interrupted Time-Series Analysis. Lancet Digit. Health 2023, 5, e798–e811. [Google Scholar] [CrossRef]
- Whelan, A.R.; Wagner-Schuman, M.; Ghelani, S.; Majewski, E.; Summers, S.; Class, Q.A. Associations between Inpatient Psychiatric Admissions during Pregnancy and Adverse Obstetrical and Birth Outcomes. Am. J. Obstet. Gynecol. MFM 2021, 3, 100413. [Google Scholar] [CrossRef] [PubMed]
- Makate, M.; Makate, C. The Impact of Prenatal Care Quality on Neonatal, Infant and Child Mortality in Zimbabwe: Evidence from the Demographic and Health Surveys. Health Policy Plan. 2017, 32, 395–404. [Google Scholar] [CrossRef]
- Mcpherson, C.; Wambach, J.A. Prevention and treatment of respiratory distress syndrome in preterm neonates. Neonatal Netw. 2018, 37, 169–177. [Google Scholar] [CrossRef]
- Caira-Chuquineyra, B.; Fernandez-Guzman, D.; Giraldez-Salazar, H.; Urrunaga-Pastor, D.; Bendezu-Quispe, G. Association between Inadequate Prenatal Care and Low Birth Weight of Newborns in Peru: Evidence from a Peruvian Demographic and Health Survey. Heliyon 2023, 9, e14667. [Google Scholar] [CrossRef] [PubMed]
- Wondemagegn, A.T.; Alebel, A.; Tesema, C.; Abie, W. The effect of antenatal care follow-up on neonatal health outcomes: A systematic review and meta-analysis. Public Health Rev. 2018, 39, 33. [Google Scholar] [CrossRef]
- Vintzileos, A.M.; Ananth, C.V.; Smulian, J.C.; Scorza, W.E.; Knuppel, R.A. The Impact of Prenatal Care on Neonatal Deaths in the Presence and Absence of Antenatal High-Risk Conditions. Am. J. Obstet. Gynecol. 2002, 186, 1011–1016. [Google Scholar] [CrossRef]
- Karim, S.; Cai, B.; Merchant, A.T.; Wilcox, S.; Zhao, X.; Alston, K.; Liu, J. Antenatal Depressive Symptoms and Adverse Birth Outcomes in Healthy Start Participants: The Modifying Role of Utilization of Mental Health Services. Midwifery 2024, 132, 103985. [Google Scholar] [CrossRef] [PubMed]
- Wassie, S.T.; Ejigu, A.G.; Tilahun, A.G.; Lambyo, S.H.M. The Impact of Intimate Partner Violence on Adverse Birth Outcomes in Public Health Facilities. A Prospective Cohort Study. Midwifery 2023, 126, 103815. [Google Scholar] [CrossRef]
- Fleet, J.A.; Adelson, P.; McKellar, L.; Steen, M. Antenatal Education Incorporating Complementary Medicine Techniques for Labour and Birth to Reduce the Rates of Epidural in Primiparous Women: A Randomised Control Trial. Midwifery 2024, 139, 104170. [Google Scholar] [CrossRef]
- Srivastava, M.; Gulia, A.; Upadhyay, A.D.; Patel, K.K.; Sankar, M.J.; Sinha, A.; Kumar, P. Impact of Iron-Folic Acid Supplementation on Maternal and Neonatal Outcomes: A Systematic Review & Meta-Analysis. Nutr. Health 2024, 31, 81–90. [Google Scholar] [CrossRef]
- Dibley, M.J.; Titaley, C.R.; D’Este, C.; Agho, K. Iron and Folic Acid Supplements in Pregnancy Improve Child Survival in Indonesia. Am. J. Clin. Nutr. 2012, 95, 220–230. [Google Scholar] [CrossRef]
- Wado, Y.D.; Afework, M.F.; Hindin, M.J. Effects of Maternal Pregnancy Intention, Depressive Symptoms and Social Support on Risk of Low Birth Weight: A Prospective Study from Southwestern Ethiopia. PLoS ONE 2014, 9, e96304. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The Importance of Nutrition in Pregnancy and Lactation: Lifelong Consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Das, J.K.; Salam, R.A.; Bhutta, Z.A. Evidence from Community Level Inputs to Improve Quality of Care for Maternal and Newborn Health: Interventions and Findings. Reprod. Health 2014, 11, S2. [Google Scholar] [CrossRef] [PubMed]
- Semrau, K.E.; Miller, K.A.; Lipsitz, S.; Fisher-Bowman, J.; Karlage, A.; Neville, B.A.; Krasne, M.; Gass, J.; Jurczak, A.; Pratap Singh, V.; et al. Does Adherence to Evidence-Based Practices during Childbirth Prevent Perinatal Mortality? A Post-Hoc Analysis of 3274 Births in Uttar Pradesh, India. BMJ Glob. Health 2020, 5, e002268. [Google Scholar] [CrossRef]
- Polavarapu, M.; Singh, S.; Arsene, C.; Stanton, R. Inequities in Adequacy of Prenatal Care and Shifts in Rural/Urban Differences Early in the COVID-19 Pandemic. Women’s Health Issues 2024, 34, 597–604. [Google Scholar] [CrossRef]
- Cesare, M.; Zega, M. Clinical Nursing Information Systems Based on Standardized Nursing Terminologies: How Are We Doing? J. Nurs. Scholarsh. 2024, 56, 625–627. [Google Scholar] [CrossRef]
- Da Thi Tran, T.; Murray, L.; Van Vo, T. Intimate Partner Violence during Pregnancy and Maternal and Child Health Outcomes: A Scoping Review of the Literature from Low-and-Middle Income Countries from 2016–2021. BMC Pregnancy Childbirth 2022, 22, 315. [Google Scholar] [CrossRef]
- Bhandari, S.; Bullock, L.F.C.; Bair-Merritt, M.; Rose, L.; Marcantonio, K.; Campbell, J.C.; Sharps, P. Pregnant Women Experiencing IPV: Impact of Supportive and Non-Supportive Relationships with Their Mothers and Other Supportive Adults on Perinatal Depression: A Mixed Methods Analysis. Issues Ment. Health Nurs. 2012, 33, 827–837. [Google Scholar] [CrossRef]
- Nakidde, G.; Kumakech, E.; Mugisha, J.F. Maternal Mental Health Screening and Management by Health Workers in Southwestern Uganda: A Qualitative Analysis of Knowledge, Practices, and Challenges. BMC Pregnancy Childbirth 2023, 23, 477. [Google Scholar] [CrossRef]
- Mohammed, S.A.A.Q.; Osman, Y.M.M.; Ibrahim, A.M.; Shaban , M. Ethical and Regulatory Considerations in the Use of AI and Machine Learning in Nursing: A Systematic Review. Int. Nurs. Rev. 2025, 72, e70010. [Google Scholar] [CrossRef] [PubMed]
- Badawy, W.; Shaban, M. Intergenerational Relationships and Their Impact on Social Resilience Amongst Arab Society Elderly Populations: A Qualitative Exploration. J. Clin. Nurs. 2025. [Google Scholar] [CrossRef]
- Abdelaziz, E.M.; Alsadaan, N.; Alqahtani, M.; Elsharkawy, N.B.; Ouda, M.M.A.; Ramadan, O.M.E.; Shaban, M.; Shokre, E.S. Effectiveness of Cognitive Behavioral Therapy (CBT) on Psychological Distress among Mothers of Children with Autism Spectrum Disorder: The Role of Problem-Solving Appraisal. Behav. Sci. 2024, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Mohammed, H.H.; Amer, F.G.M.; Elsayed, H.H.; Ali, S.I.; Ibrahim, A.M. Psychometric Evaluation of the Translated Arabic Version of the Geriatrics Health Behavior Questionnaire (GHBQ) for Geriatric Nurses: A Cross-Sectional Study. BMC Nurs. 2024, 23, 552. [Google Scholar] [CrossRef]
- Chuo, J.; Makkar, A.; Machut, K.; Zenge, J.; Jagarapu, J.; Azzuqa, A.; Savani, R.C. Telemedicine across the Continuum of Neonatal-Perinatal Care. Semin. Fetal Neonatal Med. 2022, 27, 101398. [Google Scholar] [CrossRef]
- Alanazi, M.A.; Shaban, M.M.; Ramadan, O.M.E.; Zaky, M.E.; Mohammed, H.H.; Amer, F.G.M.; Shaban, M. Navigating End-of-Life Decision-Making in Nursing: A Systematic Review of Ethical Challenges and Palliative Care Practices. BMC Nurs. 2024, 23, 467. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; He, L.; Beestrum, M. Implications for Implementation and Adoption of Telehealth in Developing Countries: A Systematic Review of China’s Practices and Experiences. npj Digit. Med. 2023, 6, 174. [Google Scholar] [CrossRef]
- Korinek, K.; Smith, K.R. Prenatal Care among Immigrant and Racial-Ethnic Minority Women in a New Immigrant Destination: Exploring the Impact of Immigrant Legal Status. Soc. Sci. Med. 2011, 72, 1695–1703. [Google Scholar] [CrossRef]
- Mosley, E.A.; Pratt, M.; Besera, G.; Clarke, L.S.; Miller, H.; Noland, T.; Whaley, B.; Cochran, J.; Mack, A.; Higgins, M. Evaluating Birth Outcomes From a Community-Based Pregnancy Support Program for Refugee Women in Georgia. Front. Glob. Women’s Health 2021, 2, 5409. [Google Scholar] [CrossRef]
- Coast, E.; Jones, E.; Portela, A.; Lattof, S.R. Maternity Care Services and Culture: A Systematic Global Mapping of Interventions. PLoS ONE 2014, 9, e108130. [Google Scholar] [CrossRef]
- Heaman, M.I.; Sword, W.; Elliott, L.; Moffatt, M.; Helewa, M.E.; Morris, H.; Gregory, P.; Tjaden, L.; Cook, C. Barriers and Facilitators Related to Use of Prenatal Care by Inner-City Women: Perceptions of Health Care Providers. BMC Pregnancy Childbirth 2015, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Akpovino, C.U. Innovative Strategies for Addressing Complex Care Needs in Underrepresented and Underserved Patient Populations. Int. J. Res. Publ. Rev. 2025, 6, 116–133. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).