Abstract
Chronic obstructive pulmonary disease (COPD) represents one of the most frequent causes of hospital readmissions and in-hospital mortality. One in five patients requires readmission within 30 days of discharge following an admission for exacerbation. These ‘early readmissions’ increase morbidity and mortality, as patients often do not recover their baseline lung function. The identification of factors associated with increased risk has been a major focus of research in recent years. Studies describe patient-related predictors, although some studies also suggest that better-resourced centres provide superior care. Objective: To describe resources, performance, and care provided in pneumology units in Spain, assessing their association with 30-day readmission for COPD and in-hospital mortality. Methods: This survey was conducted in 116 hospitals responsible for the COPD pathway in pneumology units/departments from November 2022 to March 2023. Results: Of the 116 participating hospitals, 56% had a pneumology department while 25.9% had a pneumology section. The vast majority were public and university hospitals. The number of beds allocated to pneumology/100,000 inhabitants was 6.6 (3.1–9.2) and pulmonologist staffing was 3.3 (2.6–4.1) per 100,000 inhabitants. There was an intermediate respiratory care unit (IMCU) dependent on the pneumology department in 31.9% of units and a respiratory team for 24 h emergency care in 30% of units, while only 9.5% had interventional pneumology units for bronchoscopic procedures. COPD rehabilitation programmes were offered in 58.6% of pneumology units. The average rate of patients on ventilatory support in acute failure was 13.8 (9.2–25) per 100 discharges, with a 30-day COPD readmission rate of 14.9%, with significant differences according to the level of complexity (p = 0.041), with a mean length of stay of 8.72 (1.26) days. The overall in-hospital mortality in pneumology units was 4.10 (1.18) per 100 admissions. In the adjusted model, having a discharge support programme and interventions performed during admission (number of patients with ventilatory support) were predictors of a favourable outcome. Hospital stay was also maintained as a predictor of an unfavourable outcome. Conclusions: There is significant variability in resources and the organisation of care in pneumology units in Spain. The availability of a discharge support programme and greater use of ventilatory support at discharge are factors associated with a lower 30-day COPD readmission rate in the pneumology unit. This information is relevant to improve the care of patients with COPD and as a future line of research.
1. Introduction
Chronic obstructive pulmonary disease (COPD) represents one of the most prevalent causes of hospital admissions and medical consultations [1,2], posing significant social and economic burdens that are anticipated to rise in the coming years [3,4,5]. Patients with COPD frequently encounter exacerbation episodes necessitating hospitalisation. Among those surviving hospitalisation, readmission due to acute exacerbation shortly after discharge remains a critical, unresolved issue. The high readmission rates associated with COPD correlate with increased mortality risk and substantial financial strain. Therefore, the prevention and reduction of readmissions have been prioritised as crucial management strategies. Recent research has delved into readmission rates and post-discharge risk factors. Audit studies have highlighted varied outcomes in COPD management, which are contingent upon patient characteristics and the medical care received [6,7], as evidenced in numerous studies examining the influence of treatment regimens and hospital resources on hospital mortality and readmissions post-discharge for COPD exacerbation [8,9,10].
The European COPD audit revealed significant variability in resourcing and care organisation across European hospitals treating COPD exacerbations. While some studies suggest that better-resourced facilities provide superior care [11,12], greater resources in larger hospitals does not invariably guarantee improved access to or standards of care [13]. Hence, other aspects of the care process, such as units led by qualified staff and standardised management plans to reduce practise variability, are essential elements upon which the quality of clinical care is founded.
Clinical audits are essential tools for identifying deficiencies in care, thereby raising awareness of these issues and ultimately improving the quality of care. Since 2014, the Spanish Society of Pneumology and Thoracic Surgery (SEPAR) has spearheaded a process of auditing COPD care, with the objective of using clinical audits as a mechanism for continuous quality improvement [7,14]. In 2015, the RECALAR project [15], funded by SEPAR, assessed the resources and organisational structure of respiratory disease care in the pneumology units of Spanish National Health System hospitals. Currently, there is a paucity of information regarding resources and organisational models for COPD care within pneumology units. Against this backdrop, the COPD Observatory project was conceived with the aim of analysing the resources, activities, and organisational models in COPD care within Spanish pneumology units/departments through a survey conducted by clinical managers. Additionally, the project seeks to examine the association of these factors with outcomes such as 30-day COPD readmission rates and overall in-hospital mortality in pneumology units.
2. Materials and Methods
The COPD Observatory project was conceived as a cross-sectional survey assessing the resources and organisation dedicated to COPD care, promoted by SEPAR. The Spanish Society of Pneumology and Thoracic Surgery extended an official invitation to participate in the study to all respiratory units within Spanish National Health System hospitals, as per the Ministry of Health’s 2021 registry [16]. Out of 223 hospitals invited, 116 (52%) participated. The intended respondents for the survey were those responsible for the COPD pathway in pneumology units/departments. The fieldwork was conducted from November 2022 to March 2023. The participating hospitals and investigators are detailed in Supplementary S1.
The project’s steering committee comprised 17 chest physicians experienced in COPD, representing each of the 17 regional respiratory societies in Spain. Database items were selected by the steering committee and discussed via email and in face-to-face meetings. The survey included distinct questions regarding hospital and respiratory unit resources. A detailed list of the survey items is provided in Supplementary S2. Data sources included a survey of pneumology units regarding resources and care (level of hospital complexity, number of pulmonologists and nurses, number of pneumology beds, complexity of the bronchoscopy unit, availability of an intermediate respiratory care unit, 24 h emergency care, a follow-up and discharge support programme for COPD, and rehabilitation programme) and performance (number of spirometries, length of stay, number of discharges, number of patients in a home ventilation programme and receiving non-invasive acute ventilatory support), as well as the number of 30-day COPD readmissions and overall mortality in pneumology units referred from data analysis for the year 2022 through coding of discharge reports by hospital admission departments. We analysed the data and results of the care provided to patients who were treated in these pneumology departments during the year 2022.
Data were remotely entered by each participating site onto a centrally controlled server. The web tool offered a help service with explanatory text to facilitate survey question interpretation. Surveys were administered after identifying the centres and contact persons at the 116 participating sites. For additional quality control during data collection, all completed surveys were reviewed and regular reviews of the database records were carried out to identify any issues or inconsistencies.
Statistical Analysis
Qualitative variables are presented with their frequency distribution, while quantitative variables are summarised by their mean and standard deviation (SD). Quantitative variables exhibiting an asymmetric distribution are summarised using the median and interquartile range (IQR). For the comparison of qualitative variables, the χ2 test or Fisher’s exact test was employed when necessary. Comparisons of means between two independent groups were conducted using Student’s t-test if the variables followed a normal distribution, or the nonparametric Mann–Whitney U test for asymmetric variables. To compare means across more than two independent groups, analysis of variance (ANOVA) was used, or the nonparametric Kruskal–Wallis test for asymmetric variables. To examine the correlation between quantitative variables, the Pearson correlation coefficient was utilised, or Spearman’s correlation coefficient when appropriate.
A simple linear regression model was applied to explore the association between the number of total admissions per 100 discharges in 2022 and potential individually associated factors. Additionally, a multiple linear regression model was employed to study the association jointly. A significance level of 5% was accepted for all tests. Data processing and analysis were performed using IBM SPSS Statistics v.26 and R v.4.4.1 software.
3. Results
3.1. Hospital Resources
The characteristics of the participating hospitals, categorised by their level of complexity, are summarised in Table 1. The majority of the participating centres were public institutions, with approximately three-quarters identified as university or teaching hospitals. Notably, 56% of these hospitals had a dedicated pneumology department, while 14.7% lacked an organisational structure for pneumology. The distribution of participating centres across the various autonomous communities, based on the hospital’s level of complexity and the type of organisational entity of the pneumology unit, is depicted in Figure 1.

Table 1.
Characteristics of participating hospitals, resources available, and care provided in units.
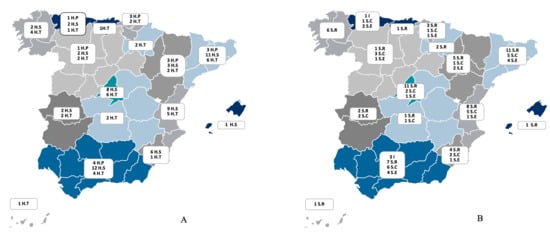
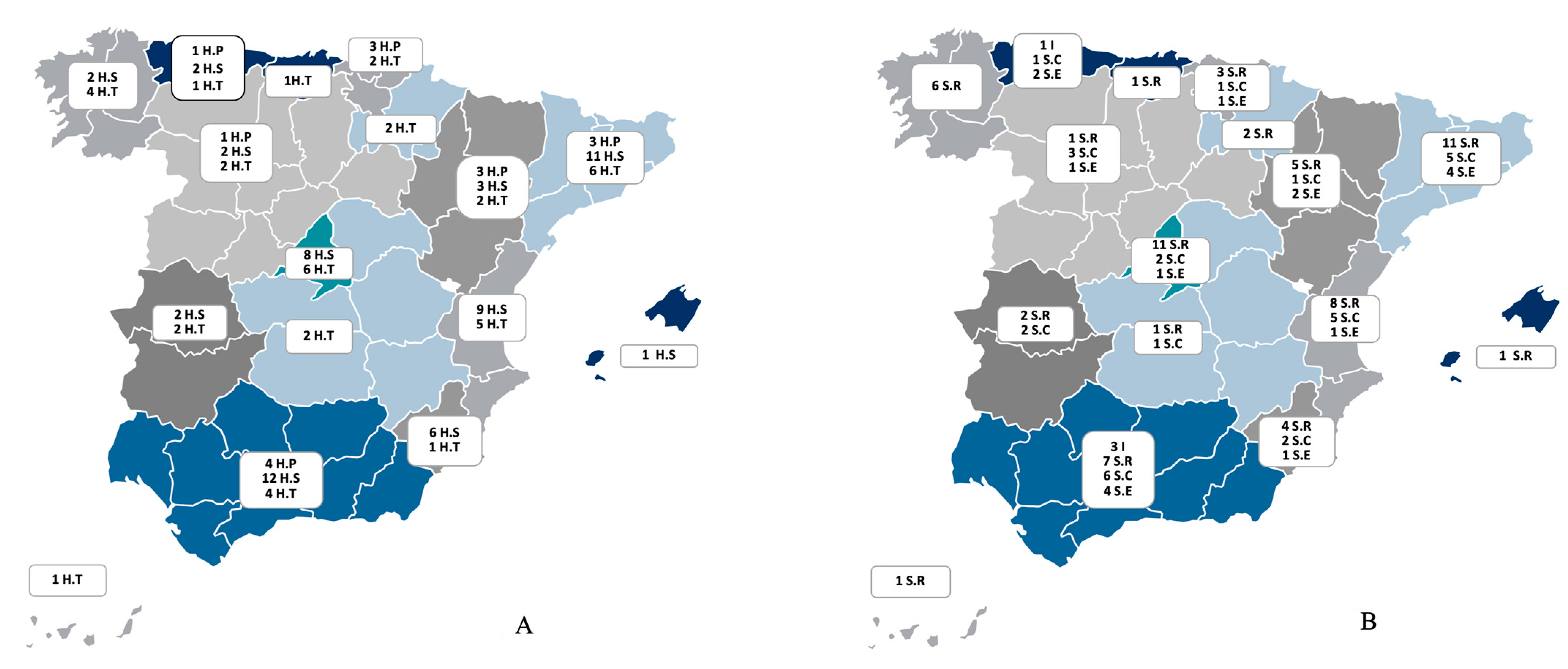
Figure 1.
(A) The distribution of the participating centres across the 16 regions of Spain (indicated by colour) according to the level of complexity of the hospital centre: level I, or primary hospital (H.P); level II, or secondary hospital (H.S); level III, or tertiary hospital (H.T). Data are presented as numbers. (B) The distribution of the participating centres across the 16 regions of Spain (indicated by colour) according to pneumology organisational entity: I (institute or area of clinical management); S.R (department); S.C (section); S.E: no entity. Data are presented as numbers.
3.2. Respiratory Unit Resources
The resources of the respiratory units are summarised in Table 1. On average, pneumology units had 6.6 beds (range 3.1–9.2) per 100,000 inhabitants. The median number of pulmonologists per 100,000 inhabitants was 3.3 (range 2.6–4.1), with variations between low-complexity centres at 2.9 (range 2.3–3.9) and high-complexity centres at 3.7 (range 3–4.6). A majority of high-complexity centres had an intermediate care unit (IMCU) (65%) and offered 24 h urgent care staffed by pulmonologists (69.8%). More than half of the centres (58.6%) had rehabilitation programmes, with 54.4% offering both inpatient and home rehabilitation. A minority of centres (9.5%) provided endoscopic volume reduction techniques for patients with COPD. Specialised COPD consultations were available in 52.6% of the units, follow-up and discharge support programmes in 55.2%, immediate COPD care devices in 44%, a specialist consultant for the COPD process in 45.6%, nursing consultations in 38.8%, and smoking prevention consultations in 48.3%. These resources were more frequently available in tertiary or level III centres. Nonetheless, follow-up and support programmes at discharge were also significantly present in lower-complexity centres.
3.3. Respiratory Unit Performance
The average number of hospital discharges from pneumology units was 266.2 (range 186.8–399.2) per 100,000 inhabitants, with a mean length of stay of 8.72 (SD 1.26) days. The average rate of patients requiring ventilatory support for acute failure was 13.8 (range 9.2–25) per 100 discharges, with a 30-day COPD readmission rate of 14.9%, showing significant differences according to the hospital’s level of complexity (p = 0.041). Overall, in-hospital mortality was 4.10 (SD 1.18) per 100 admissions to pneumology units, with no significant differences based on the hospital’s level of complexity. The average number of patients in home ventilation programmes was 57.8 (range 20.3–100.5) per 100,000 inhabitants. The average rate of spirometries performed per month per 100,000 inhabitants was 121.4 (range 70–225.7), with notable variations between low-complexity centres (170.5, range 95.8–320) and high-complexity centres (141.7, range 77.7–230.7). The number of bronchoscopic procedures performed per year per 100,000 inhabitants was 164 (range 114.8–244.8). The organisational performance of the respiratory units is summarised in Table 2.

Table 2.
Organisational performance of respiratory units.
3.4. Factors Associated with Outcomes: Overall In-Hospital Mortality and 30-Day COPD Readmissions for COPD Exacerbations
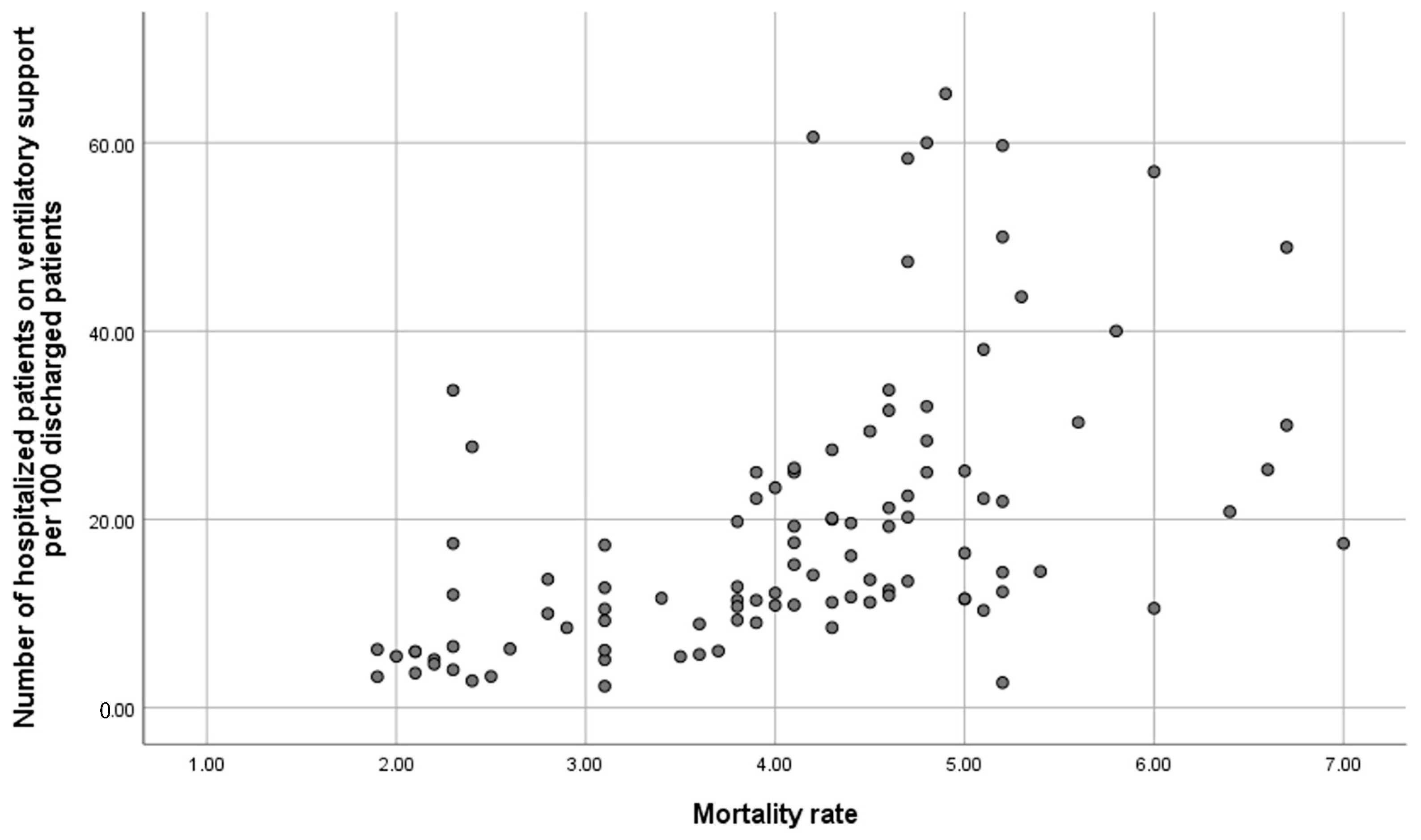
Table 3 outlines the bivariate association between 30-day readmissions for COPD and variables related to hospital resources, organisation, and interventions performed during admission and at discharge. Significant variables associated with a lower 30-day COPD readmission rate include having a follow-up and support programme at discharge, immediate care, specialised COPD consultation, nursing consultation, specialist consultants in the COPD process, hospital stay, and the number of patients receiving ventilatory support. Conversely, most resource and organisation variables were not related to in-hospital mortality (Supplementary S3), with a regression coefficient correlation of 0.622 between in-hospital mortality and the number of acutely ventilated patients per 100 discharges (Figure 2).

Table 3.
Determinants of 30-day readmission rates in patients with chronic obstructive pulmonary disease (COPD).
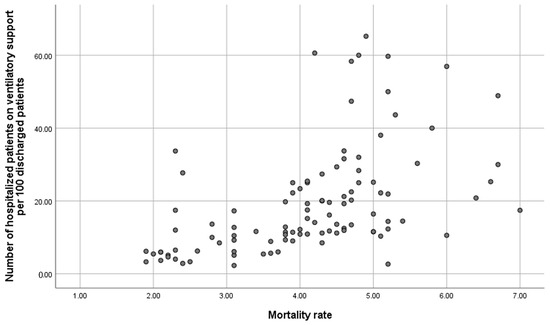
Figure 2.
Correlation between in-hospital mortality and number of acutely ventilated patients per 100 discharges.
In the multivariable analysis of 30-day COPD readmissions (Table 4), the adjusted model retained only outcome predictors linked to organisation (having a follow-up and discharge support programme) and interventions performed during admission (number of patients on ventilatory support), both predictors of favourable outcomes. Hospital stay was also identified as a predictor of unfavourable outcomes.

Table 4.
Multivariate analysis of 30-day readmissions in patients with COPD.
4. Discussion
This study provides a significant dataset from pneumology units in Spain, detailing COPD resources, activities, and care models, as well as data on readmissions and mortality for patients hospitalised due to COPD exacerbations. It also offers insights into potential interventions to enhance the quality of care during hospital admissions. Findings indicate that resources such as discharge support programmes and interventions like increased ventilatory support at discharge are associated with a reduced risk of 30-day COPD readmissions, whereas a longer hospital stay predicts unfavourable outcomes, likely reflecting COPD or patient severity.
Despite existing COPD management guidelines, there are no recommendations for minimum resources or service organisation to ensure optimal care. This study examines the variation in the provision of resources to manage patients with COPD across 116 pneumology units in Spain, exploring the correlation between hospital size, resource provision, and outcomes such as 30-day readmission rates for COPD exacerbation and in-hospital mortality. It also updates data on the organisational structure and functioning of Spanish pneumology units since the RECALAR [15] document was published in 2017.
Our data reveal a distribution of pneumology units in Spanish hospitals similar to that of 2017. Of the population assessed, over half (56%) have an institutional department designation, 25.9% are sections, and the remainder are part of internal medicine departments. According to our analysis, Spain has 6.6 pneumology beds per 100,000 inhabitants and an average of 3.3 pulmonologists per 100,000 inhabitants. These figures align closely with the 2017 survey data [15], considering the impact of the COVID-19 pandemic declared in 2020, which underscored deficiencies in respiratory care due to high morbidity and mortality. Evidence suggests that more specialists per bed correlates with reduced hospital mortality, shorter stays, and lower readmission rates, thus improving health system efficiency. The AUDIPOC audit [12] also associates a higher number of respiratory specialists per bed with lower COPD mortality. Additionally, our analysis shows minimal variation by hospital complexity level (2.9 pulmonologists/100,000 in level I or primary hospitals versus 3.7/100,000 in level III or tertiary centres). Optimal specialist allocation per bed and comprehensive activity consideration are crucial for effective, high-quality hospital care. These findings underscore the need for improvement, especially since most surveyed centres are public and university affiliated.
Hospitalisation due to COPD exacerbations is associated with high in-hospital mortality rates, which range between 2.5 and 15%, varying based on patient characteristics and the research setting [16,17]. In our study, the overall in-hospital mortality in pneumology units was 4.1% (SD 1.1%), lower in level I centres at 2.6% (SD 1.2%), and higher in high-complexity centres, averaging 4.2% (SD 1.1%). There were no significant differences in in-hospital mortality based on the complexity level of the centres or the resources of the pneumology units. Systematic reviews and meta-analyses indicate that several characteristics of patients with COPD and comorbidities significantly correlate with increased mortality [18]. A meta-analysis of over 60,000 patients with COPD revealed that non-surviving patients had more hospitalisations in the previous year, longer hospital stays, greater dyspnoea during hospitalisation, and were more likely to require ventilatory support [19]. Data from the European AUDIPOC audit demonstrated that greater resources in larger hospitals did not necessarily guarantee better access to care. Despite having more resources, larger hospitals did not show a marked difference in guideline adherence, emphasising that optimising risk stratification upon admission for COPD exacerbation, diagnosing respiratory acidosis within the first hours of admission, and timely specialist respiratory care team involvement result in better patient outcomes [20].
In the European COPD audit, ventilatory support was significantly associated with in-hospital mortality [12]. The reasons for delaying or not offering non-invasive ventilatory support may be influenced by the availability and access to intermediate respiratory care units, as initially noted by Roberts et al. [21] and corroborated by the European COPD audit data [13]. Hence, early access to specialists who can provide non-invasive support in controlled settings, such as intermediate care units (IMCUs), and having a specialised team that allows for continuous monitoring and timely intervention in case of respiratory complications is crucial for improving outcomes in critically ill patients. In the 2012 European audit, over one-third of the centres reported they could not treat all eligible patients throughout the year for both invasive and non-invasive ventilation [13,20]. In our study, intermediate respiratory care units attached to pneumology units were present in 65% of high-complexity (level III) hospitals, with a median of 7 beds (range 6–8.7) and a nurse-to-bed ratio of 4 (range 4–6). Most of these IMCUs were established during the SARS-CoV-2 pandemic. Two-thirds of level III hospitals had 24 h emergency care provided by pulmonologists. These units, being less costly than intensive care units, improve hospital efficiency by optimally utilising resources for each level of care, thus allowing better patient flow. Studies have shown that intermediate respiratory care units reduce mortality in severe exacerbations of chronic respiratory disease by administering non-invasive ventilatory support to patients with respiratory failure, a treatment proven effective in avoiding intubation in many cases and reducing the risks associated with invasive ventilation [22]. The expansion of these units within pneumology units in Spain reflects the increasing need for intermediate respiratory care and their essential role in the current hospital structure.
Ventilatory support for acidotic respiratory failure during COPD admission has been shown to be a protective factor against in-hospital mortality [23]. Our study indicates that a median of 13.8 (range 9.2–25) inpatients per 100 discharges in pneumology units benefit from acute non-invasive ventilation, with a median of 88.2 patients on long-term non-invasive ventilation per 100,000 inhabitants. These figures have doubled compared to those reported in the RECALAR [15] report in 2017, which indicated 46 patients per 100,000 inhabitants being on home ventilatory support. These data are also somewhat higher than the European average of 66 patients per 100,000 inhabitants [24]. Home ventilation for COPD has been shown to reduce the risk of COPD readmission, especially in patients with a history of respiratory acidosis and hypercapnia [25,26]. Despite the increased use of mechanical ventilation as home therapy, information on its prescription in COPD remains limited. Data from an international web survey of physicians prescribing long-term non-invasive supportive care therapy revealed that the prescription rate of home mechanical ventilation in patients with COPD was 38.5% of all prescriptions [25]. However, there is significant variability in the frequency of home mechanical ventilation prescriptions for COPD across different countries and regions [27,28].
COPD has become one of the diseases with the highest rates of early readmission within 30 days. One in five patients requires rehospitalisation within 30 days of discharge following an admission for exacerbation [29]. A recent systematic review reported that the rate of readmissions for acute exacerbations of COPD ranged from 6% to 24% within 30 days of discharge, based on an analysis of 24 studies [30]. These measurements may be confounded by variations in metric generation. Additionally, study design, age stage, WHO region, and length of hospital stay have been identified as potential sources of readmission rate heterogeneity. In our study, the median 30-day COPD readmission rate was 14.9% (range 10.1–18.7%), being lower in level I complexity centres (10.7%, range 8.2–15%) compared to level III high-complexity centres with a median of 13.1% (range 8.6–16.7%). These “early readmissions” increase morbidity and mortality, as patients often do not recover their baseline lung function [31,32]. Additionally, readmissions after COPD hospitalisation add to the economic burden, estimated to cost $13 billion and associated with poor outcomes [23]. The identification of factors associated with increased risk has been a major focus of research in recent years. Despite the heterogeneity of studies, the most frequently described predictive variables related to the patient include advanced age, comorbidities, low socioeconomic status, social situation, previous admissions, severity of illness, low adherence to treatment, the need for home ventilation, and fragility [33,34]. Additionally, factors related to the health system, such as hospital stay, lack of a defined follow-up programme, and poor health education, are also significant. Our observation indicated that longer hospital stays were associated with higher readmission rates, consistent with systematic reviews demonstrating that length of stay correlates with an increased risk of readmissions for COPD [33]. In addition, our analysis found that the 30-day readmission rate was closely related to the availability of a follow-up and support programme after the acute phase of the disease. Studies evaluating comprehensive programmes that provide multidisciplinary care from hospital to home have shown mixed results [35,36]. Approximately 50% of these readmissions could be avoided, often resulting from a fragmented healthcare system that discharges patients prematurely, poorly plans follow-up care transitions, inadequately communicates discharge instructions, fails in family education about the disease, and lacks communication with outpatient physicians responsible for future care [37]. However, it is important to recognise that there is a subset of patients with COPD who are frail, with associated social factors, lacking family support, and with multiple comorbidities. These ‘hospital-dependent patients’ represent a vulnerable population with chronic clinical conditions. Recent studies suggest that traditional resources or interventions have not effectively reduced readmissions in this high-risk group [38,39,40]. There is a pressing need to continue improving community health services for more vulnerable patients, focusing on care planning and research to identify the most vulnerable patients with COPD early.
4.1. Future Perspectives
The results of our study indicate that the availability of follow-up and discharge support programmes is associated with a lower readmission rate in patients with COPD. This is a healthcare resource that should be prioritised by identifying patients at higher risk of readmission. Research into artificial intelligence-based predictive tools can help predict 30-day readmission risk in hospitalised patients prior to discharge, allowing early implementation of personalised clinical care plans more efficiently. A recent study showed that an artificial neural network (ANN) model achieved a significant 48% reduction in readmission rates [41]. Furthermore, standardisation of hospital care with early and correct indication of ventilatory support use in a more controlled setting alongside further research into the use of non-invasive ventilation in the post-hospital management of patients with COPD may be efficient strategies to achieve an improvement in the quality of care focused on reducing readmission and mortality rates in COPD.
4.2. Study Limitations
Although this study provides new information and has a broad scope, to accurately interpret our results, several considerations must be taken into account. The information sources are reported by unit managers and have not been independently verified. Nonetheless, previous research studies on this topic, such as the 2017 RECALAR survey [15], were conducted using the same methodology, enabling comparison with results from over five years ago. It is worth noting that data collection was performed via an electronic form with explanatory texts to minimise ambiguity and interpretation issues. Data on outcomes (mortality and 30-day readmissions for COPD) and activities performed by the pneumology unit were gathered from the admission departments at the centres after coding hospitalisation reports. However, these measurements may vary due to differences in metric generation across centres, such as variations in the use of the International Classification of Diseases, and different exclusion criteria, such as transfers to other hospitals, which limit comparisons between centres.
5. Conclusions
This study has revealed significant variations in resources and performance according to the level of complexity. It highlights a stagnation in the growth of human resources, with an average of 3.3 pulmonologists per 100,000 inhabitants, and an increase in intermediate respiratory care units (IMCUs), present in 65% of pneumology departments in high-complexity centres, with an increase in the use of mechanical ventilation as home therapy. The median 30-day COPD readmission rate was 14.9%, identifying resources such as discharge support programmes and interventions like increased ventilatory support at discharge as factors associated with a reduced risk of readmission for COPD, while a longer hospital stay was an unfavourable predictor. There were no significant differences in in-hospital mortality based on the complexity level of the centres or the resources of the pneumology units. This information provides the opportunity to plan actions that will improve the quality of care and identify areas for improvement and future lines of research.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/healthcare13030317/s1, Supplementary S1: Participants in COPD Observatory study; Supplementary S2: Hospital-related variables; Supplementary S3: Determinants of in-hospital mortality.
Author Contributions
Conceptualisation, methodology, investigation, writing—review and editing M.C.R. and J.L.R.H. Resources, supervision, project administration and funding acquisition M.C.R., P.C.R., J.d.M.-D., C.E., A.F.G., J.A.G.G., R.G., J.R.H.H., J.S.L.B., J.M.F.-G., E.M., J.J.M.G., A.P.-S., J.A.R. and S.S.P. and R.S.-d.H. did the statistical analysis. All authors contributed to data analysis, results interpretation, drafting and revising the paper, and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This study has been promoted and sponsored by the Spanish Society of Pneumology and Thoracic Surgery (SEPAR). This work has received funding from AstraZeneca Spain that included logistical support for the meetings needed to develop this document. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The study was carried out according to the principles of the Declaration of Helsinki. Data confidentiality was ensured according to the 2018 Law of Data Protection. The study was approved by the Ethics Committee at the Hospital Clínico San Carlos (Madrid, Spain; internal code 21/724-E). Current research laws in Spain (Ley de Investigación Biomédica and Ley de Protección de Datos 3/2018) explicitly state that individual consent is not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors thank the centres that participated in the COPD observatory study as well as participating investigators (Supplementary S1) and José Luis López Campos as the coordinator of the COPD Area of SEPAR for his support in this project.
Conflicts of Interest
M.C.R. has received speaker fees from AstraZeneca, Bial, Chiesi, CSL Behring, GlaxoSmithKline, Menarini, Sanofi, and Grifols, and consulting fees from GlaxoSmithKline and Bial. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. P.C.R. has received speaker fees from AstraZeneca, Chiesi, GlaxoSmithKline, and Sanofi, and consulting fees from GlaxoSmithKline and AstraZeneca. J.d.M.-D. has received honoraria and funding from AstraZeneca, Bial, Boehringer, Chiesi, FAES, Gebro, GSK, Janssen, Menarini, MSD, Novartis, Pfizer, Roche, Sanofi, Teva, and Zambón. C.E. declares no conflict of interest. A.F.G. declares no conflict of interest. J.A.G.G. declares no conflict of interest. R.G. has received payments as a speaker or participant in consultancies, or financial support for participation in congresses from AstraZeneca, GSK, Novartis, FAES, Chiesi, Mundipharma, Menarini, TEVA, Grifols, Ferrer, Boehringer-Ingelheim, Rovi, Gebro, and Zambón. J.R.H.H. declares no conflict of interest. J.S.L.B. declares no conflict of interest. J.M.F.-G. has received fees and financing from (in alphabetical order) AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Faes, Ferrer, Gebro Pharma, GlaxoSmithKline, Laboratories Esteve, Menarini, MundiPharma, Novartis, and Rovi. E.M. declares no conflicts of interest. J.J.M.G. declares no conflicts of interest. A.P.-S. declares no conflicts of interest. J.A.R. reports grants and personal fees from Aflofarm, GSK, Pfizer, Novartis AG, and Menarini, as well as personal fees and non-financial support from Boehringer Ingelheim, AstraZeneca, Gebro, Rovi, and Sanofi-Regeneron outside the submitted work. S.S.P. has received speaker fees from AstraZeneca, Chiesi, GlaxoSmithKline, and Menarini, and consulting fees from GlaxoSmithKline, Sanofi, Grifols, and Bial. R.S.-d.H. declares no conflict of interest. J.L.R.H. has received speaker fees from Bial, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Chiesi, AstraZeneca, Zambon, and Grifols, and consulting fees from Bial.
Abbreviations
The following abbreviations are used in this manuscript:
| IMCU | Dependent intermediate care unit |
| COPD | Chronic obstructive pulmonary disease |
| SEPAR | Spanish Society of Pneumology and Thoracic Surgery |
References
- World Health Organization. Burden of Chronic Obstructive Pulmonary Disease. Available online: https://www.who.int/europe/health-topics/chronic-obstructive-pulmonary-disease-copd (accessed on 22 November 2024).
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Adhikari, T.B.; Advani, S.M.; Agrawal, A.; Ahmadian, E.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Iheanacho, I.; Zhang, S.; King, D.; Rizzo, M.; Ismaila, A.S. Economic Burden of Chronic Obstructive Pulmonary Disease (COPD): A Systematic Literature Review. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 439–460. [Google Scholar] [CrossRef]
- Rehman, A.U.; Hassali, M.A.A.; Muhammad, S.A.; Shah, S.; Abbas, S.; Ali, I.A.B.H.; Salman, A. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: Results from a systematic review of the literature. Expert Rev. Pharmacoecon. Outcomes Res. 2019, 20, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; López-Campos, J.L.; Soler-Cataluña, J.J.; Navarrete, B.A.; Soriano, J.B.; González-Moro, J.M.R.; Ferrer, M.E.F.; Hermosa, J.L.R. Variability in adherence to clinical practice guidelines and recommendations in COPD outpatients: A multi-level, cross-sectional analysis of the EPOCONSUL study. Respir. Res. 2017, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; López-Campos, J.L.; Miravitlles, M.; Cataluña, J.J.S.; Navarrete, B.A.; Ferrer, M.E.F.; Hermosa, J.L.R. Variations in Chronic Obstructive Pulmonary Disease Outpatient Care in Respiratory Clinics: Results From the 2021 EPOCONSUL Audit. Arch. Bronconeumol. 2023, 59, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Barnes, S.; Lowe, D.; Pearson, M.G. Evidence for a link between mortality in acute COPD and hospital type and resources. Thorax 2003, 58, 947–949. [Google Scholar] [CrossRef]
- Pozo-Rodríguez, F.; López-Campos, J.L.; Álvarez-Martínez, C.J.; Castro-Acosta, A.; Agüero, R.; Hueto, J.; Hernández-Hernández, J.; Barrón, M.; Abraira, V.; Forte, A.; et al. Clinical Audit of COPD Patients Requiring Hospital Admissions in Spain: AUDIPOC Study. PLoS ONE 2012, 7, e42156. [Google Scholar] [CrossRef] [PubMed]
- Stukel, T.A.; Fisher, E.S.; Alter, D.A.; Guttmann, A.; Ko, D.T.; Fung, K.; Wodchis, W.P.; Baxter, N.N.; Earle, C.C.; Lee, D.S. Association of Hospital Spending Intensity with Mortality and Readmission Rates in Ontario Hospitals. JAMA 2012, 307, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Romley, J.A.; Jena, A.B.; Goldman, D.P.; Gilman, B.M.; Hockenberry, J.M.; Adams, E.K.; Milstein, A.S.; Wilson, I.B.; Becker, E.R.; Hussey, P.S.; et al. Hospital Spending and Inpatient Mortality: Evidence from California. Ann. Intern. Med. 2011, 154, 160. [Google Scholar] [CrossRef] [PubMed]
- Hartl, S.; Lopez-Campos, J.L.; Pozo-Rodriguez, F.; Castro-Acosta, A.; Studnicka, M.; Kaiser, B.; Roberts, C.M. Risk of death and readmission of hospital-admitted COPD exacerbations: European COPD Audit. Eur. Respir. J. 2015, 47, 113–121. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Hartl, S.; Pozo-Rodriguez, F.; Roberts, C.M. Variability of hospital resources for acute care of COPD patients: The European COPD Audit. Eur. Respir. J. 2013, 43, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.C.; Navarrete, B.A.; Soriano, J.B.; Soler-Cataluña, J.J.; González-Moro, J.-M.R.; Ferrer, M.E.F.; Lopez-Campos, J.L. Clinical audit of COPD in outpatient respiratory clinics in Spain: The EPOCONSUL study. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Estudio RECALAR: Recursos y Calidad en Aparato Respiratorio Monografías de Arch. Bronconeumol. SEPAR 2018, 5, 1–64.
- Patil, S.P.; Krishnan, J.A.; Lechtzin, N.; Diette, G.B. In-Hospital Mortality Following Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Arch. Intern. Med. 2003, 163, 1180–1186. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Long, H.; Huang, Y.; Zeng, P.; Janssens, J.-P.; Guo, Y. Predictors of mortality in COPD exacerbation cases presenting to the respiratory intensive care unit. Respir. Res. 2021, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Singanayagam, A.; Schembri, S.; Chalmers, J.D. Predictors of Mortality in Hospitalized Adults with Acute Exacerbation of Chronic Obstructive Pulmonary Disease. A Systematic Review and Meta-analysis. Ann. Am. Thorac. Soc. 2013, 10, 81–89. [Google Scholar] [CrossRef]
- Waeijen-Smit, K.; Crutsen, M.; Keene, S.; Miravitlles, M.; Crisafulli, E.; Torres, A.; Mueller, C.; Schuetz, P.; Ringbæk, T.J.; Fabbian, F.; et al. Global mortality and readmission rates following COPD exacerbation-related hospitalisation: A meta-analysis of 65 945 individual patients. ERJ Open Res. 2024, 10, 00838-2023. [Google Scholar] [CrossRef]
- López-Campos, J.L.; Hartl, S.; Pozo-Rodriguez, F.; Roberts, C.M. European COPD Audit: Design, organisation of work and methodology. Eur. Respir. J. 2012, 41, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.M.; Stone, R.A.; Buckingham, R.J.; Pursey, N.A.; Lowe, D. Acidosis, non-invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax 2010, 66, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Confalonieri, M.; Trevisan, R.; Demsar, M.; Lattuada, L.; Longo, C.; Cifaldi, R.; Jevnikar, M.; Santagiuliana, M.; Pelusi, L.; Pistelli, R. Opening of a Respiratory Intermediate Care Unit in a General Hospital: Impact on Mortality and Other Outcomes. Respiration 2015, 90, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Lindenauer, P.K.; Dharmarajan, K.; Qin, L.; Lin, Z.; Gershon, A.S.; Krumholz, H.M. Risk Trajectories of Readmission and Death in the First Year after Hospitalization for Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018, 197, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Owen, S.J.; Donaldson, G.C.; Ambrosino, N.; Escarabill, J.; Farre, R.; Fauroux, B.; Robert, D.; Schoenhofer, B.; Simonds, A.K.; Wedzicha, J.A. Patterns of home mechanical ventilation use in Europe: Results from the Eurovent survey. Eur. Respir. J. 2005, 25, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Ergan, B.; Oczkowski, S.; Rochwerg, B.; Carlucci, A.; Chatwin, M.; Clini, E.; Elliott, M.; Gonzalez-Bermejo, J.; Hart, N.; Lujan, M.; et al. European Respiratory Society guidelines on long-term home non-invasive ventilation for management of COPD. Eur. Respir. J. 2019, 54, 1901003. [Google Scholar] [CrossRef] [PubMed]
- Macrea, M.; Oczkowski, S.; Rochwerg, B.; Branson, R.D.; Celli, B.; Coleman, J.M.; Hess, D.R.; Knight, S.L.; Ohar, J.A.; Orr, J.E.; et al. Long-Term Noninvasive Ventilation in Chronic Stable Hypercapnic Chronic Obstructive Pulmonary Disease. An Official American Thoracic Society Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, e74–e87. [Google Scholar] [CrossRef] [PubMed]
- Crimi, C.; Noto, A.; Princi, P.; Cuvelier, A.; Masa, J.F.; Simonds, A.; Elliott, M.W.; Wijkstra, P.; Windisch, W.; Nava, S. Domiciliary Non-invasive Ventilation in COPD: An International Survey of Indications and Practices. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Egea-Santaolalla, C.J.; Vives, E.C.; Lobato, S.D.; Mangado, N.G.; Tomé, M.L.; Andrés, O.M.S. Ventilación mecánica a domicilio. Open Respir. Arch. 2020, 2, 67–88. [Google Scholar] [CrossRef]
- Shams, I.; Ajorlou, S.; Yang, K. A predictive analytics approach to reducing 30-day avoidable readmissions among patients with heart failure, acute myocardial infarction, pneumonia, or COPD. Health Care Manag. Sci. 2014, 18, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Zhang, H.; Wang, J.; Zhao, H.; Han, W.; Li, J. Readmission rate for acute exacerbation of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Respir. Med. 2022, 206, 107090. [Google Scholar] [CrossRef]
- Donaldson, G.C.; Seemungal, T.A.R.; Bhowmik, A.; A Wedzicha, J. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002, 57, 847–852. [Google Scholar] [CrossRef]
- Soler-Cataluna, J.J.; Martínez-García, M.Á.; Sánchez, P.R.; Salcedo, E.; Navarro, M.; Ochando, R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005, 60, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, S.; Represas-Represas, C.; Ruano-Raviña, A.; Mouronte-Roibás, C.; Botana-Rial, M.; Ramos-Hernández, C.; Fernández-Villar, A. Social and clinical predictors of short- and long-term readmission after a severe exacerbation of copd. PLoS ONE 2020, 15, e0229257. [Google Scholar] [CrossRef]
- Bernabeu-Mora, R.; Valera-Novella, E.; Bernabeu-Serrano, E.T.; Soler-Cataluña, J.J.; Calle-Rubio, M.; Medina-Mirapeix, F. Five-Repetition Sit-to-Stand Test as Predictor of Mortality in High Risk COPD Patients. Arch. Bronconeumol. 2024, 61, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.P.; Wells, J.M.; Iyer, A.S.; Kirkpatrick, D.P.; Parekh, T.M.; Leach, L.T.; Anderson, E.M.; Sanders, J.G.; Nichols, J.K.; Blackburn, C.C.; et al. Results of a Medicare Bundled Payments for Care Improvement Initiative for Chronic Obstructive Pulmonary Disease Readmissions. Ann. Am. Thorac. Soc. 2017, 14, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.H.; Thavarajah, K.; Mendez, M.P.; Eichenhorn, M.; Kvale, P.; Yessayan, L. Predischarge Bundle for Patients with Acute Exacerbations of COPD to Reduce Readmissions and ED Visits. Chest 2015, 147, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Chassin, M.R.; Loeb, J.M.; Schmaltz, S.P.; Wachter, R.M. Accountability Measures—Using Measurement to Promote Quality Improvement. N. Engl. J. Med. 2010, 363, 683–688. [Google Scholar] [CrossRef]
- Auerbach, A.D.; Kripalani, S.; Vasilevskis, E.E.; Sehgal, N.; Lindenauer, P.K.; Metlay, J.P.; Fletcher, G.; Ruhnke, G.W.; Flanders, S.A.; Kim, C.; et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern. Med. 2016, 176, 484–493. [Google Scholar] [CrossRef]
- Finkelstein, A.; Zhou, A.; Taubman, S.; Doyle, J. Health Care Hotspotting—A Randomized, Controlled Trial. N. Engl. J. Med. 2020, 382, 152–162. [Google Scholar] [CrossRef]
- Desai, N.R.; Ross, J.S.; Kwon, J.Y.; Herrin, J.; Dharmarajan, K.; Bernheim, S.M.; Krumholz, H.M.; Horwitz, L.I. Association Between Hospital Penalty Status Under the Hospital Readmission Reduction Program and Readmission Rates for Target and Nontarget Conditions. JAMA 2016, 316, 2647–2656. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Ezeana, C.F.; Ogunti, R.; Puppala, M.; He, T.; Yu, X.; Wong, S.S.Y.; Yin, Z.; Roberts, A.W.; et al. An AI-driven clinical care pathway to reduce 30-day readmission for chronic obstructive pulmonary disease (COPD) patients. Sci. Rep. 2022, 12, 20633. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).