Patient-Centered Chronic Spinal Pain Management Using Exercise and Neuromodulation: Study Protocol for a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
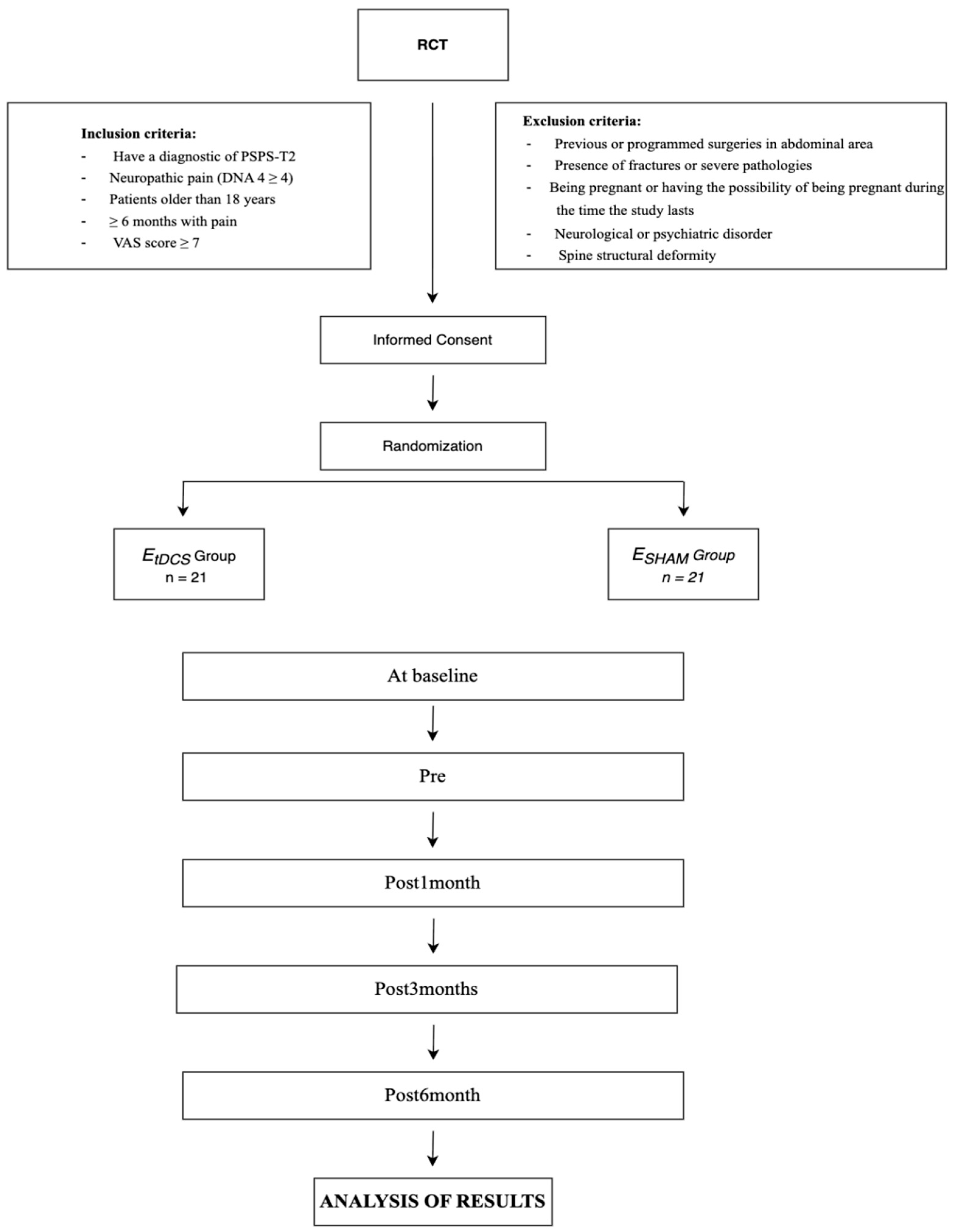
2.3. Inclusion and Exclusion Criteria
2.4. Procedure
3. Randomization and Blinding
4. Sample Size
4.1. Interventions
4.1.1. Exercise and Neuromodulation Protocol
4.1.2. Neuromodulation Protocol
4.1.3. Exercise and Sham Neuromodulation Protocol
5. Patient-Reported Outcome Measures
5.1. Baseline Characteristics
5.2. Primary Outcome
Disability
5.3. Secondary Outcomes
5.3.1. Fear of Movement
5.3.2. Self-Efficacy
5.3.3. Pain-Related Catastrophizing Thinking
5.3.4. Quality of Life
5.3.5. Central Sensitization (CS)
5.3.6. Pain Perception
5.3.7. Neuropathic Pain
5.3.8. Depressive Symptoms
6. Program Feasibility and Safety: Attendance and Compliance with Protocol
7. Oversight and Monitoring
8. Data Collection and Analysis
8.1. Data Collection
8.2. Statistical Analysis
8.2.1. Baseline Characteristics
8.2.2. Analysis of the Outcome Measures
9. Dissemination Plan
10. Discussion
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDI-II | Beck Depression Inventory Second Edition |
| CBT | Cognitive–Behavioral Therapy |
| CI | Confidence intervals |
| CS | Central Sensitization |
| CSI | Central Sensitization Inventory |
| DLPFC | Dorsolateral Prefrontal Cortex |
| FBSS | Failed Back Surgery Syndrome |
| IASP | International Association for the Study of Pain |
| LBP | Low Back Pain |
| NIBS | Noninvasive Brain Stimulation |
| ODI | Oswestry Disability Index |
| PCS | Pain Catastrophizing Scale |
| PFC | Prefrontal Cortex |
| PSPS | Persistent Spinal Pain Syndrome |
| PSPS-T2 | Persistent Spinal Pain Syndrome Type 2 |
| RCT | Randomized Controlled Trial |
| SF-12 | 12-Item Short Form Health Survey |
| tDCS | Transcranial direct current stimulation |
| TSK | Tampa Scale for Kinesiophobia |
| VAS | Visual Analogue Scale |
References
- Gore, M.; Sadosky, A.; Stacey, B.R.; Tai, K.S.; Leslie, D. The burden of chronic spinal pain: Prevalence, treatment, and costs in the US. Pain Pract. 2012, 12, 578–592. [Google Scholar]
- Nicholas, M.; Vlaeyen, J.W.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Simpson, J.C.; Christelis, N.; Russo, M. From failed back surgery syndrome to persistent spinal pain syndrome: A revised paradigm for the understanding and treatment of chronic back pain. Eur. J. Pain 2021, 25, 1943–1946. [Google Scholar] [CrossRef] [PubMed]
- Christelis, N.; Simpson, J.C.; Russo, M.; Stanton-Hicks, M. Persistent spinal pain syndrome: A proposal for a new terminology. Pain Med. 2021, 22, 807–808. [Google Scholar] [CrossRef]
- International Association for the Study of Pain (IASP). Persistent Spinal Pain Syndrome (PSPS). 2021. Available online: https://www.iasp-pain.org (accessed on 22 November 2024).
- Nie, C.; Chen, K.; Chen, J.; Zhu, Y.; Jiang, J.; Jin, X.; Xia, X.; Zheng, C. Altered central pain processing assessed by quantitative sensory testing in patients with failed back surgery syndrome. Neurophysiol. Clin. 2022, 52, 427–435. [Google Scholar] [CrossRef]
- Hutting, N.; Caneiro, J.P.; Ong’wen, O.M.; Miciak, M.; Roberts, L. Patient-centered care in musculoskeletal practice: Key elements to support clinicians to focus on the person. Musculoskelet Sci. Pract. 2022, 57, 102434. [Google Scholar] [CrossRef]
- Naiditch, N.; Billot, M.; Moens, M.; Goudman, L.; Cornet, P.; Le Breton, D.; Roulaud, M.; Ounajim, A.; Page, P.; Lorgeoux, B.; et al. Persistent Spinal Pain Syndrome Type 2 (PSPS-T2), a Social Pain? Advocacy for a Social Gradient of Health Approach to Chronic Pain. J. Clin. Med. 2021, 10, 2817. [Google Scholar] [CrossRef]
- Shiers, S.; Price, T.J. Molecular, circuit, and anatomical changes in the prefrontal cortex in chronic pain. Pain 2020, 161, 1726–1729. [Google Scholar] [CrossRef]
- Jaffal, S.M. Neuroplasticity in chronic pain: Insights into diagnosis and treatment. Korean J. Pain 2025, 38, 89–102. [Google Scholar] [CrossRef]
- Seminowicz, D.A.; Moayedi, M. The Dorsolateral Prefrontal Cortex in Acute and Chronic Pain. J. Pain 2017, 18, 1027–1035. [Google Scholar] [CrossRef]
- Baliki, M.N.; Baria, A.T.; Apkarian, A.V. The Cortical Rhythms of Chronic Back Pain. J. Neurosci. 2011, 31, 13981–13990. [Google Scholar] [CrossRef]
- Loggia, M.L.; Berna, C.; Kim, J.; Cahalan, C.M.; Gollub, R.L.; Wasan, A.D.; Harris, R.E.; Edwards, R.R.; Napadow, V. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumatol. 2014, 66, 203–212. [Google Scholar] [CrossRef]
- Kang, D.; Hesam-Shariati, N.; McAuley, J.H.; Alam, M.; Trost, Z.; Rae, C.D.; Gustin, S.M. Disruption to normal excitatory and inhibitory function within the medial prefrontal cortex in people with chronic pain. Eur. J. Pain 2021, 25, 2242–2256. [Google Scholar] [CrossRef]
- Pahapill, P.A.; Arocho-Quinones, E.V.; Chen, G.; Swearingen, B.; Tomas, C.W.; Koch, K.M.; Nencka, A.S. Distinct Functional Connectivity Patterns for Intermittent Vs Constant Neuropathic Pain Phenotypes in Persistent Spinal Pain Syndrome Type 2 Patients. J. Pain Res. 2024, 17, 1453–1460. [Google Scholar] [CrossRef]
- Cheng, J.O.S.; Cheng, S.T. Effectiveness of physical and cognitive-behavioural intervention programmes for chronic musculoskeletal pain in adults: A systematic review and meta-analysis of randomised controlled trials. PLoS ONE 2019, 14, e0223367. [Google Scholar] [CrossRef]
- Malfliet, A.; Kregel, J.; Coppieters, I.; De Pauw, R.; Meeus, M.; Roussel, N.; Cagnie, B.; Danneels, L.; Nijs, J. Effect of Pain Neuroscience Education Combined with Cognition-Targeted Motor Control Training on Chronic Spinal Pain: A Randomized Clinical Trial. JAMA Neurol. 2018, 75, 808–817. [Google Scholar] [CrossRef]
- Goudman, L.; Russo, M.; Pilitsis, J.G.; Eldabe, S.; Duarte, R.V.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Treatment modalities for patients with Persistent Spinal Pain Syndrome Type II: A systematic review and network meta-analysis. Commun. Med. 2025, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Bolognini, N.; Pascual-Leone, A.; Fregni, F. Using non-invasive brain stimulation to augment motor training-induced plasticity. J. Neuroeng. Rehabil. 2009, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rojas, A.; Pacheco-Barrios, K.; Giannoni-Luza, S.; Rivera-Torrejon, O.; Fregni, F. Noninvasive brain stimulation combined with exercise in chronic pain: A systematic review and meta-analysis. Expert. Rev. Neurother. 2020, 20, 401–412. [Google Scholar] [CrossRef]
- Straudi, S.; Buja, S.; Baroni, A.; Pavarelli, C.; Pranovi, G.; Fregni, F.; Basaglia, N. The effects of transcranial direct current stimulation (tDCS) combined with group exercise treatment in subjects with chronic low back pain: A pilot randomized control trial. Clin. Rehabil. 2018, 32, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef]
- World Medical Association (WMA). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- Van de Minkelis, J.; Peene, L.; Cohen, S.P.; Staats, P.; Al-Kaisy, A.; Van Boxem, K.; Kallewaard, J.W.; Van Zundert, J. Persistent spinal pain syndrome type 2. Pain Pract. 2024, 24, 919–936. [Google Scholar] [CrossRef]
- Corti, E.J.; Nguyen, A.T.; Marinovic, W.; Gasson, N.; Loftus, A.M. Anodal-TDCS over Left-DLPFC Modulates Motor Cortex Excitability in Chronic Lower Back Pain. Brain Sci. 2022, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.L.; Mackie, A.C.; Ribeiro, D.C. Effects of dry needling trigger point therapy in the shoulder region on patients with upper extremity pain and dysfunction: A systematic review with meta-analysis. Physiotherapy 2018, 104, 167–177. [Google Scholar] [CrossRef]
- Falla, D.; Hodges, P.W. Individualized exercise interventions for spinal pain. Exerc. Sport Sci. Rev. 2017, 45, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Mampel, J.; Falaguera-Vera, F.; Sánchez-Poveda, D.; Hernández-Zaballos, F.; Martinez-Soler, M.; Blanco-Giménez, P.; Sanchez-Montero, F.J. Spinal cord stimulation combined with exercise in patients diagnosed with persistent spinal pain syndrome. Study protocol for a randomized control trial. PLoS ONE 2024, 19, e0309935. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Nitsche, M.A.; Bolognini, N.; Bikson, M.; Wagner, T.; Merabet, L.; Edwards, D.J.; Valero-Cabre, A.; Rotenberg, A.; Pascual-Leone, A.; et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012, 5, 175–195. [Google Scholar] [CrossRef]
- Herwig, U.; Satrapi, P.; Schönfeldt-Lecuona, C. Using the International 10-20 EEG System for Positioning of Transcranial Magnetic Stimulation. Brain Topogr. 2003, 16, 95–99. [Google Scholar] [CrossRef]
- Jin, M.; Xu, X.; Zhang, Z.; Xia, W.; Lou, X.; Bai, Z. Timing of high-definition transcranial direct current stimulation to the nondominant primary motor cortex fails to modulate cortical hemodynamic activity and improve motor sequence learning. J. Neuroeng. Rehabil. 2025, 22, 17. [Google Scholar] [CrossRef]
- Ambrus, G.G.; Al-Moyed, H.; Chaieb, L.; Sarp, L.; Antal, A.; Paulus, W. The fade-in–short stimulation–fade out approach to sham tDCS–reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimul. 2012, 5, 499–504. [Google Scholar] [CrossRef]
- Mehra, A.; Baker, D.; Disney, S.; Pynsent, P.B. Oswestry Disability Index scoring made easy. Ann. R. Coll. Surg. Engl. 2008, 90, 497–499. [Google Scholar] [CrossRef]
- Fairbank, J.C.; Pynsent, P.B. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef] [PubMed]
- Turci, A.M.; Nogueira, C.G.; Nogueira Carrer, H.C.; Chaves, T.C. Self-administered stretching exercises are as effective as motor control exercises for people with chronic non-specific low back pain: A randomised trial. J. Physiother. 2023, 69, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.B.; Regidor, M.S.; Jiménez, J.B.; Jiménez, P.B. Análisis psicométrico del cuestionario de discapacidad del dolor lumbar de Oswestry. Fisioterapia 2005, 27, 250–254. [Google Scholar] [CrossRef]
- Costa, L.O.P.; Maher, C.G.; Latimer, J.; Ferreira, P.H.; Ferreira, M.L. Psychometric characteristics of the Brazilian-Portuguese versions of the functional rating index and the Roland Morris disability questionnaire. Spine 2008, 33, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Weermeijer, J.D.; Meulders, A. Clinimetrics: Tampa Scale for Kinesiophobia. J. Physiother. 2018, 64, 126. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.; Kole-Snijders, A.M.; Boeren, R.G.; van Eek, H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain 1995, 62, 363–372. [Google Scholar] [CrossRef]
- Woby, S.R.; Roach, N.K.; Urmston, M.; Watson, P.J. Psychometric properties of the TSK-11: A shortened version of the Tampa Scale for Kinesiophobia. Pain 2005, 117, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Tkachuk, G.A.; Harris, C.A. Psychometric properties of the Tampa Scale for Kinesiophobia-11 (TSK-11). J. Pain 2012, 13, 970–977. [Google Scholar] [CrossRef]
- Lamé, I.E.; Peters, M.L.; Kessels, A.G.; van Kleef, M.; Patijn, J. Test–retest stability of the Pain Catastrophizing Scale and the Tampa Scale for Kinesiophobia in chronic pain over a longer period of time. J. Health Psychol. 2008, 13, 820–826. [Google Scholar] [CrossRef]
- Martín-Aragón, S.; Gómez-Perretta, C.; Gómez-Perretta, I. Cuestionario de autoeficacia para el dolor crónico: Validación para población hispanohablante. Rev. Psicol. Salud. 1998, 10, 135–144. [Google Scholar]
- Anderson, K.O.; Dowds, B.N.; Pelletz, R.E.; Edwards, W.T.; Peeters-Asdourian, C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain 1995, 63, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.J.L.; Bishop, S.R.; Pivik, J. The Pain Catastrophizing Scale: Development and validation. Psychol. Assess. 1995, 7, 524–532. [Google Scholar] [CrossRef]
- Sturgeon, J.A.; Zautra, A.J. Psychological resilience, pain catastrophizing, and positive emotions: Perspectives on comprehensive modeling of individual pain adaptation. Curr. Pain Headache Rep. 2013, 17, 317. [Google Scholar] [CrossRef]
- Ferrando, P.J.; Morales, F.J. Spanish adaptation and psychometric properties of the Pain Catastrophizing Scale (PCS) in a general population sample. Psicothema 2017, 29, 390–395. [Google Scholar]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef]
- Huo, T.; Guo, Y.; Shenkman, E.; Muller, K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: A report from the Wellness Incentive and Navigation (WIN) study. Health Qual Life Outcomes 2018, 16, 34. [Google Scholar] [CrossRef]
- Mayer, T.G.; Neblett, R.; Cohen, H.; Howard, K.J.; Choi, Y.H.; Williams, M.J.; Perez, Y.; Gatchel, R.J. The development and psychometric validation of the Central Sensitization Inventory. Pain Pract. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Kregel, J.; Vuist, W.J.; Descheemaeker, F.; Oostendorp, R.A.; Smeets, R.J.; Coppieters, M.W. Central Sensitization Inventory: Confirmatory factor analysis and validation in a Dutch fibromyalgia population. Clin. J. Pain 2016, 32, 624–630. [Google Scholar] [CrossRef]
- Neblett, R.; Hartzell, M.; Mayer, T.G.; Cohen, H.; Gatchel, R.J. Establishing clinically significant values for the Central Sensitization Inventory. Pain Pract. 2013, 13, 685–695. [Google Scholar]
- Shen, L.; Neblett, R.; Mayer, T.G. Use of the Central Sensitization Inventory to identify central sensitivity syndromes in a chronic pain population. J. Pain Res. 2019, 12, 1307–1314. [Google Scholar]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar]
- Rodríguez-Sanz, D.; Espejo-Garcés, T.; de la Cruz-Torres, B.; López-de-Uralde-Villanueva, I.; Fernández-Carnero, J. Reliability and validity of the visual analogue scale in non-myogenic low back pain patients. In Proceedings of the International Multidisciplinary Scientific GeoConference SGEM, Albena, Bulgaria, 18–24 August 2020; pp. 109–116. [Google Scholar]
- Attal, N.; Perrot, S.; Fermanian, J.; Bouhassira, D. The neuropathic components of chronic low back pain: A prospective multicenter study using the DN4 questionnaire. J. Pain 2011, 12, 1080–1087. [Google Scholar] [CrossRef]
- Epping, R.; Verhagen, A.P.; Hoebink, E.A.; Rooker, S.; Scholten-Peeters, G.G. The diagnostic accuracy and test-retest reliability of the Dutch Pain-DETECT and the DN4 screening tools for neuropathic pain in patients with suspected cervical or lumbar radiculopathy. Musculoskelet Sci. Pract. 2017, 30, 72–79. [Google Scholar] [CrossRef]
- Dozois, D.J.A.; Dobson, K.S.; Ahnberg, J.L. A psychometric evaluation of the Beck Depression Inventory-II. Psychol. Assess. 1998, 10, 83–89. [Google Scholar] [CrossRef]
- Geisser, M.E.; Roth, R.S.; Robinson, M.E. Assessing depression among persons with chronic pain using the Beck Depression Inventory-II. Clin. J. Pain 1997, 13, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Barrios, F.X.; Kopper, B.A.; Hauptmann, W.; Jones, J.; O’Neill, E. Factor structure, reliability, and validity of the Beck Depression Inventory-II with adolescent psychiatric inpatients. Psychol. Assess. 2008, 20, 169–181. [Google Scholar]
- Slade, S.C.; Molloy, E.; Keating, J.L.; Underwood, M. What are patient beliefs and perceptions about exercise for nonspecific chronic low back pain? A systematic review of qualitative studies. Clin. J. Pain 2014, 30, 995–1005. [Google Scholar] [CrossRef]
- Gilanyi, Y.L.; Shah, B.; Cashin, A.G.; Gibbs, M.T.; Bellamy, J.; Day, R.; McAuley, J.H.; Jones, M.D. Barriers and enablers to exercise adherence in people with nonspecific chronic low back pain: A systematic review of qualitative evidence. Pain 2024, 165, 2200–2214. [Google Scholar] [CrossRef]
- Moens, M.; Goudman, L.; Van de Velde, D.; Godderis, L.; Putman, K.; Callens, J.; Lavreysen, O.; Ceulemans, D.; Leysen, L.; De Smedt, A. Personalised rehabilitation to improve return to work in patients with persistent spinal pain syndrome type II after spinal cord stimulation implantation: A study protocol for a 12-month randomised controlled trial—The OPERA study. Trials 2022, 23, 974. [Google Scholar] [CrossRef]
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; North, R. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007, 132, 179–188. [Google Scholar] [CrossRef]
- Elkins, M.R.; Moseley, A.M. Intention-to-treat analysis. J. Physiother. 2015, 61, 165–167. [Google Scholar] [CrossRef]
- Ong, W.Y.; Stohler, C.S.; Herr, D.R. Role of the prefrontal cortex in pain processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Tran, L.C.; Fitzgerald, P.B.; Hoy, K.E.; Daskalakis, Z.J. A randomized double-blind sham-controlled study of transcranial direct current stimulation for treatment-resistant major depression. Front. Psychiatry 2012, 3, 74. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Seeber, A.; Frommann, K.; Klein, C.C.; Liebetanz, D.; Lang, N.; Antal, A.; Paulus, W. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J. Physiol. 2005, 568, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Moffa, A.H.; Fregni, F.; Palm, U.; Padberg, F.; Blumberger, D.M.; Daskalakis, Z.J.; Bennabi, D.; Haffen, E.; Alonzo, A.; et al. Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data. Br. J. Psychiatry 2016, 208, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
| Study Period | |||||||
|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-Allocation | |||||
| TIMEPOINT | −ti | 0 | t1 | t2 | t3 | t4 | tX |
| ENROLMENT: | X | ||||||
| Eligibility screen | X | ||||||
| Informed consent | X | ||||||
| Randomization | X | ||||||
| INTERVENTIONS | |||||||
| [EtDCS] |  | ||||||
| [ESHAM] |  | ||||||
| ASSESSMENTS: | |||||||
| [Anthropometric variables] | X | X | |||||
| [ODI] | X | X | X | X | X | ||
| [TSK] | X | X | X | X | X | ||
| [PCS] | X | X | X | X | X | ||
| [SF-12] | X | X | X | X | X | ||
| [VAS] | X | X | X | X | X | ||
| [DN4] | X | X | X | X | X | ||
| [BDI-II] | X | X | X | X | X | ||
| [SE] | X | X | X | X | X | ||
| [CSI] | X | X | X | X | X | ||
| Phase | Days | Series | Intensity | Break | Effort | Objectives and Focus | Exercise Characteristics |
|---|---|---|---|---|---|---|---|
| Phase 1 Muscle Activation | 1–15 | 3–4 | 6/10 | 30″ | Low-Moderate | Achieve voluntary neuromuscular control |
|
| Phase 2A Posture/Alignment | 16–37 | 3–4 | 7/10 | 30″ | Moderate | Strengthen deep spinal stabilizers |
|
| Phase 2B Posture/Alignment | 38–60 | 3 | 7/10 | 30″ | Moderate | Phase 2A, with added voluntary spinal traction for enhanced postural control |
|
| Phase 2C Posture/Alignment | 61–90 | 3 | 7/10 | 60″ | Moderate | Expand ROM and reinforce control under isometric conditions |
|
| Phase 3 Movement Strategies | 90 | 3 | 7/10 | 60″ | Moderate | Integration of functional movements into daily activities |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas-Ramirez, B.; Jaenada-Carrilero, E.; Belda-Antoli, M.; Leal-Garcia, J.; Alonso-Martin, M.; Mahiques-Sanchis, A.; Benlloch-Garcia, A.; Falaguera-Vera, F.; Vicente-Mampel, J. Patient-Centered Chronic Spinal Pain Management Using Exercise and Neuromodulation: Study Protocol for a Randomized Controlled Trial. Healthcare 2025, 13, 3032. https://doi.org/10.3390/healthcare13233032
Huertas-Ramirez B, Jaenada-Carrilero E, Belda-Antoli M, Leal-Garcia J, Alonso-Martin M, Mahiques-Sanchis A, Benlloch-Garcia A, Falaguera-Vera F, Vicente-Mampel J. Patient-Centered Chronic Spinal Pain Management Using Exercise and Neuromodulation: Study Protocol for a Randomized Controlled Trial. Healthcare. 2025; 13(23):3032. https://doi.org/10.3390/healthcare13233032
Chicago/Turabian StyleHuertas-Ramirez, Borja, Eloy Jaenada-Carrilero, Mariola Belda-Antoli, Jesica Leal-Garcia, Monica Alonso-Martin, Alex Mahiques-Sanchis, Agustin Benlloch-Garcia, Francisco Falaguera-Vera, and Juan Vicente-Mampel. 2025. "Patient-Centered Chronic Spinal Pain Management Using Exercise and Neuromodulation: Study Protocol for a Randomized Controlled Trial" Healthcare 13, no. 23: 3032. https://doi.org/10.3390/healthcare13233032
APA StyleHuertas-Ramirez, B., Jaenada-Carrilero, E., Belda-Antoli, M., Leal-Garcia, J., Alonso-Martin, M., Mahiques-Sanchis, A., Benlloch-Garcia, A., Falaguera-Vera, F., & Vicente-Mampel, J. (2025). Patient-Centered Chronic Spinal Pain Management Using Exercise and Neuromodulation: Study Protocol for a Randomized Controlled Trial. Healthcare, 13(23), 3032. https://doi.org/10.3390/healthcare13233032






