Abstract
Purpose: Telehealth (TH) offers promising solutions for enhancing the management of chronic obstructive pulmonary disease (COPD), particularly in resource-limited or remote settings. However, regulatory uncertainty remains a significant barrier to adopting and integrating TH technologies into routine care. This systematic review aims to evaluate the role of regulatory guidelines in implementing and adopting TH solutions for COPD care and to identify key barriers and facilitators shaping these regulatory efforts. Methods: Following PRISMA guidelines, a comprehensive search of five databases up to 18 October 2025 (PubMed, Web of Science, Scopus, CINAHL, and JSTOR) and grey literature was conducted. Studies and governmental reports were included if they examined regulatory frameworks, stakeholder perspectives, or implementation challenges related to TH in COPD care. Study quality was assessed using the Critical Appraisal Skills Programme (CASP) tool. Narrative and data synthesis were employed. Results: From 343 identified records, 33 sources (18 peer-reviewed studies and 15 governmental/organizational reports) met the inclusion criteria. Findings revealed wide disparities in the existence, specificity, and enforcement of TH regulatory guidelines across countries. Developed nations often had more structured yet nonspecific frameworks, while emerging health systems, such as Saudi Arabia, exhibited fragmented but evolving regulatory landscapes. Common barriers included unclear stakeholder roles, inadequate funding, technological limitations, and resistance to organizational change. Conclusions: Clear, inclusive, and context-sensitive regulatory guidelines are essential to support the successful integration of TH in COPD care. Enhanced regulatory clarity can improve patient trust, engagement, and adherence by addressing safety, accountability, and accessibility concerns. Future research should focus on stakeholder-informed policies that reflect the practical realities of healthcare delivery in both developed and emerging systems.
1. Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive respiratory illness characterized by airflow limitation, commonly caused by prolonged exposure to harmful particles or gases, particularly tobacco smoke and air pollution [1]. Globally, COPD represents a significant public health burden. Managing COPD effectively requires an integrated, patient-centered approach that addresses not only pharmacologic treatment but also behavioral support, pulmonary rehabilitation, and regular monitoring. Telehealth (TH) is a collective term encompassing a range of digital technologies used to deliver and monitor healthcare remotely, and it has emerged as a promising tool in this context [2,3]. By leveraging information and communication technologies (ICTs), TH enables continuous, personalized care delivery to patients with chronic illnesses such as COPD, especially those living in underserved or remote areas [4]. Since not all TH solutions available in the market qualify as medical devices, TH overlaps with related concepts such as telemedicine, remote patient monitoring, tele-respiratory care, tele-nursing, and tele-pulmonary rehabilitation. This interchangeable usage reflects the expanding landscape of digital healthcare delivery [5,6,7].
Despite the rapid development of TH globally, the adoption of TH into routine COPD care remains inconsistent. Multiple factors contribute to this limited uptake, including unclear regulatory pathways, lack of organizational readiness, stakeholder resistance, and an absence of standardized implementation frameworks [8,9,10]. While the COVID-19 pandemic catalyzed an unprecedented expansion of TH services, the long-term sustainability of these services remains uncertain [3]. In Saudi Arabia, for instance, the Ministry of Health (MoH) launched several digital initiatives during the pandemic to strengthen healthcare delivery [11]. However, researchers have noted that these initiatives often lacked robust regulatory frameworks, suffered from fragmented implementation, and failed to include input from key stakeholders such as frontline clinicians and patients [12]. The absence of user engagement, particularly from those directly involved in care delivery, may hinder the long-term use of TH, as successful TH solutions must address end-user needs. Another barrier to the effective adoption of TH solutions in COPD care is the absence of clear regulatory guidelines that govern the initiation, operation, and management of TH systems within healthcare settings. Without such frameworks, healthcare providers may face uncertainty, inconsistent practices, and legal or ethical concerns, all of which can undermine confidence in TH and limit its integration into routine clinical care.
Central to the successful integration of TH into COPD care is the establishment of clear regulatory guidelines. Regulations in the healthcare context may take various forms, including guidelines, frameworks, policies, and legislation. These structures are essential for ensuring the safety, quality, and efficacy of TH interventions, especially TH interventions involving medical devices or the remote delivery of care [13]. Regulatory guidelines also provide clinicians with a standardized reference for adopting TH tools, thereby promoting consistency and reducing variations in care.
However, the literature highlights significant gaps in knowledge, practical, and culturally appropriate regulatory frameworks tailored to TH use in COPD care, particularly in developing and emerging health systems [13,14,15,16]. Existing regulations and guidelines are generic and lack disease-specific guidance. Empirical research on real-world regulatory guidelines implementation is limited, and the influence of infrastructure and organizational context remains unclear. Moreover, no consensus exists on the most suitable theoretical framework for guiding TH implementation in COPD care.
Therefore, this systematic literature review seeks to address these gaps by synthesizing the available literature on the regulatory aspects of TH implementation for COPD care, with a specific focus on developing and emerging healthcare systems. The literature review aims to: (1) explore the role of regulatory guidelines in shaping implementation and assess the extent to which current frameworks are responsive to contextual, organizational, and patient-level needs; (2) identify the existing barriers and facilitators associated with regulatory guidelines governing the use of TH in COPD care.
2. Materials and Methods
2.1. Search Strategy and Selection Process
This method was developed as part of doctoral research, which adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [17]. The study protocol was registered with PROSPERO under registration number CRD420251178887. We conducted an electronic search on the five databases, including sources for grey literature: Web of Science, PubMed, CINAHL, Scopus, and JSTOR. Sources of gray literature are also included in the search procedures to retrieve any relevant sources, such as governmental reports up to 18 October 2025. The search was conducted with supervision and guidance by an expert librarian at the UCL library (Name: Heather Chesters). Search strategies from all databases were provided in Table 1 and Table S1.

Table 1.
Search Strategy.
The screening process was conducted in accordance with the PRISMA 2020 guidelines [17]. Initially, the authors, NA and PT, independently screened titles and abstracts to exclude studies that did not meet the inclusion criteria. In the second stage, the remaining articles underwent a full-text review to determine final eligibility. Any discrepancies between reviewers (NA and PT) during either stage were resolved through discussion or consultation with a third reviewer (NC).
2.2. Eligibility Criteria
Inclusion Criteria and Exclusion Criteria
The criteria for including articles in this systematic review focused on studies that examined regulatory guidelines and the factors influencing the implementation of TH in COPD care. However, the review identified a scarcity of such studies. To the best of our knowledge, no scientific research has specifically addressed the implementation of regulatory guidelines for TH in patients with COPD. Therefore, the scope of our electronic search was expanded to encompass governmental and non-governmental reports that discuss regulatory guidelines for TH in COPD care. The inclusion criteria were presented in Table 2.

Table 2.
Inclusion and exclusion criteria of included studies.
2.3. Data Extraction and Quality of the Studies
We developed a standardized Microsoft Excel spreadsheet to systematically collect data from the included articles. The data extraction form was designed to capture information from qualitative studies and policy or guideline documents. It focused on elements related to TH regulations and frameworks in the context of COPD care. Extracted data included study characteristics, descriptions of TH applications, regulatory guideline content, implementation models, and identified institutional, user-level, and industry-level barriers. To ensure the methodological quality of the included studies, we applied the Critical Appraisal Skills Programme (CASP) checklist [18,19]. The CASP tool is widely recognized in evidence-based research for its structured approach to evaluating the validity, results, and relevance of qualitative and quantitative studies [18,19]. This tool was chosen due to its comprehensive nature, covering key domains such as methodological rigor, validity of findings, and relevance to the research question. In addition, grey literature was evaluated using Authority, Accuracy, Coverage, Objectivity, Date, and Significance (AACODS) checklist [20]. However, it is important to note that the quality appraisal was conducted independently of the study selection process. No studies were included or excluded based on their quality scores; rather, the appraisal was used to inform the interpretation and synthesis of the evidence [18].
2.4. Narrative and Data Synthesis
For the data analysis, we employed a narrative synthesis approach to systematically explore, organize, and interpret the extracted findings. Also, a quantitative frequency tables were applied to report barriers and facilitators identified across included studies. Then, a comparative thematic synthesis was conducted to explore differences between high-income and low-/middle-income health systems, allowing contextual interpretation of regulatory, infrastructural, and organizational variations influencing TH implementation for COPD care. A meta-analysis was not conducted, as the included studies were qualitative in nature and exhibited heterogeneity in design and outcome reporting. The included studies were summarized and synthesized under subheadings into two main groups:
- Published Articles, which were further summarized under categories into:
- Descriptions of TH Use
- Regulatory Guidelines for TH Adoption in COPD Care
- Identified Needs for Regulatory Guidelines
- Integration of TH into Health Systems
- Responsibilities Specified in Guidelines
- Implementation Frameworks
- Reported Barriers and Facilitators
- Governmental and Organizational Reports, which were summarized and critically appraised based on the region or organizational source.
3. Results
3.1. Screening and Studies Selection
The electronic search of academic databases and grey literature sources yielded a total of 343 records, including research articles and government reports. After removing 87 duplicate entries, 226 records remained for title and abstract screening. Based on the predefined inclusion and exclusion criteria, 46 records and 20 reports were deemed eligible for full-text review, comprising 18 peer-reviewed research articles and 15 government or organizational reports. All references were managed using EndNote software (version 20) to ensure accurate citation and organization. The selection process was systematically documented and illustrated using a PRISMA flow diagram (Figure 1).
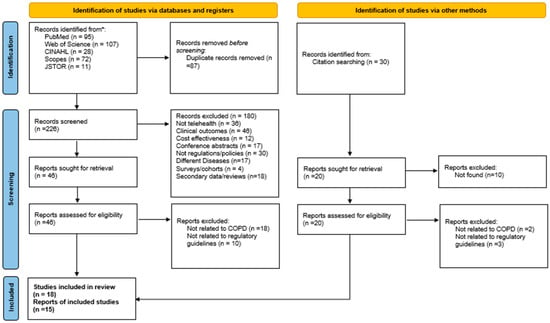
Figure 1.
PRISMA flow diagram. * means databases.
3.2. Study Characteristics
The systematic literature review included 33 records, ranging from the oldest article published in 2008 to the most recent one in 2022. Regarding the governmental reports, the oldest report was in 2015, and the latest was in 2024. Among all included articles, the countries where regulatory guidelines were discussed are the United Kingdom (n = 7), Canada (n = 4), the Netherlands (n = 3), China (n = 2), Italy (n = 2), Germany (n = 2), Scotland (n = 1), Japan (n = 1), Ireland (n = 1), Greece (n = 1), Nepal (n = 1), Spain (n = 1), Denmark (n = 1), and Norway (n = 1) (Figure 2). All included studies were qualitative studies with semi-structured interviews with different stakeholders, such as managers, administrators, informants, healthcare providers, patients, or people from industries summary of the included studies provided in Table 3. The stakeholders who directly impact implementation or market adoption of TH solutions were recruited in 13 qualitative studies out of 18.
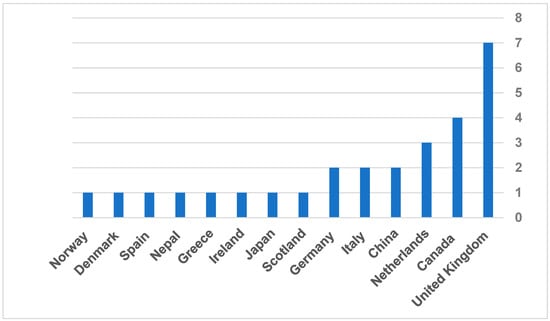
Figure 2.
Frequency of countries included in the published articles.

Table 3.
Summary of included studies.
3.3. Descriptions of TH Use Studies
Among all included studies, terminologies to describe the use of technology or new biomedical devices in COPD care were interchangeable between TH [21,23,24,25,26,27,30,32,35,37,38], digital health [31,33,34,36], and telemedicine [22,28,29]. Despite the terminology variation, all reviewed TH solutions, regardless of the label used, shared a common goal which is enable remote monitoring and management of patients with COPD. This included functionalities such as tracking symptoms, transmitting data such as vital signs, and supporting remote clinical decision-making. The interchangeable use of terms reflects the lack of standardized definitions in the digital health landscape, especially related to regulatory guidelines.
3.4. Regulatory Guidelines for TH Adoption in COPD Care
Studies have demonstrated a variation in the existence of regulatory guidelines for the adoption of TH in the management of COPD care within local practices across European countries, United Kingdom (including Wales and Scotland) [21,22,24,26,28,30,32,37], Ireland [31], Germany [28,37], Spain [28], Greece [32], Denmark [32], Norway [32], Netherlands [23,25], and Italy [29,37]. In Asian countries, China and Nepal have a regulatory guideline for adopting TH in COPD care [35,36,38].
In the included studies, regulatory guidelines commonly addressed key components such as data protection, remote patient monitoring protocols, and clinical governance. Notably, developed healthcare systems, particularly in Europe, demonstrated more advanced integration of TH into COPD care, supported by well-established regulatory guidelines and cross-regional research projects [28].
In contrast, emerging healthcare systems in Asia have shown initial progress in formulating regulations to support the adoption of TH for COPD management [35,36,38]. Although these regulatory efforts are relatively recent, they indicate a growing governmental recognition of TH as a strategic response to tackle the increasing burden of chronic diseases.
3.5. Identified Needs for Regulatory Guidelines
The included studies demonstrated several factors that can affect regulatory guidelines for TH applications in COPD care. The included studies have a growing sense of the need to establish and adopt TH solutions in COPD care. For example, between 2008 and 2012, there was an increasing demand for community-based care for chronic conditions such as COPD [21,22,23]. Evidence from this period demonstrated a pressing need to create regulatory guidelines for TH usage in primary care settings, particularly with COPD patients [21,22,23].
Since 2017, there has been a noticeable shift in focus toward ensuring the sustainability of TH interventions in the management of COPD [29,38]. Increasingly, research has recognized the critical role of regulatory frameworks, policies, and legislation in supporting the long-term viability and integration of TH solutions into healthcare systems. To ensure the sustainable implementation and adoption of TH services, developers and policymakers should consider local factors and financial constraints specific to the healthcare system [21,22,23].
Additionally, the needs for regulatory guidelines and frameworks in the current healthcare systems vary from one location to another [21,22,27,32]. In developed health systems where COPD care guidelines were a priority, the provision of regulating new TH services is much faster than in other health systems. Summary of frameworks was summarized and synthesized in Table 4.

Table 4.
Summary of the theoretical frameworks.
3.6. Integration of TH into Health Systems
Changing the health system by integrating TH into routine care practices could be a factor impacting the regulatory guidelines [23]. Also, policies and regulations of using TH must not rely only on the feasibility and effectiveness of TH solutions, including stakeholders’ needs and demands, which are important to design the guidelines [25]. Champion stakeholders (Leaders, policymakers, or payers) are required to initiate and lead the change in the health system, either in a particular project related to TH solutions or by changing the policy and regulations related to the use of TH [24,26,29,32].
3.6.1. Responsibilities Specified in Guidelines
Most of the included studies demonstrated that the current guidelines for TH are general and not specific to frontline staff, such as nurses, respiratory therapists, and physicians [22,23,24,26,27,29,31,32,33,35,36,38] or even payers such as industrial or insurance companies [25,30]. To ensure the sustainability of regulatory guidelines for adopting TH in COPD care, specific tasks and responsibilities must be clarified for targeted frontline stakeholders, such as nurses, respiratory therapists, and physicians [25,29,30,34,35,37]. This will enhance cooperation within hospitals and between hospitals and primary care structures, as well as keep each level informed about their tasks and responsibilities [25,29,30,34,35,37]. This includes the admin responsibilities and industrial responsibilities within the regulatory guidelines [25,26].
3.6.2. Reported Barriers and Facilitators
The regulatory guideline of TH faces numerous barriers that hinder its effective implementation and sustainability [29]. One of the primary challenges is resource constraints, including limited funding, workforce shortages, and infrastructure issues. These constraints make it difficult to establish and maintain guidelines for TH services. Additionally, organizational barriers contribute to fragmented communication and a lack of coordination between different policymakers, further complicating the integration of TH into existing health systems. Policy and regulation implementation also poses a significant challenge, as translating policies and regulations directly into practical service can be complex and time-consuming. Resistance to change among staff is another major barrier, with many healthcare professionals hesitant to adopt new working methods [29].
TH projects highlight additional obstacles. It often lacks clear clinical objectives, making it difficult to measure TH effectiveness. TH devices often lack practicality for sustained patient use, and data collection and analysis procedures are frequently manual rather than automated. Moreover, the implications of new interprofessional relationships and the medico-legal responsibilities associated with TH have not received sufficient attention, leading to confusion and reluctance among healthcare providers to adopt and accept TH [22].
Incomplete data capture and the absence of team dynamics also hinder the success of TH initiatives [26]. The creation and deployment of TH systems incur significant costs in terms of time and resources, necessitating project leaders (often referred to as Champions) to carefully direct these initiatives [32]. TH services often have restricted referral pathways, leading to consideration primarily for patients with significant medical needs. However, challenges remain in accurately assessing patient suitability and predicting the effects of TH on health outcomes. Staff reservations about using new technologies, concerns about increased workload, and the impact on nursing roles further complicate the adoption of TH [26].
Additionally, the regulatory guidelines frequently lack clarity regarding the duration of TH and the discontinuation process in cases where patients become dependent on remote monitoring [26,28]. Healthcare practitioners do not always prioritize TH. Finally, the absence of a shared vision and strategic ownership for investing in TH technologies further slows down the adoption of TH [26,28]. A list of 16 barriers and 16 facilitators has been identified from the included studies described in Figure 3 and Figure 4. A summary of the frequency and percentage distribution of identified barriers and facilitators is presented in Supplementary Tables S2–S5.
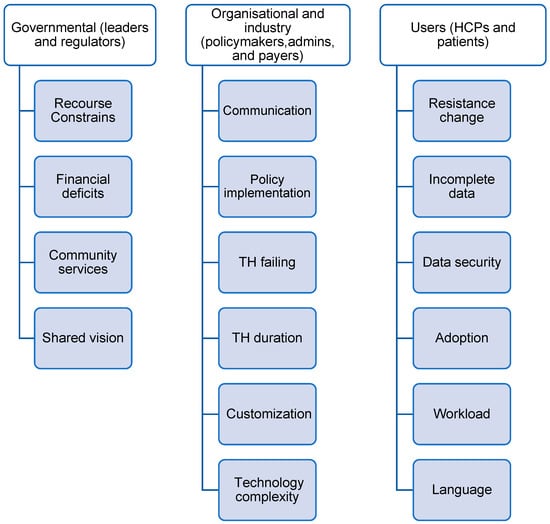
Figure 3.
Barriers identified from included studies.
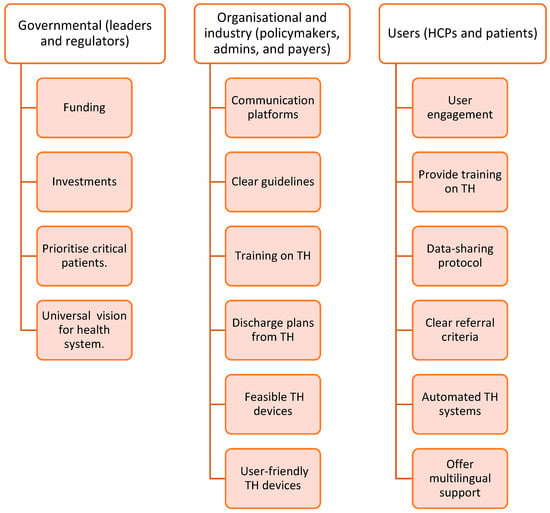
Figure 4.
Facilitators identified from the included studies.
3.7. Quality of Included Studies
The methodological quality of the included studies was assessed using the CASP checklist. Overall appraisal scores ranged from 60% to 100%, indicating moderate to high methodological rigor. Two studies scored 60% [25,33], four studies scored 70% [23,29,30,36], five studies scored 80% [21,24,27,28,37], six studies scored 90% [22,26,32,34,35,39], and one study achieved a full score of 100% [38] (Table 5).

Table 5.
Quality of studies.
3.8. Synthesis of the Reports
The external search identified 15 governmental reports from developed and emerging health systems. Among these reports, only two reports (One from Brazil and one from Southeast Asia) were published in peer-reviewed journals [39,40]. The remaining reports were described and published on the local website for the country’s health authority or MoH. Saudi Arabia has the highest governmental report to guide and regulate TH practice. The total published reports were 5 from different authorities, such as the MoH [41,42,43], Saudi Food and Drug Authority [44], and the Council of Health Insurance [45]. The WHO was the second-highest authority to publish four documents related to TH legalization [46,47,48,49], Figure 5. A summary of the grey literature appraisal is provided in Supplementary Table S6.
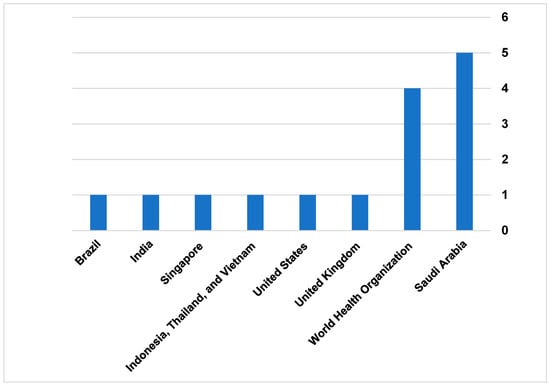
Figure 5.
Countries of governmental reports.
3.8.1. World Health Organization
The Reports from WHO mostly provide advisory guides to help local and international authorities plan TH implementation in their systems. The report provides different definitions for TH, and it was mostly general and not specific to developed or emerging countries. The reports also recommend and suggest global standards and policies for health systems to initiate regulatory guidelines to use TH, as well as encourage health systems to invest in TH solutions, particularly the reports that were published in 2012 [46,48]. The reports published in 2020 provide more technical advice on designing regulatory guidelines that consider people with disabilities who use TH on a daily basis [47]. Additionally, the report published in 2022 provides more recommendations on TH licenses and prescribing medications via TH applications [49].
3.8.2. Saudi Arabia
Different health authorities issued the report from Saudi Arabia. The report by the MoH in Saudi Arabia provides guidelines and details on how TH is regulated in healthcare practice. The report defined TH and other terms referring to TH in the health system. Also, it clarifies a statement regarding using TH as “health practitioner may conduct examinations or treatment through the services of TH in homes and workplaces, following the regulations set by the MoH.” Furthermore, there were some guides for the practice on how to obtain a license in TH, as all clinical facilities were mandated to have TH services for patients [41]. In 2021, the National Health Information Centre published the regulatory guidelines for using TH accompanying the services, as well as the stakeholders who participated in the guidelines. For example, the guidelines demonstrated the scope of using TH for screening patients, monitoring patients, consultations, and support provision of the treatment plan [41,43]. In 2022, the legal regulations for using TH were published to set standards, policies, and guidelines for using TH, and to monitor compliance of health authorities with these standards [43]. The document explains the insurance coverage as well as the liability of my malpractice for TH.
Based on the reports by the MoH, in 2023, the Council of Health Insurance [CHI] in Saudi Arabia published a document to provide the requirements for a clinical setting to be approved by CHI to provide TH services and the insurance coverages for the services for both public and private sectors. The report always regulated the conditions related to TH devices, healthcare practitioners, licensing, and data sharing policy [43]. Additionally, the Saudi Food and Drug Authority published a guideline for the TH devices approval process. The advisory guide demonstrates the step-by-step process from approving and marketing TH devices [44].
3.8.3. United Kingdom
In the United Kingdom, the guidelines published by the National Health Service (NHS) demonstrated the TH regulations in England, Scotland, Wales, and Northern Ireland. The report demonstrated that the process of using TH services by the healthcare practitioner must be regulated by the General Medical Council. Additionally, the regulatory guidelines for each healthcare country within the UK are different based on demand. The report also defined TH applications, for example, considering software as TH applications based on medical device regulations in the UK. Furthermore, the document introduced how health authorities deal with and protect data generated by or from TH solutions [50].
3.8.4. United States
In the United States, the report reviewed TH policies in all states. The primary objective of the report was to enhance healthcare access through TH. The report rated the states based on the level of extent the state aligned with the best practices as well as recommendations for optimizing and sustaining TH [51].
3.8.5. Indonesia, Thailand, and Vietnam
The reports from Indonesia, Thailand, and Vietnam were summarized in one article review. The regulatory guidelines mostly discussed a list of definitions related to TH, the infrastructure of TH in clinical practice, licensing TH services in the countries, the tasks and responsibilities of the facilities that provide TH services, and the vision plan to operate TH services in these countries [40].
3.8.6. India
The report from India was issued by the Indian Medical Council Act and the Information Technology Act. These authorities were responsible for setting regulations to govern the practice of TH and ensure its compliance with ethical and legal standards. The guidelines also offer comprehensive details about the technology platforms and tools recommended for efficient healthcare delivery [52].
3.8.7. Brazil
The article maps the regulatory framework of telemedicine in Brazil, highlighting the significant milestones and phases in its development. The study identifies 79 telemedicine-related legislations from the federal government and 31 regulations from federal health professional councils. These regulations are categorized into three historical phases: Formulation/Decision-Making, Organization/Implementation, and Expansion/Maturation [39] (Table 6).

Table 6.
Summary of governmental and organizational reports.
4. Discussion
4.1. Main Findings
The findings from the literature demonstrate that there is an effort to establish regulatory guidelines for adopting TH in COPD care in several developed countries. This effort varies from one location to another. There are general guidelines on using TH for COPD care in developed and emerging health systems, considering varying degrees of depth in regulatory guidelines and which stakeholders will be impacted by them. There is no theoretical framework for guiding adoption in most of the guidelines. The suggested frameworks explain TH adoption barriers less in depth. Organizations and user-related barriers proved to be the most common, with TH receiving less attention. Current TH regulations are often general and lack COPD-specific provisions. Since some health systems still regulate TH more broadly, comprehensive regulatory guidelines for adopting TH in COPD cannot be achieved. Various government reports from developed and emerging health systems describe TH options, stakeholders impacted by regulatory guidelines, and organizations involved in shaping TH provision. Compared to other regulatory guidelines, Saudi Arabia’s guidance was the most comprehensive. However, the standardization of COPD care regulatory guidelines needs to be improved locally and internationally.
The absence of consistent terminology poses challenges for marketing, evaluating, regulating, and implementing TH in COPD care. It also complicates the synthesis of evidence across studies and may hinder the development of unified guidelines and policies. This highlights a pressing need for consensus on terminology and classification in future research and clinical practice to enhance comparability and ensure coherent regulatory and implementation strategies.
Despite the variation in regulatory maturity across healthcare systems, the presence of regulatory guidelines remains a critical enabler for the sustainable and safe deployment of TH in clinical practice. These regulatory guidelines play an essential role in ensuring patient safety, clinical accountability, and interoperability of digital systems. Nevertheless, the heterogeneity of regulatory approaches across healthcare systems highlights the need for greater harmonization through global or regional frameworks. Such alignment would facilitate broader adoption, enhance implementation scalability, and foster international collaboration in TH applications in COPD care.
In order to effectively manage and monitor COPD patients, it is crucial to have clear and well-structured TH regulations. While TH can significantly enhance COPD care, its adoption and implementation require a deep understanding of the barriers to establishing regulatory guidelines within the health system [54,55]. In general, regulatory guidelines exist but use varying terminology. Most current regulations focus on clinical application, often omitting operational or policy-level considerations [56,57]. The obstacles related to regulatory guidelines arise from various organizational, technological, and stakeholders’ standpoints. Additionally, there are unresolved questions about how to integrate these regulatory guidelines into care models, the responsibilities of healthcare providers, and the benefits for patients. Furthermore, there is a lack of qualitative data that captures stakeholders’ perspectives on the impact of these regulatory guidelines on TH adoption. Stakeholders, individuals or groups, serve as catalysts for change, facilitating the institutionalization of TH through advocacy, leadership, and strategic influence. Their engagement ensures that policies are not only technically sound but also aligned with broader health system goals, making regulatory guidelines more practical, sustainable, and responsive to real-world challenges [58].
Several theoretical models have been proposed to guide the implementation of TH innovations. The Knowledge-to-Action (KTA) framework, for instance, offers a process-oriented view of knowledge translation but primarily focuses on institutional actors and neglects patient-level dynamics [59]. Similarly, the Technology Acceptance Model (TAM) emphasizes user attitudes and perceived ease of use but offers limited insight into systemic or regulatory challenges [60]. More recent frameworks, such as the Tool + Team + Routine model, attempt to bridge the gap between technology, users, and organizational workflows, but lack sufficient detail to explain how regulatory factors influence real-world adoption [34]. Notably, these models often fall short in addressing settings involving multiple stakeholder groups, contextual variations, and policy-level barriers that are especially pronounced in developed healthcare systems [58].
In the case of Saudi Arabia, efforts to scale TH remain hindered by a lack of regulatory clarity. Although Vision 2030 has prioritized digital health transformation as a national goal, the corresponding policies and legal instruments have not evolved at the same pace [15,16]. Many healthcare providers report uncertainty including standards compliance, data protection, and system interoperability when implementing TH for COPD care [61]. Furthermore, there is little empirical evidence examining how healthcare providers, administrators, and patients perceive existing regulations or how they navigate the regulatory guidelines. This disconnect between policy vision and clinical implementation underscores the urgent need for a regulatory framework that is locally grounded, stakeholder-informed, and adaptable to different levels of healthcare infrastructure.
International comparisons reveal similar patterns. In countries such as the UK and USA, structured TH regulations have been instrumental in expanding access and standardizing care [51,62]. Conversely, in countries like Brazil, regulatory saturation where multiple overlapping policies exist without clear implementation roadmaps has hindered widespread adoption [39]. These global experiences underscore the need for a balance between regulatory specificity and operational flexibility, particularly for chronic disease management.
Improved regulatory clarity plays a critical role in shaping patient preference, engagement, and adherence to TH services. When regulatory guidelines are transparent and well-communicated, patients are more likely to trust digital health solutions, particularly in contexts where privacy, safety, and quality of care are clearly protected [58]. Defined protocols around licensure, data security, and accountability reassure patients that the care provided is equivalent to traditional in-person services. Having this clear regulatory guideline often translates into increased patient willingness to adopt and consistently use TH platforms.
Furthermore, regulatory clarity fosters consistency in service delivery, which enhances patient satisfaction and long-term adherence. When patients experience streamlined access to TH without confusion around eligibility, insurance coverage, or technical barriers, they are more engaged and proactive in managing their health. In chronic disease care, such as for COPD, this clarity directly contributes to improved health outcomes, as patients are more likely to attend virtual follow-ups, comply with treatment plans, and communicate regularly with care teams. Overall, the alignment of regulatory policies with patient-centered care principles is essential to advancing the sustainable integration of TH in healthcare systems.
Addtionally, variation in studies quality underscores the importance of critically evaluating the evidence base used to inform TH regulatory guidelines. High-quality studies are essential for developing effective, contextually appropriate, and sustainable policies in COPD care.
4.2. Proposed Framework to Succeed TH Implementation
To address the multifaceted challenges associated with the adoption of TH, we propose a simple, novel framework aimed at enhancing the successful implementation of TH initiatives. As illustrated in Figure 6, the framework emphasizes the integration of key operational and strategic elements, including the involvement of end-users, stakeholders, and local business partners, as well as the tailoring of innovations to specific contexts. It also incorporates essential factors such as interdisciplinary collaboration, feedback mechanisms, and the availability of feasible business models. Additionally, it recognizes the importance of adapting to organizational changes and anticipating system-level transformations required for long-term integration. This framework provides a practical guide for policymakers, implementers, and healthcare organizations to structure their TH strategies effectively.
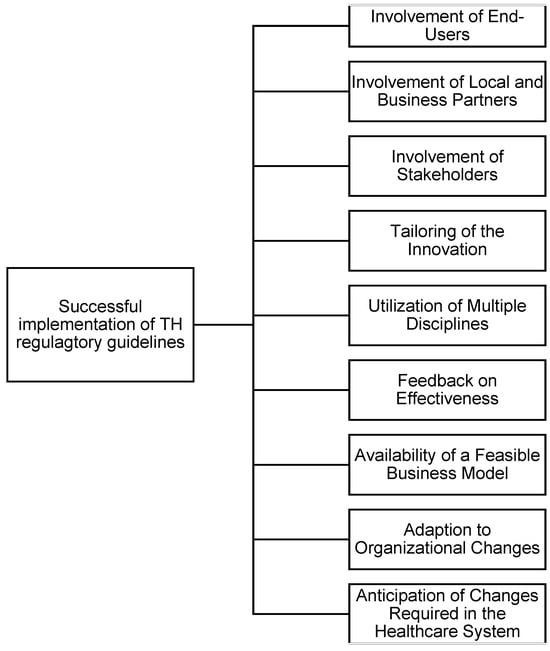
Figure 6.
Factors impacting the successful implementation of TH (Created by author).
4.3. Strengths and Limitations
This systematic review offers a comprehensive synthesis of global and regional literature on regulatory guidelines influencing TH adoption in COPD care. A key strength lies in the dual inclusion of peer-reviewed studies and grey literature, enhancing contextual relevance and policy insights. The use of the CASP tool ensures methodological rigor and appraisal consistency across qualitative studies. Additionally, the review provides a structured categorization of barriers and facilitators, supporting informed policymaking.
However, several limitations should be acknowledged. First, the lack of studies specific to Saudi Arabia (all were governmental reports) limited the depth of region-specific analysis. Second, the exclusion of non-English sources may have overlooked valuable regional reports. Third, the heterogeneity in terminology and frameworks across studies posed challenges in direct comparison. Fourth, current regulatory guidelines reports adopted by health authorities tend to emphasize general operational and technical standards, offering limited guidance for disease-specific applications such as COPD management. Finally, while narrative synthesis was appropriate, the absence of meta-analytic techniques limits generalizability. More research is needed to evaluate the impact of the current regulatory guidelines on the market adoption and implementation of TH in COPD care locally and internationally.
5. Conclusions
This review highlights the critical role of regulatory guidelines in shaping the adoption and implementation of TH solutions for COPD care. Despite global progress, the evidence suggests that existing regulations often lack specificity, stakeholder engagement, and contextual adaptation. Saudi Arabia has shown commendable progress in digital health policy under Vision 2030; however, TH guidelines remain fragmented and insufficiently tailored for chronic disease contexts such as COPD.
To promote effective TH adoption in COPD care, regulatory guidelines must move beyond generic mandates and embrace frameworks that address disease-specific, stakeholder-driven, and system-level variables. Future regulatory efforts should incorporate integrated models and frameworks, adapted to reflect regulatory interactions and policy barriers from different directions. Policymakers should also prioritize clear responsibilities, data governance, and cross-platform interoperability to bridge the gap between digital health ambitions and practical implementation. These guidelines must be co-developed with input from clinicians, patients, administrators, and technology vendors to ensure alignment with real-world clinical routines and resource constraints.
Promoting regulatory clarity begins with policymaking that centers the experiences and perspectives of all stakeholders. This includes conducting qualitative studies to capture how regulations affect provider workflows, patient trust, and organizational readiness.
Ultimately, our review calls for the development of practical, inclusive, and evidence-informed regulatory frameworks that accommodate both clinical and technological realities. Future research should prioritize qualitative studies to assess the barriers and facilitators toward TH implementation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13222858/s1.
Author Contributions
N.S.A., N.A.C. and P.T. contributed equally to this study, including its conception, design, data collection, analysis, and interpretation. All co-authors involved in drafting, revising, and critically evaluating the manuscript approved the final version and accepted responsibility for the integrity of the work. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors would like to express their appreciation to Heather Chesters of the University College London (UCL) Library Services for her assistance in guiding and updating the literature search strategy and facilitating access to key databases and resources.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, D.; Agusti, A.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Criner, G.J.; Frith, P.; Halpin, D.M.; Han, M. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: The GOLD science committee report 2019. Eur. Respir. J. 2019, 53, 1900164. [Google Scholar] [CrossRef]
- Alghamdi, S.M. Content, mechanism, and outcome of effective telehealth solutions for management of chronic obstructive pulmonary diseases: A narrative review. Healthcare 2023, 11, 3164. [Google Scholar] [CrossRef]
- Alghamdi, N.S.; Alghamdi, S.M. The role of digital technology in curbing COVID-19. Int. J. Environ. Res. Public Health 2022, 19, 8287. [Google Scholar] [CrossRef] [PubMed]
- Snoswell, C.L.; Stringer, H.; Taylor, M.L.; Caffery, L.J.; Smith, A.C. An overview of the effect of telehealth on mortality: A systematic review of meta-analyses. J. Telemed. Telecare 2023, 29, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, H.J.; Oh, J.L.; Cheong, Y.K.; Jiang, Y.; Teo, J.Y.C.; Seah, C.W.A.; Yu, M.; Wang, W. Barriers and facilitators to the adoption of digital health interventions for COPD management: A scoping review. Heart Lung 2023, 59, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.T.; Sousa, C.S.; Morais-Almeida, M.; Simões, M.J.; Mendes, P. Telemedicine in COPD: An overview by topics. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 601–617. [Google Scholar] [CrossRef]
- Almojaibel, A.A. Telerespiratory Home Care: A Guid to Success; Imam Abdulrahman Bin Faisal University: Dammam, Saudi Arabia, 2010. [Google Scholar]
- Akula, M.; Nguyen, M.; Abraham, J.; Arora, V.M.; Oladosu, F.; Sunderrajan, A.; Traeger, L.; Press, V.G. Determining if COPD Self-Management Televisit-Based Interventions are evaluated among and equitably effective across diverse patient populations to Reduce Acute Care Utilization: A Scoping Review. Chest 2024, 166, 1371–1393. [Google Scholar] [CrossRef]
- Aburub, A.; Darabseh, M.Z.; Badran, R.; Eilayyan, O.; Shurrab, A.a.M.; Degens, H. The Effects of Digital Health Interventions for Pulmonary Rehabilitation in People with COPD: A Systematic Review of Randomized Controlled Trials. Medicina 2024, 60, 963. [Google Scholar] [CrossRef]
- Alhasani, R.; Ferreira, T.J.; Valois, M.-F.; Singh, D.; Ahmed, S. Enrollment and dropout rates of individuals with chronic obstructive pulmonary disease approached for telehealth interventions: A systematic review and meta-regression analysis. Heliyon 2024, 10, e23776. [Google Scholar] [CrossRef]
- Hassounah, M.; Raheel, H.; Alhefzi, M. Digital response during the COVID-19 pandemic in Saudi Arabia. J. Med. Internet Res. 2020, 22, e19338. [Google Scholar] [CrossRef]
- Abdulaziz, A.A.; Algosaibi, A.M.; Alquhaibi, A.S.; Alali, F.N.; Almutawaa, M.S.; Roomi, M.A.; Bhatti, Y.A. Digital Healthcare Innovation and Development in Saudi Arabia During and Beyond COVID-19. Sci. Technol. Soc. 2023, 28, 370–386. [Google Scholar] [CrossRef]
- Iqbal, J.D.; Biller-Andorno, N. The regulatory gap in digital health and alternative pathways to bridge it. Health Policy Technol. 2022, 11, 100663. [Google Scholar] [CrossRef]
- Sorenson, C.; Drummond, M. Improving medical device regulation: The United States and Europe in perspective. Milbank Q. 2014, 92, 114–150. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.G.; Almutairi, S.A.; Almutairi, A.A.; Althobaiti, N.N.H.; Alrashedi, K.A.T.; Alotaibi, M.F. Telehealth in Saudi Arabia: Its Evolution, Present Infrastructure, and Forward-Looking Implications. Glob. J. Health Sci. 2023, 15, 53–57. [Google Scholar] [CrossRef]
- Sheerah, H.A.; AlSalamah, S.; Alsalamah, S.A.; Lu, C.-T.; Arafa, A.; Zaatari, E.; Alhomod, A.; Pujari, S.; Labrique, A. The Rise of Virtual Health Care: Transforming the Health Care Landscape in the Kingdom of Saudi Arabia: A Review Article. Telemed. E-Health 2024, 30, 2545–2554. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Long, H.A.; French, D.P.; Brooks, J.M. Optimising the value of the critical appraisal skills programme (CASP) tool for quality appraisal in qualitative evidence synthesis. Res. Methods Med. Health Sci. 2020, 1, 31–42. [Google Scholar] [CrossRef]
- Singh, J. Critical appraisal skills programme. J. Pharmacol. Pharmacother. 2013, 4, 76–77. [Google Scholar] [CrossRef]
- Tyndall, J. AACODS Checklist. 2010. Available online: http://dspace.flinders.edu.au/dspace/ (accessed on 4 November 2025).
- Hamilton, S.; Huby, G.; Tierney, A.; Powell, A.; Kielmann, T.; Sheikh, A.; Pinnock, H. Mind the gap between policy imperatives and service provision: A qualitative study of the process of respiratory service development in England and Wales. BMC Health Serv. Res. 2008, 8, 248. [Google Scholar] [CrossRef]
- Elwyn, G.; Hardisty, A.R.; Peirce, S.C.; May, C.; Evans, R.; Robinson, D.K.; Bolton, C.E.; Yousef, Z.; Conley, E.C.; Rana, O.F.; et al. Detecting deterioration in patients with chronic disease using telemonitoring: Navigating the ‘trough of disillusionment’. J. Eval. Clin. Pract. 2012, 18, 896–903. [Google Scholar] [CrossRef]
- Walters, B.H.; Adams, S.A.; Nieboer, A.P.; Bal, R. Disease management projects and the Chronic Care Model in action: Baseline qualitative research. BMC Health Serv. Res. 2012, 12, 114. [Google Scholar] [CrossRef]
- Odeh, B.; Kayyali, R.; Nabhani-Gebara, S.; Philip, N. Implementing a telehealth service: Nurses’ perceptions and experiences. Br. J. Nurs. 2014, 23, 1133–1137. [Google Scholar] [CrossRef]
- Dirven, J.A.; Moser, A.; Tange, H.J.; Muris, J.W.; Van Schayck, O.C. An innovative COPD early detection programme in general practice: Evaluating barriers to implementation. npj Prim. Care Respir. Med. 2014, 24, 14055. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Coates, E.; Brewster, L.; Mountain, G.; Wessels, B.; Hawley, M.S. Examining the use of telehealth in community nursing: Identifying the factors affecting frontline staff acceptance and telehealth adoption. J. Adv. Nurs. 2015, 71, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Hunting, G.; Shahid, N.; Sahakyan, Y.; Fan, I.; Moneypenny, C.R.; Stanimirovic, A.; North, T.; Petrosyan, Y.; Krahn, M.D.; Rac, V.E. A multi-level qualitative analysis of Telehomecare in Ontario: Challenges and opportunities. BMC Health Serv. Res. 2015, 15, 544. [Google Scholar] [CrossRef] [PubMed]
- Rojahn, K.; Laplante, S.; Sloand, J.; Main, C.; Ibrahim, A.; Wild, J.; Sturt, N.; Areteou, T.; Johnson, K.I. Remote monitoring of chronic diseases: A landscape assessment of policies in four European countries. PLoS ONE 2016, 11, e0155738. [Google Scholar] [CrossRef]
- Segato, F.; Masella, C. Telemedicine services: How to make them last over time. Health Policy Technol. 2017, 6, 268–278. [Google Scholar] [CrossRef]
- Keene, O.N.; Ruberg, S.; Schacht, A.; Akacha, M.; Lawrance, R.; Berglind, A.; Wright, D. What matters most? Different stakeholder perspectives on estimands for an invented case study in COPD. Pharm. Stat. 2020, 19, 370–387. [Google Scholar] [CrossRef]
- Slevin, P.; Kessie, T.; Cullen, J.; Butler, M.; Donnelly, S.; Caulfield, B. Exploring the barriers and facilitators for the use of digital health technologies for the management of COPD: A qualitative study of clinician perceptions. QJM Int. J. Med. 2020, 113, 163–172. [Google Scholar] [CrossRef]
- Gaveikaite, V.; Grundstrom, C.; Lourida, K.; Winter, S.; Priori, R.; Chouvarda, I.; Maglaveras, N. Developing a strategic understanding of telehealth service adoption for COPD care management: A causal loop analysis of healthcare professionals. PLoS ONE 2020, 15, e0229619. [Google Scholar] [CrossRef]
- Alwashmi, M.F.; Fitzpatrick, B.; Davis, E.; Farrell, J.; Gamble, J.-M.; Hawboldt, J. Features of a mobile health intervention to manage chronic obstructive pulmonary disease: A qualitative study. Ther. Adv. Respir. Dis. 2020, 14, 1753466620951044. [Google Scholar] [CrossRef]
- van Lieshout, F.; Yang, R.; Stamenova, V.; Agarwal, P.; Cornejo Palma, D.; Sidhu, A.; Engel, K.; Erwood, A.; Bhatia, R.S.; Bhattacharyya, O.; et al. Evaluating the implementation of a Remote-Monitoring program for chronic obstructive pulmonary disease: Qualitative methods from a service design perspective. J. Med. Internet Res. 2020, 22, e18148. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.N.; Lloyd, J.; Baral, K.P.; Bhatta, N.; Mehata, S.; Harris, M. Evaluating the feasibility and acceptability of a co-design approach to developing an integrated model of care for people with multi-morbid COPD in rural Nepal: A qualitative study. BMJ Open 2021, 11, e045175. [Google Scholar] [CrossRef]
- An, Q.; Kelley, M.M.; Yen, P.-Y. Stakeholder mapping on the development of digital health interventions for self-management among patients with chronic obstructive pulmonary disease in China. Stud. Health Technol. Inform. 2022, 290, 1106–1107. [Google Scholar] [CrossRef] [PubMed]
- Meiwald, A.; Gara-Adams, R.; Rowlandson, A.; Ma, Y.; Watz, H.; Ichinose, M.; Scullion, J.; Wilkinson, T.; Bhutani, M.; Weston, G.; et al. Qualitative validation of COPD evidenced care pathways in Japan, Canada, England, and Germany: Common barriers to optimal COPD care. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, P.; Chen, Z.; Guo, J.; Wang, S.; Liu, F.; Li, J. Patients’ and healthcare providers’ perceptions and experiences of telehealth use and online health information use in chronic disease management for older patients with chronic obstructive pulmonary disease: A qualitative study. BMC Geriatr. 2022, 22, 9. [Google Scholar] [CrossRef]
- Silva, A.B.; da Silva, R.M.; Ribeiro, G.d.R.; Guedes, A.C.C.M.; Santos, D.L.; Nepomuceno, C.C.; Caetano, R. Three decades of telemedicine in Brazil: Mapping the regulatory framework from 1990 to 2018. PLoS ONE 2020, 15, e0242869. [Google Scholar] [CrossRef]
- Intan Sabrina, M.; Defi, I.R. Telemedicine guidelines in South East Asia—A scoping review. Front. Neurol. 2021, 11, 581649. [Google Scholar] [CrossRef]
- MoH. The Governing Rules of Telehealth in the Kingdom of Saudi Arabia; Ministry of Health: Victoria, BC, Canada, 2021. [Google Scholar]
- HSA. Regulatory Guideline for Telehealth Products; The Health Sciences Authority: Singapore, 2021. [Google Scholar]
- MoH. Legal Regulations for Telehealth Services; Ministry of Health: Victoria, BC, Canada, 2022. [Google Scholar]
- SFDA. Reguirments for Telehaelth Devices Martking; Saudi Food and Drug Authority: Riyadh, Saudi Arabia, 2023. [Google Scholar]
- CHI. Telehealth Policies and Procedures; Council Health Insurance: Riyadh, Saudi Arabia, 2023. [Google Scholar]
- WHO. National eHealth Strategy Toolkit; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- WHO. WHO-ITU Global Standard for Accessibility of Telehealth Services; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Digital Implementation Investment Guide (DIIG); World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- WHO. Recommendations on Digital Interventions for Health System Strengthening; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Dennis, A. Issues with Regulation of Telemedicine in the UK; TaylorWessing: New York, NY, USA, 2022. [Google Scholar]
- Archambault, J.; Nastasi, V.; Perkins, A. State Policy Agenda for Telehealth Innovation; Cicero Institute: Austin, TX, USA, 2024. [Google Scholar]
- MCI. Telemedicine Practice Guidelines Enabling Registered Medical Practitioners to Provide Healthcare Using Telemedicine; Medical Council of India: New Delhi, India, 2023. [Google Scholar]
- SFDA. Requirements for Approving Telehealth Devices in Clinical Settings; Saudi Food and Drug Authority: Riyadh, Saudi Arabia, 2021. [Google Scholar]
- Ben-Assuli, O. Cost-effectiveness, use and implementation of telehealth solutions for CHF and COPD: A systematic review using the PRISMA method. Health Policy Technol. 2025, 14, 101023. [Google Scholar] [CrossRef]
- Hu, C.; Liao, X.; Fang, Y.; Zhu, S.; Lan, X.; Cheng, G. Clinical and Cost-Effectiveness of Telehealth-Supported Home Oxygen Therapy on Adherence, Hospital Readmission, and Health-Related Quality of Life in Patients with Chronic Obstructive Pulmonary Disease: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2025, 27, e73010. [Google Scholar]
- Alghamdi, S.M.; Alqahtani, J.S.; Aldhahir, A.M. Current status of telehealth in Saudi Arabia during COVID-19. J. Fam. Community Med. 2020, 27, 208–211. [Google Scholar] [CrossRef]
- Alqahtani, M.M.; Alanazi, A.M.; Algarni, S.S.; Aljohani, H.; Alenezi, F.K.; Alotaibi, T.F.; Alotaibi, M.; Alqahtani, M.K.; Alahmari, M.; Alwadeai, K.S.; et al. Unveiling the Influence of AI on Advancements in Respiratory Care: Narrative Review. Interact. J. Med. Res. 2024, 13, e57271. [Google Scholar] [CrossRef]
- Alghamdi, S.M.; Chikwendu, O.C.; Chukwuma, O.U.; Okech, D.O.; Okwu, M.O.; Khalid, S.; Vlachostergiou, A. Navigating ethical challenges in digital transformation: Insights on climate adaptation, microbiology, healthcare, robotics, and AI under the EU AI act: An experts panel discussion. Glob. Bioeth. 2025, 36, 2550823. [Google Scholar] [CrossRef]
- Field, B.; Booth, A.; Ilott, I.; Gerrish, K. Using the Knowledge to Action Framework in practice: A citation analysis and systematic review. Implement. Sci. 2014, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, B.; Nadri, H.; Afshar, H.L.; Timpka, T. A systematic review of the technology acceptance model in health informatics. Appl. Clin. Inform. 2018, 9, 604–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hao, J.; Sun, H.; Li, M.; Zhang, Y.; Qian, Q. Applications of digital health technologies and artificial intelligence algorithms in COPD: Systematic review. BMC Med. Inform. Decis. Mak. 2025, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- NHS. Technology Enabled Care Services Resource for Commissioners; National Health Service: London, UK, 2015. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).