“Diving into the Gray Zone”: A Case Report of a 19-Year-Old Patient Treated with Tooth-Borne Rapid Maxillary Expansion
Abstract
1. Introduction
2. Case Presentation, Diagnostics and Intervention
2.1. Patient Information and History
2.2. Diagnostic Assessment and Etiology
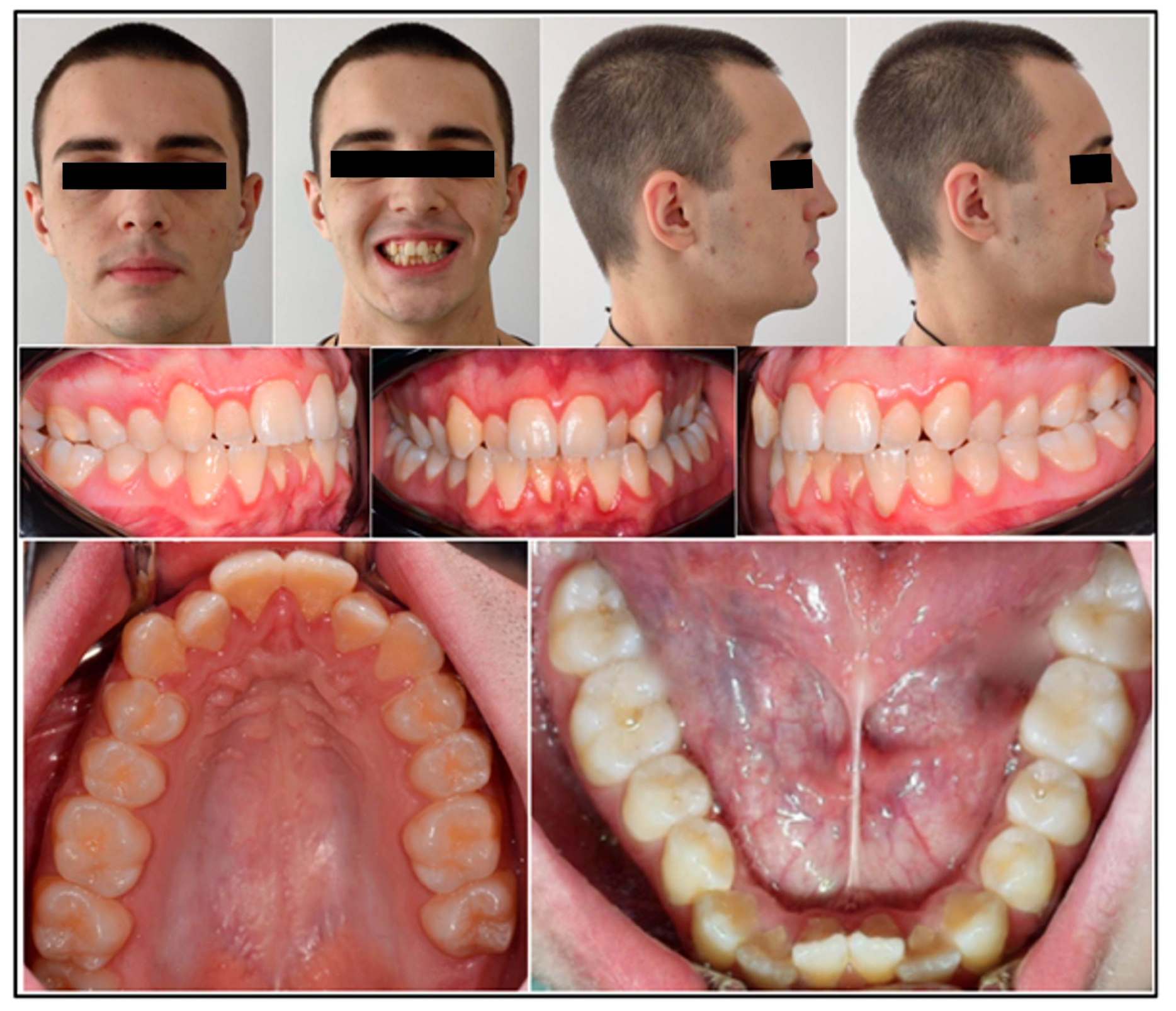
2.3. Clinical Findings and Imaging
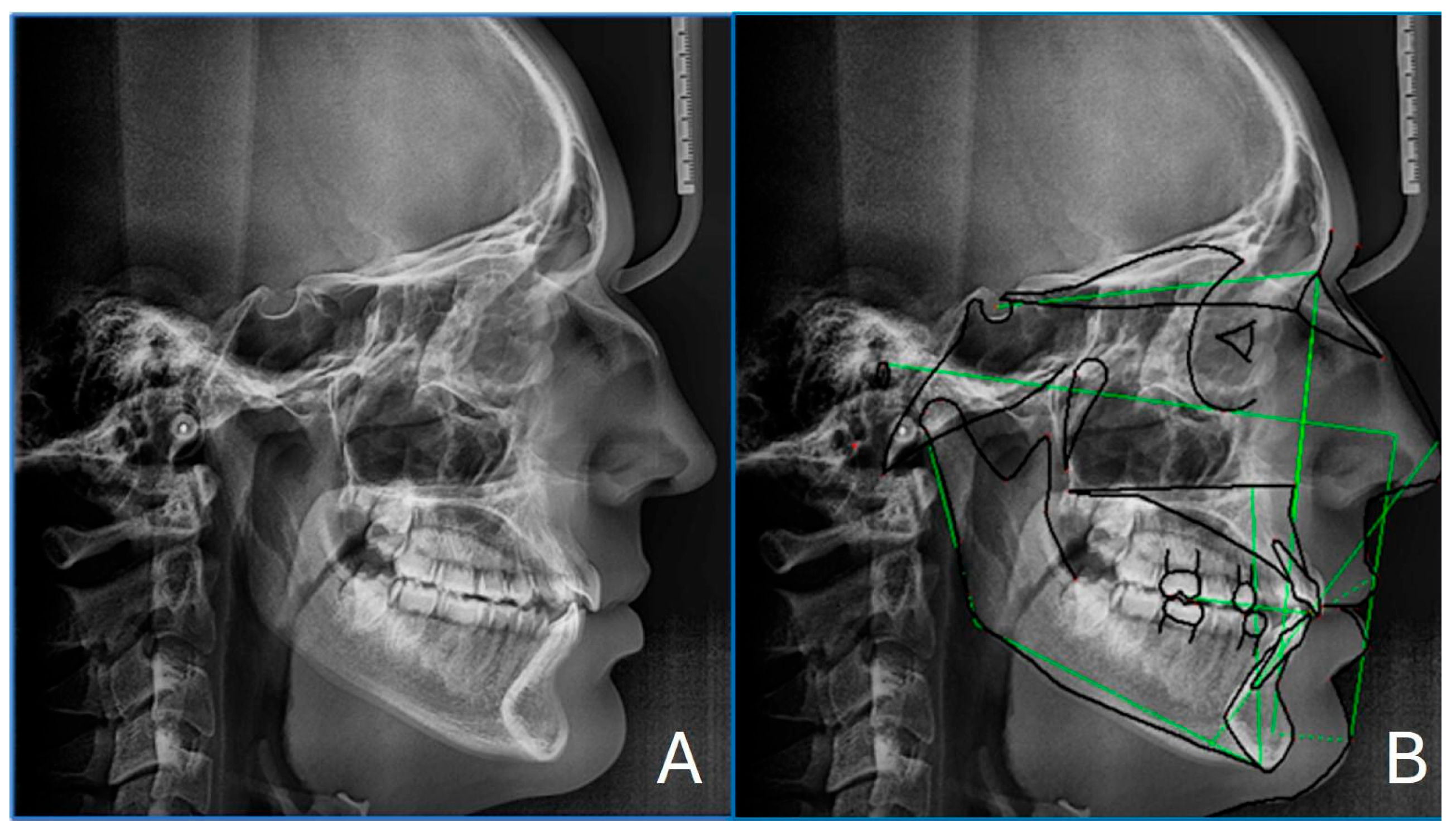
2.4. Cephalometry
2.5. Periodontal Status
2.6. Evaluation of the Skeletal Maturation
2.7. Treatment Objectives and Treatment Plan
Treatment Alternatives
2.8. Treatment Progress
2.9. Measurements
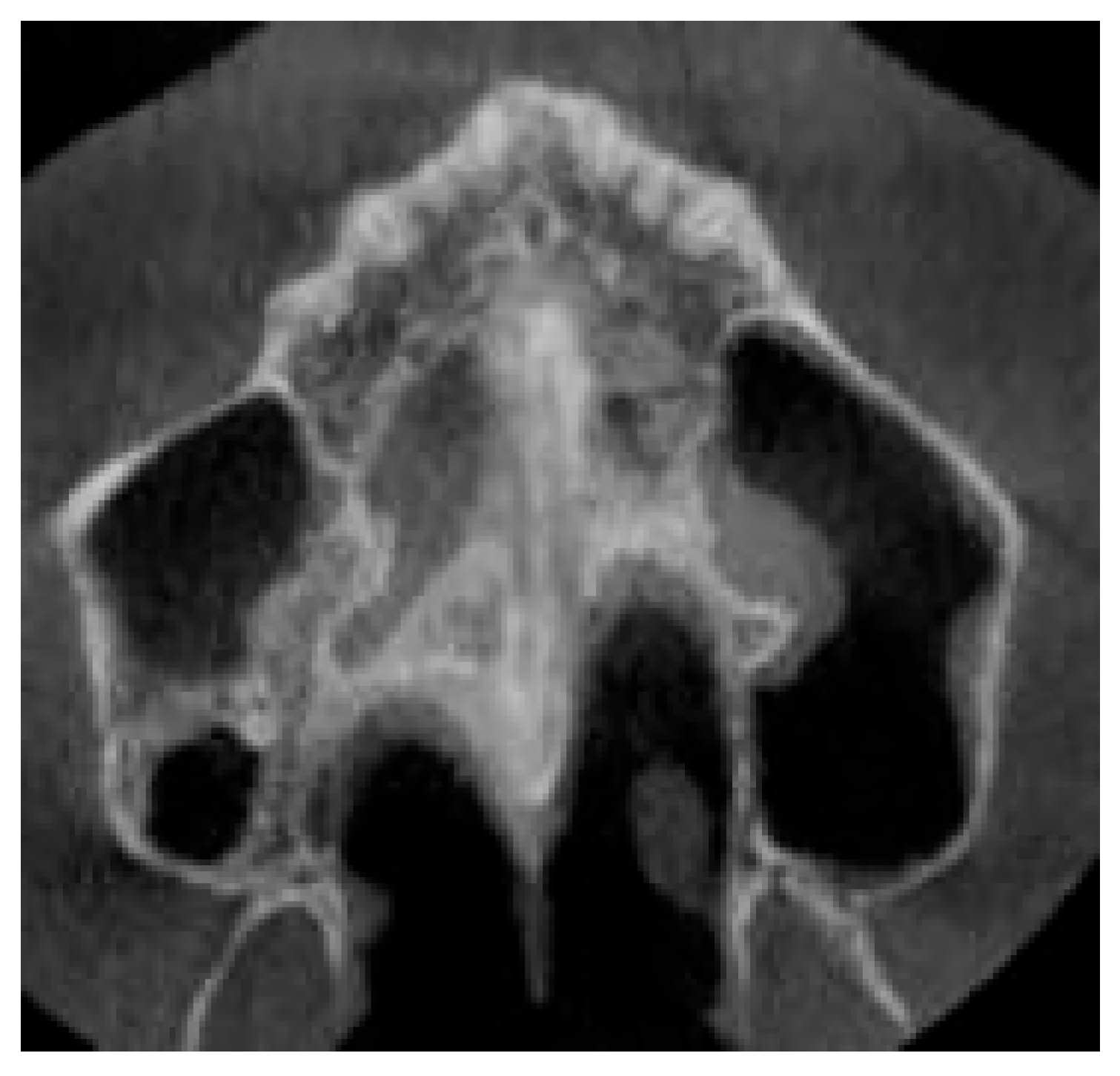
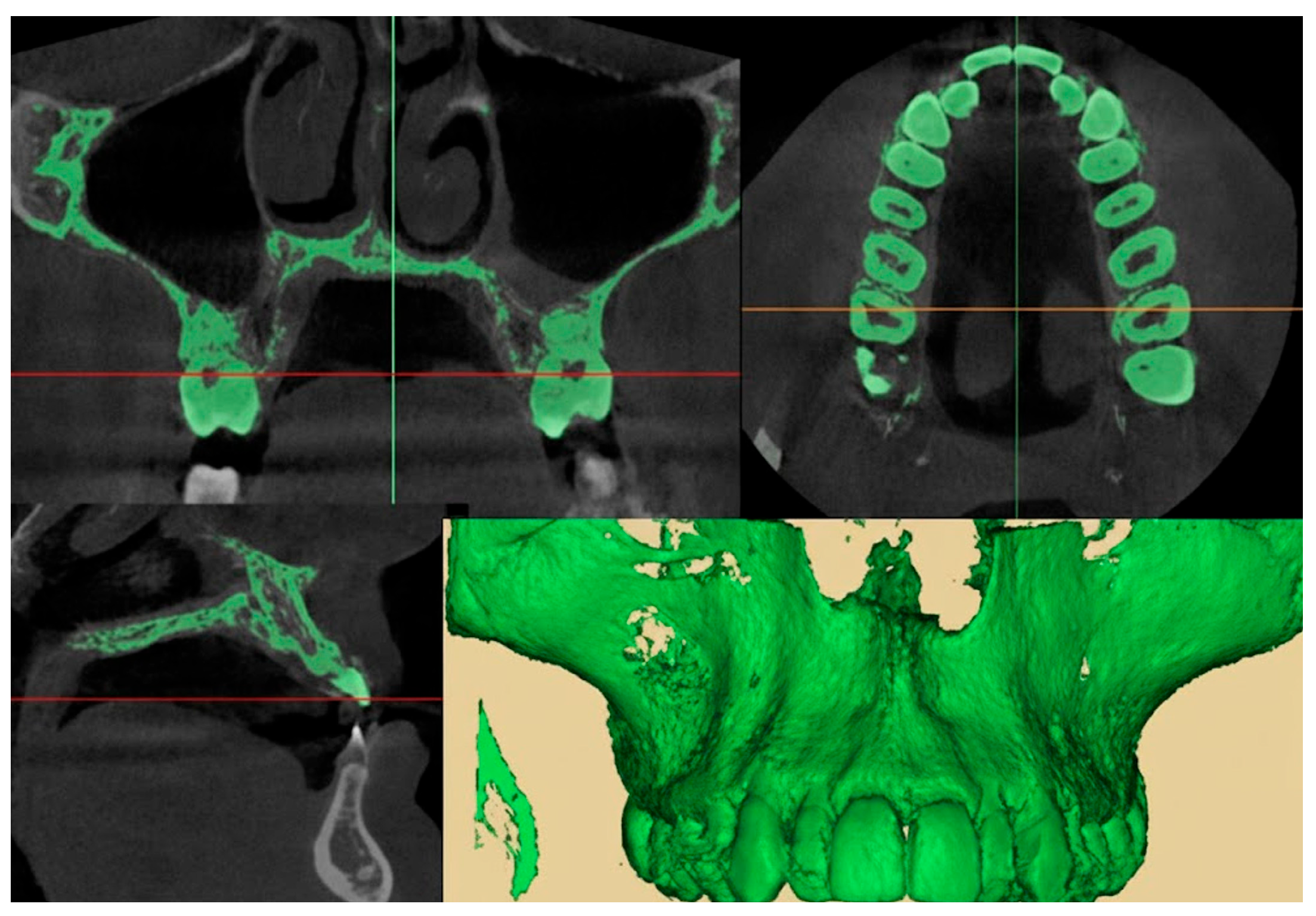
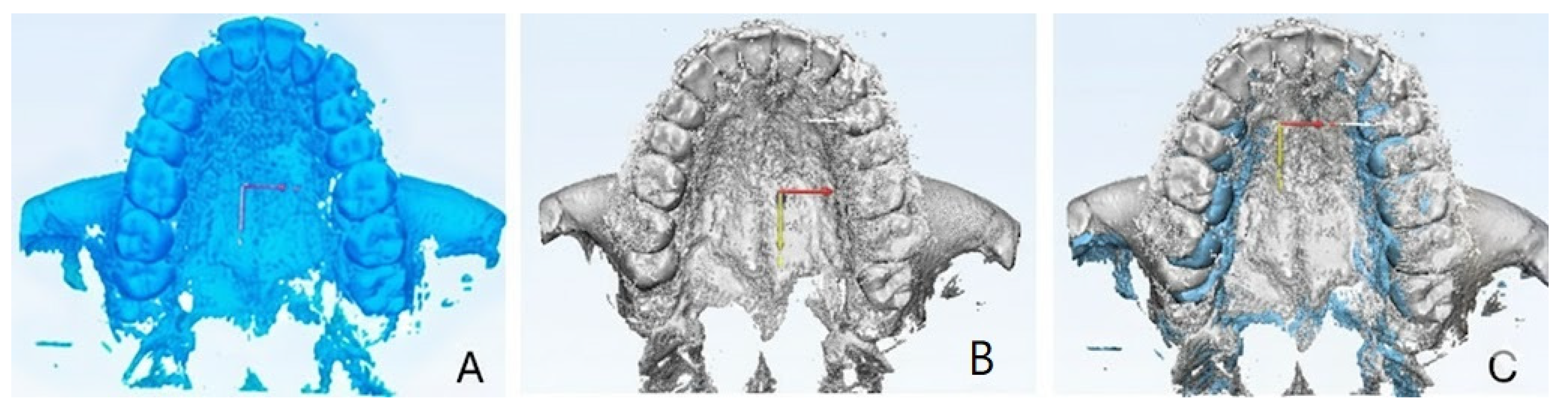
2.9.1. Qualitative Evaluation of Maxillary Expansion on Cone Beam Computed Tomography
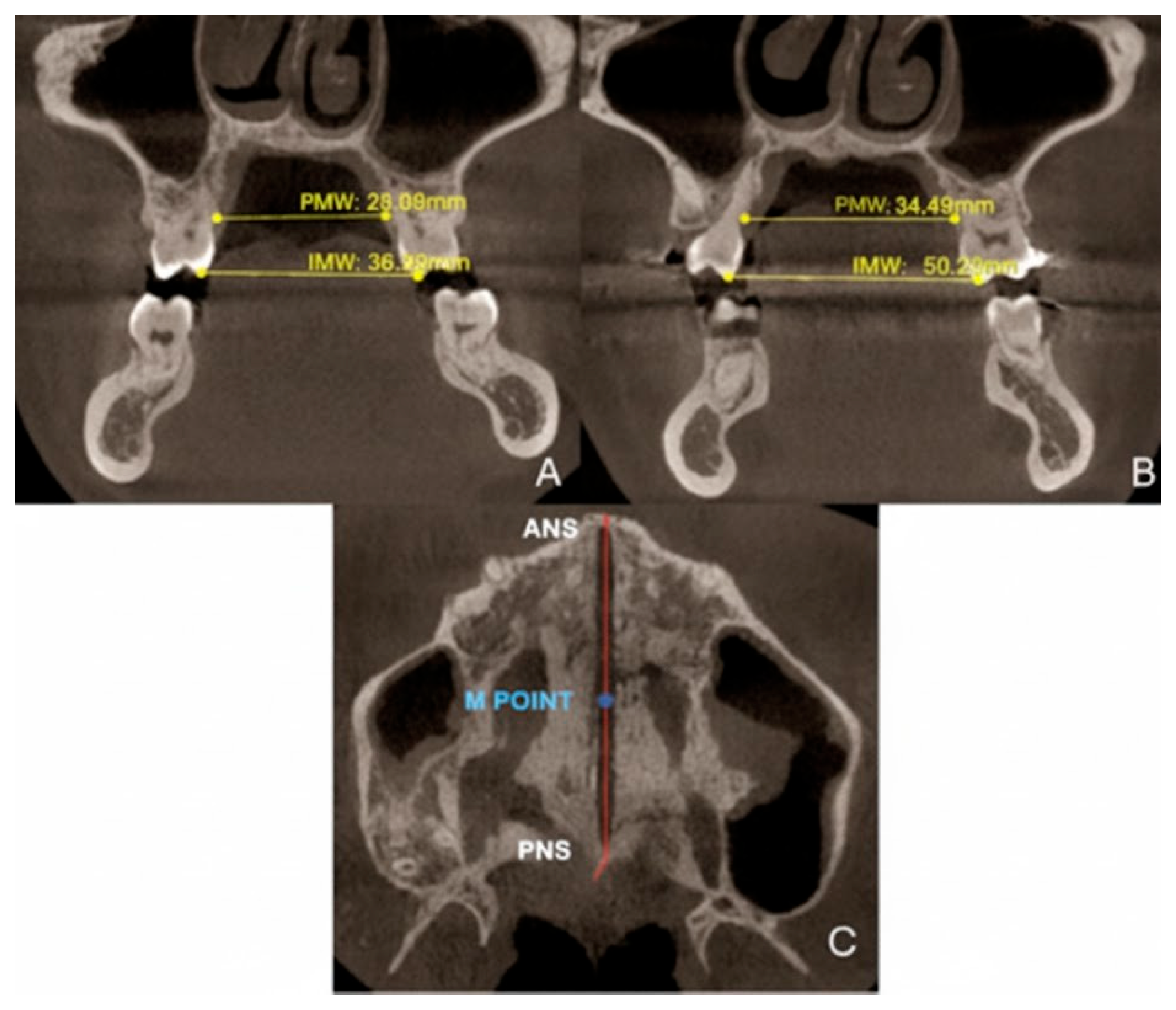
2.9.2. Quantitative Two-Dimensional Analysis of Upper Palatal Expansion on CBCT
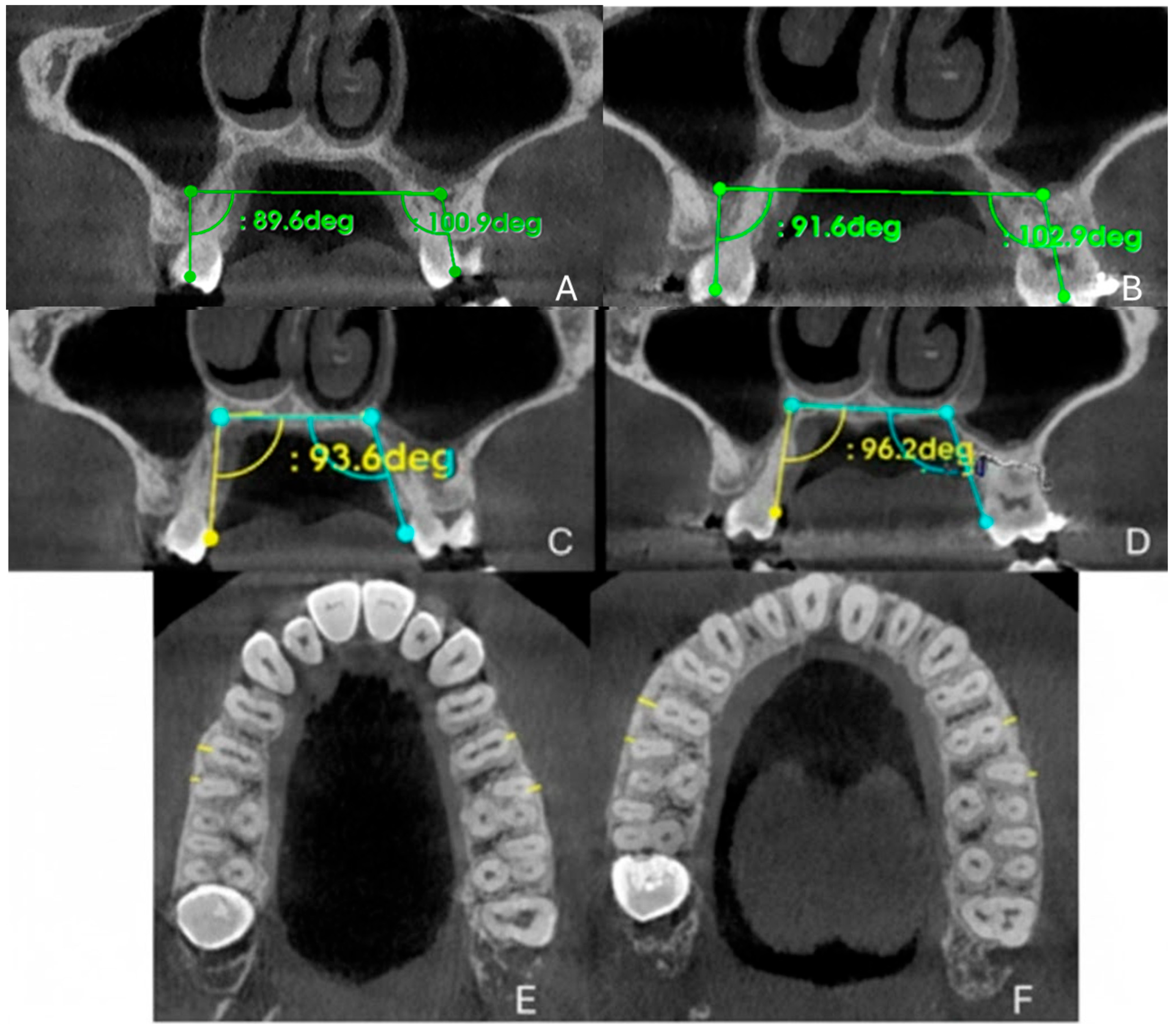
2.9.3. Evaluation of Dental Effects: Alveolar Bending, Palatal Alveolar Angle, Dental Tipping and Bone Dehiscence
3. Results
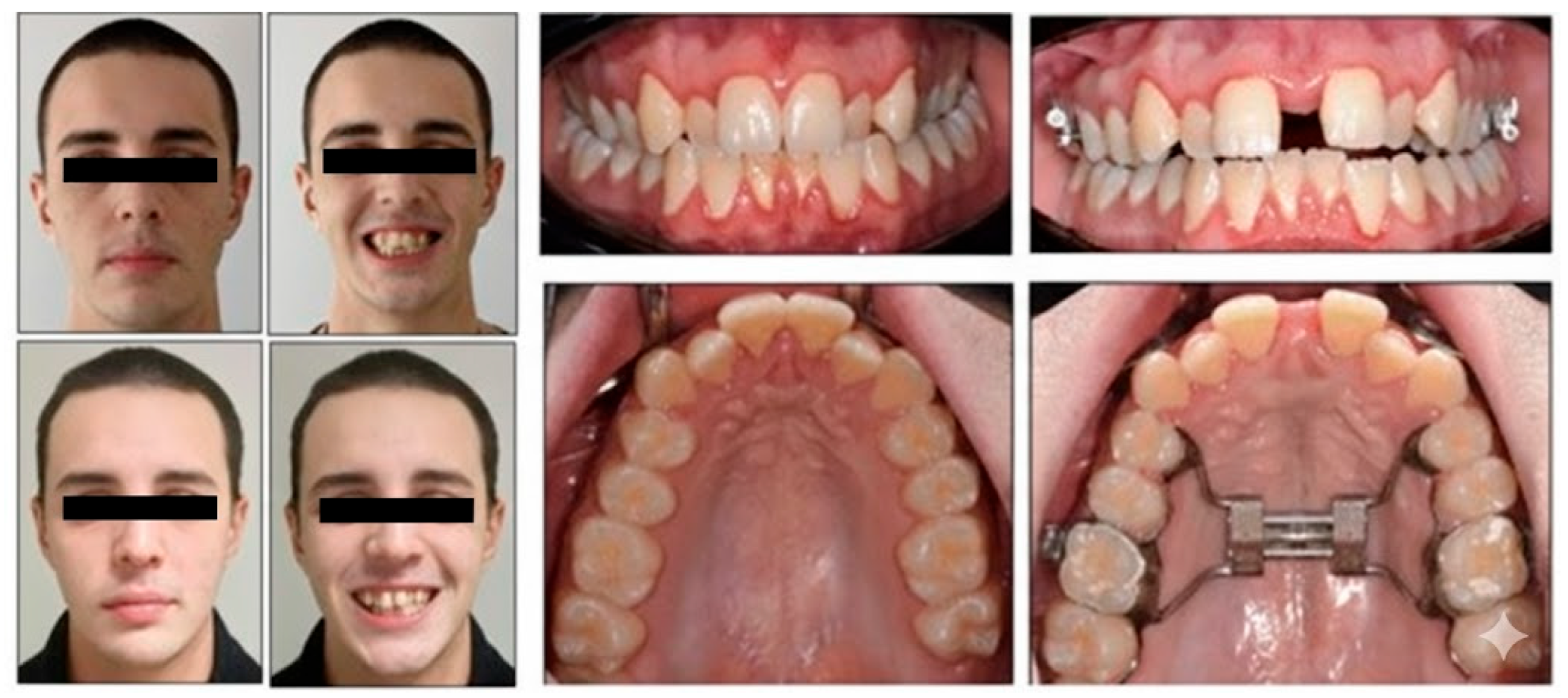
3.1. Orthodontic Results
3.2. Qualitative Evaluation of Maxillary Expansion on Cone Beam Computed Tomography
3.3. Quantitative Two-Dimensional Analysis of Upper Palatal Expansion on CBCT
3.4. Evaluation of Dental Effects: Palatal Alveolar Angle, Buccal Tipping Angle, Bone Dehiscences and Fenestrations
3.5. Clinical Evaluation of the Periodontal Status
3.6. Follow-Up and Outcome Evaluation
4. Discussion
4.1. Interpretation of the Results
4.2. Generalization of Results in the Context of the Literature
4.3. Strengths and Limitations
4.4. Clinical Implications
4.5. Implications for Future Research
5. Conclusions
5.1. Patient Perspective
5.2. Hypothesis
5.3. Take-Home Messages
- The present case showed that the tooth-borne RPE can induce a predominantly skeletal effect even in a young adult patient.
- The assessment of midpalatal suture morphology and maturation through CBCT-scan should be integrated with a clinical screening of the periodontal status to guide case selection in the post-pubertal “gray zone.”
- A conservative, non-surgical expansion in adults who decline skeletal anchorage or surgery can provide a clinically meaningful improvement in sleep quality (PSQI 7 → 3) and stable periodontal health.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, R.; Ma, X.; Wamalwa, P.; Zou, S.J. Nonsurgical treatment of an adult patient with bilateral posterior crossbite. Am. J. Orthod. Dentofac. Orthop. 2011, 140, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Franchi, L.; Cameron, C.G.; McNamara, J.A., Jr. Treatment timing for rapid maxillary expansion. Angle Orthod. 2001, 71, 343–350. [Google Scholar]
- Franchi, L.; Statie, M.D.; Clauser, T.; Migliorati, M.; Ugolini, A.; Bucci, R.; Rongo, R.; Nucera, R.; Portelli, M.; McNamara, J.A.; et al. Skeletal versus conventional anchorage in dentofacial orthopedics: An international modified Delphi consensus study. Prog. Orthod. 2025, 26, 9. [Google Scholar] [CrossRef] [PubMed]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.; McNamara, J.A., Jr. Diagnostic performance of skeletal maturity for the assessment of midpalatal suture maturation. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 1010–1016. [Google Scholar] [CrossRef]
- Brunetto, D.P.; Sant’Anna, E.F.; Machado, A.W.; Moon, W. Non-surgical rapid maxillary expansion in adults: A clinical, radiographic, and tomographic study. Dental Press J. Orthod. 2017, 22, 110–125. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Greene, J.C.; Vermillion, J.R. The Simplified Oral Hygiene Index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Horner, K.; Islam, M.; Flygare, L.; Tsiklakis, K.; Whaites, E. Basic principles for use of dental cone beam computed tomography: Consensus guidelines of the European Academy of Dental and Maxillofacial Radiology. Dentomaxillofacial Radiol. 2009, 38, 187–195. [Google Scholar] [CrossRef]
- Pauwels, R. Cone beam CT for dental and maxillofacial imaging: Dose matters. Radiat. Prot. Dosim. 2015, 165, 156–161. [Google Scholar] [CrossRef]
- Angelieri, F.; Franchi, L.; Cevidanes, L.H.; Bueno-Silva, B.; McNamara, J.A., Jr. Prediction of rapid maxillary expansion by assessing the maturation of the midpalatal suture on cone beam CT. Dent. Press J. Orthod. 2016, 21, 115–125. [Google Scholar] [CrossRef]
- Watted, N.; Lone, I.M.; Midlej, K.; Zohud, O.; Awadi, O.; Masarwa, S.; Watted, A.; Paddenberg, E.; Krohn, S.; Kirschneck, C.; et al. The Complexity of Skeletal Transverse Dimension: From Diagnosis, Management, and Treatment Strategies to the Application of Collaborative Cross (CC) Mouse Model. J. Funct. Morphol. Kinesiol. 2024, 14, 51. [Google Scholar] [CrossRef]
- Gurani, S.F.; Cattaneo, P.M.; Rafaelsen, S.R.; Pedersen, M.R.; Thorn, J.J.; Pinholt, E.M. The effect of altered head and tongue posture on upper airway volume based on a validated upper airway analysis-An MRI pilot study. Orthod. Craniofac. Res. 2020, 23, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Aiello, D.; Nucera, R.; Costa, S.; Figliuzzi, M.M.; Paduano, S. A Simplified Digital Approach to the Treatment of a Postpuberty Patient with a Class III Malocclusion and Bilateral Crossbite. Case Rep. Dent. 2021, 2021, 3883187. [Google Scholar] [CrossRef]
- Walter, A.; Winsauer, H.; Crespo, E.; Walter, D.; Winsauer, C.; Schwärzler, A.; Mojal, S.; Arcos, I.; Puigdollers, A. Adult maxillary expansion: CBCT evaluation of skeletal changes and determining an efficiency factor between force-controlled polycyclic slow activation and continuous rapid activation for mini-screw-assisted palatal expansion—MASPE vs. MARPE. Head. Face Med. 2024, 20, 70. [Google Scholar] [CrossRef]
- Baysal, A.; Uysal, T.; Veli, I.; Ozer, T.; Karadede, I.; Hekimoglu, S. Evaluation of alveolar bone loss following rapid maxillary expansion using cone-beam computed tomography. Korean J. Orthod. 2013, 43, 83–95. [Google Scholar] [CrossRef]
- Di Carlo, G.; Saccucci, M.; Luzzi, V.; Ierardo, G.; Vozza, I.; Sfasciotti, G.L.; Polimeni, A. Prevalence of maxillary canine impaction in skeletal Class III malocclusions compared to Class I malocclusions. J. Clin. Exp. Dent. 2019, 11, e264–e268. [Google Scholar] [CrossRef]
- Baratieri, C.d.L.; Alves, M., Jr.; Mattos, C.T.; Lau, G.W.; Nojima, L.I.; de Souza, M.M. Transverse effects on the nasomaxillary complex one year after rapid maxillary expansion as the only intervention: A controlled study. Dent. Press J. Orthod. 2014, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pirelli, P.; Saponara, M.; Guilleminault, C. Rapid maxillary expansion in children with obstructive sleep apnea syndrome. Sleep Med. 2004, 5, 495–500. [Google Scholar] [CrossRef]
- Gauthier, C.; Voyer, R.; Paquette, M.; Rompré, P.; Papadakis, A. Periodontal effects of surgically assisted rapid palatal expansion evaluated clinically and with cone-beam computerized tomography: 6-month preliminary results. Am. J. Orthod. Dentofac. Orthop. 2011, 139, S117–S128. [Google Scholar] [CrossRef] [PubMed]
- Calil, R.C.; Marin Ramirez, C.M.; Otazu, A.; Torres, D.M.; Gurgel, J.A.; Oliveira, R.C.; de Oliveira, R.C.G.; Valarelli, F.P.; Freitas, K.M.S. Maxillary dental and skeletal effects after treatment with self-ligating appliance and miniscrew-assisted rapid maxillary expansion. Am. J. Orthod. Dentofac. Orthop. 2021, 159, e93–e101. [Google Scholar] [CrossRef]
- Annarumma, F.; Posadino, M.; De Mari, A.; Drago, S.; Aghazada, H.; Gravina, G.M.; Qorri, E.; Silvestrini-Biavati, A.; Migliorati, M. Skeletal and dental changes after maxillary expansion with a bone-borne appliance in young and late adolescent patients. Am. J. Orthod. Dentofac. Orthop. 2021, 159, e363–e375. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.H.; Park, J.H.; Lee, K.J. Nonsurgical maxillary expansion in a 60-year-old patient with gingival recession and crowding. Korean J. Orthod. 2021, 51, 217–227. [Google Scholar] [CrossRef]
- Gurani, S.F.; Di Carlo, G.; Thorn, J.J.; Ingerslev, J.; Cattaneo, P.M.; Pinholt, E.M. Two-Year Postoperative Upper Airway Cone-Beam Computed Tomographic Outcomes Based on a Verified Upper Airway Analysis Following Bimaxillary Orthognathic Surgery. J. Oral Maxillofac. Surg. 2019, 77, 1435–1445. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Jiang, C.; Zhao, Y.; Zhang, Y.; Zhang, Q.; Fan, C.; Liu, Y. The short- and long-term changes of upper airway and alar in nongrowing patients treated with Mini-Implant Assisted Rapid Palatal Expansion (MARPE): A systematic review and meta-analysis. BMC Oral Health 2023, 23, 820. [Google Scholar] [CrossRef]
- Lagravere, M.O.; Major, P.W.; Flores-Mir, C. Long-term skeletal changes with rapid maxillary expansion: A systematic review. Angle Orthod. 2006, 76, 201–210. [Google Scholar]
- Handelman, C.S.; Wang, L.; BeGole, E.A.; Haas, A.J. Nonsurgical rapid maxillary expansion in adults: Report on 47 cases using the Haas expander. Angle Orthod. 2000, 70, 129–144. [Google Scholar] [PubMed]
- Camacho, M.; Chang, E.T.; Song, S.A.; Abdullatif, J.; Zaghi, S.; Pirelli, P.; Certal, V.; Guilleminault, C. Rapid maxillary expansion for pediatric obstructive sleep apnea: A systematic review and meta-analysis. Sleep 2017, 40, zsx015. [Google Scholar] [CrossRef]
- Cantarella, D.; Dominguez-Mompell, R.; Moschik, C.; Mallya, S.M.; Pan, H.C.; Alkahtani, M.R.; Elkenawy, I.; Moon, W. Midfacial changes in the coronal plane induced by microimplant-supported skeletal expander, studied with cone-beam computed tomography images. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 539–549. [Google Scholar] [CrossRef]
- Kaya, N.; Seker, E.D.; Yücesoy, T. Comparison of the effects of different rapid maxillary expansion techniques on craniofacial structures: A finite element analysis study. Prog. Orthod. 2023, 24, 7. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Patient Value | Normal Range | Interpretation |
|---|---|---|---|
| SNA (°) | 80.0 | 82 ± 2 | Normal maxillary position |
| SNB (°) | 80.2 | 80 ± 2 | Normal mandibular position |
| ANB (°) | −0.1 | 2 ± 2 | Skeletal class I |
| Wits appraisal (mm) | −0.1 | 0 ± 1 | Skeletal class I |
| FMA (°) | 20.1 | 25 ± 4 | Hypodivergent growth pattern |
| Interincisal angle (°) | 120.4 | 128 ± 5 | Proclined incisors |
| A to N–Perp (mm) | −3.5 | 1 ± 2 | Retruded maxilla (McNamara) |
| Pog to N–Perp (mm) | −3.1 | 0 ± 2 | Retruded chin point (McNamara) |
| FH to AB (°) | 86.5 | 81 ± 3 | Skeletal Class III (McNamara) |
| Scoring System/Teeth and Surfaces | T0 | T1 | |
|---|---|---|---|
| Modified gingival index | 0: healthy 1: mild inflammation (partial unit) 2: mild inflammation (entire unit) 3: moderate inflammation 4: severe inflammation | 2 | 2 |
| Calculus index | 0: no calculus 1: calculus covering up to 1/3 of the tooth surface 2: calculus covering up to 2/3 of the tooth surface and/or separate flecks of subgingival calculus 3: calculus covering more than 2/3 of the tooth surface and/or a continuous band of subgingival calculus | 1/3 | 0 |
| Plaque index | 0: no plaque 1: separate flecks of plaque at the cervical margin of the tooth 2: thin continuous band of plaque (up to 1 mm) at the cervical margin of the tooth 3: band of plaque wider than 1 mm covering less than 1/3 of the crown of the tooth 4: plaque covering at least 1/3 but less than 2/3 of the crown of the tooth 5: plaque covering 2/3 or more of the crown of the tooth | 1 | 1 |
| Clinical attachment level | Distance from the cemento-enamel junction (CEJ) to the base of the pocket. | 1 | 1 |
| Bleeding on probing | resent/absent | P | A |
| Measurements | Pre-Treatment | Post-Treatment |
|---|---|---|
| PMW (mm) | 28.04 | 34.5 |
| IMW (mm) | 36.08 | 50.02 |
| SE (mm) | 0.32 | 7.82 |
| M16 (mm) | 0.6 | 0.7 |
| M15 (mm) | 1.6 | 2.3 |
| M25 (mm) | 0.7 | 1.3 |
| M26 (mm) | 1.2 | 0.9 |
| PAA 16 (°) | 93.6 | 96.2 |
| PAA 26 (°) | 109.6 | 112.2 |
| DTA 16 (°) | 89.06 | 91.6 |
| DTA 26 (°) | 100.9 | 102.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coviello, V.; Gentile, D.; Staderini, E.; Camodeca, A.; Guarino, A.; Cordaro, M. “Diving into the Gray Zone”: A Case Report of a 19-Year-Old Patient Treated with Tooth-Borne Rapid Maxillary Expansion. Healthcare 2025, 13, 2854. https://doi.org/10.3390/healthcare13222854
Coviello V, Gentile D, Staderini E, Camodeca A, Guarino A, Cordaro M. “Diving into the Gray Zone”: A Case Report of a 19-Year-Old Patient Treated with Tooth-Borne Rapid Maxillary Expansion. Healthcare. 2025; 13(22):2854. https://doi.org/10.3390/healthcare13222854
Chicago/Turabian StyleCoviello, Valentina, Davide Gentile, Edoardo Staderini, Andrea Camodeca, Angela Guarino, and Massimo Cordaro. 2025. "“Diving into the Gray Zone”: A Case Report of a 19-Year-Old Patient Treated with Tooth-Borne Rapid Maxillary Expansion" Healthcare 13, no. 22: 2854. https://doi.org/10.3390/healthcare13222854
APA StyleCoviello, V., Gentile, D., Staderini, E., Camodeca, A., Guarino, A., & Cordaro, M. (2025). “Diving into the Gray Zone”: A Case Report of a 19-Year-Old Patient Treated with Tooth-Borne Rapid Maxillary Expansion. Healthcare, 13(22), 2854. https://doi.org/10.3390/healthcare13222854







