Changes in Smoking Patterns and Cervical Cancer Risk: Preventive Implications from a Nationwide Japanese Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement and Data Source
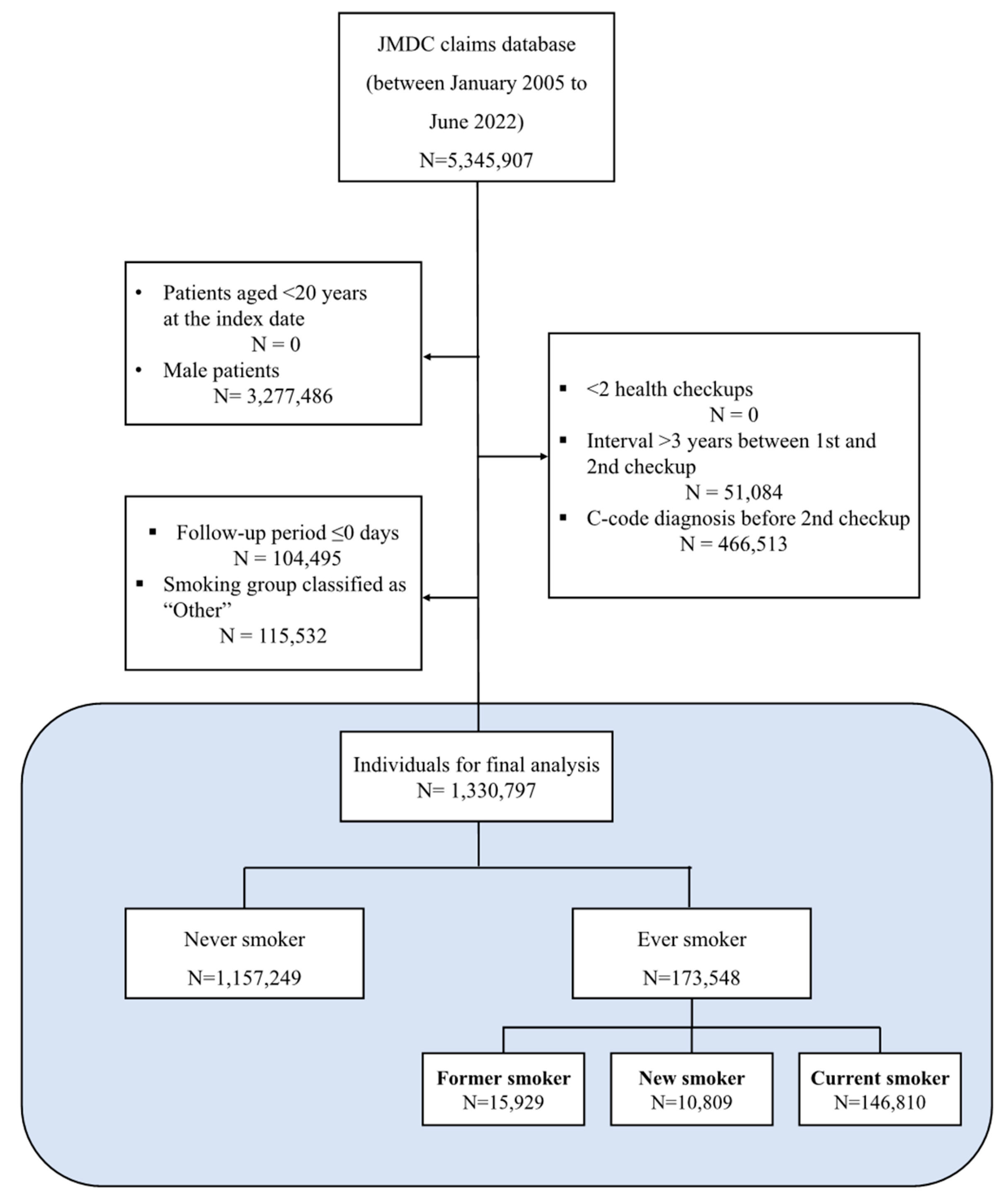
2.2. Study Population
2.3. Definitions of Smoking Exposure
2.4. Definition of Outcome
2.5. Follow-Up and Censoring
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Participants by Smoking Status
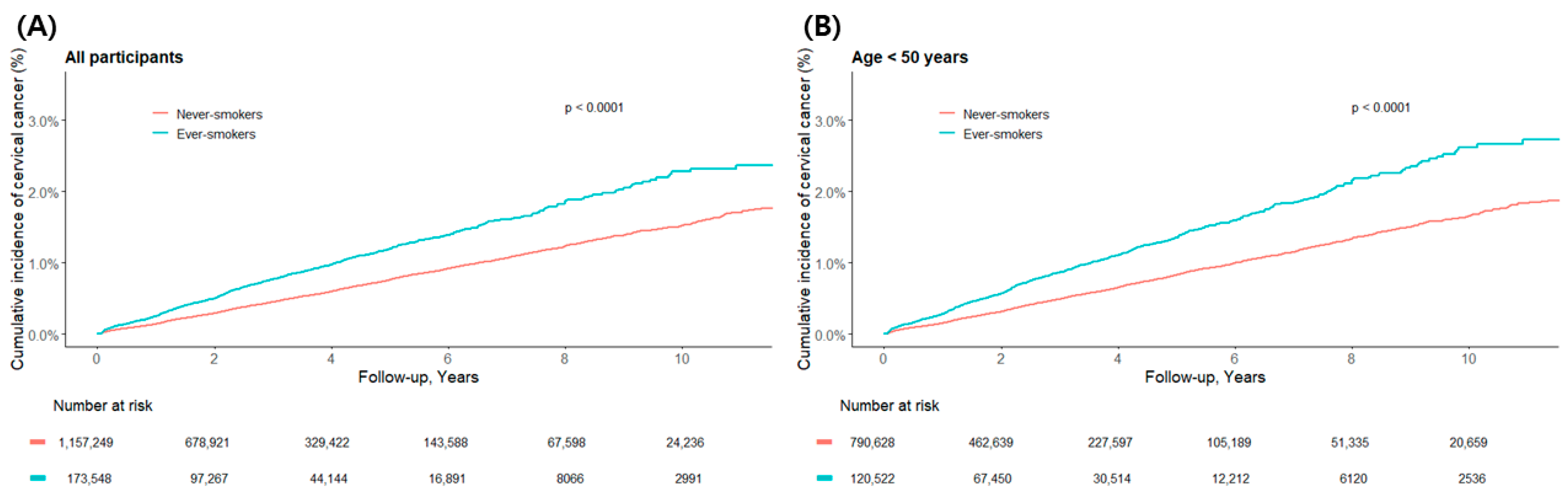
3.2. Cervical Cancer Incidence by Smoking Status
3.3. Association Between Smoking Exposure and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| HPV | Human Papillomavirus |
| HR | Hazard Ratio |
| ICD-10 | International Classification of Diseases, 10th Revision |
| IRB | Institutional Review Board |
| JMDC | Japan Medical Data Center |
| SD | Standard Deviation |
| WHO | World Health Organization |
| WPRO | Western Pacific Regional Office |
References
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/bitstream/handle/10665/342712/9789240030961-eng.pdf (accessed on 5 July 2025).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhu, B.; Gu, H.; Mao, Z.; Beeraka, N.M.; Zhao, X.; Anand, M.P.; Zheng, Y.; Zhao, R.; Li, S.; Manogaran, P.; et al. Global burden of gynaecological cancers in 2022 and projections to 2050. J. Glob. Health 2024, 14, 04155. [Google Scholar] [CrossRef]
- Hanley, S.J.B.; Yoshioka, E.; Ito, Y.; Kishi, R. HPV vaccination crisis in Japan. Lancet 2015, 385, 2571. [Google Scholar] [CrossRef]
- Roura, E.; Castellsague, X.; Pawlita, M.; Travier, N.; Waterboer, T.; Margall, N.; Bosch, F.X.; De Sanjosé, S.; Dillner, J.; Gram, I.T. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. Int. J. Cancer 2014, 135, 453–466. [Google Scholar] [CrossRef]
- Ma, K.; Li, S.; Wu, S.; Zhu, J.; Yang, Y. Impact of smoking exposure on human papillomavirus clearance among Chinese women: A follow-up propensity score matching study. Tob. Induc. Dis. 2023, 21, 42. [Google Scholar] [CrossRef]
- Castle, P.E. How does tobacco smoke contribute to cervical carcinogenesis? J. Virol. 2008, 82, 6084–6086. [Google Scholar] [CrossRef] [PubMed]
- Prokopczyk, B.; Cox, J.E.; Hoffmann, D.; Steven, E.S.E. Identification of tobacco-specific carcinogen in the cervical mucus of smokers and nonsmokers. J. Natl. Cancer Inst. 1997, 89, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Koshiol, J.; Schroeder, J.; Jamieson, D.J.; Marshall, S.W.; Duerr, A.; Heilig, C.M.; Shah, K.V.; Klein, R.S.; Cu-Uvin, S.; Schuman, P. Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am. J. Epidemiol. 2006, 164, 176–183. [Google Scholar] [CrossRef]
- Kayar, I.; Goc, G.; Cetin, F.; Birge, Ö. Impact of Smoking on Cervical Histopathological Changes in High-Risk HPV-Positive Women: A Matched Case-Control Study. Medicina 2025, 61, 235. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Tsuji, I.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; Kitamura, Y.; et al. Cigarette smoking and cervical cancer risk: An evaluation based on a systematic review and meta-analysis among Japanese women. Jpn. J. Clin. Oncol. 2019, 49, 77–86. [Google Scholar] [CrossRef]
- Fujita, M.; Tase, T.; Kakugawa, Y.; Hoshi, S.; Nishino, Y.; Nagase, S.; Ito, K.; Niikura, H.; Yaegashi, N.; Minami, Y. Smoking, Earlier Menarche and Low Parity as Independent Risk Factors for Gynecologic Cancers in Japanese: A Case-Control Study. Tohoku J. Exp. Med. 2008, 216, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Vidrine, J.I.; Sutton, S.K.; Wetter, D.W.; Shih, Y.T.; Ramondetta, L.M.; Elting, L.S.; Walker, J.L.; Smith, K.M.; Frank-Pearce, S.G.; Li, Y.; et al. Efficacy of a Smoking Cessation Intervention for Survivors of Cervical Intraepithelial Neoplasia or Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2023, 41, 2779–2788. [Google Scholar] [CrossRef]
- Levy, D.T.; Issabakhsh, M.; Warner, K.E.; Liber, A.; Meza, R.; Cummings, M. Evaluating trends in cigarette and HTP use in Japan and measurement issues in the National Health and Nutrition Survey. Tob. Control 2024, 34, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Arrington, C.L. Regulating smoking in Japan: From manners to rules. Int. J. Asian Stud. 2024, 1–22. [Google Scholar] [CrossRef]
- Ito, Y.; Katanoda, K.; Yamamoto, S.; Hamajima, N.; Mochizuki, Y.; Matsuo, K. Trends in smoking prevalence and attitude toward tobacco control among members of the JCA in 2004–2017. Cancer Sci. 2022, 113, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Galani, A.; Zikopoulos, A.; Moustakli, E.; Potiris, A.; Paraskevaidi, M.; Arkoulis, I.; Machairoudias, P.; Stavrakaki, S.M.; Kyrgiou, M.; Stavros, S. Cervical Cancer Screening in the HPV-Vaccinated and Digital Era: Reassessing Strategies in Light of Artificial Intelligence and Evolving Risk. Cancers 2025, 17, 3179. [Google Scholar] [CrossRef]
- Nagai, K.; Tanaka, T.; Kodaira, N.; Kimura, S.; Takahashi, Y.; Nakayama, T. Data resource profile: JMDC claims database sourced from health insurance societies. J. Gen. Fam. Med. 2021, 22, 118–127. [Google Scholar] [CrossRef]
- Tajima, T.; Kanehara, R.; Fujii, M.; Tanaka, S.; Umezawa, J.; Ohno, Y.; Inoue, M. Trajectory Patterns of Three Lifestyle Behaviors and Subsequent Health Conditions in Japanese Adults: A Retrospective Longitudinal Study Using a Health Checkup Database. JMA J. 2024, 7, 506–517. [Google Scholar] [CrossRef]
- Kaneko, H.; Itoh, H.; Yotsumoto, H.; Kiriyama, H.; Kamon, T.; Fujiu, K.; Morita, K.; Kashiwabara, K.; Michihata, N.; Jo, T.; et al. Cardiovascular Health Metrics of 87,160 Couples: Analysis of a Nationwide Epidemiological Database. J. Atheroscler. Thromb. 2021, 28, 535–543. [Google Scholar] [CrossRef]
- Takeuchi, M.; Shinozaki, T.; Kawakami, K. Universal Health Checkups and Risk of Incident Diabetes and Hypertension. Jama Netw. Open 2024, 7, e2451813. [Google Scholar] [CrossRef]
- Yamana, H.; Moriwaki, M.; Horiguchi, H.; Kodan, M.; Fushimi, K.; Yasunaga, H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 2017, 27, 476–482. [Google Scholar] [CrossRef]
- Luu, M.N.; Han, M.; Bui, T.T.; Tran, P.T.T.; Lim, M.K.; Oh, J.K. Smoking trajectory and cancer risk: A population-based cohort study. Tob. Induc. Dis. 2022, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.; Jung, K.J.; Back, J.H.; Lee, S.M.; Lee, S.H. Trajectory of smoking and early bladder cancer risk among Korean young adult men. Cancer Causes Control 2020, 31, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Gillet, V.G.; Feldman, S.; Lii, H.; Toh, S.; Brown, J.S.; Katz, J.N.; Solomon, D.H.; Schneeweiss, S. Validation of claims-based algorithms for identification of high-grade cervical dysplasia and cervical cancer. Pharmacoepidemiol. Drug Saf. 2013, 22, 1239–1244. [Google Scholar] [CrossRef]
- de Luise, C.; Sugiyama, N.; Morishima, T.; Higuchi, T.; Katayama, K.; Nakamura, S.; Chen, H.; Nonnenmacher, E.; Hase, R.; Jinno, S. Validity of claims-based algorithms for selected cancers in Japan: Results from the VALIDATE-J study. Pharmacoepidemiol. Drug Saf. 2021, 30, 1153–1161. [Google Scholar] [CrossRef]
- Ogawa, T.; Takahashi, H.; Saito, H.; Sagawa, M.; Aoki, D.; Matsuda, K.; Nakayama, T.; Kasahara, Y.; Kato, K.; Saitoh, E. Novel algorithm for the estimation of cancer incidence using claims data in Japan: A feasibility study. JCO Glob. Oncol. 2023, 9, e2200222. [Google Scholar] [CrossRef]
- Kim, J.; Jo, H.; Ha, M.C.; Kim, H.; Lee, J.K.; Han, J.H.; Lee, S.-H.; Kang, D.R.; Kim, S.Y.; Kim, H.-S. Elevated risk of cervical cancer in elderly women with incident ulcerative colitis in South Korea. Sci. Rep. 2023, 13, 8323. [Google Scholar] [CrossRef]
- Castle, P.E.; Wacholder, S.; Lorincz, A.T.; Scott, D.R.; Sherman, M.E.; Glass, A.G.; Rush, B.B.; Schussler, J.E.; Schiffman, M. A prospective study of high-grade cervical neoplasia risk among human papillomavirus-infected women. J. Natl. Cancer Inst. 2002, 94, 1406–1414. [Google Scholar] [CrossRef]
- Fonseca-Moutinho, J.A. Smoking and cervical cancer. Int. Sch. Res. Not. 2011, 2011, 847684. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Conway, M.J.; Chen, H.-S.; Meyers, C. The cigarette smoke carcinogen benzo [a] pyrene enhances human papillomavirus synthesis. J. Virol. 2008, 82, 1053–1058. [Google Scholar] [CrossRef]
- Malevolti, M.C.; Lugo, A.; Scala, M.; Gallus, S.; Gorini, G.; Lachi, A.; Carreras, G. Dose-risk relationships between cigarette smoking and cervical cancer: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2023, 32, 171–183. [Google Scholar] [CrossRef]
- Lacey, J.V.; Frisch, M.; Brinton, L.A.; Abbas, F.M.; Barnes, W.A.; Gravitt, P.E.; Greenberg, M.D.; Greene, S.M.; Hadjimichael, O.C.; McGowan, L. Associations between smoking and adenocarcinomas and squamous cell carcinomas of the uterine cervix (United States). Cancer Causes Control 2001, 12, 153–161. [Google Scholar] [CrossRef]
- Rajkumar, T.; Appleby, P.; Beral, V.; Berrington, D.A.; Bull, D.; Crossley, B.; Green, J.; Reeves, G.; Sweetland, S.; Kjaer, S.; et al. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies—International collaboration of epidemiological studies of cervical cancer. Int. J. Cancer 2006, 118, 1481–1495. [Google Scholar] [CrossRef]
- Adams, J.M. Smoking cessation—Progress, barriers, and new opportunities: The Surgeon General’s report on smoking cessation. Jama 2020, 323, 2470–2471. [Google Scholar] [CrossRef]
- Flynn, J.R.; Bartlett, E.K.; Lichtman, S.M.; Panageas, K.S. Employing competing risks analysis in an aging population where many patients die from other causes. J. Geriatr. Oncol. 2022, 13, 245–248. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Vaccarella, S.; Herrero, R.; Snijders, P.J.; Dai, M.; Thomas, J.O.; Hieu, N.T.; Ferreccio, C.; Matos, E.; Posso, H.; de Sanjosé, S. Smoking and human papillomavirus infection: Pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int. J. Epidemiol. 2008, 37, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.-B.; Nayar, R. 2019 ASCCP risk-based management consensus guidelines: Updates through 2023. J. Low. Genit. Tract Dis. 2024, 28, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Malevolti, M.C.; Maci, C.; Lugo, A.; Possenti, I.; Gallus, S.; Gorini, G.; Carreras, G. Secondhand smoke exposure and cervical cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 14353–14363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishii, K.; Tabuchi, T.; Iso, H. Trends in socioeconomic inequalities in cervical, breast, and colorectal cancer screening participation among women in Japan, 2010–2019. Cancer Epidemiol. 2023, 84, 102353. [Google Scholar] [CrossRef]
- Mitoma, T.; Maki, J.; Ooba, H.; Ogawa, C.; Masuyama, H.; Tabuchi, T. Association of Regular Cervical Cancer Screening with Socioeconomic, COVID-19 Infection and Vaccine Status Among Japanese Population: Cohort Observational Study. Int. J. Gen. Med. 2024, 17, 541–551. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Framework Convention on Tobacco Control (FCTC) Implementation Database: Japan. Available online: https://extranet.who.int/fctcapps/fctcapps/fctc/implementation-database/parties/japan (accessed on 29 October 2025).


| 1 Ever Smoker (N =173,548) | 1 Never Smoker | 2 p-Value | |||
|---|---|---|---|---|---|
| Former Smoker (N = 15,929) | New Smoker (N = 10,809) | Current Smoker (N = 146,810) | (N = 1,157,249) | ||
| Age/mean (sd) | 41.3 (11.5) | 39.0 (11.6) | 44.4 (10.6) | 43.7 (11.7) | |
| Age | <0.0001 | ||||
| <50 | 11,873 (74.54) | 8702 (80.51) | 99,947 (68.08) | 790,628 (68.32) | |
| 50–64 | 3721 (23.36) | 1906 (17.63) | 43,552 (29.67) | 325,623 (28.14) | |
| ≥65 | 335 (2.10) | 201 (1.86) | 3311 (2.26) | 40,998 (3.54) | |
| 3 BMI | <0.0001 | ||||
| Obese | 745 (4.68) | 470 (4.35) | 7959 (5.42) | 42,926 (3.71) | |
| Overweight | 2291 (14.38) | 1383 (12.79) | 20,905 (14.24) | 140,635 (12.15) | |
| Normal | 10,838 (68.04) | 7211 (66.71) | 93,951 (63.99) | 794,552 (68.66) | |
| Underweight | 2055 (12.90) | 1745 (16.14) | 23,995 (16.34) | 179,136 (15.48) | |
| Missing | |||||
| Drinking Habit | <0.0001 | ||||
| Daily | 2715 (17.04) | 2058 (19.04) | 35,793 (24.38) | 112,453 (9.72) | |
| Sometimes | 5041 (31.65) | 4132 (38.23) | 43,151 (29.39) | 379,940 (32.83) | |
| None | 6671 (41.87) | 3697 (34.20) | 57,808 (39.38) | 589,155 (50.91) | |
| Missing | 1502 (9.44) | 922 (8.53) | 10,058 (6.85) | 75,701 (6.54) | |
| Regular Exercise | <0.0001 | ||||
| Yes | 2102 (13.20) | 1550 (14.34) | 19,669 (13.40) | 97,180 (8.40) | |
| No | 13,827 (86.80) | 9259 (85.66) | 127,141 (86.60) | 1,060,069 (91.60) | |
| Comorbidities | <0.0001 | ||||
| Hypertension | 1361 (8.54) | 683 (6.32) | 12,811 (8.73) | 97,180 (8.40) | |
| Diabetes mellitus | 2125 (13.34) | 1187 (10.98) | 16,580 (11.29) | 136,993 (11.84) | |
| Cerebrovascular disease | 878 (5.51) | 458 (4.24) | 5986 (4.08) | 56,005 (4.84) | |
| Cardiovascular disease | 697 (4.38) | 327 (3.03) | 4750 (3.24) | 42,892 (3.71) | |
| Cholangitis | 19 (0.12) | 8 (0.07) | 186 (0.13) | 1403 (0.12) | |
| Year of Follow-up (Mean (sd) | 3.00 ± 2.60 | 2.89 ± 2.40 | 2.9 ± 2.37 | 3.14 ± 2.53 | <0.0001 |
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Events | Person-Years | Incidence Rate per 100,000 Person Years (95% CI) | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value |
| Total | |||||||||
| Never smoker | 5481 | 3,619,696.8 | 151.4 (147.4–155.4) | Ref | Ref | Ref | |||
| Ever smoker | 1247 | 509,067.5 | 244.9 (231.3–258.5) | 1.61 (1.52–1.72) | <0.0001 | 1.61 (1.52–1.72) | <0.0001 | 1.53 (1.43–1.62) | <0.0001 |
| <50 | |||||||||
| Never smoker | 4130 | 2,510,776.3 | 164.4 (159.4–169.5) | Ref | Ref | Ref | |||
| Ever smoker | 993 | 356,055.7 | 278.8 (261.5–296.2) | 1.69 (1.58–1.81) | <0.0001 | 1.67 (1.56–1.79) | <0.0001 | 1.58 (1.47–1.70) | <0.0001 |
| 50–64 | |||||||||
| Never smoker | 1254 | 1,011,613.8 | 123.9 (117.0–130.8) | Ref | Ref | Ref | |||
| Ever smoker | 248 | 144,292.2 | 171.8 (150.4–193.2) | 1.38 (1.20–1.58) | <0.0001 | 1.37 (1.19–1.57) | <0.0001 | 1.35 (1.17–1.55) | <0.0001 |
| ≥65 | |||||||||
| Never smoker | 97 | 97,306.7 | 99.6 (79.8–119.5) | Ref | Ref | Ref | |||
| Ever smoker | 6 | 8719.6 | 68.8 (13.7–123.8) | 0.69 (0.30–1.57) | 0.971 | 0.67 (0.29–1.54) | 0.963 | 0.69 (0.30–1.59) | 0.6180 |
| Incidence Rate per 100,000 Person Years (95% CI) | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Events | Person-Years | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | |
| Total | |||||||||
| Never smoker | 5481 | 3,619,696.82 | 151.4 (147.41–155.43) | Ref | Ref | Ref | |||
| Former smoker | 109 | 47,579.26 | 229.09 (186.08–272.09) | 1.51 (1.25–1.83) | <0.0001 | 1.52 (1.26–1.84) | <0.0001 | 1.44 (1.15–1.79) | <0.0001 |
| New smoker | 75 | 31,072.83 | 241.36 (186.74–295.99) | 1.59 (1.27–2.00) | <0.0001 | 1.61 (1.28–2.02) | <0.0001 | 1.51 (1.20–1.90) | <0.0001 |
| Current smoker | 1063 | 430,415.39 | 246.97 (232.12–261.81) | 1.63 (1.52–1.74) | <0.0001 | 1.63 (1.52–1.74) | <0.0001 | 1.54 (1.44–1.64) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Kim, S.Y.; Lee, N.K.; Lee, S.W. Changes in Smoking Patterns and Cervical Cancer Risk: Preventive Implications from a Nationwide Japanese Cohort. Healthcare 2025, 13, 2852. https://doi.org/10.3390/healthcare13222852
Lee YJ, Kim SY, Lee NK, Lee SW. Changes in Smoking Patterns and Cervical Cancer Risk: Preventive Implications from a Nationwide Japanese Cohort. Healthcare. 2025; 13(22):2852. https://doi.org/10.3390/healthcare13222852
Chicago/Turabian StyleLee, Yun Jeong, Sun Yeup Kim, Nang Kyeong Lee, and Seung Won Lee. 2025. "Changes in Smoking Patterns and Cervical Cancer Risk: Preventive Implications from a Nationwide Japanese Cohort" Healthcare 13, no. 22: 2852. https://doi.org/10.3390/healthcare13222852
APA StyleLee, Y. J., Kim, S. Y., Lee, N. K., & Lee, S. W. (2025). Changes in Smoking Patterns and Cervical Cancer Risk: Preventive Implications from a Nationwide Japanese Cohort. Healthcare, 13(22), 2852. https://doi.org/10.3390/healthcare13222852









