Abstract
Background/Objectives: Canada’s Food Guide 2019 includes advice such as “Cook more often” and “Eat meals with others”, which are considered healthy eating practices. However, mothers with a history of gestational diabetes mellitus (GDM) may face specific barriers to adopting healthy eating practices. This study aimed to compare eating practices between mothers with (GDM+) and without (GDM−) a history of GDM, and to explore the associations between eating practices, diet quality, and the anthropometric and cardiometabolic profile of these mothers. Methods: The cross-sectional study was conducted in Quebec (Canada) between 2012 and 2017. Eating practices were assessed using a self-administered questionnaire. Diet quality was evaluated by the Healthy Eating Food Index 2019 through a validated food frequency questionnaire. Weight, height, and waist circumference were measured, and body composition was obtained by absorptiometry. Results: Data from 105 GDM+ and 38 GDM− mothers were analyzed (mean age 37.5 years ± 4.9). GDM+ mothers were more likely to prepare a greater proportion of dinners (≥1 per week) using pre-prepared or processed foods than GDM− mothers (49.0% vs. 34.2%; p = 0.016). Among GDM+ mothers, those who prepared ≥1 dinners per week using pre-prepared or processed foods showed lower adherence to the “Whole-grain foods” (1.1 ± 0.8 vs. 1.9 ± 1.2; p = 0.002) and “Sodium” (4.9 ± 2.0 vs. 5.8 ± 2.0, p = 0.013) recommendations, had a higher percentage of total body fat (37.5% ± 7.6 vs. 34.0% ± 7.7; p = 0.041), a higher waist circumference (91.6 cm ± 13.9 vs. 87.1 cm ± 16.3; p = 0.030), and a higher glycated hemoglobin (5.6% ± 0.5 vs. 5.5% ± 0.3; p = 0.025) than those who used less pre-prepared or processed foods. Conclusions: GDM+ mothers were more likely than GDM− mothers to prepare dinners using pre-prepared or processed foods, an eating practice associated with less favorable components of diet quality and some altered anthropometric and cardiometabolic variables. Further investigation into the factors influencing cooking from scratch within this population is warranted.
1. Introduction
In alignment with the statement “Healthy Eating is More Than the Foods You Eat”, Canada’s Food Guide (CFG) 2019 includes advice such as “Be mindful of your eating habits”, “Enjoy your food”, “Cook more often”, and “Eat meals with others” [1]. Beyond the content of our plates, the context in which food is consumed has garnered increasing interest, referred to as eating practices [2]. In the general population, these eating practices have been linked to a better diet quality and, ultimately, improved overall health [2,3,4,5]. However, like other high-risk populations, mothers with a history of gestational diabetes mellitus (GDM) may encounter specific barriers to adopting healthy eating practices, such as a lack of information about healthy eating after their complicated pregnancy [6,7,8], which in turn may influence the food environment of their high-risk offspring [9].
GDM is defined as hyperglycemia with onset or first recognition during pregnancy [10]. In Canada, the prevalence of GDM was 10.4% in 2019, according to the most recent data—a rate that has doubled in one decade [11]. Mothers with a history of GDM (GDM+) are at an increased risk of developing chronic diseases in the years following delivery [12,13,14,15]. They are ten times more likely to develop type 2 diabetes (T2D) and twice as likely to suffer from cardiovascular diseases (CVD) compared to mothers with no history of GDM (GDM−) [12,13].
Knowledge about eating practices among mothers with a history of GDM is scarce. According to a previous study by our group, based on the Theory of Planned Behavior, the disapproval of a family member other than the partner was a subjective norm associated with a lower intention to adopt a healthy diet among GDM+ mothers [6]. Social influence also appears to be an important determinant of diet among individuals at risk for T2D or CVD [16,17], highlighting the importance of studying the eating context specifically among GDM+ mothers.
To our knowledge, unlike diet quality, eating practices have not yet been explored in mothers with a history of GDM. Multiple studies showed that a better diet quality was associated with a more favorable anthropometric and cardiometabolic profile among GDM+ mothers [14,18,19,20,21]. According to a recent scoping review on the association between specific dietary patterns and cardiometabolic outcomes in GDM+ mothers, providing simple and adaptable dietary guidance could make any proposed intervention easier to implement and improve adherence within this population [19]. Since GDM+ mothers often struggle to maintain a healthy diet after delivery [7,22,23,24,25], it is essential to analyze their diet in a more comprehensive way, focusing on the “how” of eating rather than just the “what”. Eating practices offer an opportunity for developing more practical and specific dietary recommendations for these at-risk mothers [7,26]. The first objective of this study was to compare eating practices between GDM+ and GDM− mothers. The second objective was to explore the associations between eating practices and (1) diet quality, and (2) anthropometric and cardiometabolic profiles of GDM+ and GDM− mothers.
2. Materials and Methods
2.1. Study Population
Recruitment of the participants for the GDM2 study took place between 2012 and 2017. The aim of this cross-sectional study was to evaluate the impact of GDM on maternal and offspring health. A total of 143 mothers (105 GDM+ and 38 GDM−) enrolled in this study. More details on the study design can be found elsewhere [6,27]. Briefly, mothers were recruited through medical records from the two main hospitals with a neonatal care unit in Quebec City (Hôpital Saint-François d’Assise and Centre Hospitalier de l’Université Laval), the provincial health plan registry (Régie de l’assurance maladie du Québec), emails sent to the Laval University community, and posts on healthcare websites and social networks. French-speaking mothers from the Quebec City metropolitan area aged ≥18 years who carried a pregnancy between 2003 and 2013 were invited to participate. Those who were pregnant at the time of the study or had pre-existing diabetes (type 1 or type 2) were excluded. Mothers were invited for a single visit (lasting 3 to 4 h) at the Institute of Nutrition and Functional Foods (INAF) in Quebec City, Canada, during which all data were collected. GDM was diagnosed between 24 and 28 weeks of gestation using the sequential two-step approach [28,29,30]. Mothers were recruited on average 6.1 ± 2.4 years after their last pregnancy complicated by GDM. Written consent was obtained from all participants, and ethical approval was obtained from the Université Laval Ethics Committee (2011-196-A-4 R-3) and the Centre Hospitalier Universitaire de Québec Ethics Committee (2015–2031, B14-07-2031-21). This study was registered in the Clinical Trials.gov registry (NCT01340924).
2.2. Eating Practices
Eating practices were collected through a self-administered questionnaire, which mothers completed with a provided electronic device during their visit to the research center. If needed, mothers could receive assistance with completing the questionnaire. Since data collection was conducted before the publication of the CFG 2019, the questions that better reflect the practices targeted in the guide were included in this study. Three categories of eating practices were documented as part of the GDM2 study: “Be mindful of your eating habits”, “Eat meals with others” and “Cook more often” [1].
One question was related to the “Be mindful of your eating habits” practice: “How many times have you had meals in front of/with a screen in the past seven days?”. This question was open-ended (i.e., answers given as the number of breakfasts, lunches, and dinners per week). A “screen” was defined as television, computer, gaming console, iPad, etc. Another open-ended question allowed the analysis of “Eat meals with others” practice: “How many times have you had family meals in the past seven days?”. Family meals were defined as meals taken with children and at least one parent. The “Cook more often” practice was assessed using this open-ended question: “In the past seven days, how many times was dinner (out of a total of 7)…” (1) “…cooked from scratch?”, (2) “…cooked with pre-prepared or processed foods?”, (3) “…ready-to-eat?” or (4) “…take-out/restaurant?”. Some examples were provided to help mothers select the best answer. Dinners cooked with pre-prepared or processed foods were defined with examples such as the use of marinated meat or fish, packaged sauces, or ready-to-cook items from the grocery store. Ready-to-eat dinners were defined as frozen meals, canned soup or ready-to-eat lasagna, as examples.
2.3. Diet Quality
Mothers completed a validated web-based food frequency questionnaire (web-FFQ) with a provided electronic device during their visit to the research center [31]. This tool is an online self-administered quantitative FFQ that has been validated to assess dietary intakes over the last month among the French-speaking Canadian population [31]. Food, nutrient, and energy intake data were derived from a food composition database developed and validated for this web-FFQ, based on the Nutrition Data System for Research (software version 4.03, Food and Nutrient Database 31, Minneapolis, MN, USA) and the Canadian Nutrient File (CNF, version 2007b, Ottawa, ON, Canada) [31]. The diet quality was evaluated by the Healthy Eating Food Index (HEFI)-2019 and its ten components [32]. HEFI-2019 is a score of adherence to the 2019 CFG recommendations [32]. Seven of the HEFI-2019 components are “adequacy” components (i.e., greater adherence is defined by greater relative consumption): Vegetables and fruits, Whole-grain foods, Grain foods ratio, Protein foods, Plant-based protein foods, Beverages, and Fatty acids ratio [32]. HEFI-2019 also includes three “moderation” components (i.e., greater adherence is defined by lower relative consumption) that relate to nutrients of concern: Saturated fats, Free sugars, and Sodium [32].
Since healthy eating practices can be associated with the use of less calorie-dense foods [26,33,34], adjusting for energy intake in our analyses helps connect the “what” and the “how” of eating, provided that energy intake is accurately reported. An additional adjustment for the reporting status allows for handling misreporting [35], which was determined using the ratio of self-reported energy intake to predicted energy requirements [36,37]. Mothers with a ratio of <0.78, 0.78 to 1.22, or >1.22 were categorized as under-, plausible, and over-reporters, respectively [36].
2.4. Anthropometric and Cardiometabolic Profile
Mothers’ weight was measured in light clothes and without shoes using a calibrated balance to the nearest 0.1 kg (Tanita BC-418, Tanita Corporation of America Inc., Arlington Heights, IL, USA). Their height was measured with a stadiometer to the nearest millimeter. Body mass index (BMI) was calculated (kg/m2). Waist circumference was measured twice, under the clothes, following a standardized procedure at the midpoint between the iliac crest and the last palpable rib [38]. Mothers’ body composition and fat distribution were also measured using a dual-energy X-ray absorptiometry scanner (DXA, GE Lunar Prodigy Bone Densitometer, GE Healthcare Lunar, Madison, WI, USA). Two variables were considered: total fat mass (%), and android fat mass (%).
Fasting blood samples were collected, and a 75 g, 2 h oral glucose tolerance test (OGTT) was performed. During this test, blood samples were collected at −15, 0, 15, 30, 60, 90, and 120 min to measure glucose and insulin concentrations. Plasma glucose was measured enzymatically, and insulin was measured by radioimmunoassay [14]. Glycated hemoglobin (HbA1C) was determined using the Cobas Integra 800 analyzer (Roche Diagnostics, Switzerland) standardized to the National Glycated Hemoglobin Standardization Program [39]. HbA1C reflects the average glucose level over the past 2–3 months. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from fasting glucose and insulin concentrations to evaluate insulin resistance [40]. Lipids were measured using automated enzymatic methods [41]. The cholesterol ratio was obtained by dividing total cholesterol (mmol/L) by HDL cholesterol (mmol/L); a higher ratio indicates a higher risk of CVD [42].
2.5. Maternal Data
Sociodemographic characteristics were collected through self-administered questionnaires. Mothers were asked about their age, ethnicity, household annual income, highest level of education, age of their youngest child (classified as preschool-aged if ≤5 years old or school-aged if 6–12 years old), and number of children. Mothers were not asked about their gender. Of note, the term “mothers”, as used in this manuscript, refers to the parent whose biological sex is female and who has given birth to one or more children.
Mothers’ lifestyle habits were also used as covariates for the second objective of this study (physical activity, smoking status, and alcohol consumption). Physical activity was objectively assessed using ActiGraph GT3X triaxial accelerometers (ActiGraph, Pensacola, FL, USA). Mothers were instructed to wear it over the hip on an elasticized belt during 7 consecutive days after their visit to the research center. More details on the use of accelerometers in the GDM2 study can be found elsewhere [43]. The number of minutes of moderate-to-vigorous physical activity per day was extracted. Current smoking status (yes or no) and average alcohol consumption (number of consumptions per week) were also collected though a self-administered questionnaire during their visit to the research center.
2.6. Statistical Analyses
Mothers’ characteristics according to their history of GDM were compared using Chi-square tests (or Fisher exact tests) for categorical variables. Results on eating practices were presented as dichotomous variables; categories were created according to the mean of each eating practice. For example, the number of dinners cooked from scratch was categorized as <5 dinners per week or ≥5 dinners per week (mean of 5.3 ± 1.3 per week).
Eating practices were compared between GDM+ and GDM− mothers using log-binomial regression, adjusting for age, household annual income, highest maternal level of education, age of the youngest child, and number of children [44,45,46,47]. Further adjustment for energy intake and reporting status was also performed. Eating practices that differed between the two groups were selected for further analyses. Generalized linear models (GLMs) were performed to examine the association between these eating practices and diet quality (HEFI-2019) among GDM+ and GDM− mothers separately, adjusting for the same covariates previously described.
In addition, GLMs were performed to examine the relationship between eating practices and anthropometric and cardiometabolic measures in GDM+ and GDM− mothers separately. Two models were used: Model 1, which adjusted for potential covariates (age, household annual income, highest maternal level of education, moderate-to-vigorous physical activity, smoking, and alcohol consumption), and Model 2, which further adjusted for energy intake and reporting status. These covariates were selected based on their influence on mothers’ eating practices, anthropometric measures, and cardiometabolic measures according to the current literature [45,46,47,48,49]. Variables that were not normally distributed were transformed using the Box–Cox procedure when necessary. The statistical software SAS OnDemand for Academics (SAS Studio Version 3.82) was used for the analyses. Results presented in this study are from exploratory analyses and were not pre-registered.
3. Results
The mean age of mothers was 37.5 ± 4.9 years, and more than 90% identified as White. Mothers’ characteristics according to prior GDM status are presented in Table 1. GDM+ mothers were older than GDM− mothers; with 37.5% of GDM+ mothers being 40 years old or older, compared to 29.0% of GDM− mothers. Other sociodemographic characteristics were similar between GDM+ and GDM− mothers.

Table 1.
Mothers’ characteristics according to history of gestational diabetes.
3.1. Eating Practices
Eating practices are shown in Table 2. GDM+ and GDM− mothers shared a similar proportion of family meals and meals eaten in front of a screen. However, GDM+ mothers were more likely to prepare a greater proportion of dinners (≥1 per week) using pre-prepared or processed foods compared to GDM− mothers (49.0% vs. 34.2%, p = 0.016), after adjusting for age, household annual income, highest maternal level of education, age of the youngest child, and number of children. Similar results were obtained after further adjustment for energy intake and reporting status. Other methods of preparation (number of dinners cooked from scratch, ready-to-eat, or purchased at a take-out restaurant) were similar between GDM+ and GDM− mothers.

Table 2.
Eating practices of mothers according to history of gestational diabetes.
3.2. Diet Quality
Among GDM+ mothers, those who prepared ≥1 dinners per week using pre-prepared or processed foods had a mean energy intake of 2287 ± 641 kcal/d vs. 2099 ± 460 kcal/d for those who prepared no dinner of this type (p = 0.681, Table S1). Similar results were observed among GDM− mothers (2737 ± 919 kcal/d vs. 2404 ± 688 kcal/d, p = 0.371, Table S2). The proportion of under-, plausible, and over-reporters was not different between the two groups for GDM+ mothers (p = 0.386; Table S1), but was different for GDM− mothers with more over-reporters among those who prepared ≥1 dinners per week using pre-prepared or processed foods compared to those without dinners using pre-prepared or processed foods (53.9% vs. 44.0%, respectively; p = 0.030; Table S2).
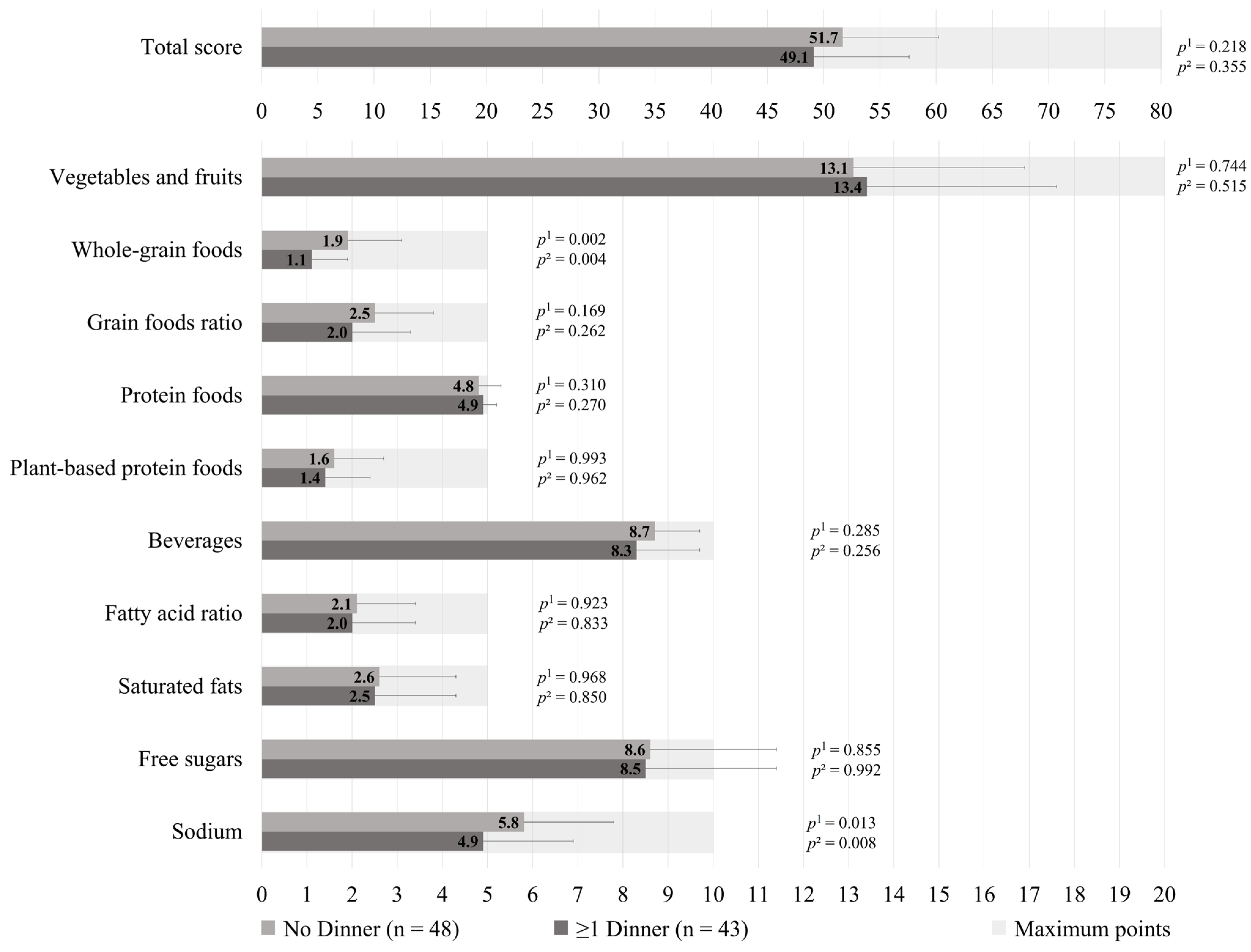
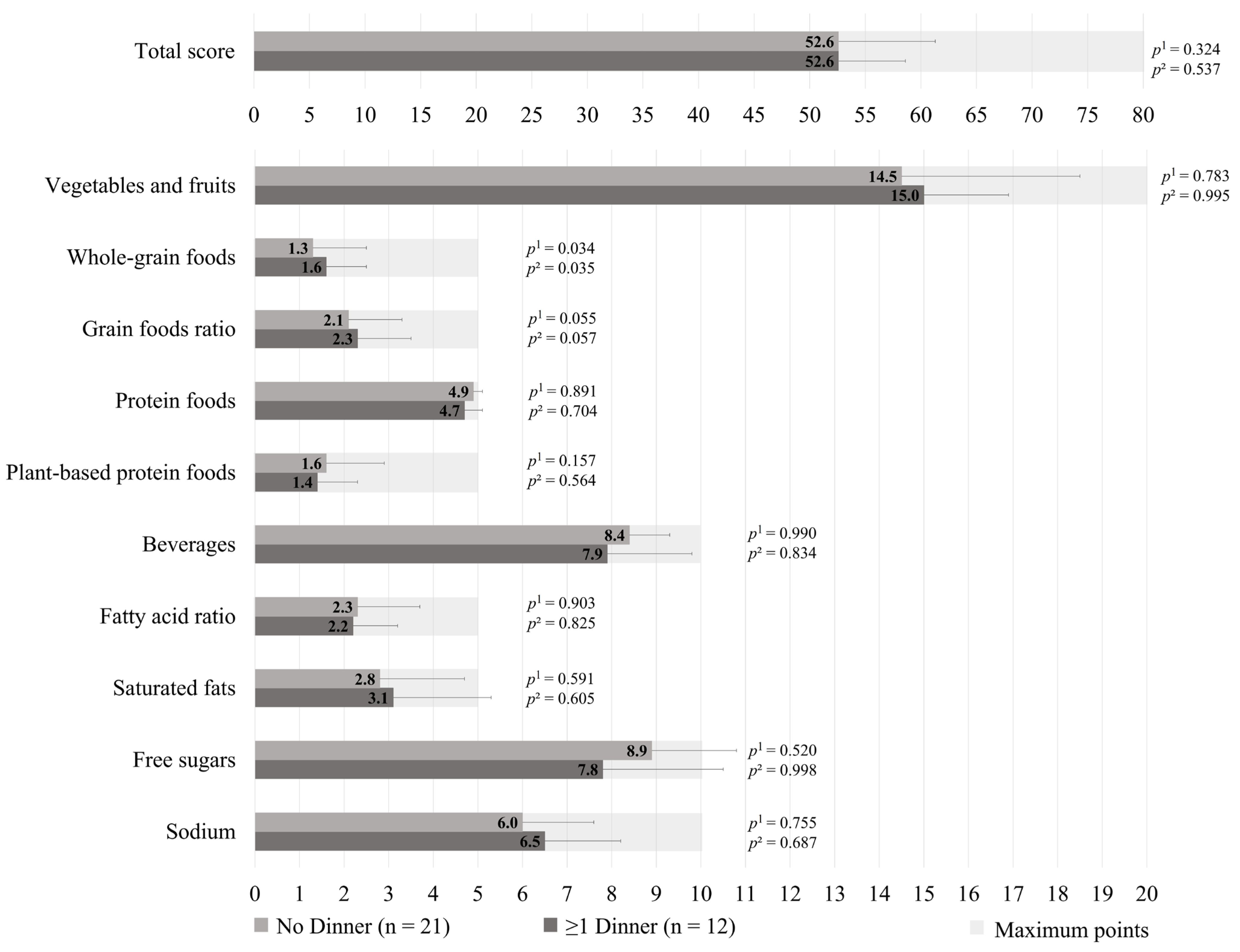
The HEFI-2019 score was similar between those who prepared ≥1 dinners per week vs. no dinner using pre-prepared or processed foods among GDM+ mothers (49.1 ± 8.5 vs. 51.7 ± 8.5, respectively; p = 0.218), as shown in Figure 1. More specifically, the first group adhered less to the “Whole-grain foods” (1.1 ± 0.8 vs. 1.9 ± 1.2, respectively; p = 0.002) and “Sodium” (4.9 ± 2.0 vs. 5.8 ± 2.0, respectively, p = 0.013) components of the HEFI-2019 score compared to the latter. Among GDM− mothers, those who prepared ≥1 dinners per week vs. no dinner using pre-prepared or processed foods had a similar HEFI-2019 score (52.6 ± 6.0 vs. 52.6 ± 8.7, respectively; p = 0.324), as shown in Figure 2. Conversely to GDM+ mothers, GDM− mothers who prepared ≥1 dinners per week using pre-prepared or processed foods adhered more to the “Whole-grain foods” (1.6 ± 0.9 vs. 1.3 ± 1.2, respectively; p = 0.034) component compared to those who prepared no dinner of this type. The HEFI-2019 results are also presented in Supplementary Table S3 for GDM+ mothers and in Supplementary Table S4 for GDM− mothers.
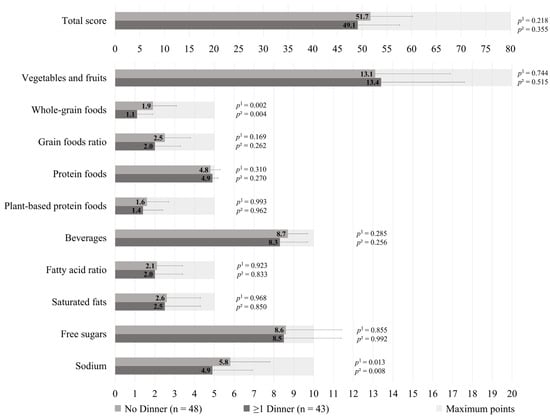
Figure 1.
Healthy Eating Food Index—2019 of GDM+ mothers according to the number of dinners using pre-prepared or processed foods per week. Note: GDM+: history of gestational diabetes. 1 Adjustment for age, household annual income, highest maternal level of education, age of the youngest child, and number of children. 2 Further adjustment for energy intake and reporting status.
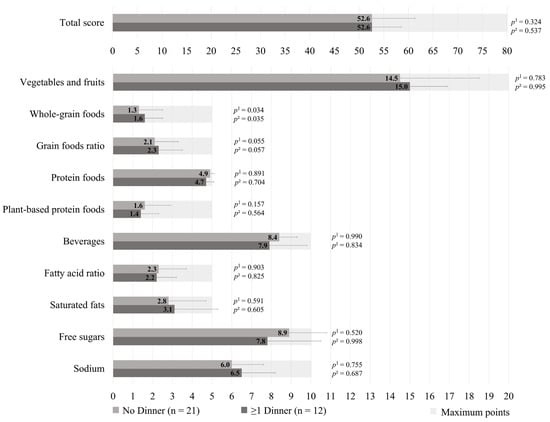
Figure 2.
Healthy Eating Food Index—2019 of GDM− mothers according to the number of dinners using pre-prepared or processed foods per week. Note: GDM−: no history of gestational diabetes. 1 Adjustment for age, household annual income, highest maternal level of education, age of the youngest child, and number of children. 2 Further adjustment for energy intake and reporting status.
3.3. Anthropometric and Cardiometabolic Profile
Anthropometric and cardiometabolic profiles among GDM+ mothers according to the proportion of dinners per week using pre-prepared or processed foods are shown in Table 3. Those who prepared ≥1 dinners per week using pre-prepared or processed foods had a higher percentage of total body fat (37.5% ± 7.6 vs. 34.0% ± 7.7; p = 0.041), a higher waist circumference (91.6 cm ± 13.9 vs. 87.1 cm ± 16.3; p = 0.030) and a higher glycated hemoglobin (5.6% ± 0.5 vs. 5.5% ± 0.3; p = 0.025) than those who prepared a lower proportion, after adjustment for age, household annual income, highest maternal level of education, moderate-to-vigorous physical activity, smoking, and alcohol consumption. A similar result for glycated hemoglobin was obtained after further adjustment for energy intake and reporting status. However, differences in total body fat and waist circumference were no longer significant (p = 0.090 and p = 0.088, respectively). Among GDM− mothers, as presented in Table 4, no significant anthropometric and cardiometabolic differences were observed between those who prepared ≥1 dinners per week using pre-prepared or processed foods and those who prepared no dinners of this type.

Table 3.
Anthropometric and cardiometabolic profile of GDM+ mothers according to the number of dinners using pre-prepared or processed foods per week.

Table 4.
Anthropometric and cardiometabolic profile of GDM− mothers according to the number of dinners using pre-prepared or processed foods per week.
4. Discussion
Results of this study showed that mothers with a history of GDM adopted less favorable eating practices compared to mothers without a history of GDM according to CFG 2019 recommendations [1]. Specifically, a greater proportion of GDM+ mothers cooked dinners using pre-prepared or processed foods compared to GDM− mothers. This eating practice was associated with less favorable components of diet quality and with altered anthropometric and cardiometabolic measures among GDM+ mothers.
We showed that GDM+ mothers were more likely to prepare a greater proportion of dinners using pre-prepared or processed foods than GDM− mothers. Half (49%) of GDM+ mothers cooked one or more dinners per week using pre-prepared or processed foods, compared to a third (34%) of GDM− mothers. To our knowledge, this is the first study focusing on eating practices among mothers with a history of GDM. GDM+ mothers represent a unique high-risk population for T2D. They encounter multiple barriers to healthy eating that are common to all mothers—such as lack of time, children’s food preferences, and work schedule [26,50,51]—but they also encounter specific barriers: no systematic postpartum follow-up after a pregnancy complicated by GDM in Canada’s healthcare system, lack of information about healthy eating after childbirth, lack of direct impact of the maternal diet on the child’s health (unlike during pregnancy), and possible return to a sub-optimal diet similar to the pre-pregnancy diet [6,8,25,52,53,54]. Numerous studies have also shown that a large proportion of GDM+ mothers are unaware that this pregnancy complication is associated with an increased risk of T2D and CVD after delivery, which may partly explain why they do not consistently adopt healthy lifestyle habits [55,56,57]. The proportion of cooked dinners using pre-prepared or processed foods per week among GDM− mothers (7.5% ± 14.0) was similar to findings from a study of 150 ethnically and socioeconomically diverse families with young children in the United States: 7% of meals were partly home-cooked [58]. In comparison, among GDM+ mothers, the proportion of dinners using pre-prepared or processed foods per week was nearly doubled (14.1% ± 18.3).
Globally, our results highlight the complexity of healthy eating, where both the “what” and the “how” of eating interact synergistically and are difficult to separate. Home-cooked meals are known to be more nutritious than ready-to-eat meals or those purchased at a takeout restaurant [4,5,59]. However, considerable variability exists in how meals can be cooked [58,60]. It is likely that cooking more frequently with pre-prepared or processed foods is associated with some unfavorable dietary components, such as a higher energy intake and a lower diet quality [33,61]. Indeed, GDM+ mothers who prepared ≥1 dinners using pre-prepared or processed foods per week had less favorable components of diet quality (i.e., a lower consumption of whole grains and a higher consumption of sodium) than those without dinners using pre-prepared or processed foods. Consistent with our findings, some studies showed that cooking less often from scratch was associated with some unfavorable dietary components, such as a lower vegetable and fruit intake and a higher ultra-processed food consumption [58,60,62].
The results of our study also showed that, among GDM+ mothers, those who prepared ≥1 dinners per week using pre-prepared or processed foods had a higher percentage of total body fat, a higher waist circumference, and a higher HbA1C than those without dinners using pre-prepared or processed foods. Since pre-prepared and processed foods are generally more calorie-dense than whole foods [33], we made further adjustments for energy intake and reporting status, and observed that the results were attenuated. However, differences in HbA1C remained significant even after these adjustments. Since GDM+ mothers are ten times more likely to develop T2D later in life compared to GDM− mothers [13] and that even small changes in HbA1C matter regarding cardiometabolic risk [10,63], this result is important from a clinical standpoint.
In parallel, in the context of this study, the anthropometric and cardiometabolic profile among GDM− mothers between those who prepared ≥1 dinners per week vs. no dinner using pre-prepared or processed foods were similar. GDM− mothers who prepared ≥1 dinners per week using pre-prepared or processed foods had a similar HEFI-2019 and a favorable dietary component (i.e., a higher consumption of whole grains) compared to those without dinners using pre-prepared or processed foods, which may explain the lack of association with anthropometric and cardiometabolic variables. Pre-prepared and processed foods fall within a broad and heterogeneous category [58,60]. It is possible that GDM+ mothers make less optimal choices within this category compared to GDM− mothers, which aligns with findings related to the HEFI-2019 components. This hypothesis highlights an opportunity for nutrition education targeting GDM+ mothers, who may lack the time or skills to prepare meals from scratch [22]. Providing them with guidance to make healthier choices among pre-prepared or processed foods—while respecting their family’s realities—could support more effective dietary interventions [19,25].
The limitations of this study include the fact that the socioeconomic status of the GDM2 study was high, despite efforts to recruit from a more vulnerable area of Quebec City, which may limit the generalizability of the results. The limited number of GDM− mothers in this study may increase the risk of false-negative results. Since our results were obtained from cross-sectional data, our findings should be interpreted with caution and confirmed in longitudinal studies. The Canadian Eating Practices Screener remains the validated tool for measuring eating practices according to the CFG 2019; however, this screener was not available at the time of the study [1,2]. The method of preparation for other meals of the day (i.e., breakfast and lunch) was also not documented. Culturally, dinner remains the most important meal of the day in Canada—the one most likely to be cooked and shared with family [45,64]. The strengths of this study include the exclusion of other types of diabetes or pregnancy complications associated with various outcomes for mothers, compared to GDM [65,66]. The presence of a control group also enabled a comparison of eating practices between GDM+ and GDM− mothers. One of the main strengths of this study was our approach to studying diet in a more global way, by including the analysis of eating practices among GDM+ mothers. Finally, the OGTT provides a more comprehensive glycemic profile, while the DXA scan is one of the most reliable methods for body composition analysis [67].
5. Conclusions
Mothers with a history of GDM had less favorable eating practices than mothers without prior GDM. More specifically, GDM+ mothers were more likely to prepare dinners using pre-prepared or processed foods, an eating practice associated with less favorable components of diet quality and altered anthropometric and cardiometabolic profiles. This study highlighted the importance of analyzing diet in a more comprehensive way among this high-risk population, focusing not only on the “what” but also on the “how” of eating. Future studies in this population should explore a broader range of eating practices and how they evolve from the complicated pregnancy through the years following delivery [68,69]. Given the higher risk of chronic diseases among GDM+ mothers and their offspring, the factors influencing cooking from scratch within this population should be further investigated.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/healthcare13212792/s1, Table S1: Energy intake and reporting status of GDM+ mothers according to the number of dinners using pre-prepared or processed foods per week. Table S2: Energy intake and reporting status of GDM− mothers according to the number of dinners using pre-prepared or processed foods per week. Table S3: Healthy Eating Food Index—2019 of GDM+ mothers according to the number of dinners using pre-prepared or processed foods per week. Table S4: Healthy Eating Food Index—2019 of GDM− mothers according to the number of dinners using pre-prepared or processed foods per week.
Author Contributions
Conceptualization, J.R.; Methodology, J.R.; Validation, J.R.; Formal Analysis, M.B.; Investigation, M.B. and J.P.; Writing—Original Draft Preparation, M.B.; Writing—Review and Editing, M.B., C.S., J.P., S.L. and J.R.; Visualization, M.B.; Supervision, C.S., J.P. and J.R.; Project Administration, J.R.; Funding Acquisition, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Canadian Institutes of Health Research [OOP-98026, 2009–2010], Fonds de recherche du Québec—Santé [#16225, 2009–2012], Diabetes Canada [OG-3-14-4543-JR, 2014–2017] and the Danone Institute of Canada [2012–2014]. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Université Laval Ethics Committee (approval code: 2011-196-A-4 R-3, approval date: 14 December 2011) and the Centre Hospitalier Universitaire de Québec Ethics Committee (approval code: 2015–2031, B14-07-2031-21, approval date: 17 December 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author, as they are not publicly available due to ethical restrictions.
Acknowledgments
Mélissa Bélanger received a scholarship from the Canadian Institutes of Health Research and the Fonds de recherche du Québec—Santé. Julie Robitaille is the Chair of Nutrition at Université Laval. The authors would like to sincerely thank all participants in the study for their devoted time.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BMI | Body mass index |
| CFG | Canada’s Food Guide |
| CVD | Cardiovascular diseases |
| DXA | dual-energy X-ray absorptiometry |
| FFQ | Food frequency questionnaire |
| GDM | Gestational diabetes mellitus |
| GDM+ | History of gestational diabetes mellitus |
| GDM− | No history of gestational diabetes mellitus |
| GLMs | Generalized linear models |
| HbA1C | Glycated hemoglobin |
| HEFI-2019 | Healthy Eating Food Index—2019 |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| INAF | Institute of Nutrition and Functional Foods |
| NUTRISS | Centre Nutrition, Santé et Société |
| OGTT | Oral glucose tolerance test |
| T2D | Type 2 diabetes |
References
- Health Canada. Canada’s Food Guide. Available online: https://food-guide.canada.ca/en/ (accessed on 28 August 2025).
- Perreault, M.; Wallace, A.; Martin, A.; Sadowski, A.; Laila, A.; Lemieux, S.; Hutchinson, J.M.; Kirkpatrick, S.I.; Simpson, J.R.; Guenther, P.M.; et al. Construct validity and reliability of the Canadian Eating Practices Screener to assess eating practices based on 2019 Canada’s Food Guide recommendations. Appl. Physiol. Nutr. Metab. 2023, 48, 919–931. [Google Scholar] [CrossRef]
- Ducrot, P.; Mejean, C.; Aroumougame, V.; Ibanez, G.; Alles, B.; Kesse-Guyot, E.; Hercberg, S.; Peneau, S. Meal planning is associated with food variety, diet quality and body weight status in a large sample of French adults. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 12. [Google Scholar] [CrossRef]
- Marquis, M.; Jobin, N.; Aubé, J.; Côté, S.; Soucy, M.D. Comportements, préoccupations et priorités liés à l’utilisation du temps entourant les repas familiaux au Québec. Cah. Nutr. Diététique 2018, 53, 151–160. [Google Scholar] [CrossRef]
- Fernandez, M.A.; Maximova, K.; Fulkerson, J.A.; Raine, K.D. Associations between cooking skills, cooking with processed foods, and health: A cross-sectional study. Appl. Physiol. Nutr. Metab. 2024, 49, 330–339. [Google Scholar] [CrossRef]
- Bélanger, M.; Dugas, C.; Perron, J.; St-Yves, A.; Rancourt-Bouchard, M.; John Weisnagel, S.; Robitaille, J. Intention to adopt a healthy diet among women with and without a history of gestational diabetes: Constructs and beliefs from the theory of planned behavior. Prev. Med. Rep. 2023, 35, 102328. [Google Scholar] [CrossRef] [PubMed]
- Dennison, R.A.; Griffin, S.J.; Usher-Smith, J.A.; Fox, R.A.; Aiken, C.E.; Meek, C.L. “Post-GDM support would be really good for mothers”: A qualitative interview study exploring how to support a healthy diet and physical activity after gestational diabetes. PLoS ONE 2022, 17, e0262852. [Google Scholar] [CrossRef]
- Zulfiqar, T.; Lithander, F.E.; Banwell, C.; Young, R.; Boisseau, L.; Ingle, M.; Nolan, C.J. Barriers to a healthy lifestyle post gestational-diabetes: An Australian qualitative study. Women Birth 2017, 30, 319–324. [Google Scholar] [CrossRef]
- Persson, M.; Winkvist, A.; Mogren, I. Lifestyle and health status in a sample of Swedish women four years after pregnancy: A comparison of women with a history of normal pregnancy and women with a history of gestational diabetes mellitus. BMC Pregnancy Childbirth 2015, 15, 57. [Google Scholar] [CrossRef]
- Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2018, 42, S1–S325. [Google Scholar] [CrossRef]
- Nelson, C.R.; Dzakpasu, S.; Moore, A.M.; Darling, E.K.; Edwards, W.; Murphy, P.; Scott, H.; Van Den Hof, M.; Ray, J.G. Diabetes mellitus in pregnancy across Canada. BMC Pregnancy Childbirth 2024, 24, 349. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.K.; Campbell, S.; Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: A systematic review and meta-analysis. Diabetologia 2019, 62, 905–914. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.C.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m1361. [Google Scholar] [CrossRef] [PubMed]
- Gingras, V.; Paradis, A.M.; Tchernof, A.; Weisnagel, S.J.; Robitaille, J. Relationship between the adoption of preventive practices and the metabolic profile of women with prior gestational diabetes mellitus. Appl. Physiol. Nutr. Metab. 2012, 37, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Cormier, H.; Vigneault, J.; Garneau, V.; Tchernof, A.; Vohl, M.C.; Weisnagel, S.J.; Robitaille, J. An explained variance-based genetic risk score associated with gestational diabetes antecedent and with progression to pre-diabetes and type 2 diabetes: A cohort study. BJOG 2015, 122, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Blue, C.L. Does the Theory of Planned Behavior Identify Diabetes-Related Cognitions for Intention to Be Physically Active and Eat a Healthy Diet? Public Health Nurs. 2007, 24, 141–150. [Google Scholar] [CrossRef]
- Lakerveld, J.; Bot, S.D.; Chinapaw, M.J.; Knol, D.L.; de Vet, H.C.; Nijpels, G. Measuring pathways towards a healthier lifestyle in the Hoorn Prevention Study: The Determinants of Lifestyle Behavior Questionnaire (DLBQ). Patient Educ. Couns. 2011, 85, e53–e58. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Chavarro, J.E.; Bao, W.; Tobias, D.K.; Ley, S.H.; Forman, J.P.; Liu, A.; Mills, J.; Bowers, K.; et al. Healthful Dietary Patterns and the Risk of Hypertension Among Women with a History of Gestational Diabetes Mellitus: A Prospective Cohort Study. Hypertension 2016, 67, 1157–1165. [Google Scholar] [CrossRef]
- O’Hara, H.; Taylor, J.; Woodside, J.V. The Association of Specific Dietary Patterns with Cardiometabolic Outcomes in Women with a History of Gestational Diabetes Mellitus: A Scoping Review. Nutrients 2023, 15, 1613. [Google Scholar] [CrossRef]
- Mercier, R.; Perron, J.; Weisnagel, S.J.; Robitaille, J. Associations between fruit and vegetables intake and abnormal glucose tolerance among women with prior gestational diabetes mellitus. Eur. J. Nutr. 2019, 58, 689–696. [Google Scholar] [CrossRef]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Olsen, S.; Bao, W.; Bjerregaard, A.A.; Fung, T.T.; Manson, J.E.; Hu, F.B. Healthful dietary patterns and long-term weight change among women with a history of gestational diabetes mellitus. Int. J. Obes. 2016, 40, 1748–1753. [Google Scholar] [CrossRef]
- Evans, M.K.; Patrick, L.J.; Wellington, C.M. Health Behaviours of Postpartum Women with a History of Gestational Diabetes. Can. J. Diabetes 2010, 34, 227–232. [Google Scholar] [CrossRef]
- Hoedjes, M.; Berks, D.; Vogel, I.; Franx, A.; Duvekot, J.J.; Oenema, A.; Steegers, E.A.; Raat, H. Motivators and barriers to a healthy postpartum lifestyle in women at increased cardiovascular and metabolic risk: A focus-group study. Hypertens. Pregnancy 2012, 31, 147–155. [Google Scholar] [CrossRef]
- Diedrichsen, K.H.; Pico, M.L.; Rasmussen, E.S.; Nielsen, K.K.; Dahl-Petersen, I.K.; Ovesen, P.; Damm, P.; Jensen, D.M.; Kampmann, U.; Mathiesen, E.R.; et al. Health literacy, perceived stress, and dietary quality among Danish women with recent gestational diabetes mellitus. Public Health 2025, 245, 105787. [Google Scholar] [CrossRef]
- Dennison, R.A.; Ward, R.J.; Griffin, S.J.; Usher-Smith, J.A. Women’s views on lifestyle changes to reduce the risk of developing Type 2 diabetes after gestational diabetes: A systematic review, qualitative synthesis and recommendations for practice. Diabet. Med. 2019, 36, 702–717. [Google Scholar] [CrossRef]
- Laila, A.; Leme, A.C.; Hou, S.; Ma, D.W.L.; Haines, J. Perceived challenges and strategies to achieve Canada’s Food Guide recommendation to “Cook more often”: Findings from parents of young children. Appetite 2023, 182, 106413. [Google Scholar] [CrossRef]
- Dugas, C.; Kearney, M.; Mercier, R.; Perron, J.; Tchernof, A.; Marc, I.; Weisnagel, S.J.; Robitaille, J. Early life nutrition, glycemic and anthropometric profiles of children exposed to gestational diabetes mellitus in utero. Early Hum. Dev. 2018, 118, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Canadian Diabetes Association. The Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2013, 37, S168–S183. Available online: https://www.sciencedirect.com/journal/canadian-journal-of-diabetes/vol/37/suppl/S1 (accessed on 28 August 2025).
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can. J. Diabetes 2003, 27, S1–S152. [Google Scholar]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2008 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diabetes 2008, 32 (Suppl. 1), S1–S201. [Google Scholar]
- Labonte, M.E.; Cyr, A.; Baril-Gravel, L.; Royer, M.M.; Lamarche, B. Validity and reproducibility of a web-based, self-administered food frequency questionnaire. Eur. J. Clin. Nutr. 2012, 66, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Brassard, D.; Elvidge Munene, L.A.; St-Pierre, S.; Guenther, P.M.; Kirkpatrick, S.I.; Slater, J.; Lemieux, S.; Jessri, M.; Haines, J.; Prowse, R.; et al. Development of the Healthy Eating Food Index (HEFI)-2019 measuring adherence to Canada’s Food Guide 2019 recommendations on healthy food choices. Appl. Physiol. Nutr. Metab. 2022, 47, 595–610. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Lawrence, M.; Costa Louzada, M.L.; Pereira Machado, P. Ultra-Processed Foods, Diet Quality, and Health Using the NOVA Classification System; FAO: Rome, Italy, 2019. [Google Scholar]
- Moubarac, J.C.; Batal, M.; Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520. [Google Scholar] [CrossRef]
- Jessri, M.; Lou, W.Y.; L’Abbe, M.R. Evaluation of different methods to handle misreporting in obesity research: Evidence from the Canadian national nutrition survey. Br. J. Nutr. 2016, 115, 147–159. [Google Scholar] [CrossRef]
- Huang, T.T.; Roberts, S.B.; Howarth, N.C.; McCrory, M.A. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes. Res. 2005, 13, 1205–1217. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids; National Academy Press: Washington, DC, USA, 2005. [Google Scholar]
- Lohman, T.G.; Roche, A.F.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics: Chicago, IL, USA, 1988. [Google Scholar]
- Little, R.R.; Rohlfing, C.; Sacks, D.B. The National Glycohemoglobin Standardization Program: Over 20 Years of Improving Hemoglobin A(1c) Measurement. Clin. Chem. 2019, 65, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- McNarnara, J.R.; Schaefer, E.J. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta 1987, 166, 1–8. [Google Scholar] [CrossRef]
- Després, J.P.; Lemieux, I.; Dagenais, G.R.; Cantin, B.; Lamarche, B. HDL-cholesterol as a marker of coronary heart disease risk: The Québec cardiovascular study. Atherosclerosis 2000, 153, 263–272. [Google Scholar] [CrossRef]
- Belanger, M.; Dugas, C.; Perron, J.; Ruchat, S.M.; Weisnagel, S.J.; Marc, I.; Tchernof, A.; Robitaille, J. Association between lifestyle habits and adiposity values among children exposed and unexposed to gestational diabetes mellitus in utero. Diabetes Metab. Syndr. 2019, 13, 2947–2952. [Google Scholar] [CrossRef] [PubMed]
- Bassett-Gunter, R.L.; Levy-Milne, R.; Naylor, P.J.; Downs, D.S.; Benoit, C.; Warburton, D.E.R.; Blanchard, C.M.; Rhodes, R.E. Oh baby! Motivation for healthy eating during parenthood transitions: A longitudinal examination with a theory of planned behavior perspective. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L. Making time for family meals: Parental influences, home eating environments, barriers and protective factors. Physiol. Behav. 2018, 193, 248–251. [Google Scholar] [CrossRef]
- Reczek, C.; Beth Thomeer, M.; Lodge, A.C.; Umberson, D.; Underhill, M. Diet and Exercise in Parenthood: A Social Control Perspective. J. Marriage Fam. 2014, 76, 1047–1062. [Google Scholar] [CrossRef]
- Carbonneau, E.; Lamarche, B.; Provencher, V.; Desroches, S.; Robitaille, J.; Vohl, M.C.; Begin, C.; Belanger, M.; Couillard, C.; Pelletier, L.; et al. Associations Between Nutrition Knowledge and Overall Diet Quality: The Moderating Role of Sociodemographic Characteristics-Results From the PREDISE Study. Am. J. Health Promot. 2021, 35, 38–47. [Google Scholar] [CrossRef]
- Blake, C.E.; Wethington, E.; Farrell, T.J.; Bisogni, C.A.; Devine, C.M. Behavioral contexts, food-choice coping strategies, and dietary quality of a multiethnic sample of employed parents. J. Am. Diet. Assoc. 2011, 111, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Colpani, V.; Baena, C.P.; Jaspers, L.; van Dijk, G.M.; Dhana, K.; Tielemans, M.J.; Voortman, T.; Freak-Poli, R.; Veloso, G.G.V.; Kavousi, M.; et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderlywomen: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Ortenblad, L.; Hotoft, D.; Krogh, R.H.; Lynggaard, V.; Juel Christiansen, J.; Vinther Nielsen, C.; Hedeager Momsen, A.M. Women’s perspectives on motivational factors for lifestyle changes after gestational diabetes and implications for diabetes prevention interventions. Endocrinol. Diabetes Metab. 2021, 4, e00248. [Google Scholar] [CrossRef]
- Nicklas, J.M.; Zera, C.A.; Seely, E.W.; Abdul-Rahim, Z.S.; Rudloff, N.D.; Levkoff, S.E. Identifying postpartum intervention approaches to prevent type 2 diabetes in women with a history of gestational diabetes. BMC Pregnancy Childbirth 2011, 11, 23. [Google Scholar] [CrossRef]
- Lambert, V.; Munoz, S.E.; Gil, C.; Roman, M.D. Maternal dietary components in the development of gestational diabetes mellitus: A systematic review of observational studies to timely promotion of health. Nutr. J. 2023, 22, 15. [Google Scholar] [CrossRef]
- Dubois, N.; Giroux, I. Gestational Diabetes Mellitus: Efficacy of Non-Pharmacological Interventions for Management and Prevention. Healthcare 2025, 13, 2261. [Google Scholar] [CrossRef]
- Lim, S.; Makama, M.; Ioannou, E.; Skouteris, H.; Montanaro, C.; Taye, M.; Kodapally, B.; Moran, L.J.; Chirp; Reja, A.; et al. Values, principles and research priorities for the implementation of type 2 diabetes prevention after gestational diabetes: A global consensus from Asia, Africa, Americas, Europe and Oceania. Diabet. Med. 2025, 42, e70017. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, E.P.; Narayan, K.M.; Reilly, C.M.; Foster, J.; McCullough, M.; Ziegler, T.R.; Guo, Y.; Dunbar, S.B. Dietary self-efficacy predicts AHEI diet quality in women with previous gestational diabetes. Diabetes Educ. 2014, 40, 688–699. [Google Scholar] [CrossRef]
- Vu, A.; Turk, N.; Duru, O.K.; Mangione, C.M.; Panchal, H.; Amaya, S.; Castellon-Lopez, Y.; Norris, K.; Moin, T. Association of Type 2 Diabetes Risk Perception with Interest in Diabetes Prevention Strategies Among Women with a History of Gestational Diabetes. Diabetes Spectr. 2022, 35, 335–343. [Google Scholar] [CrossRef]
- Sharma, M.; Purewal, T.S.; Fallows, S.; Kennedy, L. The low-risk perception of developing type 2 diabetes among women with a previous history of gestational diabetes: A qualitative study. Pract. Diabetes 2019, 36, 15–19b. [Google Scholar] [CrossRef]
- Fertig, A.R.; Loth, K.A.; Trofholz, A.C.; Tate, A.D.; Miner, M.; Neumark-Sztainer, D.; Berge, J.M. Compared to Pre-prepared Meals, Fully and Partly Home-Cooked Meals in Diverse Families with Young Children Are More Likely to Include Nutritious Ingredients. J. Acad. Nutr. Diet. 2019, 119, 818–830. [Google Scholar] [CrossRef]
- Larson, N.I.; Perry, C.L.; Story, M.; Neumark-Sztainer, D. Food preparation by young adults is associated with better diet quality. J. Am. Diet. Assoc. 2006, 106, 2001–2007. [Google Scholar] [CrossRef]
- Martins, C.A.; Andrade, G.C.; Oliveira, M.F.B.; Rauber, F.; Castro, I.R.R.; Couto, M.T.; Levy, R.B. “Healthy”, “usual” and “convenience” cooking practices patterns: How do they influence children’s food consumption? Appetite 2021, 158, 105018. [Google Scholar] [CrossRef]
- Nansel, T.R.; Cummings, J.R.; Burger, K.; Siega-Riz, A.M.; Lipsky, L.M. Greater Ultra-Processed Food Intake during Pregnancy and Postpartum Is Associated with Multiple Aspects of Lower Diet Quality. Nutrients 2022, 14, 3933. [Google Scholar] [CrossRef] [PubMed]
- Laska, M.N.; Hearst, M.O.; Lust, K.; Lytle, L.A.; Story, M. How we eat what we eat: Identifying meal routines and practices most strongly associated with healthy and unhealthy dietary factors among young adults. Public Health Nutr. 2015, 18, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Aubé, J.; Marquis, M. Attitudes et habitudes de Canadiens relativement à la planification des repas et à la cuisine maison. Can. J. Diet. Pract. Res. 2011, 72, 70–75. [Google Scholar] [CrossRef]
- Fisher, M.; Smith, G.; Potter, B.K.; Arbuckle, T.E.; Little, J.; Weiler, H.; Morisset, A.S.; Lanphear, B.; Braun, J.M.; Kumarathasan, P.; et al. The Association between Pregnancy Complications and Long-Term Maternal Cardiometabolic Health in the MIREC Cohort Study. J. Clin. Endocrinol. Metab. 2025, 110, 2879–2891. [Google Scholar] [CrossRef]
- Khan, S.S.; Cameron, N.A.; Lindley, K.J. Pregnancy as an Early Cardiovascular Moment: Peripartum Cardiovascular Health. Circ. Res. 2023, 132, 1584–1606. [Google Scholar] [CrossRef]
- Imboden, M.T.; Welch, W.A.; Swartz, A.M.; Montoye, A.H.K.; Finch, H.W.; Harber, M.P.; Kaminsky, L.A. Reference standards for body fat measures using GE dual energy x-ray absorptiometry in Caucasian adults. PLoS ONE 2017, 12, e0175110. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.; Gupta, Y.; Hernandez, T.L.; Levitt, N.; van Poppel, M.; Yang, X.; Zarowsky, C.; Backman, H.; Feghali, M.; Nielsen, K.K. Call to action for a life course approach. Lancet 2024, 404, 193–214. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).