Abstract
Background: Gestational diabetes mellitus (GDM) is a type of diabetes diagnosed during pregnancy and its prevalence is on the rise around the world. GDM increases the risk of serious adverse health outcomes for the mother and child. Multiple types of non-pharmacological interventions have been developed for the management and prevention of GDM; however, there is a lack of clarity regarding their effectiveness. Objective: To summarize the evidence on the efficacy of non-pharmacological interventions in the management and prevention of GDM. Methods: For this integrative review, a comprehensive literature search was conducted in the databases MEDLINE, CINAHL, Embase, Scopus, and Web of Science. The methodology followed the integrative approach outlined by Whittemore and Knafl’s, and study quality was evaluated using the Mixed Methods Assessment Tool. Results: A total of 44 relevant studies were included. Key themes identified for GDM management were (1) nutrition therapy and physical activity, (2) social and psychological support, (3) digital tools, and (4) barriers and facilitators. For GDM prevention, themes were categorized into individual-level approaches, (5) lifestyle and supplements, and population-level approaches: (6) environmental factors, and (7) health in all policies. Conclusions: The growing prevalence of GDM is a major public health concern that requires the implementation of effective multi-level evidence-based strategies. Environmental, socioeconomic, and racial determinants of health have substantial impacts on GDM, highlighting the need to address the root causes of the illness. Further research is needed to support effective preventive and management measures beyond standard pharmacological treatment, so that evidence-based solutions can be applied to enhance and safeguard the health of current and future generations.
Keywords:
gestational diabetes; management; prevention; intervention; pregnancy; health; lifestyle; nutrition; physical activity 1. Introduction
Gestational diabetes mellitus (GDM) has become more prevalent globally over the past few decades and is anticipated to continue its upward trend [1,2]. This rise is driven by higher rates of obesity in women of reproductive age, delayed childbearing, and the implementation of revised diagnostic criteria [2]. GDM is characterized by any level of glucose intolerance that arises or is first detected during pregnancy [3]. The prevalence of GDM among pregnant women typically ranges from 3% to 20% [4]. This condition is transient and develops during gestation but subsides after delivery [5]. For women with a prior history of the illness, research indicates that the probability of having GDM in future pregnancies ranges from 30 to 80%, with prevalence rates differing according to risk factors [6]. Furthermore, the likelihood of developing type 2 diabetes (T2D) in the future is estimated to be more than seven times greater for women who have had GDM compared to those with normal glucose levels during pregnancy [7]. Risk factors for GDM include pre-pregnancy overweight or obesity, being 35 years or older, a personal or family history of diabetes, and belonging to high-risk ethnic groups such as South Asian, Hispanic/Latino, Indigenous, Black/African American, Pacific Islander, and Middle Eastern [4,8,9]. Fetal complications related to GDM include macrosomia, congenital abnormalities, growth retardation, premature birth, and stillbirth [10,11]. GDM also increases the risk of developing obesity and T2D for the offspring [12,13]. Overall, the healthcare cost for an individual with GDM is estimated to be at least 25% more than for a woman without the disease, indicating that the economic burden associated with this condition is substantial [14,15]. GDM has far-reaching implications on public health as it perpetuates the detrimental cycle of diabetes and obesity over successive generations [16,17]. This highlights the importance of effective interventions to prevent the development of GDM and facilitate its management to help reduce or delay its recurrence and complications.
Although various interventions have been developed to prevent and manage GDM beyond standard pharmacological treatments typically involving insulin therapy, there is a lack of clarity regarding their effectiveness. Therefore, this integrative review aims to summarize the evidence on the efficacy of these non-pharmacological interventions in the management and prevention of GDM.
2. Methods
This study used an integrative review methodology to systematically synthesize the existing body of literature. The integrative review approach is particularly valuable for exploring complex research topics, as it allows for the inclusion of studies with varying methodologies and thus enables the development of a comprehensive understanding of the phenomenon being studied [18,19]. The review was conducted in alignment with the five-step framework proposed by Whittemore and Knalf (2005) [19], which includes: (1) identification of the research problem, involving a clear articulation of the issue and objective of the review; (2) conduct of a structured literature search using a strategy that incorporate databases and additional sources as needed to ensure an exhaustive retrieval of relevant studies; (3) selection and appraisal of data, where studies are assessed according to methodological criteria appropriate to their design; (4) data analysis, which entail synthesizing results to identify central themes and patterns across different types of studies; and (5) presentation of the findings, which involve summarizing key results using tools such as tables to support comparison and interpretation across studies.
2.1. Search Strategy
This integrative review was conducted to address the research question: “What is known from the current literature regarding the efficacy of non-pharmacological interventions for the management and prevention of GDM?” A literature search was performed across the databases MEDLINE, CINAHL, Embase, PsycINFO, Scopus, and Web of Science, covering all available publications up to August 2024. In addition to searches in electronic databases, manual searches of reference lists from relevant sources were conducted to identify further material that might not have been captured through database queries. Inclusion criteria were peer-reviewed scientific articles available in full text and published in either English or French, consistent with the authors’ language proficiency. No restrictions were imposed on the year of publication or type of study design. Following the principles of integrative review methodology, which seeks to integrate diverse sources of evidence to achieve a comprehensive understanding of a phenomenon, both primary and review-level studies were included in the search. This encompassed systematic reviews and meta-analyses, which provide valuable synthesized evidence and highlight broader trends, thereby strengthening and complementing other types of evidence [20,21]. Exclusion criteria included commentaries, editorials, gray literature, unpublished works, and dissertations. Articles that did not contain information relevant to the research question were also excluded. To maximize the scope and depth of the search, a wide range of keywords and database-specific Medical Subject Headings (MeSH) were utilized in various combinations. The terms included “gestational diabetes”, “GDM”, “maternal diabetes”, “diabetes in pregnancy”, “intervention”, “program”, “approach”, “prevention”, “management”, “individual intervention”, “community based”, “population based”, “non-pharmacological”, “self-management”, “self-care”, “efficacy”, “efficiency”, and other relevant synonyms. Boolean operators “AND” and “OR” were employed to refine the search, and truncation and wildcard were applied where appropriate to broaden the yield. All search results were first processed through Covidence “www.covidence.org (accessed on 5 August 2024)” for duplicate removal and preliminary screening of titles and abstracts. Articles deemed potentially eligible were then exported to Zotero (version 6.0.36), where full-text screening was carried out in accordance with the inclusion and exclusion criteria.
2.2. Data Analysis and Quality Assessment
Data analysis was guided by thematic analysis using an inductive methodology based on the frameworks proposed by Popenoe et al. (2021) and Dwyer (2020) [22,23]. This strategy enables researchers to consider multiple perspectives, identify unanticipated themes, and minimize bias by grounding findings directly in the literature [22,23]. This is particularly valuable for integrative reviews, which synthesize evidence from diverse sources and require an approach capable of capturing complexity while revealing overarching patterns [22,23].
The quality of the studies included in the review was assessed collaboratively by both authors using the Mixed Methods Appraisal Tool (MMAT) version 2018. The MMAT was chosen for its ability to evaluate a variety of research designs including quantitative, qualitative, and mixed methods, making it especially suitable for integrative reviews [24]. This tool allows to assess study quality across five domains: clarity of research questions, suitability of data collection methods, adequacy of the study sample, rigor of analysis, and alignment of findings with data, with higher scores indicating stronger methodological quality [24]. This assessment tool is also recognized for its balanced combination of reliability, convenience, and methodological rigor [25].
3. Results
3.1. Characteristics of Included Studies
From the literature search, a total of 323 studies were identified through database searches, with an additional 12 studies found via hand-searching relevant materials and bibliographic references. All retrieved articles were imported into Covidence for the removal of duplicates. Title and abstract screening were conducted within Covidence, after which the remaining 80 articles were exported to Zotero for full-text review. Based on the defined inclusion and exclusion criteria, 36 articles were removed, leaving 44 studies for inclusion in the review (see Figure 1). The characteristics of these included studies are detailed in Table 1. All retrieved studies were written in English; therefore, only English written articles were kept. Most included studies (39/44, 89%) have been published in the past 10 years (2014 to 2024). A large share of the selected articles are systematic reviews (22/44, 50%), including 15 meta-analyses. Regarding the quality assessment, all included studies demonstrated medium to high methodological quality. Among the 44 articles appraised, 25 (57%) obtained a score of 4/5, while 19 (43%) achieved the maximum score of 5/5. No study was rated below 4/5, reflecting the generally high quality of evidence (see Table 1).
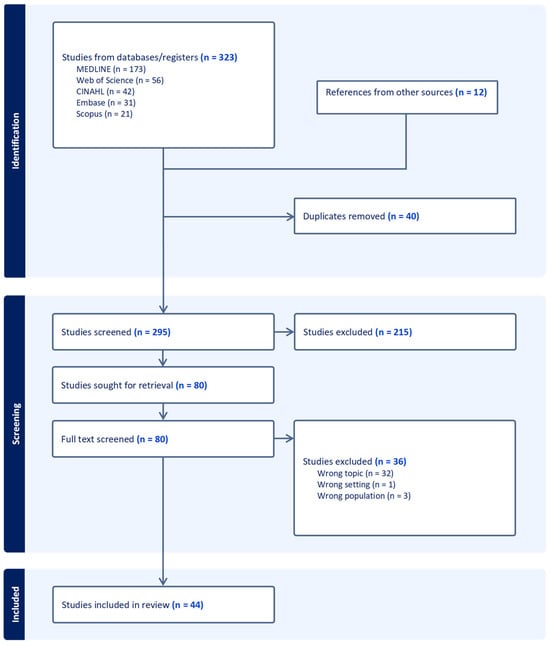
Figure 1.
PRISMA flowchart.

Table 1.
Characteristics of the studies included from the literature search.
3.2. Themes Identified from the Literature Search
The following themes were identified through inductive analysis and are discussed below:
- Section 3.2.1. Interventions for GDM management beyond standard pharmacological treatment:
- Nutrition therapy and physical activity;
- Social and psychological support;
- Digital tools;
- Barriers and facilitators.
- Section 3.2.2. Interventions for GDM prevention:
- Individual-level approaches:
- Lifestyle and supplements.
- Population-level approaches:
- Environmental factors;
- Health in All Policies (HiAP).
3.2.1. Interventions for GDM Management Beyond Standard Pharmacological Treatment
Nutrition Therapy and Physical Activity
Dietary recommendations for women with GDM appear to play a key role in controlling maternal gestational weight gain (GWG) and enhancing blood glucose management, resulting in better health outcomes for the mother and baby. Physical activity during pregnancy seems to promote blood glucose control and improve delivery outcomes for women diagnosed with GDM.
Evidence from systematic reviews suggested that dietary interventions during pregnancy can significantly reduce GWG and lower the risk of GDM, as well as lower the likelihood of cesarean sections, preeclampsia, and preterm birth [53,61,63]. Intensive dietary counseling combined with a low-glycemic index (LGI) diet, particularly when culturally tailored, has been shown to improve blood glucose levels and reduce pregnancy complications among Hispanic women with GDM [32]. According to findings from meta-analyses, dietary and lifestyle modifications helped reduce GWG without adversely impacting fetal growth, with dietary interventions demonstrating the greatest effect compared to physical activity or combined approaches [63]. Adopting a LGI diet and engaging in regular physical activity has also been found to reduce the risk of macrosomia [53]. Personalized nutrition therapy appeared most effective for optimizing blood glucose management, although general nutrition education was still beneficial for women with GDM [43]. Improved glycemic control has been associated with LGI and fiber-enriched diets among Chinese women with GDM [65]. According to meta-analyses data, physical activity during pregnancy was found to have a small protective effect against the onset of GDM but may assist in managing fasting and postprandial blood glucose levels, as well as hemoglobin A1c, in women already diagnosed with the condition [49,57]. Additionally, customized exercise regimens, particularly in the second and third trimesters, combined with dietary guidance, have been shown to improve blood glucose control, self-management, and delivery outcomes in women with GDM according to cross-sectional data [69].
Social and Psychological Support
Evidence indicates that women with GDM often experience depression, which may hinder effective disease management; however, psychological and social support interventions have been shown to improve health outcomes.
Pregnant women with GDM were 3.79 times (OR = 3.79, 95% CI: 1.07–13.45, p = 0.04) more likely to have a history of depression than women without GDM, even after controlling for age, income, marital status, body mass index (BMI), and gravida, according to cross-sectional data [29]. Results from a prospective study indicated that women who lacked adequate social support during pregnancy reported higher rates of depression and a diminished quality of life [36]. Integrating comprehensive psychological support services in conjunction with treatment may enhance pregnant women’s ability to manage GDM more effectively [45]. An experimental study revealed that women with GDM reported experiencing less stress and enhanced control of their blood glucose levels after receiving mindfulness counseling during their pregnancy [68]. Moreover, improvement in GDM self-management as well as a decrease in GWG and macrosomia in infants was observed in a randomized controlled trial (RCT) where both the mother and her partner were involved in learning about GDM and its management [38]. Cross-sectional research further highlighted a strong positive correlation between social capital and self-efficacy in women with GDM [41].
Digital Tools
Mobile health (mHealth) and other digital resources seem beneficial in assisting and facilitating behavior modification and blood glucose level management for women with GDM.
Findings from a systematic review highlighted that digital tools were useful for women diagnosed with GDM by supporting them to adhere to their treatment, engaging in physical activities, and making healthier food choices, however research remains scarce [26]. A meta-analysis further indicated that the addition of mHealth therapies were more effective than standard care for patients with GDM and led to positive outcomes for mothers and infants [66]. Users of mHealth technology among GDM patients reported benefiting from motivational tools (goal setting, risk awareness, and problem solving) which helped them with behavior change and glycemic control according to a qualitative meta-synthesis [52]. Peer support via mHealth was also appreciated, such as being part of a network of women navigating similar challenges, allowing them to encourage one another throughout the process of GDM management and lifestyle modification [52]. However, participants also expressed that meaningful engagement with healthcare professionals remained a crucial component of effective support [52].
Barriers and Facilitators
For pregnant women with GDM, barriers to management often include negative psychosocial effects of the disease, difficulties dealing with the dietary and medical challenges involved, and adapting it to their unique family circumstances. Facilitating factors encompass social support, and the provision of culturally sensitive recommendations tailored to their health literacy and socioeconomic level.
Evidence from a scoping review highlighted that key facilitators of effective GDM management included support from family, friends, and broader social networks [28]. Conversely, common barriers involved inadequate knowledge, financial constraints, low motivation to adopt lifestyle changes, the high cost of healthy foods, and cultural or religious food practices [28]. Barriers to self-management have also been found to include mental strain, social isolation, stigma, and other adverse social and psychological effects associated with GDM [55]. Additional challenges included competing work and family responsibilities including childcare, as well as limited social capital [28]. A systematic review found that pregnant individuals with GDM who had limited knowledge of the condition exhibited higher blood glucose levels and demonstrated poorer self-care attitudes compared to those with a better understanding of GDM, its associated health risks, and effective management strategies [40]. Migrant women with low income and low literacy level were reported to struggle particularly with understanding and following nutritional and medical recommendations for the self-management of GDM, according to findings from qualitative studies and systematic reviews [30,31,32,43,44]. Furthermore, findings from a systematic review revealed that ethnic minority patients were more likely to trust healthcare providers when care was delivered in their preferred language, either directly or via interpreters, and adapted to their health literacy and socioeconomic level [48]. Interventions delivered in culturally familiar and accessible locations helped foster a sense of safety and comfort, thereby enhancing engagement, and served as facilitating factors [48]. Moreover, evidence indicated that women often struggle to prioritize their own dietary needs when these conflict with the interests of their families [64]. Results from a systematic review suggested that barriers to self-management and self-efficacy among women with GDM include a lack of individualized care, limited treatment options, inadequate follow-up options with their healthcare providers, and a feeling of abandonment after childbirth, which can negatively impact the adoption of lasting lifestyle changes [46]. Among facilitating factors, GDM mothers were more likely to be open to interventions when they understood they were making a positive difference for their baby [31,32].
3.2.2. Interventions for GDM Prevention
Individual-Level Approaches
- Lifestyle and Supplements
A growing body of evidence supports the use of both lifestyle modifications and targeted supplements, such as probiotics, myo-inositol and vitamin D, in reducing the risk of GDM, although findings remain inconsistent and at times contradictory. While no single strategy has proven universally effective, interventions that are personalized, initiated early, and supported by healthcare professionals or peer groups seem to yield the best outcomes.
Several recent meta-analyses have examined the impact of physical activity, dietary interventions, and supplements on GDM prevention. Studies reported that combined diet and physical activity interventions, as well as inositol and vitamin D supplementation, significantly reduced GDM incidence in high-risk women identified early in pregnancy [54]. Subgroup analyses suggested that lifestyle interventions were most effective among women with multiple GDM risk factors, while inositol supplementation appeared particularly beneficial for women with overweight or obesity [54]. Likewise, interventions involving lifestyle modification and myo-inositol were found to reduce GDM risk, especially when initiated preconception or during the early first trimester [51]. These strategies demonstrated greater efficacy in women without polycystic ovary syndrome (PCOS) and in those without a prior history of GDM [51]. Results from another recent meta-analysis found a decrease in the incidence of GDM when dietary, physical activity, or diet combined with physical activity therapies were compared to control measures [59]. In contrast, other meta-analytic findings indicated that while interventions targeting a single behavior, either diet or physical activity, can effectively reduce the risk of GDM, combining both into a comprehensive lifestyle intervention unexpectedly did not yield the same benefit [27]. On the other hand, physical activity and probiotic supplementation were found to significantly reduce GDM risk compared to placebo, whereas dietary changes, combined dietary and physical activity interventions, and inositol supplementation did not show significant effects, according to other meta-analytic data [62]. Other evidence suggests that physical activity-only therapies reduced the likelihood of developing GDM compared to no exercise at all, and exercise alone was more effective than programs combining it with other therapies [34]. Focused intervention with high-risk women utilizing a combination of dietary changes and exercises was found effective at reducing GDM risk, according to meta-analyses [39,51,54,58]. However, evidence from another meta-analysis showed that preventing GDM in overweight and obese women was not significantly helped by diet, physical activity or a combination of diet and exercise, even though those interventions were associated with lower GWG [67]. Pregnancy-related GDM risk appeared to be reduced in those who followed healthy eating habits before pregnancy such as the Mediterranean diet, according to a systematic review [35]. However, dietary interventions implemented during pregnancy were found to be less effective to prevent the development of GDM, particularly in high-risk groups [35]. Lastly, findings from a meta-analysis suggested that physical activity interventions carried out in healthcare facilities or in group-setting with supervision and feedback from professionals were found to be more effective at preventing GDM than those in community or individual settings [59].
Population-Level Approaches
- Environmental Factors
The way urban spaces are designed can influence lifestyle habits, which in turn can promote physical and mental well-being and reduce the prevalence of certain diseases. Green space exposure during pregnancy is linked to better maternal glucose regulation, while low access to green space is associated with increased risks of GDM and other pregnancy complications, including preeclampsia, preterm birth, and depression.
Findings from a prospective birth cohort study (N = 6807) indicated that greater access to green space was significantly associated with reduced maternal blood glucose levels and a lower risk of developing impaired glucose tolerance and GDM [50]. Similarly, a birth cohort study (N = 5814) reported that higher residential exposure to greenery was linked to lower levels of the hyperglycemic biomarker hemoglobin (Hb) A1c during mid-to-late pregnancy in women diagnosed with GDM [42]. A retrospective birth cohort study (N = 238,922) found that women living in areas with the lowest levels of green space faced the highest risks of developing GDM and mental health disorders [56]. Additionally, pregnant women with limited or poor access to green space within walking distance were more likely to experience preeclampsia, preterm birth, or depression [56]. Furthermore, in a prospective cohort study (N = 9155), researchers found that pregnant women residing in food deserts or less walkable neighborhoods had higher odds of developing GDM, after adjusting for known covariates [37].
- 2.
- Health in All Policies
To effectively address the growing burden of GDM, it is essential to acknowledge that health is influenced by factors beyond the healthcare system. The HiAP approach, which emphasizes collaboration across sectors to address the determinants of health, promotes comprehensive and preventive strategies addressing the root causes of diseases to improve population health and well-being.
Research highlights the potential of cross-sectoral collaboration in tackling persistent societal challenges such as perinatal health inequities [33]. These disparities arise from a complex interplay of social determinants, which requires coordinated action among professionals from local governments, healthcare, social services, and public health sectors [33]. The HiAP framework supports this by recognizing that health is deeply connected to social, cultural, environmental, and economic factors, and therefore requires cooperation across multiple sectors [33,60]. Active community engagement is also a key component of multisectoral strategies, particularly in addressing social determinants of health [47]. Consistent involvement of diverse sectors, agencies, and civil society can help co-develop culturally appropriate and effective interventions, while also fostering stronger communication and trust between communities and service providers [47]. Additionally, evidence indicates that achieving the HiAP agenda requires strategic leadership, strong data systems, sufficient financial investment, and well-coordinated intersectoral efforts [60].
4. Discussion
4.1. Discussion of Main Findings
The purpose of this integrative review was to summarize the evidence on the efficacy of non-pharmacological interventions in the management and prevention of GDM.
4.1.1. Interventions for GDM Management Beyond Standard Pharmacological Treatment
Nutrition Therapy and Physical Activity
In terms of GDM management, which aims to control blood glucose levels within appropriate targets during pregnancy, the first line of treatment typically consists of dietary modifications and increased physical activity, along with regular blood glucose self-monitoring [4]. Insulin therapy is usually introduced only if blood glucose levels remain elevated despite these lifestyle modifications [4]. Findings from this review indicate that nutritional therapy can effectively prevent excess maternal gestational weight gain and improve glycemic control, leading to better outcomes for both mother and baby [32,43,53,61,63,65]. In addition, pregnant women with GDM who exercise regularly may have enhanced control of their blood glucose levels and safer delivery [49,57,69]. Research suggests that addressing systemic obstacles at the organizational level, such as offering childcare services, proved successful in facilitating increased physical activity among women with GDM [70].
Psychological Support
A growing body of evidence indicates that individuals diagnosed with GDM are particularly vulnerable to developing depression, which can negatively affect their overall treatment of the condition and blood glucose levels [29,71,72,73,74,75,76,77]. Research highlights that women with GDM have reported encountering stigma, often in the form of negative stereotypes that overlap with those associated with obesity and T2D [78,79,80,81]. However, GDM is uniquely perceived as a deviation from a normal pregnancy and is closely tied to societal expectations about the concept of being a “good mother” [79,80,81]. The stigma surrounding GDM is often amplified by these cultural expectations of ideal motherhood [79,80,81]. In many cultures, women are expected to put the well-being of the whole family before their own needs and to be responsible for domestic tasks and childcare, which can create major barriers to self-care and GDM management [82,83,84,85,86,87]. This often leads to internalized stigma, including guilt, self-blame, shame, and anxiety, and may negatively influence both physical and mental health, potentially leading to psychological distress and self-isolation [78,79,80,81]. It can also contribute to maladaptive coping behaviors, including avoidance of blood glucose monitoring, and low engagement with healthcare services [78,79,81,88,89,90]. Preexisting mental health issues can further complicate GDM self-management, which is concerning given the increased prevalence of past depression among this population [36,89,90]. Additionally, women affected by both depression and GDM tend to experience worse perinatal outcomes compared to those diagnosed only with GDM, according to findings from a cohort study in the United States, (N = 170,572) [75]. Evidence from a meta-analysis reported that GDM also contributes to an elevated risk of postpartum depression, with relative risk of 1.59 (95% CI: 1.22–2.07, p = 0.001) [71]. These findings underscore the need for routine psychological screening and individualized interventions to address GDM-related mental health challenges through specialized care.
Social Support
Findings from this review suggest that peer support is effective in helping individuals with GDM self-management and depressive symptoms [38,41,45]. In addition, social support networks have been found to influence not only maternal emotional well-being and quality of life, but also health behaviors and lifestyle choices during pregnancy, including dietary habits [36]. The size and quality of one’s social network were closely linked to health outcomes, as strong relationships provide emotional support that fosters better mental well-being and more effective decision-making in GDM self-management [41]. Low social support in pregnant women has been associated with weakened psychosocial resources, including diminished social integration and stability, which often translates into inadequate assistance during their pregnancy [36]. Disease management of GDM improved among women when their partners were actively engaged, offering both emotional encouragement and practical assistance [38]. Peers can also provide guidance on accessing affordable, nutritious food options and motivate one another to participate in group exercise activities within a safe environment [91,92]. Additionally, strengthening social capital among individuals with diabetes was found to improve blood glucose control, as those with cohesive family relationships, robust social networks, and frequent communication tend to have greater self-efficacy in managing and preventing the condition [93,94].
Digital Tools
According to the World Health Organization (WHO), the use of digital technologies is rapidly expanding in both the public and private sectors of healthcare [95]. Among the objectives of digital health is the promotion and support of sustainable lifestyle behaviors that contribute to the effective prevention and management of various health conditions [26]. Additionally, these platforms aim to reduce the need for unnecessary in-person healthcare visits by providing timely health information and guidance [26]. The widespread adoption of mHealth in perinatal care enables healthcare providers to support pregnant women through various digital means, including mobile phones, text messaging, emails, applications, online health journals, and integrated digital networks [96,97]. Findings from this review indicate that mHealth and other digital resources appear to be beneficial in promoting treatment adherence and facilitating behavior change among women with GDM [26,52,66]. Similarly, results from a RCT showed that GDM patients assigned to smartphone-supported care displayed improved compliance with blood glucose monitoring, better glycemic outcomes, and a reduced need for insulin therapy [98]. Furthermore, meta-analyses reported that mHealth interventions positively influenced glycemic control and reduced adverse pregnancy outcomes in individuals with GDM, and contributed to a lower incidence of neonatal intensive care unit admissions [66,99,100]. In contrast, evidence on the effectiveness of mHealth in supporting dietary management for GDM remains inconsistent [26,101]. Research suggests that effective digital solutions for nutritional therapy in GDM should be user-friendly for diet tracking, adaptable to individual dietary needs and cultural preferences, and grounded in an evidence-based framework [26,101]. In addition, data from a meta-analysis indicate that mHealth initiatives often prioritize behavioral change and treatment adherence, but frequently fail to acknowledge the impact of mental health challenges on self-care [102]. On the other hand, a meta-analysis found that lifestyle therapies based on mHealth may help alleviate symptoms of depression and anxiety in women with GDM [103]. Similarly, other studies have reported inconsistent effects of mHealth on psychosocial well-being during pregnancy, and the wide variation in research designs, settings, and assessment tools makes it difficult to generalize findings [104]. Existing research suggests that mHealth and digital tools may be especially beneficial for pregnant women from socioeconomically disadvantaged backgrounds, those with pre-existing health conditions, or those managing complex perinatal issues [104,105]. Nevertheless, there is a need for more research to evaluate the long-term implications of mHealth strategies for both maternal and child health [66].
Barriers and Facilitators
According to findings from this review, the management of GDM can be hindered by several barriers, including the negative psychosocial consequences of the condition, challenges in adhering to dietary therapy, competing work and family responsibilities, financial hardship, low health literacy, and a lack of individualized care from healthcare professionals. Facilitating factors include culturally sensitive approaches that are tailored to a woman’s literacy and income level, family and social support, and motivation to prevent negative health outcomes for the baby. Similar findings have been reported in the literature where cultural beliefs, food practices, and familial obligations can substantially influence GDM management [31,87,106,107,108].
- Self-Management Challenges
Evidence suggests that many women feel overwhelmed and anxious following a GDM diagnosis, as they must quickly adapt to intensive lifestyle changes [89,109,110,111,112]. These include multiple daily finger pricks for blood glucose monitoring, which can be painful for some women, along with dietary modifications, increased physical activity, regular healthcare appointments, and, when necessary, insulin therapy requiring self-injection, more frequent blood testing, and a deeper understanding of dietary management as well as hypoglycemia prevention and treatment [89,109,110,111,112]. Conversely, some women perceived a GDM diagnosis as a positive opportunity to improve their lifestyle and make beneficial changes during pregnancy [113]. In addition, qualitative research indicates that participants with GDM often believed dietary restrictions and exercise during pregnancy could be potentially harmful to the baby, particularly in cultures where eating for two and limiting physical activity during pregnancy is the norm [107,114]. Moreover, evidence suggests that a lower level of education and poor health literacy are linked to a diminished understanding of the severity of GDM and the importance of its self-management [115,116,117]. Additionally, social customs, such as feeling obliged to accept food offered by friends or family, can sometimes conflict with clinical recommendations [85,87,118,119]. For many women, food carries deep emotional, cultural and social significance that extends far beyond its nutritional value, as research shows it often serves as a powerful cultural symbol that strengthens family bonds through social gatherings centered around traditional dishes, making dietary changes especially challenging [90,114,120]. In addition, women with GDM often report receiving conflicting or culturally insensitive nutritional advice from healthcare professionals, leading them to seek alternative information within their families and communities [65,90,107,114,118,121]. Studies revealed that to comply with GDM dietary advice, women frequently adapt their traditional recipes or cooking methods, and sometimes even prepare separate meals for themselves and their family, which quickly adds to their daily workload [113,118,120]. Additionally, evidence indicates that women’s perceptions of dietary advice are strongly shaped by the quality of their relationship with their healthcare providers [120]. Empathy, individualized care, and consistent follow-up build positive patient–provider relationships, while a lack of understanding leads some women to feel mistrust and seek information elsewhere [114,120].
- 2.
- Fatalistic Attitude
Research also indicates that fatalism, a belief that life events are supernaturally predetermined, which is prevalent in many cultures, has been identified as a determinant of GDM self-management [122]. This mindset can lead to a sense of powerlessness over health problems, making individuals feel that their condition cannot be changed [122]. Moreover, in cultures and communities with high diabetes prevalence, the widespread acceptance and normalization of diabetes and GDM can lead to fatalistic views, where diabetes development is seen as predetermined and unavoidable [89,117,123,124]. This outlook is linked to reduced perceived control over diabetic conditions and decreased adherence to recommended self-care routines, as people believe their actions have minimal impact on outcomes, which limits their self-efficacy and expectations for positive results [89,117,123,124]. To address these barriers, researchers advocate involving family members, friends, and community networks in diabetes education to counteract fatalistic beliefs and support both GDM management and T2D prevention [123].
- 3.
- Financial Hardship
Furthermore, qualitative evidence suggests that women with GDM from low socioeconomic backgrounds often face financial hardship and food insecurity, limiting their access to nutritious foods such as fresh fruits and vegetables [90,115]. As a result, they may rely on inexpensive energy-dense alternatives, particularly when their primary concern is ensuring their children do not go hungry [89,90]. Women with variable work schedules, irregular eating habits, and insufficient social support for childcare face challenges in maintaining consistent routines necessary for effective glycemic control [89,90]. These factors create substantial barriers to both blood glucose monitoring and treatment adherence [89,90]. The social and economic burdens of living in deprived areas can also intersect with family conflict, violence, unstable housing, and trauma, making lifestyle changes even more difficult while contributing to psychosocial stress that ultimately limits the time, energy, and resources needed to manage the condition effectively [90,115].
- 4.
- Disordered Eating
Additionally, stress, prenatal cravings, depression, and complicated relationship with food often contribute to emotional eating, such as indulging in comfort foods [89,90,125]. For women with a history of disordered eating, managing GDM can exacerbate dysregulated eating behaviors and lead to unhealthy choices [89,90,112,125,126]. Qualitative findings highlight that some women engage in overeating or binging on energy-dense foods, self-induced vomiting to control weight gain and glycemia, or overly restrictive eating to regulate blood glucose levels [89,90,112,125,126].
- 5.
- Culturally Appropriate Care
The literature indicates that important facilitators of GDM management include one-on-one culturally sensitive education tailored to individual needs, ideally delivered by healthcare professionals from similar cultural backgrounds who speak the patient’s preferred language, or with assistance of a trained professional interpreter [111,121,127,128,129]. In contrast, a one-size-fits-all approach is usually viewed as ineffective, reinforcing the need to involve healthcare providers and communities in tailoring culturally appropriate care, including support for sustained lifestyle changes postpartum to reduce the long-term risk of T2D [118,127,130].
4.1.2. Interventions for GDM Prevention
Individual-Level Approaches
In terms of individual interventions to prevent the onset of GDM, this review found conflicting evidence from systematic reviews and meta-analysis regarding the most effective lifestyle strategies to adopt. The heterogeneity of results might be explained by the need for interventions to be compared within similar contexts and targeted groups, considering both individual and structural factors of influence. Although no single strategy has demonstrated universal efficacy, a multifaceted, personalized and culturally relevant approach may provide the most benefit in preventing GDM. Overall, the evidence from this review suggests that individualized interventions, particularly those initiated preconception or in early pregnancy, offer the greatest potential for reducing the risk of GDM [35,51]. Strategies that incorporate dietary modification and structured physical activity appear sometimes effective, especially when tailored to high-risk populations [34,39,51,54,58,59,62]. In contrast, recent research suggests that focusing exclusively on high-risk groups may unintentionally contribute to social stigma, potentially undermining individuals’ efforts to prevent the condition, whereas structural interventions that address the social and environmental determinants of GDM and T2D may represent a safer preventive strategy [131].
- Diet
Findings from meta-analyses indicate that dietary patterns resembling the Mediterranean diet or the Dietary Approaches to Stop Hypertension (DASH) diet, when adopted before or early in pregnancy, are associated with a reduced risk of GDM [132,133,134,135,136,137,138]. The DASH diet, initially developed for hypertension management, promotes the consumption of fruits, vegetables, legumes, nuts, and moderate amounts of low-fat dairy, while limiting excess sodium, animal protein, and sweets [132,139,140]. Beyond blood pressure control, it has demonstrated benefits for cardiovascular health, metabolic syndrome, and glycemic control [141,142,143,144,145,146,147,148]. Similarly, the Mediterranean diet is rich in plant-based, fiber-dense foods and is associated with reduced chronic disease burden [149,150,151,152,153]. Although its precise definition varies by region, the traditional Mediterranean diet is generally characterized by high consumption of fruits, vegetables, legumes, nuts, whole grains, and olive oil, with moderate intake of fish and wine, and minimal amounts of red or processed meat [136,151,152,153,154]. The emphasis on complex carbohydrates and the resulting lower glycemic index may also mediate their protective effects against GDM [155,156]. High intake of dietary fiber and phytochemicals from fruits and vegetables have been linked to improved insulin sensitivity and reduced risk of T2D [157,158,159,160,161]. In contrast, diets high in animal protein, saturated fats, refined sugars, and low in fiber are associated with inflammation, obesity, insulin resistance, and increased cardiovascular risk [162,163,164,165,166,167,168,169,170,171,172]. A growing body of evidence indicates that oxidative stress and pathologic level of inflammation play a central role in pregnancy complications, including GDM, preeclampsia, and poor fetal outcomes [173,174,175,176,177,178,179,180,181,182,183,184]. The Mediterranean, DASH, and similar diets may help prevent GDM by modulating low-grade inflammation, according to research findings [170,185,186,187,188,189,190,191,192]. Obesity is also associated with disrupted inflammatory responses in maternal and fetal tissues and is an important risk factor for GDM [193,194,195,196,197,198,199]. Foods rich in antioxidants such as olive oil, fruits, and vegetables have been shown to reduce inflammatory markers [154,200,201,202]. Polyphenols and flavonoids found in olive oil and other plant-based foods may lower inflammation by downregulating pro-inflammatory gene expression [203,204,205,206,207]. Thus, dietary choices play a crucial role in modulating oxidative stress and inflammation, which is linked to the prevention of GDM [177,178,179,180,183,184,198,202]. It is worth noting that although moderate alcohol intake is traditionally part of the Mediterranean diet, its inclusion conflicts with pregnancy guidelines, as alcohol poses teratogenic and other health risks, with no known safe threshold at any stage [208,209,210]. Consequently, complete abstinence from alcohol is now recommended in many countries and should be clearly emphasized by healthcare providers advising women who are pregnant, breastfeeding, or planning to conceive [211,212,213,214,215,216].
- 2.
- Nutritional Supplements
Other findings from this review indicate that supplements of probiotics, myo-inositol, and vitamin D may be helpful to reduce the likelihood of developing GDM, although more clinical research is needed [51,54,62].
- Probiotics
Findings from meta-analyses indicate that probiotic supplementation may lower the risk of developing GDM, enhance insulin sensitivity, and support glycemic control during pregnancy, while also potentially improving neonatal outcomes in women with GDM [217,218,219,220,221,222,223,224,225,226,227,228]. Emerging evidence further suggests that probiotics may be particularly effective in helping to prevent GDM when introduced early in pregnancy and administered over an extended duration [222,225,229]. These benefits are thought to result from favorable alterations in the gut microbiota, which modulate metabolic and inflammatory pathways, regulate lipid metabolism, and reduce oxidative stress [217,221,227,228,230]. However, the precise mechanisms through which probiotics influence insulin resistance and GDM risk remain unclear [231]. Moreover, substantial heterogeneity across studies contributes to inconsistent results. Further research is needed to determine the most effective strains, dosages, timing, and intervention models of the use of probiotics for the prevention and management of GDM.
- Myo-Inositol
Myo-inositol is a nutriment essential for the formation of cell membranes and for mediating cellular responses to environmental stimuli [232]. It is an isomer of inositol, one of the intracellular mediators involved in insulin signaling, and is recognized as an insulin-sensitizing agent that supports glucose homeostasis [232,233,234,235,236]. According to findings from meta-analyses, myo-inositol supplementation may lower the incidence of GDM and its related complications [232,234,236,237,238,239,240,241,242,243,244,245]. While the beneficial effects of myo-inositol supplementation in the context of GDM appear promising, further investigation is needed to determine the optimal timing for initiation and appropriate dosage and intake frequency.
- Vitamin D
Vitamin D deficiency appears to be linked to an increased risk of GDM, according to meta-analytic evidence, and supplementation may help support glycemic control, improve blood lipid profiles, and reduce adverse neonatal outcomes in individuals with GDM [246,247,248,249,250,251,252,253,254,255,256]. Nonetheless, the underlying biological processes remain to be clearly defined through additional studies.
- 3.
- Physical Activity
Evidence suggests that physical activity interventions are generally safe and beneficial for women with GDM and should be encouraged in the absence of obstetric contraindications [4]. The American College of Obstetricians and Gynecologists (ACOG) recommends that pregnant women with diabetes receive individualized exercise prescriptions to ensure safety and effectiveness [257]. Women using insulin or other glucose-lowering agents need to be informed of the risk of hypoglycemia, as medication doses may require adjustments based on physical activity levels [4,258]. Dietitians with expertise in prenatal care and GDM play a key role in aligning dietary recommendations with exercise regimens and insulin therapy [4,258]. A growing body of evidence from systematic reviews and meta-analyses indicates that supervised, low-to-moderate-intensity exercise initiated in the first trimester can reduce the incidence of GDM, particularly among women who are not overweight or obese [132,138,259,260,261,262]. In contrast, the effectiveness of physical activity alone in women with overweight or obesity remains inconclusive. While some studies highlight a potential benefit when adherence to exercise is high, others report limited or no effect on GDM risk in this group [263,264]. Nonetheless, early implementation of individualized lifestyle interventions focusing on realistic goal setting, a low-glycemic/hypocaloric diet, and regular moderate-intensity exercise, has been associated with reduced GWG and GDM risk in women with elevated BMI [265,266,267,268]. According to findings from a cohort study of 14,451 women, the likelihood of developing GDM increased consistently with higher pre-pregnancy BMI [269]. Compared to women with normal weight, those who were overweight or obese prior to pregnancy had nearly twice and more than twice the odds of developing GDM, with odds ratios of 1.91 and 2.55, respectively [269]. In pregnancies complicated by GDM, both pre-pregnancy obesity and excessive GWG are contributors to complications such as fetal growth disturbances, hypertensive disorders, congenital malformations, macrosomia, stillbirth, and an increased likelihood of cesarean delivery [269,270,271,272,273,274,275,276,277]. These risks extend beyond pregnancy, with associations noted between maternal GDM and postpartum weight retention as well as increased BMI in offspring during childhood and adulthood [278,279,280,281,282,283,284,285,286]. Research also highlights that any participation in physical activity before or during early pregnancy has been associated with a 21–46% reduction in the odds of developing GDM, compared to no activity at all [34,136,287]. Beyond GDM prevention, prenatal exercise is linked to additional maternal and neonatal health benefits, such as modest reductions in maternal weight gain, and significantly lower risks of adverse outcomes including macrosomia, preterm birth, cesarean delivery, fetal growth restriction, and birth trauma in women with GDM [288,289,290]. Maternal benefits also include improved fitness and enhanced psychological well-being [291]. GDM shares many pathophysiological features with T2D, such as insulin resistance, systemic inflammation, and increased adiposity [284,287,292]. Physical activity may counteract these mechanisms by reducing body fat and enhancing insulin sensitivity [287,293,294]. Furthermore, it promotes glucose uptake in skeletal muscle through upregulation of glucose transporter type 4 (GLUT4) and glycogen synthase activity [293,294,295]. Moreover, exercise-induced secretion of interleukin-6 (IL-6) from muscle cells has anti-inflammatory effects by inhibiting cytokines like tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), contributing to reduced insulin resistance [294,296,297,298].
Evidence suggests that women diagnosed with GDM are often motivated to adopt healthier lifestyle behaviors during pregnancy, viewing the diagnosis as a catalyst to protect their unborn child and improve their health habits [113,299,300]. Nutrition has been recognized as the cornerstone of effective diabetes prevention and management during pregnancy, and the literature highlights the importance of personalized nutrition counseling in the context of GDM [4,258,301,302,303,304,305]. However, although the benefits of physical activity during pregnancy are well documented, many women encounter barriers, including time constraints, fatigue, nausea, physical discomfort, and uncertainty about exercise safety [113,299,300]. To address these issues, exercise prescriptions should integrate culturally adapted behavioral strategies and take into account key social and environmental factors, such as access to safe places to exercise, family responsibilities, employment conditions, income level, and social support networks, that influence physical activity behaviors [113,299,300,306,307]. Although staying active before and during pregnancy offers clear benefits, research indicates that it is usually not sufficient on its own to control GWG and manage GDM effectively [4]. Thus, despite promising evidence, high heterogeneity across studies makes it challenging to interpret the true impact of exercise in the context of GDM prevention. This variability may be attributed to differences in study design, physical activity measurement methods, and intervention characteristics, including the type of exercise (e.g., aerobic activities like walking, cycling, or swimming; resistance training including weightlifting and pelvic floor exercises), as well as the timing, frequency, intensity, supervision, and setting of exercise sessions. Other methodological concerns include poor participant compliance, unmeasured confounding factors such as diet, and inconsistent control of these variables across studies. Consequently, further high-quality research is needed to clarify the characteristics of optimal exercise interventions for GDM, how these can be tailored to individual and cultural contexts, and their true effectiveness when accounting for potential confounding factors.
Population-Level Approaches
- Environmental Factors
Research indicates that city policies and environmental planning can help prevent obesity and chronic diseases [308,309,310,311,312]. Community layout is recognized as an important determinant of population health as it can influence dietary choices, physical activity, and leisure time by ensuring, for example, walking distance to facilities and shops, as well as the availability of safe bicycle paths [309,310,311,312,313]. Evidence suggests that the design of buildings and cities might also influence people’s social integration and isolation, impacting their mental health [309]. Research shows that environmental influences can benefit body and mind, including access to green spaces, and having safe and friendly streets [309,314]. Living in greener neighborhoods has been associated with better mental health, with specific improvements observed in symptoms of anxiety and depression [315]. This may be due to the mental restoration provided by natural settings and their role in facilitating social contact and community engagement [315]. A prospective cohort study in the UK (N = 23,865) with a mean follow-up of 11.3 years found that individuals living in the greenest neighborhoods had a 19% lower relative risk of developing T2D compared to those in the least green environments; this association remained statistically significant after adjusting for age, sex, BMI, parental diabetes, and socioeconomic status [310]. Similarly, data from a systematic review and meta-analysis indicated that while urban residence was associated with a higher risk and prevalence of T2D, walkable environments and access to green space were consistently linked to reduced risk and prevalence [311]. Findings from a Danish cohort study (N = 42,775) reported that both commuter and recreational cycling were associated with a reduced risk of T2D [313]. Additionally, cross-sectional research revealed that individuals living in more walkable neighborhoods engaged in higher levels of physical activity, drove less, and exhibited slightly lower obesity rates, regardless of demographic factors [312].
According to findings from this review, exposure to green space during pregnancy supports healthier maternal glucose regulation, whereas limited access is linked to higher risks of GDM and related complications such as preeclampsia, preterm birth, and depression. Multiple cohort studies consistently show that greater proximity to green environments corresponds with lower blood glucose markers in pregnant women, while those in areas with sparse green space, poor neighborhood walkability, or food deserts (areas with limited access to affordable, healthy food options), face elevated odds of metabolic and mental health challenges [37,42,50,56]. Similar findings have been reported in the literature supporting the benefits of green space and walkability on GDM risk and glycemic control. A prospective cohort found that pregnant individuals with pregestational diabetes residing in highly walkable neighborhoods achieved better glycemic control in both early and late pregnancy [316]. Similarly, a retrospective cohort indicated that greater residential green space exposure during the second trimester was significantly linked to a lower risk of developing GDM [317]. Data from a randomized, cluster-controlled trial further revealed that the protective effect of urban greenness on GDM risk was especially strong among socioeconomically disadvantaged women, underscoring the heightened value of green spaces for vulnerable groups [318]. Nevertheless, neighborhoods with lower socioeconomic status frequently have limited green space availability, and residents often worry about the safety and quality of nearby parks and recreational facilities [56]. Ensuring more equitable distribution, access, and safety to green space could serve as a strategy to address health disparities linked to economic disadvantage in underserved communities. Therefore, thoughtful urban design and community planning can promote well-being both before and during pregnancy while offering the advantage of not requiring individual behavioral modifications, ultimately leading to sustained public health benefits. This perspective has been increasingly embraced at national levels, including by Canada’s Chief Public Health Officer, as part of a broader initiative to prevent disease by fostering healthier living environments [314].
- 2.
- Health in All Policies
Finally, findings from this review highlight the usefulness of multisector collaboration in enhancing population health and addressing persistent societal challenges, such as perinatal health inequities. These disparities stem from complex interconnections between medical and social determinants, necessitating coordinated efforts among local authorities and professionals across the medical, social, and public health sectors [33]. The WHO defines HiAP as an approach that acknowledges the profound influence of non-health sectors on population well-being, emphasizing that health outcomes are largely shaped by social and economic conditions governed outside the healthcare system [319]. By integrating health considerations into policymaking at all levels, national, regional, and local, HiAP shifts the focus from individual responsibility to systemic and policy-related drivers of health [320]. This approach aims to implement policies that impact areas such as public safety, housing, transportation, agriculture, education, and marketing, to promote health equity and prevent diseases [319,321,322,323,324]. Accordingly, evidence shows that environmental and socioeconomic determinants of health have a substantial impact on the incidence, morbidity, and mortality rates of chronic diseases, including diabetes and perinatal health outcomes [33,37,131,325,326,327,328]. As explained by WHO, social determinants of health are non-medical factors that influence well-being, encompassing the conditions in which people are born, grow, live, work, and age, shaped by the distribution of money, power, and resources [329]. Key determinants include income and social status, education and literacy, employment and working conditions, social support networks, culture, race and ethnicity, gender, and access to healthcare and healthy environments [329,330,331]. A focus on HiAP requires cooperation with the healthcare system and all stakeholders to reverse the growing prevalence of GDM and its consequences [33,332]. By targeting upstream determinants of health, HiAP promotes more aligned and effective policymaking, linking policies from various sectors to health outcomes [320]. Improving health, therefore, involves, for example, reducing unemployment and social exclusion, ensuring income security, improving housing, enhancing education, and implementing policies on the marketing of unhealthy foods and beverages [320]. As outlined in Frieden’s framework for public health action [333], preventive interventions that target the broader determinants of health typically have the greatest population-level impact while requiring the least individual effort, compared to downstream strategies that focus on individual behavior change after a disease diagnosis, such as GDM [333]. This underscores the importance of addressing the underlying causes of GDM to halt its increasing prevalence and associated outcomes. However, despite the recognized value of intersectoral collaboration in advancing HiAP, research indicates that implementation often remains challenging due to weak institutional frameworks, unclear accountability across sectors, and limited financial resources [334,335]. Evidence also highlights that such collaboration is frequently hindered by structural, cultural, and practical barriers; however it can be overcome through strong political backing, a shared vision, and the ability to align interests across sectors [33,60,332,334,335]. Insights from Finland’s long-standing HiAP initiative demonstrate that this framework can effectively advance public health in contemporary societies [336,337]. Its success in Finland has relied on sustained intersectoral collaboration, which requires health sector personnel to have the time, expertise, and resources to engage with other sectors, along with access to comprehensive data on health and its determinants, and the capacity to analyze policy impacts across all levels of governance [336,337]. Broad public support also depends on ensuring that the general population, political leaders, civil servants, and the media understand health literacy concepts and their wider societal implications [336,337]. In addition, legislative foundations have proven especially valuable for maintaining the long-term consistency and sustainability of HiAP initiatives [336,337].
4.2. Recommendations for Practice, Policy, and Research
To improve the management of GDM, healthcare providers should adopt holistic, person-centered approaches that consider each individual’s unique circumstances, including socioeconomic status, cultural background, and health literacy level, and how these factors influence adherence to treatment. Recommendations for nutrition and physical activity need to be tailored to reflect considerations like affordability, accessibility, cultural and religious preferences, and family responsibilities. Psychological support should also be offered from the beginning of pregnancy and continue through the postpartum period to help prevent and manage emotional distress and depression. Additionally, integrating mHealth digital tools into standard care may help enhance women’s ability to self-manage their condition. Postpartum services need to be offered to support women in sustaining positive long-term lifestyle changes to prevent T2D.
Preventing GDM requires moving beyond individual-level interventions to address the upstream social and environmental determinants of health. Preventive strategies should include investment in preconception care, especially for underserved populations, to address disparities and improve maternal and neonatal health outcomes. Policies must acknowledge that GDM risk is shaped by broader conditions like poverty, neighborhood safety, food security, access to green spaces, housing quality, and job stability. This means rethinking policy priorities and investing in initiatives such as healthy food programs, safe and walkable infrastructure, equitable employment practices, and affordable housing; factors that collectively create environments supportive of metabolic and reproductive health. To achieve this, policy frameworks like HiAP can guide intersectoral strategies to ensure health considerations are integrated across sectors like transportation, housing, urban planning, labor, and education.
Further research is essential to address knowledge gaps in the prevention and management of GDM. There is a growing need to study effective training models for healthcare providers, including strategies for interprofessional collaboration in the culturally competent care of patients with GDM, both before and after professional licensure. Well-designed trials are also needed to evaluate the effectiveness of non-pharmacological interventions, including physical activity, diet, and supplementation. Special attention should be given to high-risk groups such as migrants and individuals with low socioeconomic status, to identify effective, culturally appropriate strategies. Furthermore, research should investigate the role of GDM-related stigma, its prevalence across various populations including ethnic minorities, and its impact on mental health, glycemic control, and overall maternal and fetal health. Understanding how social determinants of health, especially those linked to racial, ethnic, and socioeconomic inequalities, affect outcomes in women with GDM should be research priority. Finally, studies must assess the impact of broader policy changes on maternal health and identify how best to adapt interventions for diverse communities.
4.3. Strengths and Limitations
This integrative review has both strengths and limitations. Among its strengths are the inclusion of numerous peer-reviewed studies, many of which are systematic reviews and meta-analyses, and the inclusion of varied methodological approaches allowing for a more comprehensive and multifaceted examination of the research topic. All included studies were assessed to be of medium to high methodological quality. Nevertheless, synthesizing findings from multiple study designs can be complex and potentially introduce bias. This was mitigated through a structured, rigorous approach based on established frameworks. However, the review only included literature published in English, which may have excluded relevant studies in other languages. Furthermore, as most included articles originated from high-income countries, the generalizability of the findings to developing nations may be limited.
5. Conclusions
The rising prevalence of GDM, with its profound impact on maternal and child health and substantial burden on healthcare systems, underscores the need for a comprehensive, multi-level public health response. This integrative review highlights that effective GDM management requires interventions that are culturally appropriate, adapted to individual literacy levels, and incorporate psychological and social support. Digital health tools also offer a valuable avenue for improving patient adherence.
Prevention efforts should focus on pre-pregnancy lifestyle modifications, including plant-rich diets and regular physical activity, while some nutritional supplements appear promising but need further clinical validation. Importantly, addressing the root causes of GDM, including its environmental, socioeconomic, and racial determinants of health, is essential for mitigating disease incidence and severity. A multisectoral approach, such as the HiAP framework, can be helpful in fostering collaboration across sectors to build supportive environments, promote healthy behaviors, and reduce inequities and disease burden. Further research is needed to support effective preventive and management measures beyond standard pharmacological treatment, so that evidence-based solutions can be applied to enhance and safeguard the health of current and future generations.
Author Contributions
Conceptualization, N.D.; methodology, N.D.; formal analysis, N.D. and I.G.; investigation, N.D.; writing—original draft preparation, N.D.; writing—review and editing, N.D. and I.G.; supervision, I.G.; project administration, N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACOG | American College of Obstetricians and Gynecologists |
| BMI | Body mass index |
| DASH | Dietary Approaches to Stop Hypertension |
| GDM | Gestational Diabetes Mellitus |
| GLUT | Glucose transporter |
| GWG | Gestational weight gain |
| Hb | Hemoglobin |
| HiAP | Health in All Policies |
| IL | Interleukin |
| LGI | Low-glycemic index |
| MeSH | Medical Subject Headings |
| MMAT | Mixed Methods Appraisal Tool |
| mHealth | Mobile health |
| PCOS | Polycystic ovary syndrome |
| RCT | Randomized controlled trial |
| TNF-α | Tumor necrosis factor alpha |
| T2D | Type 2 diabetes |
| UK | United Kingdom |
| U.S. | United States |
| WHO | World Health Organization |
References
- Saeedi, M.; Cao, Y.; Fadl, H.; Gustafson, H.; Simmons, D. Increasing Prevalence of Gestational Diabetes Mellitus When Implementing the IADPSG Criteria: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 172, 108642. [Google Scholar] [CrossRef]
- Sweeting, A.; Wong, J.; Murphy, H.R.; Ross, G.P. A Clinical Update on Gestational Diabetes Mellitus. Endocr. Rev. 2022, 43, 763–793. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2025. Diabetes Care 2024, 48, S27–S49. [Google Scholar] [CrossRef]
- Diabetes Canada Diabetes and Pregnancy. Clinical Practice Guidelines. Available online: https://guidelines.diabetes.ca/cpg/chapter36 (accessed on 10 August 2024).
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Kim, C.; Berger, D.K.; Chamany, S. Recurrence of Gestational Diabetes Mellitus: A Systematic Review. Diabetes Care 2007, 30, 1314–1319. [Google Scholar] [CrossRef]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 Diabetes Mellitus after Gestational Diabetes: A Systematic Review and Meta-Analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Lynch, C.D.; Powe, C.E.; Costantine, M.M.; Thung, S.F.; Gabbe, S.G.; Grobman, W.A.; Landon, M.B. Risk of Adverse Pregnancy Outcomes among Pregnant Individuals with Gestational Diabetes by Race and Ethnicity in the United States, 2014–2020. JAMA 2022, 327, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, E.; Eliner, Y.; Chervenak, F.A.; Grünebaum, A. Racial Disparity in Pregnancy Risks and Complications in the US: Temporal Changes during 2007–2018. J. Clin. Med. 2020, 9, 1414. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.M.; Arnegard, M.E.; Maric-Bilkan, C. Dysglycemia in Pregnancy and Maternal/Fetal Outcomes. J. Women’s Health 2021, 30, 187–193. [Google Scholar] [CrossRef]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef]
- Guan, J.; Qiu, J.; Li, L.; Fu, M.; Zhang, M.; Wu, Y.; Xu, Y.; Ding, H.; Gao, Q. A Meta-analysis of Adverse Offspring Health Outcomes in Patients with Gestational Diabetes Mellitus. Diabetes Obes. Metab. 2025, 27, 3555–3567. [Google Scholar] [CrossRef]
- Gao, M.; Cao, S.; Li, N.; Liu, J.; Lyu, Y.; Li, J.; Yang, X. Risks of Overweight in the Offspring of Women with Gestational Diabetes at Different Developmental Stages: A Meta-Analysis with More than Half a Million Offspring. Obes. Rev. 2022, 23, e13395. [Google Scholar] [CrossRef]
- Gillespie, P.; Cullinan, J.; O’Neill, C.; Dunne, F. Modeling the Independent Effects of Gestational Diabetes Mellitus on Maternity Care and Costs. Diabetes Care 2013, 36, 1111–1116. [Google Scholar] [CrossRef]
- Kolu, P.; Raitanen, J.; Rissanen, P.; Luoto, R. Health Care Costs Associated with Gestational Diabetes Mellitus among High-Risk Women—Results from a Randomised Trial. BMC Pregnancy Childbirth 2012, 12, 71. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Mamerto, T.P.; Chung, G.; Villavieja, A.; Gaus, N.L.; Morgan, E.; Pineda-Cortel, M.R.B. Gestational Diabetes Mellitus: A Harbinger of the Vicious Cycle of Diabetes. Int. J. Mol. Sci. 2020, 21, 5003. [Google Scholar] [CrossRef] [PubMed]
- Thornton, J.M.; Shah, N.M.; Lillycrop, K.A.; Cui, W.; Johnson, M.R.; Singh, N. Multigenerational Diabetes Mellitus. Front. Endocrinol. 2024, 14, 1245899. [Google Scholar] [CrossRef]
- Toronto, C.E.; Remington, R. (Eds.) A Step-by-Step Guide to Conducting an Integrative Review; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-37503-4. [Google Scholar]
- Whittemore, R.; Knafl, K. The Integrative Review: Updated Methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef]
- Dhollande, S.; Taylor, A.; Meyer, S.; Scott, M. Conducting Integrative Reviews: A Guide for Novice Nursing Researchers. J. Res. Nurs. 2021, 26, 427–438. [Google Scholar] [CrossRef]
- Ghosh, A.; Choudhury, S. Understanding Different Types of Review Articles: A Primer for Early Career Researchers. Indian J. Psychiatry 2025, 67, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, P.A. Analysis and Synthesis. In A Step-by-Step Guide to Conducting an Integrative Review; Toronto, C.E., Remington, R., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 57–70. ISBN 978-3-030-37504-1. [Google Scholar]
- Popenoe, R.; Langius-Eklöf, A.; Stenwall, E.; Jervaeus, A. A Practical Guide to Data Analysis in General Literature Reviews. Nord. J. Nurs. Res. 2021, 41, 175–186. [Google Scholar] [CrossRef]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) Version 2018. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Pace, R.; Pluye, P.; Bartlett, G.; Macaulay, A.C.; Salsberg, J.; Jagosh, J.; Seller, R. Testing the Reliability and Efficiency of the Pilot Mixed Methods Appraisal Tool (MMAT) for Systematic Mixed Studies Review. Int. J. Nurs. Stud. 2012, 49, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Adesina, N.; Dogan, H.; Green, S.; Tsofliou, F. Effectiveness and Usability of Digital Tools to Support Dietary Self-Management of Gestational Diabetes Mellitus: A Systematic Review. Nutrients 2021, 14, 10. [Google Scholar] [CrossRef]
- Bennett, C.J.; Walker, R.E.; Blumfield, M.L.; Gwini, S.-M.; Ma, J.; Wang, F.; Wan, Y.; Dickinson, H.; Truby, H. Interventions Designed to Reduce Excessive Gestational Weight Gain Can Reduce the Incidence of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetes Res. Clin. Pract. 2018, 141, 69–79. [Google Scholar] [CrossRef]
- Breuing, J.; Pieper, D.; Neuhaus, A.L.; Heß, S.; Lütkemeier, L.; Haas, F.; Spiller, M.; Graf, C. Barriers and Facilitating Factors in the Prevention of Diabetes Type 2 and Gestational Diabetes in Vulnerable Groups: A Scoping Review. PLoS ONE 2020, 15, e0232250. [Google Scholar] [CrossRef]
- Byrn, M.; Penckofer, S. The Relationship between Gestational Diabetes and Antenatal Depression. J. Obstet. Gynecol. Neonatal Nurs. 2015, 44, 246–255. [Google Scholar] [CrossRef]
- Carolan, M. Diabetes Nurse Educators’ Experiences of Providing Care for Women, with Gestational Diabetes Mellitus, from Disadvantaged Backgrounds. J. Clin. Nurs. 2014, 23, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Carolan, M.; Gill, G.K.; Steele, C. Women’s Experiences of Factors That Facilitate or Inhibit Gestational Diabetes Self-Management. BMC Pregnancy Childbirth 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J. A Systematic Review of Interventions for Hispanic Women with or at Risk of Gestational Diabetes Mellitus (GDM). Sex. Reprod. Health 2017, 13, 14–22. [Google Scholar] [CrossRef]
- Daalderop, L.A.; Barsties, L.S.; van Steenbergen, F.; Waelput, A.J.M.; Lagendijk, J.; Been, J.V.; Steegers, E.A.P.; Loorbach, D. Building Cross-Sectoral Collaborations to Address Perinatal Health Inequities: Insights from the Dutch Healthy Pregnancy 4 All-3 Program. Int. J. Health Policy Manag. 2024, 13, 8115. [Google Scholar] [CrossRef]
- Davenport, M.H.; Ruchat, S.-M.; Poitras, V.J.; Garcia, A.J.; Gray, C.E.; Barrowman, N.; Skow, R.J.; Meah, V.L.; Riske, L.; Sobierajski, F.; et al. Prenatal Exercise for the Prevention of Gestational Diabetes Mellitus and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Br. J. Sports Med. 2018, 52, 1367. [Google Scholar] [CrossRef]
- Donazar-Ezcurra, M.; Lopez-Del Burgo, C.; Bes-Rastrollo, M. Primary Prevention of Gestational Diabetes Mellitus through Nutritional Factors: A Systematic Review. BMC Pregnancy Childbirth 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Elsenbruch, S.; Benson, S.; Rücke, M.; Rose, M.; Dudenhausen, J.; Pincus-Knackstedt, M.K.; Klapp, B.F.; Arck, P.C. Social Support during Pregnancy: Effects on Maternal Depressive Symptoms, Smoking and Pregnancy Outcome. Human Reprod. 2007, 22, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Field, C.; Grobman, W.A.; Yee, L.M.; Johnson, J.; Wu, J.; McNeil, B.; Mercer, B.; Simhan, H.; Reddy, U.; Silver, R.M.; et al. Community-Level Social Determinants of Health and Pregestational and Gestational Diabetes. Am. J. Obstet. Gynecol. Matern. Fetal Med. 2024, 6, 101249. [Google Scholar] [CrossRef]
- Guo, M.; Shi, W.-X.; Parsons, J.; Forbes, A.; Kong, M.; Zhang, Y.-P.; Yang, H.; Forde, R. The Effects of a Couple-Based Gestational Diabetes Mellitus Intervention on Self-Management and Pregnancy Outcomes: A Randomised Controlled Trial. Diabetes Res. Clin. Pract. 2023, 205, 110947. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Shu, J.; Fu, X.-H.; Chen, X.-P.; Zhang, L.; Ji, M.-X.; Liu, X.-M.; Yu, T.-T.; Sheng, J.-Z.; Huang, H.-F. Improving the Effectiveness of Lifestyle Interventions for Gestational Diabetes Prevention: A Meta-Analysis and Meta-Regression. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 311–320. [Google Scholar] [CrossRef]
- Haron, Z.; Sutan, R.; Zakaria, R.; Abdullah Mahdy, Z. Self-Care Educational Guide for Mothers with Gestational Diabetes Mellitus: A Systematic Review on Identifying Self-Care Domains, Approaches, and Their Effectiveness. Belitung Nurs. J. 2023, 9, 6–16. [Google Scholar] [CrossRef]
- Hasani Moghadam, S.; Yousefi Abdolmaleki, E.; Alijani, F.; Bagherian Afrakoti, N.; Ganji, J. The Relationship between Social Capital and Self-Efficacy in Women with Gestational Diabetes Mellitus: A Cross Sectional Study. Ethiop. J. Health Sci. 2020, 30, 541–548. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Lin, Q.; Su, X.; Liu, Y.; Luo, W.; Zhang, Z.; Zeng, H.; Lin, H.; Guo, X.; Yang, Y. Residential Greenness Exposure and Repeatedly Measured Hyperglycemic Markers in Women with Gestational Diabetes Mellitus: A Birth Cohort Study in Foshan, China. Public Health 2025, 242, 304–310. [Google Scholar] [CrossRef]
- Helm, M.M.; Izuora, K.; Basu, A. Nutrition-Education-Based Interventions in Gestational Diabetes: A Scoping Review of Clinical Trials. Int. J. Environ. Res. Public Health 2022, 19, 2926. [Google Scholar] [CrossRef]
- Huang, S.; Magny-Normilus, C.; McMahon, E.; Whittemore, R. Systematic Review of Lifestyle Interventions for Gestational Diabetes Mellitus in Pregnancy and the Postpartum Period. J. Obstet. Gynecol. Neonatal Nurs. 2022, 51, 115–125. [Google Scholar] [CrossRef]
- Jung, S.; Kim, Y.; Park, J.; Choi, M.; Kim, S. Psychosocial Support Interventions for Women with Gestational Diabetes Mellitus: A Systematic Review. Korean J. Women Health Nurs. 2021, 27, 75–92. [Google Scholar] [CrossRef]
- Karavasileiadou, S.; Almegwely, W.; Alanazi, A.; Alyami, H.; Chatzimichailidou, S. Self-Management and Self-Efficacy of Women with Gestational Diabetes Mellitus: A Systematic Review. Glob. Health Action 2022, 15, 2087298. [Google Scholar] [CrossRef]
- Khatri, R.B.; Erku, D.; Endalamaw, A.; Wolka, E.; Nigatu, F.; Zewdie, A.; Assefa, Y. Multisectoral Actions in Primary Health Care: A Realist Synthesis of Scoping Review. PLoS ONE 2023, 18, e0289816. [Google Scholar] [CrossRef] [PubMed]
- Lagisetty, P.A.; Priyadarshini, S.; Terrell, S.; Hamati, M.; Landgraf, J.; Chopra, V.; Heisler, M. Culturally Targeted Strategies for Diabetes Prevention in Minority Population: A Systematic Review and Framework. Diabetes Educ. 2017, 43, 54–77. [Google Scholar] [CrossRef] [PubMed]
- Laredo-Aguilera, J.A.; Gallardo-Bravo, M.; Rabanales-Sotos, J.A.; Cobo-Cuenca, A.I.; Carmona-Torres, J.M. Physical Activity Programs during Pregnancy Are Effective for the Control of Gestational Diabetes Mellitus. Int. J. Environ. Res. Public Health 2020, 17, 6151. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Chen, X.; Xu, S.; Li, Y.; Zhang, B.; Cao, Z.; Zhang, Y.; Liang, S.; Hu, K.; Xia, W. Effect of Residential Exposure to Green Space on Maternal Blood Glucose Levels, Impaired Glucose Tolerance, and Gestational Diabetes Mellitus. Environ. Res. 2019, 176, 8526. [Google Scholar] [CrossRef]
- Lim, S.; Takele, W.W.; Vesco, K.K.; Redman, L.; Josefson, J. A Systematic Review and Meta-Analysis of Participant Characteristics in the Prevention of Gestational Diabetes: A Summary of Evidence for Precision Medicine. medRxiv 2023. [Google Scholar] [CrossRef]
- McGovern, L.; O’Toole, L.; Houshialsadat, Z.; O’Reilly, S.L. Women’s Perspectives on mHealth Behavior Change Interventions for the Management of Overweight, Obesity, or Gestational Diabetes: A Qualitative Meta-Synthesis. Obes. Rev. 2024, 25, e13761. [Google Scholar] [CrossRef]
- Oostdam, N.; van Poppel, M.N.M.; Wouters, M.G.A.J.; van Mechelen, W. Interventions for Preventing Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Women’s Health 2011, 20, 1551–1563. [Google Scholar] [CrossRef]
- Quotah, O.F.; Andreeva, D.; Nowak, K.G.; Dalrymple, K.V.; Almubarak, A.; Patel, A.; Vyas, N.; Cakir, G.S.; Heslehurst, N.; Bell, Z.; et al. Interventions in Preconception and Pregnant Women at Risk of Gestational Diabetes; a Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetol. Metab. Syndr. 2024, 16, 8. [Google Scholar] [CrossRef]
- Roesler, A.; Butten, K.; Taylor, P.; Morrison, M.; Varnfield, M.; Holmes-Truscott, E. The Experiences of Individuals Who Have Had Gestational Diabetes: A Qualitative Exploration. Diabet. Med. 2024, 41, e15374. [Google Scholar] [CrossRef]
- Runkle, J.D.; Matthews, J.L.; Sparks, L.; McNicholas, L.; Sugg, M.M. Racial and Ethnic Disparities in Pregnancy Complications and the Protective Role of Greenspace: A Retrospective Birth Cohort Study. Sci. Total Environ. 2022, 808, 152145. [Google Scholar] [CrossRef]
- Russo, L.M.; Nobles, C.; Ertel, K.A.; Chasan-Taber, L.; Whitcomb, B.W. Physical Activity Interventions in Pregnancy and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2015, 125, 576–582. [Google Scholar] [CrossRef]
- Sampathkumar, S.; Parkhi, D.; Ghebremichael-Weldeselassie, Y.; Sukumar, N.; Saravanan, P. Effectiveness of Pre-Pregnancy Lifestyle in Preventing Gestational Diabetes Mellitus-a Systematic Review and Meta-Analysis of 257,876 Pregnancies. Nutr. Diabetes 2023, 13, 22. [Google Scholar] [CrossRef]
- Takele, W.W.; Vesco, K.K.; Josefson, J.; Redman, L.M.; Hannah, W.; Bonham, M.P.; Chen, M.; Chivers, S.C.; Fawcett, A.J.; Grieger, J.A.; et al. Effective Interventions in Preventing Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Commun. Med. 2024, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Tancred, T.; Caffrey, M.; Falkenbach, M.; Raven, J. The Pathway to Health in All Policies through Intersectoral Collaboration on the Health Workforce: A Scoping Review. Health Policy Plan. 2024, 39, i54–i74. [Google Scholar] [CrossRef]
- Tanentsapf, I.; Heitmann, B.L.; Adegboye, A.R.A. Systematic Review of Clinical Trials on Dietary Interventions to Prevent Excessive Weight Gain during Pregnancy among Normal Weight, Overweight and Obese Women. BMC Pregnancy Childbirth 2011, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Zhong, Y.; Xu, C.; Li, W.; Wang, H.; Hou, Y. Effectiveness of Five Interventions Used for Prevention of Gestational Diabetes: A Network Meta-Analysis. Medicine 2022, 101, e29126. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Rogozinska, E.; Jolly, K.; Glinkowski, S.; Roseboom, T.; Tomlinson, J.W.; Kunz, R.; Mol, B.W.; Coomarasamy, A.; Khan, K.S. Effects of Interventions in Pregnancy on Maternal Weight and Obstetric Outcomes: Meta-Analysis of Randomised Evidence. BMJ (Clin. Res. Ed.) 2012, 344, e2088. [Google Scholar] [CrossRef]
- Wah, Y.Y.E.; McGill, M.; Wong, J.; Ross, G.P.; Harding, A.-J.; Krass, I. Self-Management of Gestational Diabetes among Chinese Migrants: A Qualitative Study. Women Birth 2019, 32, e17–e23. [Google Scholar] [CrossRef]
- Wan, C.S.; Nankervis, A.; Teede, H.; Aroni, R. Dietary Intervention Strategies for Ethnic Chinese Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutr. Diet. 2019, 76, 211–232. [Google Scholar] [CrossRef]
- Wei, H.X.; Yang, Y.L.; Luo, T.Y.; Chen, W.Q. Effectiveness of Mobile Health Interventions for Pregnant Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Obstet. Gynaecol. 2023, 43, 2245906. [Google Scholar] [CrossRef]
- Wu, S.; Jin, J.; Hu, K.-L.; Wu, Y.; Zhang, D. Prevention of Gestational Diabetes Mellitus and Gestational Weight Gain Restriction in Overweight/Obese Pregnant Women: A Systematic Review and Network Meta-Analysis. Nutrients 2022, 14, 2383. [Google Scholar] [CrossRef]
- Zeinabeh, M.Z.; Atefeh, A.; Masumeh, G.H.P.; Tania, D.; Mojgan, S.; Katayoun, A. The Effect of Mindfulness-Based Stress Reduction Counseling on Blood Glucose and Perceived Stress in Women with Gestational Diabetes. Rev. Bras. Ginecol. Obstet. 2023, 45, e517–e523. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, Y.; Dong, Q. The Effect of Individualized Exercise Prescriptions Combined with Dietary Management on Blood Glucose in the Second-and-Third Trimester of Gestational Diabetes Mellitus. Am. J. Transl. Res. 2021, 13, 7388–7393. [Google Scholar]
- Ioannou, E.; Humphreys, H.; Homer, C.; Purvis, A. Preventing Type 2 Diabetes after Gestational Diabetes: A Systematic Review Mapping Physical Activity Components Using the Socio-Ecological Model. Matern. Child Health J. 2024, 28, 1354–1379. [Google Scholar] [CrossRef]
- Azami, M.; Badfar, G.; Soleymani, A.; Rahmati, S. The Association between Gestational Diabetes and Postpartum Depression: A Systematic Review and Meta-Analysis. Diabetes Res. Clin. Pract. 2019, 149, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Morales-Suárez-Varela, M. The Bidirectional Relationship between Gestational Diabetes and Depression in Pregnant Women: A Systematic Search and Review. Healthcare 2023, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, S.N.; Buck Louis, G.M.; Rawal, S.; Zhu, Y.; Albert, P.S.; Zhang, C. A Longitudinal Study of Depression and Gestational Diabetes in Pregnancy and the Postpartum Period. Diabetologia 2016, 59, 2594–2602. [Google Scholar] [CrossRef] [PubMed]
- OuYang, H.; Chen, B.; Abdulrahman, A.-M.; Li, L.; Wu, N. Associations between Gestational Diabetes and Anxiety or Depression: A Systematic Review. J. Diabetes Res. 2021, 2021, 9959779. [Google Scholar] [CrossRef]
- Packer, C.H.; Pilliod, R.A.; Chatroux, L.R.; Caughey, A.B.; Valent, A.M. Increased Rates of Adverse Perinatal Outcomes in Women with Gestational Diabetes and Depression. J. Matern. Fetal Neonatal Med. 2021, 34, 3862–3866. [Google Scholar] [CrossRef]
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.-F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and Management of Gestational Diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef]
- Yu, C.; Hung, C.; Wang, Y. The Impact of Prenatal Depression and Diabetes Management Self-efficacy on Postpartum Stress and Depression in Women with Gestational Diabetes Mellitus. J. Clin. Nurs. 2022, 31, 2867–2873. [Google Scholar] [CrossRef]
- Davidsen, E.; Maindal, H.T.; Rod, M.H.; Olesen, K.; Byrne, M.; Damm, P.; Nielsen, K.K. The Stigma Associated with Gestational Diabetes Mellitus: A Scoping Review. eClinicalMedicine 2022, 52, 101614. [Google Scholar] [CrossRef]
- Davidsen, E.; Terkildsen Maindal, H.; Byrne, M.; Kelstrup, L.; Ovesen, P.; Damm, P.; Kragelund Nielsen, K. A Qualitative Investigation into the Perceptions and Experiences of the Stigma Attached to Gestational Diabetes Mellitus among Women in Denmark. Diabetes Res. Clin. Pract. 2023, 203, 110858. [Google Scholar] [CrossRef]
- Wazqar, D.Y.; Evans, M.K. Socio-Cultural Aspects of Self-Management in Gestational Diabetes. J. Diabetes Nurs. 2012, 16, 62–67. [Google Scholar]
- Sun, S.; Pellowski, J.; Pisani, C.; Pandey, D.; Go, M.; Chu, M.; Ruan, J.; Werner, E.F. Experiences of Stigma, Psychological Distress, and Facilitative Coping among Pregnant People with Gestational Diabetes Mellitus. BMC Pregnancy Childbirth 2023, 23, 643. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.R.; Gringle, M.R.; Pulliam, R.M. “You Have to Put Your Children’s Needs First or You’re Really Not a Good Mother”: Black Motherhood and Self-Care Practices. Women Gend. Fam. Color 2015, 3, 165–189. [Google Scholar] [CrossRef]
- Devsam, B.U.; Bogossian, F.E.; Peacock, A.S. An Interpretive Review of Women’s Experiences of Gestational Diabetes Mellitus: Proposing a Framework to Enhance Midwifery Assessment. Women Birth 2013, 26, e69–e76. [Google Scholar] [CrossRef]
- Carolan-Olah, M.; Duarte-Gardea, M.; Lechuga, J.; Salinas-Lopez, S. The Experience of Gestational Diabetes Mellitus (GDM) among Hispanic Women in a U.S. Border Region. Sex. Reprod. Healthc. 2017, 12, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Neven, A.C.H.; Lake, A.J.; Williams, A.; O’Reilly, S.L.; Hendrieckx, C.; Morrison, M.; Dunbar, J.A.; Speight, J.; Teede, H.; Boyle, J.A.; et al. Barriers to and Enablers of Postpartum Health Behaviours among Women from Diverse Cultural Backgrounds with Prior Gestational Diabetes: A Systematic Review and Qualitative Synthesis Applying the Theoretical Domains Framework. Diabet. Med. 2022, 39, e14945. [Google Scholar] [CrossRef]
- Britton, L.E.; Kaur, G.; Zork, N.; Marshall, C.J.; George, M. ‘We Tend to Prioritise Others and Forget Ourselves’: How Women’s Caregiving Responsibilities Can Facilitate or Impede Diabetes Self-Management. Diabet. Med. 2023, 40, e15030. [Google Scholar] [CrossRef]
- Razee, H.; van der Ploeg, H.P.; Blignault, I.; Smith, B.J.; Bauman, A.E.; McLean, M.; Wah Cheung, N. Beliefs, Barriers, Social Support, and Environmental Influences Related to Diabetes Risk Behaviours among Women with a History of Gestational Diabetes. Health Promot. J. Aust. 2010, 21, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, D.; Bi, X.; Feng, Y.; Zhang, K.; Jiang, J.; Wang, Y. Barriers and Facilitators to Self-Management among Women with Gestational Diabetes: A Systematic Review Using the COM-B Model. Midwifery 2024, 138, 104141. [Google Scholar] [CrossRef]
- Yee, L.M.; McGuire, J.M.; Taylor, S.M.; Niznik, C.M.; Simon, M.A. “I Was Tired of All the Sticking and Poking”: Identifying Barriers to Diabetes Self-Care among Low-Income Pregnant Women. J. Health Care Poor Underserved 2015, 26, 926–940. [Google Scholar] [CrossRef]
- Neufeld, H.T. Food Perceptions and Concerns of Aboriginal Women Coping with Gestational Diabetes in Winnipeg, Manitoba. J. Nutr. Educ. Behav. 2011, 43, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Brownson, C.A.; Heisler, M. The Role of Peer Support in Diabetes Care and Self-Management. Patient Patient Centered Outcome Res. 2009, 2, 5–17. [Google Scholar] [CrossRef]
- Fisher, E.B.; Bloch, P.; Sherlaw, W. The Ecological Approach to Self-Management in Diabetes. In The Diabetes Textbook: Clinical Principles, Patient Management and Public Health Issues; Rodriguez-Saldana, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 49–76. ISBN 978-3-031-25519-9. [Google Scholar]
- Farajzadegan, Z.; Jafari, N.; Nazer, S.; Keyvanara, M.; Zamani, A. Social Capital—A Neglected Issue in Diabetes Control: A Cross-sectional Survey in Iran. Health Social Care Community 2013, 21, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Nishigori, H.; Sugiyama, T.; Takahashi, F.; Iwama, N.; Watanabe, Z.; Sakurai, K.; Ishikuro, M.; Obara, T.; Tatsuta, N.; et al. Association between Social Capital and the Prevalence of Gestational Diabetes Mellitus: An Interim Report of the Japan Environment and Children’s Study. Diabetes Res. Clin. Pract. 2016, 120, 132–141. [Google Scholar] [CrossRef]
- WHO. World Health Organization mHealth: Use of Appropriate Digital Technologies for Public Health: Report by the Director-General; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Bakker, C.J.; Wyatt, T.H.; Breth, M.C.; Gao, G.; Janeway, L.M.; Lee, M.A.; Martin, C.L.; Tiase, V.L. Nurses’ Roles in Mhealth App Development: Scoping Review. JMIR Nurs. 2023, 6, e46058. [Google Scholar] [CrossRef]
- Kusyanti, T.; Wirakusumah, F.F.; Rinawan, F.R.; Muhith, A.; Purbasari, A.; Mawardi, F.; Puspitasari, I.W.; Faza, A.; Stellata, A.G. Technology-Based (Mhealth) and Standard/Traditional Maternal Care for Pregnant Woman: A Systematic Literature Review. Healthcare 2022, 10, 1287. [Google Scholar] [CrossRef]
- Miremberg, H.; Ben-Ari, T.; Betzer, T.; Raphaeli, H.; Gasnier, R.; Barda, G.; Bar, J.; Weiner, E. The Impact of a Daily Smartphone-Based Feedback System among Women with Gestational Diabetes on Compliance, Glycemic Control, Satisfaction, and Pregnancy Outcome: A Randomized Controlled Trial. Am. J. Obstet. Gynecol. 2018, 218, 453.e1–453.e7. [Google Scholar] [CrossRef]
- Guo, P.; Chen, D.; Xu, P.; Wang, X.; Zhang, W.; Mao, M.; Zheng, Q.; Jin, Y.; Feng, S. Web-Based Interventions for Pregnant Women with Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2023, 25, e36922. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Wiley, J.; Ge, L. Effectiveness of Smartphone-Based Lifestyle Interventions on Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Psychol. Res. Behav. Manag. 2022, 15, 3541–3559. [Google Scholar] [CrossRef]
- Surendran, S.; Lim, C.S.; Koh, G.C.H.; Yew, T.W.; Tai, E.S.; Foong, P.S. Women’s Usage Behavior and Perceived Usefulness with Using a Mobile Health Application for Gestational Diabetes Mellitus: Mixed-Methods Study. Int. J. Environ. Res. Public Health 2021, 18, 6670. [Google Scholar] [CrossRef]
- El-Gayar, O.; Ofori, M.; Nawar, N. On the Efficacy of Behavior Change Techniques in mHealth for Self-Management of Diabetes: A Meta-Analysis. J. Biomed. Inform. 2021, 119, 3839. [Google Scholar] [CrossRef]
- Liao, R.; Li, Y.; Yang, H.; Luo, Y. Influence of mHealth-Based Lifestyle Interventions on Symptoms of Anxiety and Depression of Women with Gestational Diabetes: A Meta-Analysis. Clin. Nurs. Res. 2024, 33, 448–459. [Google Scholar] [CrossRef]
- Sakamoto, J.L.; Carandang, R.R.; Kharel, M.; Shibanuma, A.; Yarotskaya, E.; Basargina, M.; Jimba, M. Effects of mHealth on the Psychosocial Health of Pregnant Women and Mothers: A Systematic Review. BMJ Open 2022, 12, e056807. [Google Scholar] [CrossRef] [PubMed]
- Leziak, K.; Birch, E.; Jackson, J.; Strohbach, A.; Niznik, C.; Yee, L.M. Identifying Mobile Health Technology Experiences and Preferences of Low-Income Pregnant Women with Diabetes. J. Diabetes Sci. Technol. 2021, 15, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, M.; Small, R.; Davey, M.-A.; Oats, J.J.N.; Forster, D.A.; Aylward, A. Lived Experience of Gestational Diabetes Mellitus among Immigrant South Asian Women in Australia. Aust. N. Z. J. Obstet. Gynaecol. 2011, 51, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Clinch, M.; Afsar, N.; Choudhury, Y.; Sudra, R.; Campbell-Richards, D.; Claydon, A.; Hitman, G.A.; Hanson, P.; Finer, S. Socio-Cultural Influences on the Behaviour of South Asian Women with Diabetes in Pregnancy: Qualitative Study Using a Multi-Level Theoretical Approach. BMC Med. 2015, 13, 120. [Google Scholar] [CrossRef]
- Hjelm, K.; Bard, K.; Nyberg, P.; Apelqvist, J. Swedish and Middle-Eastern-Born Women’s Beliefs about Gestational Diabetes. Midwifery 2005, 21, 44–60. [Google Scholar] [CrossRef]
- Carolan, M. Women’s Experiences of Gestational Diabetes Self-Management: A Qualitative Study. Midwifery 2013, 29, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; O’Brien, B. Gestational Diabetes: The Meaning of an at-Risk Pregnancy. Qual. Health Res. 2005, 15, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Helmersen, M.; Sørensen, M.; Lukasse, M.; Laine, H.K.; Garnweidner-Holme, L. Women’s Experience with Receiving Advice on Diet and Self-Monitoring of Blood Glucose for Gestational Diabetes Mellitus: A Qualitative Study. Scand. J. Prim. Health Care 2021, 39, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.; Silverio, S.A.; Ismail, K. “It Feels like Medically Promoted Disordered Eating”: The Psychosocial Impact of Gestational Diabetes Mellitus in the Perinatal Period. PLoS ONE 2023, 18, e0288395. [Google Scholar] [CrossRef]
- Kaptein, S.; Evans, M.; McTavish, S.; Banerjee, A.T.; Feig, D.S.; Lowe, J.; Lipscombe, L.L. The Subjective Impact of a Diagnosis of Gestational Diabetes among Ethnically Diverse Pregnant Women: A Qualitative Study. Can. J. Diabetes 2015, 39, 117–122. [Google Scholar] [CrossRef]
- Bandyopadhyay, M. Gestational Diabetes Mellitus: A Qualitative Study of Lived Experiences of South Asian Immigrant Women and Perspectives of Their Health Care Providers in Melbourne, Australia. BMC Pregnancy Childbirth 2021, 21, 500. [Google Scholar] [CrossRef]
- Yee, L.M.; McGuire, J.M.; Taylor, S.M.; Niznik, C.M.; Simon, M.A. Social and Environmental Barriers to Nutrition Therapy for Diabetes Management among Underserved Pregnant Women: A Qualitative Analysis. J. Nutr. Educ. Behav. 2016, 48, 170–180.e1. [Google Scholar] [CrossRef]
- Carolan, M.; Steele, C.; Margetts, H. Attitudes towards Gestational Diabetes among a Multiethnic Cohort in Australia. J. Clin. Nurs. 2010, 19, 2446–2453. [Google Scholar] [CrossRef]
- Chertok, I.R.A.; Silk, J.J.; Kulasa, K.A. Perspectives on Barriers and Facilitators in Caring for Women with Gestational Diabetes in Rural Appalachia. MCN Am. J. Matern. Child Nurs. 2019, 44, 289–295. [Google Scholar] [CrossRef]
- Draffin, C.R.; Alderdice, F.A.; McCance, D.R.; Maresh, M.; Harper, M.; McSorley, O.; Holmes, V.A. Exploring the Needs, Concerns and Knowledge of Women Diagnosed with Gestational Diabetes: A Qualitative Study. Midwifery 2016, 40, 141–147. [Google Scholar] [CrossRef]
- Ndwiga, D.W.; McBride, K.A.; Thompson, R.; Simmons, D.; MacMillan, F. “We Are Competing with Culture” the Chasm between Healthcare Professionals and Australian Samoan Women in the Prevention and Management of Gestational Diabetes Mellitus. Aust. J. Adv. Nurs. 2023, 40, 5–14. [Google Scholar] [CrossRef]
- Lawrence, R.L.; Ward, K.; Wall, C.R.; Bloomfield, F.H. New Zealand Women’s Experiences of Managing Gestational Diabetes through Diet: A Qualitative Study. BMC Pregnancy Childbirth 2021, 21, 819. [Google Scholar] [CrossRef]
- Kandasamy, S.; Nguyen, L.; Desai, D.; Anand, S.S.; Sherifali, D.; de Souza, R.J. Barriers to, and Facilitators of, Lifestyle Changes to Prevent Gestational Diabetes: An Interpretive Description of South Asian Women and Health-Care Providers Living and Working in Southern Ontario, Canada. Can. J. Diabetes 2021, 45, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Anataca, G.; Celik, S.; Taskin Yilmaz, F. Fatalism in Turkish Women with Gestational Diabetes: Its Relationship with Diabetes Self-Management and Perception of Risk to Maternal and Fetal Health in Pregnancy. J. Health Psychol. 2024, 30, 1977–1989. [Google Scholar] [CrossRef] [PubMed]
- Power, T.; Wilson, D.; East, L.; Cashman, B.; Wannell, J.; Jackson, D. Indigenous Women’s Experiences of Diabetes in Pregnancy: A Thematic Synthesis. Collegian 2021, 28, 541–550. [Google Scholar] [CrossRef]
- Reid, J.; Anderson, A.; Cormack, D.; Reid, P.; Harwood, M. The Experience of Gestational Diabetes for Indigenous Māori Women Living in Rural New Zealand: Qualitative Research Informing the Development of Decolonising Interventions. BMC Pregnancy Childbirth 2018, 18, 478. [Google Scholar] [CrossRef]
- Silvani, J.; Schmidt, M.I.; Zajdenverg, L.; Galliano, L.M.; Antunes Nunes, M.A. Impact of Binge Eating during Pregnancy on Gestational Weight Gain and Postpartum Weight Retention among Women with Gestational Diabetes Mellitus: LINDA-Brasil. Int. J. Eat. Disord. 2020, 53, 1818–1825. [Google Scholar] [CrossRef]
- Siddiqui, R.; McAdams, C.J. Challenges in the Management of Gestational Diabetes Mellitus in Anorexia Nervosa. Psychiatry Res. Case Rep. 2024, 3, 100215. [Google Scholar] [CrossRef]
- Oxlad, M.; Whitburn, S.; Grieger, J.A. The Complexities of Managing Gestational Diabetes in Women of Culturally and Linguistically Diverse Backgrounds: A Qualitative Study of Women’s Experiences. Nutrients 2023, 15, 1053. [Google Scholar] [CrossRef]
- Wan, C.S.; Teede, H.; Nankervis, A.; Aroni, R. Ethnic Differences in Dietary Management of Gestational Diabetes Mellitus: A Mixed Methods Study Comparing Ethnic Chinese Immigrants and Australian Women. J. Acad. Nutr. Diet. 2020, 120, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Flores, G. The Impact of Medical Interpreter Services on the Quality of Health Care: A Systematic Review. Med. Care Res. Rev. 2005, 62, 255–299. [Google Scholar] [CrossRef]
- Tzotzis, L.; Hooper, M.-E.; Douglas, A.; Kurz, E.; Atchan, M.; Spiller, S.; Davis, D. The Needs and Experiences of Women with Gestational Diabetes Mellitus from Minority Ethnic Backgrounds in High-Income Nations: A Systematic Integrative Review. Women Birth 2023, 36, 205–216. [Google Scholar] [CrossRef]
- Hashimoto, H. Social Determinants of Health and Diabetes Outcomes. In The Diabetes Textbook: Clinical Principles, Patient Management and Public Health Issues; Rodriguez-Saldana, J., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 77–85. ISBN 978-3-031-25519-9. [Google Scholar]
- Altemani, A.H.; Alzaheb, R.A. The Prevention of Gestational Diabetes Mellitus (The Role of Lifestyle): A Meta-Analysis. Diabetol. Metab. Syndr. 2022, 14, 83. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Riahi, R.; Safari, S.; Heidari, Z. Dose–Response Association between Dietary Patterns and Gestational Diabetes Mellitus Risk: A Systematic Review and Meta-Analysis of Observational Studies. Food Sci. Nutr. 2023, 11, 57–92. [Google Scholar] [CrossRef]
- Nasab, S.J.; Ghanavati, M.; Clark, C.C.; Nasirian, M. Adherence to Mediterranean Dietary Pattern and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Diabetes 2024, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; He, L. Mediterranean Diet for the Prevention of Gestational Diabetes: A Meta-Analysis of Randomized Controlled Trials. J. Matern. Fetal Neonatal Med. 2022, 35, 10247–10252. [Google Scholar] [CrossRef]
- Mijatovic-Vukas, J.; Capling, L.; Cheng, S.; Stamatakis, E.; Louie, J.; Cheung, N.W.; Markovic, T.; Ross, G.; Senior, A.; Brand-Miller, J.C.; et al. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 698. [Google Scholar] [CrossRef]
- Pham, N.M.; Do, V.V.; Lee, A.H. Polyphenol-Rich Foods and Risk of Gestational Diabetes: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2019, 73, 647–656. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, F.; Tashiro, S.; Liu, P.J. Effects of Dietary Approaches and Exercise Interventions on Gestational Diabetes Mellitus: A Systematic Review and Bayesian Network Meta-Analysis. Adv. Nutr. 2024, 15, 100330. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.P. DASH Eating Plan: An Eating Pattern for Diabetes Management. Diabetes Spectr. 2017, 30, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Arablou, T.; Jayedi, A.; Salehi-Abargouei, A. Adherence to the Dietary Approaches to Stop Hypertension (DASH) Diet in Relation to All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutr. J. 2020, 19, 37. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Sabihi, S.; Esmaillzadeh, A. A Randomized Controlled Clinical Trial Investigating the Effect of DASH Diet on Insulin Resistance, Inflammation, and Oxidative Stress in Gestational Diabetes. Nutrition 2013, 29, 619–624. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Esmaillzadeh, A. The Effect of DASH Diet on Pregnancy Outcomes in Gestational Diabetes: A Randomized Controlled Clinical Trial. Eur. J. Clin. Nutr. 2014, 68, 490–495. [Google Scholar] [CrossRef]
- Liese, A.D.; Bortsov, A.; Günther, A.L.B.; Dabelea, D.; Reynolds, K.; Standiford, D.A.; Liu, L.; Williams, D.E.; Mayer-Davis, E.J.; D’Agostino, R.B.; et al. Association of DASH Diet with Cardiovascular Risk Factors in Youth with Diabetes Mellitus: The SEARCH for Diabetes in Youth Study. Circulation 2011, 123, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.M.; Steck, S.E.; Fung, T.T.; Zhang, J.; Hazlett, L.J.; Han, K.; Lee, S.-H.; Kwon, H.-S.; Merchant, A.T. Mediterranean Diet, Dietary Approaches to Stop Hypertension (DASH) Style Diet, and Metabolic Health in U.S. Adults. Clin. Nutr. 2017, 36, 1301–1309. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Salehi-Abargouei, A.; Maghsoudi, Z.; Shirani, F.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH)-Style Diet on Fatal or Nonfatal Cardiovascular Diseases—Incidence: A Systematic Review and Meta-Analysis on Observational Prospective Studies. Nutrition 2013, 29, 611–618. [Google Scholar] [CrossRef]
- Shirani, F.; Salehi-Abargouei, A.; Azadbakht, L. Effects of Dietary Approaches to Stop Hypertension (DASH) Diet on Some Risk for Developing Type 2 Diabetes: A Systematic Review and Meta-Analysis on Controlled Clinical Trials. Nutrition 2013, 29, 939–947. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Chowdhury, S.; Ashor, A.; Oggioni, C.; Mathers, J.C. Effects of the Dietary Approach to Stop Hypertension (DASH) Diet on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2015, 113, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nahm, S.; Marchesoni, J.; Maity, A.; Maguire, R.L.; House, J.S.; Tucker, R.; Atkinson, T.; Murphy, S.K.; Hoyo, C. Maternal Mediterranean Diet Adherence and Its Associations with Maternal Prenatal Stressors and Child Growth. Curr. Dev. Nutr. 2022, 6, nzac146. [Google Scholar] [CrossRef]
- Izadi, V.; Tehrani, H.; Haghighatdoost, F.; Dehghan, A.; Surkan, P.J.; Azadbakht, L. Adherence to the DASH and Mediterranean Diets Is Associated with Decreased Risk for Gestational Diabetes Mellitus. Nutrition 2016, 32, 1092–1096. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean Diet and Health Status: Meta-Analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Macchi, C.; Abbate, R.; Gensini, G.F.; Casini, A. Mediterranean Diet and Health. BioFactors 2013, 39, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Annunziata, G.; Tenore, G.C.; Palmas, V.; Manzin, A.; Velluzzi, F. Diet-Derived Antioxidants and Their Role in Inflammation, Obesity and Gut Microbiota Modulation. Antioxidants 2021, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Soltani, S.; Jenkins, D.; Sievenpiper, J.; Shab-Bidar, S. Dietary Glycemic Index, Glycemic Load, and Chronic Disease: An Umbrella Review of Meta-Analyses of Prospective Cohort Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 2460–2469. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Duan, B.; Zhang, Q.; Zhou, Z.; Liu, W. Effects of a Low Glycemic Index or Low Glycemic Load Diet on Pregnant Women at High Risk of Gestational Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 2006–2018. [Google Scholar] [CrossRef]
- Carlos, S.; De La Fuente-Arrillaga, C.; Bes-Rastrollo, M.; Razquin, C.; Rico-Campà, A.; Martínez-González, M.A.; Ruiz-Canela, M. Mediterranean Diet and Health Outcomes in the SUN Cohort. Nutrients 2018, 10, 439. [Google Scholar] [CrossRef]
- Kotzakioulafi, E.; Bakaloudi, D.R.; Chrysoula, L.; Theodoridis, X.; Antza, C.; Tirodimos, I.; Chourdakis, M. High versus Low Adherence to the Mediterranean Diet for Prevention of Diabetes Mellitus Type 2: A Systematic Review and Meta-Analysis. Metabolites 2023, 13, 779. [Google Scholar] [CrossRef]
- Mao, T.; Huang, F.; Zhu, X.; Wei, D.; Chen, L. Effects of Dietary Fiber on Glycemic Control and Insulin Sensitivity in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. J. Funct. Foods 2021, 82, 104500. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-Analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef]
- Post, R.E.; Mainous, A.G.; King, D.E.; Simpson, K.N. Dietary Fiber for the Treatment of Type 2 Diabetes Mellitus: A Meta-Analysis. J. Am. Board. Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef]
- Due, A.; Larsen, T.M.; Hermansen, K.; Stender, S.; Holst, J.J.; Toubro, S.; Martinussen, T.; Astrup, A. Comparison of the Effects on Insulin Resistance and Glucose Tolerance of 6-Mo High-Monounsaturated-Fat, Low-Fat, and Control Diets1. Am. J. Clin. Nutr. 2008, 87, 855–862. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary Patterns, Insulin Resistance, and Prevalence of the Metabolic Syndrome in Women2. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Iwase, M.; Ohkuma, T.; Ogata-Kaizu, S.; Ide, H.; Kikuchi, Y.; Idewaki, Y.; Joudai, T.; Hirakawa, Y.; Uchida, K.; et al. Impact of Dietary Fiber Intake on Glycemic Control, Cardiovascular Risk Factors and Chronic Kidney Disease in Japanese Patients with Type 2 Diabetes Mellitus: The Fukuoka Diabetes Registry. Nutr. J. 2013, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, T.; Han, K.; Peng, P.; Zhang, G.; Wang, H.; Zhao, L.; Liang, H.; Lv, X.; Du, Y. Impact of Anti-Inflammatory Diets on Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis. Front. Nutr. 2025, 12, 1549831. [Google Scholar] [CrossRef]
- Kopp, W. How Western Diet and Lifestyle Drive the Pandemic of Obesity and Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Dietary Inflammatory Index and Its Association with the Risk of Cardiovascular Diseases, Metabolic Syndrome, and Mortality: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2018, 50, 345–358. [Google Scholar] [CrossRef]
- Pavillard, L.E.; Marín-Aguilar, F.; Bullon, P.; Cordero, M.D. Cardiovascular Diseases, NLRP3 Inflammasome, and Western Dietary Patterns. Pharmacol. Res. 2018, 131, 44–50. [Google Scholar] [CrossRef]
- Yeh, K.-L.; Kautz, A.; Lohse, B.; Groth, S.W. Associations between Dietary Patterns and Inflammatory Markers during Pregnancy: A Systematic Review. Nutrients 2021, 13, 834. [Google Scholar] [CrossRef]
- Zhong, X.; Guo, L.; Zhang, L.; Li, Y.; He, R.; Cheng, G. Inflammatory Potential of Diet and Risk of Cardiovascular Disease or Mortality: A Meta-Analysis. Sci. Rep. 2017, 7, 6367. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Templin, T.N. Association of Depressive Symptoms with Inflammatory Biomarkers among Pregnant African-American Women. J. Reprod. Immunol. 2012, 94, 202–209. [Google Scholar] [CrossRef]
- Cotechini, T.; Graham, C.H. Aberrant Maternal Inflammation as a Cause of Pregnancy Complications: A Potential Therapeutic Target? Placenta 2015, 36, 960–966. [Google Scholar] [CrossRef]
- Gustafsson, H.C.; Sullivan, E.L.; Nousen, E.K.; Sullivan, C.A.; Huang, E.; Rincon, M.; Nigg, J.T.; Loftis, J.M. Maternal Prenatal Depression Predicts Infant Negative Affect via Maternal Inflammatory Cytokine Levels. Brain Behav. Immun. 2018, 73, 470–481. [Google Scholar] [CrossRef]
- Hantsoo, L.; Kornfield, S.; Anguera, M.C.; Epperson, C.N. Inflammation: A Proposed Intermediary between Maternal Stress and Offspring Neuropsychiatric Risk. Biol. Psychiatry 2019, 85, 97–106. [Google Scholar] [CrossRef]
- Jiménez-Osorio, A.S.; Carreón-Torres, E.; Correa-Solís, E.; Ángel-García, J.; Arias-Rico, J.; Jiménez-Garza, O.; Morales-Castillejos, L.; Díaz-Zuleta, H.A.; Baltazar-Tellez, R.M.; Sánchez-Padilla, M.L.; et al. Inflammation and Oxidative Stress Induced by Obesity, Gestational Diabetes, and Preeclampsia in Pregnancy: Role of High-Density Lipoproteins as Vectors for Bioactive Compounds. Antioxidants 2023, 12, 1894. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Prins, J.R.; Schoots, M.H.; Wessels, J.I.; Campmans-Kuijpers, M.J.E.; Navis, G.J.; van Goor, H.; Robertson, S.A.; van der Beek, E.M.; Sobrevia, L.; Gordijn, S.J. The Influence of the Dietary Exposome on Oxidative Stress in Pregnancy Complications. Mol. Asp. Med. 2022, 87, 101098. [Google Scholar] [CrossRef]
- Ray, G.W.; Zeng, Q.; Kusi, P.; Zhang, H.; Shao, T.; Yang, T.; Wei, Y.; Li, M.; Che, X.; Guo, R. Genetic and Inflammatory Factors Underlying Gestational Diabetes Mellitus: A Review. Front. Endocrinol. 2024, 15, 1399694. [Google Scholar] [CrossRef]
- Scholl, T.O.; Chen, X.; Goldberg, G.S.; Khusial, P.R.; Stein, T.P. Maternal Diet, c-Reactive Protein, and the Outcome of Pregnancy. J. Am. Coll. Nutr. 2011, 30, 233–240. [Google Scholar] [CrossRef]
- Soysal, C.; Sarı, H.; Işıkalan, M.M.; Özkaya, E.B.; Ulaş, Ö.; Taşçı, Y.; Keskin, N. Role of the Systemic Immune-inflammation Index in Threatened Abortion Patients and Predicting of Abortion. J. Obstet. Gynaecol. Res. 2023, 49, 1723–1728. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. The Influence of Lifestyle and Treatment on Oxidative Stress and Inflammation in Diabetes. Int. J. Mol. Sci. 2022, 23, 15743. [Google Scholar] [CrossRef]
- Zheng, Z.-X.; Feng, X.; Zhuang, L. The Effect of Oxidative Stress and Antioxidants Treatment on Gestational Diabetes Mellitus Outcome: A Scoping Review. Cell Biochem. Biophys. 2024, 82, 3003–3013. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chrysoula, L.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 1514. [Google Scholar] [CrossRef]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between Adherence to the Mediterranean Diet and Oxidative Stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar] [CrossRef]
- Esposito, K.; Kastorini, C.-M.; Panagiotakos, D.B.; Giugliano, D. Mediterranean Diet and Metabolic Syndrome: An Updated Systematic Review. Rev. Endocr. Metab. Disord. 2013, 14, 255–263. [Google Scholar] [CrossRef]
- Koloverou, E.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Georgousopoulou, E.N.; Grekas, A.; Christou, A.; Chatzigeorgiou, M.; Skoumas, I.; Tousoulis, D.; et al. Adherence to Mediterranean Diet and 10-year Incidence (2002–2012) of Diabetes: Correlations with Inflammatory and Oxidative Stress Biomarkers in the ATTICA Cohort Study. Diabetes/Metab. Res. Rev. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Quetglas-Llabrés, M.M.; Monserrat-Mesquida, M.; Bouzas, C.; Gómez, C.; Mateos, D.; Ripoll-Vera, T.; Tur, J.A.; Sureda, A. Inflammatory and Oxidative Stress Markers Related to Adherence to the Mediterranean Diet in Patients with Metabolic Syndrome. Antioxidants 2022, 11, 901. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; KÖchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.-M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Tranidou, A.; Dagklis, T.; Magriplis, E.; Apostolopoulou, A.; Tsakiridis, I.; Chroni, V.; Tsekitsidi, E.; Kalaitzopoulou, I.; Pazaras, N.; Chourdakis, M. Pre-Pregnancy Adherence to Mediterranean Diet and Risk of Gestational Diabetes Mellitus: A Prospective Cohort Study in Greece. Nutrients 2023, 15, 848. [Google Scholar] [CrossRef]
- Bodnaruc, A.M.; Roberge, M.; Giroux, I.; Aguer, C. The Bidirectional Link between Major Depressive Disorder and Type 2 Diabetes: The Role of Inflammation. Endocrines 2024, 5, 478–500. [Google Scholar] [CrossRef]
- Abell, S.K.; De Courten, B.; Boyle, J.A.; Teede, H.J. Inflammatory and Other Biomarkers: Role in Pathophysiology and Prediction of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2015, 16, 13442–13473. [Google Scholar] [CrossRef]
- Dennedy, M.C.; Dunne, F. The Maternal and Fetal Impacts of Obesity and Gestational Diabetes on Pregnancy Outcome. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 573–589. [Google Scholar] [CrossRef]
- Musa, E.; Salazar-Petres, E.; Arowolo, A.; Levitt, N.; Matjila, M.; Sferruzzi-Perri, A.N. Obesity and Gestational Diabetes Independently and Collectively Induce Specific Effects on Placental Structure, Inflammation and Endocrine Function in a Cohort of South African Women. J. Physiol. 2023, 601, 1287–1306. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Retnakaran, R.; Hanley, A.J.G.; Raif, N.; Connelly, P.W.; Sermer, M.; Zinman, B. C-Reactive Protein and Gestational Diabetes: The Central Role of Maternal Obesity. J. Clin. Endocrinol. Metab. 2003, 88, 3507–3512. [Google Scholar] [CrossRef]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Díaz-Velázquez, M.F.; Meixueiro-Calderón, C.; Valencia-Ortega, J. Role of Oxidative Stress and Inflammation in Gestational Diabetes Mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef]
- Schmatz, M.; Madan, J.; Marino, T.; Davis, J. Maternal Obesity: The Interplay between Inflammation, Mother and Fetus. J. Perinatol. 2010, 30, 441–446. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxidative Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef]
- Mangge, H.; Becker, K.; Fuchs, D.; Gostner, J.M. Antioxidants, Inflammation and Cardiovascular Disease. World J. Cardiol. 2014, 6, 462–477. [Google Scholar] [CrossRef]
- Muñoz, A.; Costa, M. Nutritionally Mediated Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2013, 2013, 610950. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is Olive Oil Good for You? A Systematic Review and Meta-Analysis on Anti-Inflammatory Benefits from Regular Dietary Intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.-I.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; del Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In Vivo Nutrigenomic Effects of Virgin Olive Oil Polyphenols within the Frame of the Mediterranean Diet: A Randomized Controlled Trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Cohen, A.A.; Heshmati, J.; Alami, M.; Berrougui, H.; Zoubdane, N.; Pizarro, A.B.; Khalil, A. Effect of Extra Virgin Olive Oil on Anthropometric Indices, Inflammatory and Cardiometabolic Markers: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Nutr. 2024, 154, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Pourrajab, B.; Fotros, D.; Asghari, P.; Shidfar, F. Effect of the Mediterranean Diet Supplemented with Olive Oil versus the Low-Fat Diet on Serum Inflammatory and Endothelial Indexes among Adults: A Systematic Review and Meta-Analysis of Clinical Controlled Trials. Nutr. Rev. 2024, 83, e1421–e1440. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of Olive Oil on Markers of Inflammation and Endothelial Function—A Systematic Review and Meta-Analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Lees, B.; Mewton, L.; Jacobus, J.; Valadez, E.A.; Stapinski, L.A.; Teesson, M.; Tapert, S.F.; Squeglia, L.M. Association of Prenatal Alcohol Exposure with Psychological, Behavioral, and Neurodevelopmental Outcomes in Children from the Adolescent Brain Cognitive Development Study. AJP 2020, 177, 1060–1072. [Google Scholar] [CrossRef]
- Oei, J.L. Alcohol Use in Pregnancy and Its Impact on the Mother and Child. Addiction 2020, 115, 2148–2163. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Manzano, S.; González-Paramás, A.M. Wine, Polyphenols, and Mediterranean Diets. What Else Is There to Say? Molecules 2021, 26, 5537. [Google Scholar] [CrossRef]
- Bruguera, C.; Segura-García, L.; Okulicz-Kozaryn, K.; Gandin, C.; Matrai, S.; Braddick, F.; Zin-Sędek, M.; Slodownik, L.; Scafato, E.; Colom, J. Prevention of Alcohol Exposed Pregnancies in Europe: The FAR SEAS Guidelines. BMC Pregnancy Childbirth 2024, 24, 246. [Google Scholar] [CrossRef]
- Canadian Centre on Substance Use and Addiction Canada’s Guidance on Alcohol and Health. Available online: https://www.ccsa.ca/en/guidance-tools-resources/substance-use-and-addiction/alcohol/canadas-guidance-alcohol-and-health (accessed on 29 April 2025).
- Dejong, K.; Olyaei, A.; Lo, J.O. Alcohol Use in Pregnancy. Clin. Obstet. Gynecol. 2019, 62, 142. [Google Scholar] [CrossRef] [PubMed]
- Green, P.P. Vital Signs: Alcohol-Exposed Pregnancies—United States, 2011–2013. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Haber, P.S.; Riordan, B.C.; Winter, D.T.; Barrett, L.; Saunders, J.; Hides, L.; Gullo, M.; Manning, V.; Day, C.A.; Bonomo, Y.; et al. New Australian Guidelines for the Treatment of Alcohol Problems: An Overview of Recommendations. Med. J. Aust. 2021, 215, S3–S32. [Google Scholar] [CrossRef]
- National Health Service, United Kingdom Drinking Alcohol While Pregnant. Available online: https://www.nhs.uk/pregnancy/keeping-well/drinking-alcohol-while-pregnant/ (accessed on 2 May 2025).
- Han, M.-M.; Sun, J.-F.; Su, X.-H.; Peng, Y.-F.; Goyal, H.; Wu, C.-H.; Zhu, X.-Y.; Li, L. Probiotics Improve Glucose and Lipid Metabolism in Pregnant Women: A Meta-Analysis. Ann. Transl. Med. 2019, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Hasain, Z.; Che Roos, N.A.; Rahmat, F.; Mustapa, M.; Raja Ali, R.A.; Mokhtar, N.M. Diet and Pre-Intervention Washout Modifies the Effects of Probiotics on Gestational Diabetes Mellitus: A Comprehensive Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3045. [Google Scholar] [CrossRef]
- Łagowska, K.; Malinowska, A.M.; Zawieja, B.; Zawieja, E. Improvement of Glucose Metabolism in Pregnant Women through Probiotic Supplementation Depends on Gestational Diabetes Status: Meta-Analysis. Sci. Rep. 2020, 10, 17796. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; He, Y.; Zhang, D.; Zhang, S. Probiotics for the Prevention of Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Biomol. Biomed. 2024, 24, 1092–1104. [Google Scholar] [CrossRef]
- Mu, J.; Guo, X.; Zhou, Y.; Cao, G. The Effects of Probiotics/Synbiotics on Glucose and Lipid Metabolism in Women with Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2023, 15, 1375. [Google Scholar] [CrossRef]
- Pakmehr, A.; Ejtahed, H.-S.; Shirzad, N.; Hemmatabadi, M.; Farhat, S.; Larijani, B. Preventive Effect of Probiotics Supplementation on Occurrence of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2022, 9, 1031915. [Google Scholar] [CrossRef]
- Pan, Y.-Q.; Zheng, Q.-X.; Jiang, X.-M.; Chen, X.-Q.; Zhang, X.-Y.; Wu, J.-L. Probiotic Supplements Improve Blood Glucose and Insulin Resistance/Sensitivity among Healthy and GDM Pregnant Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Altern. Med. 2021, 2021, 9830200. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Hou, H.; Yang, S. The Effect of Probiotics on Gestational Diabetes Mellitus: An Umbrella Meta-Analysis. BMC Endocr. Disord. 2024, 24, 253. [Google Scholar] [CrossRef]
- Taylor, B.L.; Woodfall, G.E.; Sheedy, K.E.; O’Riley, M.L.; Rainbow, K.A.; Bramwell, E.L.; Kellow, N.J. Effect of Probiotics on Metabolic Outcomes in Pregnant Women with Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2017, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Luan, J.; Hu, J.; Li, Z. Effect of Probiotics on Pregnancy Outcomes in Gestational Diabetes: Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2024, 310, 769–781. [Google Scholar] [CrossRef]
- Yefet, E.; Bar, L.; Izhaki, I.; Iskander, R.; Massalha, M.; Younis, J.S.; Nachum, Z. Effects of Probiotics on Glycemic Control and Metabolic Parameters in Gestational Diabetes Mellitus: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1633. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Wu, S.; Guo, C.; Long, S.; Tan, H. Effects of Probiotic Supplement in Pregnant Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Diabetes Res. 2019, 2019, 5364730. [Google Scholar] [CrossRef]
- Gomez Arango, L.F.; Barrett, H.L.; Callaway, L.K.; David McIntyre, H.; Nitert, M.D. Probiotics in the Prevention of Gestational Diabetes Mellitus (GDM). In Nutrition and Diet in Maternal Diabetes; Humana Press: Totowa, NJ, USA; Springer: Cham, Switzerland, 2018; pp. 275–288. ISBN 978-3-319-56440-1. [Google Scholar]
- Mahdizade Ari, M.; Teymouri, S.; Fazlalian, T.; Asadollahi, P.; Afifirad, R.; Sabaghan, M.; Valizadeh, F.; Ghanavati, R.; Darbandi, A. The Effect of Probiotics on Gestational Diabetes and Its Complications in Pregnant Mother and Newborn: A Systematic Review and Meta-analysis during 2010–2020. J. Clin. Lab. Anal. 2022, 36, e24326. [Google Scholar] [CrossRef] [PubMed]
- Dallanora, S.; Medeiros de Souza, Y.; Deon, R.G.; Tracey, C.A.; Freitas-Vilela, A.A.; Wurdig Roesch, L.F.; Hack Mendes, R. Do Probiotics Effectively Ameliorate Glycemic Control during Gestational Diabetes? A Systematic Review. Arch. Gynecol. Obstet. 2018, 298, 477–485. [Google Scholar] [CrossRef]
- Crawford, T.J.; Crowther, C.A.; Alsweiler, J.; Brown, J. Antenatal Dietary Supplementation with Myo-inositol in Women during Pregnancy for Preventing Gestational Diabetes. Cochrane Database Syst. Rev. 2015, 2015, CD011507. [Google Scholar] [CrossRef]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-Chiro-Inositol Glycans in Insulin Signaling and Insulin Resistance. Mol. Med. 2010, 16, 543–552. [Google Scholar] [CrossRef]
- Mashayekh-Amiri, S.; Mohammad-Alizadeh-Charandabi, S.; Abdolalipour, S.; Mirghafourvand, M. Myo-Inositol Supplementation for Prevention of Gestational Diabetes Mellitus in Overweight and Obese Pregnant Women: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2022, 14, 93. [Google Scholar] [CrossRef]
- Noventa, M.; Vitagliano, A.; Quaranta, M.; Borgato, S.; Abdulrahim, B.; Gizzo, S. Preventive and Therapeutic Role of Dietary Inositol Supplementation in Periconceptional Period and during Pregnancy: A Summary of Evidences and Future Applications. Reprod. Sci. 2016, 23, 278–288. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.; Li, Z.; Sun, L.; Guo, W. The Efficacy of Myo-Inositol Supplementation to Prevent Gestational Diabetes Onset: A Meta-Analysis of Randomized Controlled Trials. J. Matern. Fetal Neonatal Med. 2019, 32, 2249–2255. [Google Scholar] [CrossRef]
- Chan, K.Y.; Wong, M.M.H.; Pang, S.S.H.; Lo, K.K.H. Dietary Supplementation for Gestational Diabetes Prevention and Management: A Meta-Analysis of Randomized Controlled Trials. Arch. Gynecol. Obstet. 2021, 303, 1381–1391. [Google Scholar] [CrossRef]
- Chen, H.; Xiong, J.; Li, Z.; Chen, Y.; Zhang, M.; Chen, D.; Liu, B. Influence of Myo-Inositol on Metabolic Status for Gestational Diabetes: A Meta-Analysis of Randomized Controlled Trials. J. Matern. Fetal Neonatal Med. 2024, 37, 2228450. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, S.; Miao, Z.; Li, Z.; Zhang, H. Myo-Inositol Lowers the Risk of Developing Gestational Diabetic Mellitus in Pregnancies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with Trial Sequential Analysis. J. Diabetes Its Complicat. 2018, 32, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, J. Myo-Inositol Supplementation for the Prevention of Gestational Diabetes: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 273, 38–43. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z. The Efficacy of Myo-Inositol Supplementation to Reduce the Incidence of Gestational Diabetes: A Meta-Analysis. Gynecol. Endocrinol. 2022, 38, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Rogozińska, E.; Chamillard, M.; Hitman, G.A.; Khan, K.S.; Thangaratinam, S. Nutritional Manipulation for the Primary Prevention of Gestational Diabetes Mellitus: A Meta-Analysis of Randomised Studies. PLoS ONE 2015, 10, e0115526. [Google Scholar] [CrossRef]
- Vitagliano, A.; Saccone, G.; Cosmi, E.; Visentin, S.; Dessole, F.; Ambrosini, G.; Berghella, V. Inositol for the Prevention of Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Arch. Gynecol. Obstet. 2019, 299, 55–68. [Google Scholar] [CrossRef]
- Wei, J.; Yan, J.; Yang, H. Inositol Nutritional Supplementation for the Prevention of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2022, 14, 2831. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, Z.; Zhang, Y.; Lin, Y.; Song, J.; Zheng, L.; Lin, S. Relationship between Myo-Inositol Supplementary and Gestational Diabetes Mellitus. Medicine 2015, 94, e1604. [Google Scholar] [CrossRef] [PubMed]
- Amraei, M.; Mohamadpour, S.; Sayehmiri, K.; Mousavi, S.F.; Shirzadpour, E.; Moayeri, A. Effects of Vitamin D Deficiency on Incidence Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Wang, X.; You, L.; Xu, P.; Cui, X.; Zhu, L.; Ji, C.; Guo, X.; Wen, J. Maternal Vitamin D Status and Risk of Gestational Diabetes: A Meta-Analysis. Cell. Physiol. Biochem. 2018, 45, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Jahanjoo, F.; Farshbaf-Khalili, A.; Shakouri, S.K.; Dolatkhah, N. Maternal and Neonatal Metabolic Outcomes of Vitamin D Supplementation in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Ann. Nutr. Metab. 2018, 73, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Xu, Y.; Lv, L.; Zhang, M. Association between Vitamin D Status and the Risk of Gestational Diabetes Mellitus: A Meta-Analysis. Arch. Gynecol. Obstet. 2016, 293, 959–966. [Google Scholar] [CrossRef]
- Milajerdi, A.; Abbasi, F.; Mousavi, S.M.; Esmaillzadeh, A. Maternal Vitamin D Status and Risk of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Clin. Nutr. 2021, 40, 2576–2586. [Google Scholar] [CrossRef]
- Ojo, O.; Weldon, S.M.; Thompson, T.; Vargo, E.J. The Effect of Vitamin D Supplementation on Glycaemic Control in Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Int. J. Environ. Res. Public Health 2019, 16, 1716. [Google Scholar] [CrossRef]
- Poel, Y.H.M.; Hummel, P.; Lips, P.; Stam, F.; van der Ploeg, T.; Simsek, S. Vitamin D and Gestational Diabetes: A Systematic Review and Meta-Analysis. Eur. J. Intern. Med. 2012, 23, 465–469. [Google Scholar] [CrossRef]
- Sadeghian, M.; Asadi, M.; Rahmani, S.; Akhavan Zanjani, M.; Sadeghi, O.; Hosseini, S.A.; Zare Javid, A. Circulating Vitamin D and the Risk of Gestational Diabetes: A Systematic Review and Dose-Response Meta-Analysis. Endocrine 2020, 70, 36–47. [Google Scholar] [CrossRef]
- Tripathi, P.; Rao, Y.; Pandey, K.; Gautam, K. Significance of Vitamin D on the Susceptibility of Gestational Diabetes Mellitus—A Meta-Analysis. Indian J. Endocrinol. Metab. 2019, 23, 514–524. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Song, Y.; Zhang, Z. Serum Vitamin D Deficiency and Risk of Gestational Diabetes Mellitus: A Meta-Analysis. Arch. Med. Sci. AMS 2020, 16, 742–751. [Google Scholar] [CrossRef]
- Wu, C.; Yang, S.; Wang, X. Vitamin D Supplementation for the Outcomes of Patients with Gestational Diabetes Mellitus and Neonates: A Meta-Analysis and Systematic Review. Int. J. Clin. Pract. 2023, 2023, 1907222. [Google Scholar] [CrossRef] [PubMed]
- Artal, R.; O’Toole, M. Guidelines of the American College of Obstetricians and Gynecologists for Exercise during Pregnancy and the Postpartum Period. Br. J. Sports Med. 2003, 37, 6–12. [Google Scholar] [CrossRef]
- Egan, A.M.; Dunne, F.P. Excessive Gestational Weight Gain and Pregnancy Outcomes in Gestational and Pre-Gestational Diabetes. In Nutrition and Diet in Maternal Diabetes; Humana Press: Cham, Switzerland, 2018; pp. 401–411. ISBN 978-3-319-56440-1. [Google Scholar]
- Martínez-Vizcaíno, V.; Sanabria-Martínez, G.; Fernández-Rodríguez, R.; Cavero-Redondo, I.; Pascual-Morena, C.; Álvarez-Bueno, C.; Martínez-Hortelano, J.A. Exercise during Pregnancy for Preventing Gestational Diabetes Mellitus and Hypertensive Disorders: An Umbrella Review of Randomised Controlled Trials and an Updated Meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 264–275. [Google Scholar] [CrossRef]
- Ming, W.-K.; Ding, W.; Zhang, C.J.P.; Zhong, L.; Long, Y.; Li, Z.; Sun, C.; Wu, Y.; Chen, H.; Chen, H.; et al. The Effect of Exercise during Pregnancy on Gestational Diabetes Mellitus in Normal-Weight Women: A Systematic Review and Meta-Analysis. BMC Pregnancy Childbirth 2018, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, C.P.; Bandak, E.; Edemann-Callesen, H.; Juhl, C.B.; Händel, M.N. The Effects of Exercise during Pregnancy on Gestational Diabetes Mellitus, Preeclampsia, and Spontaneous Abortion among Healthy Women—A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 6069. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, H.; Ren, M. Influence of Exercise Intervention on Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J. Endocrinol. Investig. 2017, 40, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Nasiri-Amiri, F.; Sepidarkish, M.; Shirvani, M.A.; Habibipour, P.; Tabari, N.S.M. The Effect of Exercise on the Prevention of Gestational Diabetes in Obese and Overweight Pregnant Women: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2019, 11, 72. [Google Scholar] [CrossRef]
- Santa Cruz, T.E.; Sarasqueta, C.; Muruzábal, J.C.; Ansuategui, E.; Sanz, O. A Systematic Review and Meta-Analysis of Exercise-Based Intervention to Prevent Gestational Diabetes in Women with Overweight or Obesity. BMC Pregnancy Childbirth 2025, 25, 5. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Sinclair, M.; Liddle, D.; Hill, A.J.; Madden, E.; Stockdale, J. A Systematic Review Investigating Healthy Lifestyle Interventions Incorporating Goal Setting Strategies for Preventing Excess Gestational Weight Gain. PLoS ONE 2012, 7, e39503. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Petrella, E.; Bertarini, V.; Pedrielli, G.; Neri, I.; Facchinetti, F. Adherence to a Lifestyle Programme in Overweight/Obese Pregnant Women and Effect on Gestational Diabetes Mellitus: A Randomized Controlled Trial. Matern. Child Nutr. 2017, 13, e12333. [Google Scholar] [CrossRef]
- Petrella, E.; Malavolti, M.; Bertarini, V.; Pignatti, L.; Neri, I.; Battistini, N.C.; Facchinetti, F. Gestational Weight Gain in Overweight and Obese Women Enrolled in a Healthy Lifestyle and Eating Habits Program. J. Matern. Fetal Neonatal Med. 2014, 27, 1348–1352. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Y.; Zhang, X.; Zhang, Y.; Xu, Q.; Sun, Y.; Su, S.; Zhang, L.; Liu, C.; Feng, Y.; et al. A Randomized Clinical Trial of Exercise during Pregnancy to Prevent Gestational Diabetes Mellitus and Improve Pregnancy Outcome in Overweight and Obese Pregnant Women. Am. J. Obstet. Gynecol. 2017, 216, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.M.; Yang, H.X.; Zhu, W.W.; Liu, X.Y.; Meng, W.Y.; Wang, Y.Q.; Shang, L.X.; Cai, Z.Y.; Ji, L.P.; Wang, Y.F.; et al. Risk of Adverse Pregnancy Outcomes Stratified for Pre-Pregnancy Body Mass Index. J. Matern. Fetal Neonatal Med. 2016, 29, 2205–2209. [Google Scholar] [CrossRef]
- Cnattingius, S.; Bergström, R.; Lipworth, L.; Kramer, M.S. Prepregnancy Weight and the Risk of Adverse Pregnancy Outcomes. N. Engl. J. Med. 1998, 338, 147–152. [Google Scholar] [CrossRef]
- García-Patterson, A.; Erdozain, L.; Ginovart, G.; Adelantado, J.M.; Cubero, J.M.; Gallo, G.; Leiva, A.; Corcoy, R. In Human Gestational Diabetes Mellitus Congenital Malformations Are Related to Pre-Pregnancy Body Mass Index and to Severity of Diabetes. Diabetologia 2004, 47, 509–514. [Google Scholar] [CrossRef]
- Ke, J.-F.; Liu, S.; Ge, R.-L.; Ma, L.; Li, M.-F. Associations of Maternal Pre-Pregnancy BMI and Gestational Weight Gain with the Risks of Adverse Pregnancy Outcomes in Chinese Women with Gestational Diabetes Mellitus. BMC Pregnancy Childbirth 2023, 23, 414. [Google Scholar] [CrossRef]
- Li, N.; Liu, E.; Guo, J.; Pan, L.; Li, B.; Wang, P.; Liu, J.; Wang, Y.; Liu, G.; Baccarelli, A.A.; et al. Maternal Prepregnancy Body Mass Index and Gestational Weight Gain on Pregnancy Outcomes. PLoS ONE 2013, 8, e82310. [Google Scholar] [CrossRef]
- Lyu, Y.; Cui, M.; Zhang, L.; Zheng, G.; Zuo, H.; Xiu, Q.; Shah, P.S. Pre-Pregnancy Body Mass Index, Gestational Diabetes Mellitus, and Gestational Weight Gain: Individual and Combined Effects on Fetal Growth. Front. Public Health 2024, 12, 1354355. [Google Scholar] [CrossRef]
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal Obesity and Pregnancy Outcome: A Study of 287 213 Pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1175. [Google Scholar] [CrossRef]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of Pre-Pregnancy Body Mass Index and Gestational Weight Gain on Maternal and Infant Complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef]
- Thamban, V.; Venkatappa, K.G. Gestational Diabetes Mellitus and Its Relation to Pre-Pregnancy Body Mass Index. J. Clin. Diagn. Res. 2020, 14, CC01–CC03. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic Syndrome in Childhood: Association with Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Choi, M.J.; Yu, J.; Choi, J. Maternal Pre-Pregnancy Obesity and Gestational Diabetes Mellitus Increase the Risk of Childhood Obesity. Children 2022, 9, 928. [Google Scholar] [CrossRef]
- Leng, J.; Li, W.; Zhang, S.; Liu, H.; Wang, L.; Liu, G.; Li, N.; Redman, L.M.; Baccarelli, A.A.; Hou, L.; et al. GDM Women’s Pre-Pregnancy Overweight/Obesity and Gestational Weight Gain on Offspring Overweight Status. PLoS ONE 2015, 10, e0129536. [Google Scholar] [CrossRef]
- Mamun, A.A.; O’Callaghan, M.; Callaway, L.; Williams, G.; Najman, J.; Lawlor, D.A. Associations of Gestational Weight Gain with Offspring Body Mass Index and Blood Pressure at 21 Years of Age: Evidence from a Birth Cohort Study. Circulation 2009, 119, 1720–1727. [Google Scholar] [CrossRef]
- Moreira, P.; Padez, C.; Mourão-Carvalhal, I.; Rosado, V. Maternal Weigh Gain during Pregnancy and Overweight in Portuguese Children. Int. J. Obes. 2007, 31, 608–614. [Google Scholar] [CrossRef]
- Oken, E.; Rifas-Shiman, S.L.; Field, A.E.; Frazier, A.L.; Gillman, M.W. Maternal Gestational Weight Gain and Offspring Weight in Adolescence. Obstet. Gynecol. 2008, 112, 999–1006. [Google Scholar] [CrossRef]
- Robitaille, J.; Grant, A.M. The Genetics of Gestational Diabetes Mellitus: Evidence for Relationship with Type 2 Diabetes Mellitus. Genet. Med. 2008, 10, 240–250. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Pawliczak, J.; Passow, D.; Hartmann, R.; Rossi, R.; Bührer, C.; Harder, T.; Plagemann, A.; Vetter, K.; Kordonouri, O. Birth Weight and Parental BMI Predict Overweight in Children from Mothers with Gestational Diabetes. Diabetes Care 2005, 28, 1745–1750. [Google Scholar] [CrossRef]
- Wrotniak, B.H.; Shults, J.; Butts, S.; Stettler, N. Gestational Weight Gain and Risk of Overweight in the Offspring at Age 7 y in a Multicenter, Multiethnic Cohort Study. Am. J. Clin. Nutr. 2008, 87, 1818–1824. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Henriksen, T.; Saugstad, O.D.; Tonstad, S. Physical Activity and the Risk of Gestational Diabetes Mellitus: A Systematic Review and Dose–Response Meta-Analysis of Epidemiological Studies. Eur. J. Epidemiol. 2016, 31, 967–997. [Google Scholar] [CrossRef]
- Cui, H.; Li, H.; Huang, J.; Wu, Y.; Wei, Y.; Li, M. The Effect of Exercise on the Adverse Neonatal Outcomes Related to Women with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Front. Clin. Diabetes Healthc. 2025, 6, 1566577. [Google Scholar] [CrossRef]
- Sanabria-Martínez, G.; García-Hermoso, A.; Poyatos-León, R.; Álvarez-Bueno, C.; Sánchez-López, M.; Martínez-Vizcaíno, V. Effectiveness of Physical Activity Interventions on Preventing Gestational Diabetes Mellitus and Excessive Maternal Weight Gain: A Meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 1167–1174. [Google Scholar] [CrossRef]
- Teede, H.J.; Bailey, C.; Moran, L.J.; Bahri Khomami, M.; Enticott, J.; Ranasinha, S.; Rogozińska, E.; Skouteris, H.; Boyle, J.A.; Thangaratinam, S.; et al. Association of Antenatal Diet and Physical Activity–Based Interventions with Gestational Weight Gain and Pregnancy Outcomes: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2022, 182, 106–114. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Ong, M.J.; Crisp, N.A.; Fournier, P.A.; Wallman, K.E.; Grove, J.R.; Doherty, D.A.; Newnham, J.P. Regular Exercise to Prevent the Recurrence of Gestational Diabetes Mellitus: A Randomized Controlled Trial. Obstet. Gynecol. 2016, 128, 819–827. [Google Scholar] [CrossRef]
- Ben-Haroush, A.; Yogev, Y.; Hod, M. Epidemiology of Gestational Diabetes Mellitus and Its Association with Type 2 Diabetes. Diabet. Med. 2004, 21, 103–113. [Google Scholar] [CrossRef]
- Holten, M.K.; Zacho, M.; Gaster, M.; Juel, C.; Wojtaszewski, J.F.P.; Dela, F. Strength Training Increases Insulin-Mediated Glucose Uptake, GLUT4 Content, and Insulin Signaling in Skeletal Muscle in Patients with Type 2 Diabetes. Diabetes 2004, 53, 294–305. [Google Scholar] [CrossRef]
- Venkatasamy, V.V.; Pericherla, S.; Manthuruthil, S.; Mishra, S.; Hanno, R. Effect of Physical Activity on Insulin Resistance, Inflammation and Oxidative Stress in Diabetes Mellitus. J. Clin. Diagn. Res. 2013, 7, 1764–1766. [Google Scholar] [CrossRef]
- Kraniou, G.N.; Cameron-Smith, D.; Hargreaves, M. Acute Exercise and GLUT4 Expression in Human Skeletal Muscle: Influence of Exercise Intensity. J. Appl. Physiol. 2006, 101, 934–937. [Google Scholar] [CrossRef]
- Bgeginski, R.; Ribeiro, P.A.B.; Mottola, M.F.; Ramos, J.G.L. Effects of Weekly Supervised Exercise or Physical Activity Counseling on Fasting Blood Glucose in Women Diagnosed with Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Trials. J. Diabetes 2017, 9, 1023–1032. [Google Scholar] [CrossRef]
- Cremona, A.; O’Gorman, C.; Cotter, A.; Saunders, J.; Donnelly, A. Effect of Exercise Modality on Markers of Insulin Sensitivity and Blood Glucose Control in Pregnancies Complicated with Gestational Diabetes Mellitus: A Systematic Review. Obes. Sci. Pract. 2018, 4, 455–467. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Fischer, C.P. Beneficial Health Effects of Exercise—The Role of IL-6 as a Myokine. Trends Pharmacol. Sci. 2007, 28, 152–156. [Google Scholar] [CrossRef]
- Ortenblad, L.; Hotoft, D.; Krogh, R.H.; Lynggaard, V.; Juel Christiansen, J.; Vinther Nielsen, C.; Hedeager Momsen, A.-M. Women’s Perspectives on Motivational Factors for Lifestyle Changes after Gestational Diabetes and Implications for Diabetes Prevention Interventions. Endocrinol. Diabetes Metab. 2021, 4, e00248. [Google Scholar] [CrossRef]
- Tierney, M.; O’Dea, A.; Danyliv, A.; Noctor, E.; McGuire, B.; Glynn, L.; Al-Imari, H.; Dunne, F. Factors Influencing Lifestyle Behaviours during and after a Gestational Diabetes Mellitus Pregnancy. Health Psychol. Behav. Med. 2015, 3, 204–216. [Google Scholar] [CrossRef]
- Absalom, G.; Zinga, J.; Margerison, C.; van der Pligt, P. Associations of Dietetic Management with Maternal and Neonatal Health Outcomes in Women Diagnosed with Gestational Diabetes: A Retrospective Cohort Study. J. Human Nutr. Diet. 2019, 32, 728–736. [Google Scholar] [CrossRef]
- Fagen, C.; King, J.D.; Erick, M. Nutrition Management in Women with Gestational Diabetes Mellitus: A Review by ADA’s Diabetes Care and Education Dietetic Practice Group. J. Am. Diet. Assoc. 1995, 95, 460–467. [Google Scholar] [CrossRef]
- Madhuvrata, P.; Govinden, G.; Bustani, R.; Song, S.; Farrell, T. Prevention of Gestational Diabetes in Pregnant Women with Risk Factors for Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomised Trials. Obstet. Med. 2015, 8, 68–85. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Mauricio, D.; Hernandez, M. Role of Medical Nutrition Therapy in the Management of Gestational Diabetes Mellitus. Curr. Diabetes Rep. 2016, 16, 22. [Google Scholar] [CrossRef]
- Reader, D.; Splett, P.; Gunderson, E.P. Impact of Gestational Diabetes Mellitus Nutrition Practice Guidelines Implemented by Registered Dietitians on Pregnancy Outcomes. J. Am. Diet. Assoc. 2006, 106, 1426–1433. [Google Scholar] [CrossRef]
- Muhwava, L.S.; Murphy, K.; Zarowsky, C.; Levitt, N. Experiences of Lifestyle Change among Women with Gestational Diabetes Mellitus (GDM): A Behavioural Diagnosis Using the COM-B Model in a Low-Income Setting. PLoS ONE 2019, 14, e0225431. [Google Scholar] [CrossRef]
- Hussien, S.; Mcmanus, R.; Prapavessis, H.; Sopper, M.M.; Mottola, M.F. Effect of Lifestyle on Reducing Risk Factors for Type 2 Diabetes among Arab Canadian Muslim Women: A Randomized Control Trial. Med. Sci. Sports Exerc. 2024, 56, 249–256. [Google Scholar] [CrossRef]
- Astell-Burt, T.; Feng, X.; Kolt, G.S. Is Neighborhood Green Space Associated with a Lower Risk of Type 2 Diabetes? Evidence from 267,072 Australians. Diabetes Care 2014, 37, 197–202. [Google Scholar] [CrossRef]
- Barton, H. City of Well-Being: A Radical Guide to Planning; Routledge: London, UK, 2016; ISBN 978-1-315-43868-9. [Google Scholar]
- Dalton, A.M.; Jones, A.P.; Sharp, S.J.; Cooper, A.J.M.; Griffin, S.; Wareham, N.J. Residential Neighbourhood Greenspace Is Associated with Reduced Risk of Incident Diabetes in Older People: A Prospective Cohort Study. BMC Public Health 2016, 16, 1171. [Google Scholar] [CrossRef]
- Den Braver, N.R.; Lakerveld, J.; Rutters, F.; Schoonmade, L.J.; Brug, J.; Beulens, J.W.J. Built Environmental Characteristics and Diabetes: A Systematic Review and Meta-Analysis. BMC Med. 2018, 16, 12. [Google Scholar] [CrossRef]
- Frank, L.D.; Saelens, B.E.; Powell, K.E.; Chapman, J.E. Stepping towards Causation: Do Built Environments or Neighborhood and Travel Preferences Explain Physical Activity, Driving, and Obesity? Social Sci. Med. 2007, 65, 1898–1914. [Google Scholar] [CrossRef]
- Rasmussen, M.G.; Grøntved, A.; Blond, K.; Overvad, K.; Tjønneland, A.; Jensen, M.K.; Østergaard, L. Associations between Recreational and Commuter Cycling, Changes in Cycling, and Type 2 Diabetes Risk: A Cohort Study of Danish Men and Women. PLoS Med. 2016, 13, e1002076. [Google Scholar] [CrossRef]
- Government of Canada. The Chief Public Health Officer’s Report on the State of Public Health in Canada 2017—Designing Healthy Living. Available online: https://www.canada.ca/en/public-health/services/publications/chief-public-health-officer-reports-state-public-health-canada/2017-designing-healthy-living.html (accessed on 13 August 2024).
- Maas, J.; Verheij, R.A.; de Vries, S.; Spreeuwenberg, P.; Schellevis, F.G.; Groenewegen, P.P. Morbidity Is Related to a Green Living Environment. J. Epidemiol. Community Health 2009, 63, 967. [Google Scholar] [CrossRef]
- Field, C.; Lynch, C.D.; Fareed, N.; Joseph, J.J.; Wu, J.; Thung, S.F.; Gabbe, S.G.; Landon, M.B.; Grobman, W.A.; Venkatesh, K.K. Association of Community Walkability and Glycemic Control among Pregnant Individuals with Pregestational Diabetes Mellitus. Am. J. Obstet. Gynecol. MFM 2023, 5, 100898. [Google Scholar] [CrossRef]
- Yu, Z.; Feng, Y.; Chen, Y.; Zhang, X.; Zhao, X.; Chang, H.; Zhang, J.; Gao, Z.; Zhang, H.; Huang, C. Green Space, Air Pollution and Gestational Diabetes Mellitus: A Retrospective Cohort Study in Central China. Ecotoxicol. Environ. Saf. 2023, 249, 114457. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, W.; Huang, Y.; Wang, C.; Cao, J.; Qiu, W.; Huang, R.; Zhang, J.; Ye, W.; Zhang, Q. The Influence of Socioeconomic Status on the Association between Residential Greenness and Gestational Diabetes Mellitus in an Urban Setting: A Multicenter Study. BMC Public Health 2025, 25, 1708. [Google Scholar] [CrossRef]
- WHO. Promoting Health in All Policies and Intersectoral Action Capacities. Available online: https://www.who.int/activities/promoting-health-in-all-policies-and-intersectoral-action-capacities (accessed on 10 August 2024).
- Ståhl, T.; Wismar, M.; Ollila, E.; Lahtinen, E.; Leppo, K. (Eds.) Health in All Policies: Prospects and Potentials; Ministry of Social Affairs and Health: Helsinki, Finland, 2006; ISBN 978-952-00-1964-8. [Google Scholar]
- Cain, R.; Greece, J.A.; Galea, S.; Knight, E.K.; Manco, A.; Parikh, A.; Jones, D.K. Improving Health across Sectors: Best Practices for the Implementation of Health in All Policies Approaches. Prev. Med. Rep. 2022, 29, 101961. [Google Scholar] [CrossRef]
- ASTHO. Association of State and Territorial Health Officials Health in All Policies. 2013. Available online: https://www.astho.org/globalassets/pdf/hiap/hiap-strategies-to-promote-innovative-leadership.pdf (accessed on 11 August 2024).
- Sing, F.; Backholer, K. Strengthening Global Legislative Actions to Protect Children from the Harmful Impacts of Unhealthy Food and Non-Alcoholic Beverage Marketing. Curr. Obes. Rep. 2023, 12, 1–9. [Google Scholar] [CrossRef]
- Chung, A.; Zorbas, C.; Riesenberg, D.; Sartori, A.; Kennington, K.; Ananthapavan, J.; Backholer, K. Policies to Restrict Unhealthy Food and Beverage Advertising in Outdoor Spaces and on Publicly Owned Assets: A Scoping Review of the Literature. Obes. Rev. 2022, 23, e13386. [Google Scholar] [CrossRef]
- Castillo-Castrejon, M.; Powell, T.L. Placental Nutrient Transport in Gestational Diabetic Pregnancies. Front. Endocrinol. 2017, 8, 306. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2021, 44, 258–279. [Google Scholar] [CrossRef]
- Sharifi, N.; Dolatian, M.; Mahmoodi, Z.; Mohammadi Nasrabadi, F. Gestational Diabetes and Its Relationship with Social Determinants of Health According to World Health Organization Model: Systematic Review. Iran. J. Obstet. Gynecol. Infertil. 2017, 19, 6–18. [Google Scholar] [CrossRef]
- Walker, R.J.; Strom Williams, J.; Egede, L.E. Influence of Race, Ethnicity and Social Determinants of Health on Diabetes Outcomes. Am. J. Med. Sci. 2016, 351, 366–373. [Google Scholar] [CrossRef]
- WHO. Social Determinants of Health. Available online: https://www.who.int/health-topics/social-determinants-of-health (accessed on 29 July 2024).
- CDC Social Determinants of Health. Available online: https://www.cdc.gov/public-health-gateway/php/about/social-determinants-of-health.html (accessed on 18 May 2025).
- Government of Canada. Social Determinants of Health and Health Inequalities. Available online: https://www.canada.ca/en/public-health/services/health-promotion/population-health/what-determines-health.html (accessed on 13 August 2024).
- Timm, A.; Nielsen, K.K.; Christensen, U.; Maindal, H.T. Healthcare Professionals’ Perspectives on the Cross-Sectoral Treatment Pathway for Women with Gestational Diabetes during and after Pregnancy—A Qualitative Study. J. Clin. Med. 2021, 10, 843. [Google Scholar] [CrossRef]
- Frieden, T.R. A Framework for Public Health Action: The Health Impact Pyramid. Am. J. Public Health 2010, 100, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Brygger Venø, L.; Pedersen, L.B.; Søndergaard, J.; Ertmann, R.K.; Jarbøl, D.E. Challenges in the Cross-Sectoral Collaboration on Vulnerable Pregnant Women: A Qualitative Study among Danish General Practitioners. BMC Prim. Care 2022, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Bryson, J.M.; Crosby, B.C.; Stone, M.M. Designing and Implementing Cross-sector Collaborations: Needed and Challenging. Public Adm. Rev. 2015, 75, 647–663. [Google Scholar] [CrossRef]
- Puska, P.; Ståhl, T. Health in All Policies—The Finnish Initiative: Background, Principles, and Current Issues. Annu. Rev. Public Health 2010, 31, 315–328. [Google Scholar] [CrossRef]
- Ståhl, T. Health in All Policies: From Rhetoric to Implementation and Evaluation—The Finnish Experience. Scand. J. Public Health 2018, 46, 38–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).