Abstract
Background/Objectives: The Internet of Things (IoT), integrated with application software, has increasingly been used to support health management through monitoring indicators like physical activity, sleep, and heart rate, in pregnant and postpartum women. However, limited evidence exists regarding its effectiveness in improving health outcomes for pregnant and postpartum women. The objective of this systematic review and meta-analysis was to evaluate and synthesize the role of IoT in enhancing the health outcomes of pregnant and postpartum women. Methods: A systematic search was conducted on 13 February 2023, across CENTRAL, CINAHL, ClinicalTrials.gov, Embase, MEDLINE, PsycINFO, PubMed, and WHO ICTRP to identify all randomized controlled trials. Studies were included if they involved pregnant or postpartum women in high-income countries and used sensor-based data collection via smartphones or wearable devices. Two reviewers independently selected the studies, extracted data, and assessed the risk of bias using the Cochrane Collaboration’s risk of bias assessment tool 2.0. We performed a pairwise meta-analysis using a random effects model. The findings were reported according to PRISMA guidelines. Results: Seven studies with 1638 pregnant and postpartum women were included in this review. Of the seven included studies, half targeted women with gestational diabetes and the other half targeted obese women. A meta-analysis revealed that IoT interventions may reduce gestational weight gain in women with obesity with a mean difference of −3.35 kg (95% confidence interval (CI): −5.23 to−1.46; I2 = 36%; two studies; 242 women; moderate certainty of evidence). Conclusions: This review suggested that IoT interventions may limit gestational weight gain in pregnant women with obesity. Future studies should evaluate the long-term effects of IoT-based interventions on maternal and neonatal health outcomes.
1. Introduction
Globally, the prevalence of obesity among women has been increasing since the 1970s [1]. Approximately one in five pregnant women in high-income countries is classified as obese during antenatal appointments [2]. Overweight and obesity are associated with adverse pregnancy outcomes like maternal mortality, childbirth complications, gestational diabetes, preterm birth, and intrauterine growth restriction [3,4,5]. Additionally, women with gestational diabetes have a high risk of developing type 2 diabetes mellitus within 5 years after delivery [6].
These pregnancy and postpartum complications are often associated with lifestyle behaviors such as diet, exercise, and sleep [7,8,9]. Recent studies have utilized the Internet of Things (IoT) integrated with application software to improve women’s lifestyle behaviors and health management [10,11,12]. The IoT is a technological phenomenon arising from the advancement in information and communication technologies [13]. Tsirmpas et al. defined IoT as the ability of smart devices to communicate with each other and create interconnected networks [13]. Previously, patient health and behavior-related data were limited to intermittent measurements. However, advancements in wearable sensors and systems have made real time, remote data collection and analysis of patient information feasible, facilitating applications in medical fields such as early disease detection, chronic condition prevention, and rapid emergency response [14].
Various types of IoT devices have been developed, including smartwatches, smart rings, smart clothing, and smart glasses. These devices are accompanied by a diverse array of applications aimed at fostering behavioral change, e.g., step-counting smartphone applications to improve postpartum weight among women with gestational diabetes [15,16] and smart wristbands that monitor health indicators, such as physical activity, sleep patterns, and heart rate among pregnant women [17,18].
Previous systematic reviews have examined IoT interventions targeting maternal and child health. These include reviews of wearable sensor technologies for monitoring the health of pregnant women and fetuses [19]; IoT architectures, systems, models, and devices for monitoring and managing complications during pregnancy, the postpartum period, and neonatal care [20]; and remote monitoring via digital glucose meters to aid glycemic control in women with gestational diabetes [21]. However, these studies summarized the findings narratively and did not conduct meta-analyses.
In recent years, randomized controlled trials (RCTs) have been conducted to evaluate the effectiveness of IoT interventions using wearable devices to improve the health of pregnant and postpartum women. By defining IoT interventions as sensor-driven technologies capable of tracking physiological and behavioral health indicators, it becomes feasible to synthesize evidence exclusively from RCTs. In high-income countries, the adoption of IoT technologies, such as smartphones and wearable devices, is widespread, and their integration with healthcare services and internet infrastructure is well established [22]. Therefore, this review aimed to evaluate the clinical effectiveness of IoT-based interventions on maternal and neonatal outcomes among pregnant and postpartum women in high-income countries.
2. Materials and Methods
2.1. Study Design
The systematic review protocol was registered on PROSPERO (register number: CRD42022384620) and published in a journal [23]. Initially intended as a network meta-analysis, the study was subsequently conducted as a systematic review and meta-analysis owing to the insufficient number of studies and limited variety of interventions. This study was conducted in accordance with the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions [24]. The findings are reported (Table S1) according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [25].
2.2. Inclusion and Exclusion Criteria
The eligibility criteria are defined in the following PICOS framework (P—Participants, I—Interventions, C—Comparator, O—Outcomes, and S—Study design):
2.2.1. Participants
The study participants were pregnant or postpartum women in high-income countries. For the definition of high income, we used the World Bank classification [26].
2.2.2. Intervention
Studies on IoT interventions designed to improve the health of pregnant or postpartum women were included. To evaluate the effectiveness of sensor-driven IoT applications in tracking physiological and behavioral health indicators, this review encompasses various types of IoT, with a special focus on data collection and monitoring through sensors in smartphones and wearable devices. Thus, mHealth interventions—which involve the use of mobile technologies such as mobile phones, patient monitoring tools, PDAs, and other wireless devices to facilitate the delivery of medical care and public health services [27]—and IoT interventions primarily designed for health education purposes, were excluded from the study.
2.2.3. Comparators
Studies that evaluated the effectiveness of the IoT intervention compared to usual care, interventions that did not use IoT, or alternative approaches such as education or exercise without IoT, were included.
2.2.4. Outcomes
Studies that reported maternal and neonatal health outcomes were included and categorized into primary and secondary outcomes. The primary outcomes were maternal and neonatal health outcomes, as they were directly related to the overall purpose of this review, which was to evaluate the effectiveness of IoT interventions in improving clinically significant health outcomes during pregnancy and the postpartum period. The secondary outcomes included lifestyle and behavioral changes, which serve as intermediate indicators that facilitate the achievement of the primary health outcomes.
Although defined as secondary outcomes, behavioral and lifestyle changes like increased physical activity, dietary improvements, and regular self-monitoring act as crucial mediators of the clinical effects of IoT interventions. These behavioral modifications represent key mechanisms through which improvements in maternal and neonatal outcomes are achieved. Therefore, evaluating these mediators is crucial to fully understand the mechanisms of impact and the effectiveness of the intervention beyond the direct clinical endpoint. This hierarchical classification aligns with the Cochrane Handbook for Systematic Reviews of Interventions [24], which emphasizes prioritizing clinically significant outcomes that directly impact patient care.
- Primary maternal health outcomes included health status, such as the number of cases diagnosed or treated for high-risk pregnancies (e.g., hypertensive disorders of pregnancy, gestational diabetes, and preterm delivery). Neonatal health outcomes included low birth weight, defined as birth weight <2.5 kg, and perinatal death.
- Secondary outcomes included lifestyle and behavioral changes, maintenance of a healthy weight, and other indicators such as body mass index (BMI), body composition, waist circumference, and increased physical activity.
2.2.5. Study Designs
Individual and cluster RCTs were included to evaluate the effectiveness of IoT interventions on the health of pregnant and postnatal women. Reviews, qualitative studies, observational studies, cross-sectional studies, case studies, commentaries, editorials, expert opinions, and letters were excluded from this study.
2.3. Search Methods for Study Identification
The CENTRAL, CINAHL, ClinicalTrials.gov, Embase, MEDLINE, PsycINFO, PubMed, and WHO ICTRP databases were searched from inception to 13 February 2023, with no restrictions on date/time, language, document type, or publication status. The core search terms included combinations of keywords and MeSH terms related to “pregnancy,” “postpartum,” “Internet of Things,” “mHealth,” and “wearable devices,” with filters applied to identify RCTs. The detailed strategies are provided in Supplementary Table S2. The Cochrane Handbook for Systematic Reviews of Interventions [24] and Cochrane’s MECIR [28] were utilized to conduct the search. The PRISMA-S [29] and PRISMA guidelines [25] were followed for reporting the search, and the PRESS guidelines were applied for peer-reviewing the search strategies [30]. Keywords were collected through expert opinions, literature reviews, controlled vocabulary, and an examination of the primary search results (Table S2).
2.4. Study Selection
Duplicate records identified from the databases were excluded. Pairs of reviewers were selected from among the twelve authors (EN, NY, KS, PPT, MRO, MN, GM, KDSL, RS, DS, AN, HH) and subsequently independently screened the titles and abstracts of the records using the predefined study eligibility criteria. The full texts of the studies included in the initial screening were independently assessed by the two authors. The Rayyan tool was used during these screening processes [31]. Any disagreements between reviewers during the screening were resolved through discussion. If an agreement could not be reached, the reviewers discussed the issue with a third reviewer who made the final decision.
2.5. Data Extraction
The two authors used a predesigned data extraction form to extract data from the included studies. The data extraction form was designed to use Excel to extract the following information: study setting, participants, interventions, comparisons, study design, and outcome measures, including primary and secondary outcomes.
2.6. Risk of Bias
Two authors used the Cochrane Collaboration’s risk of bias assessment tool 2.0 to independently assess the risk of bias for each study outcome [32]. The Cochrane Collaboration’s risk of bias assessment tool 2.0 provides a framework for considering the risk of bias in the findings of any type of randomized trial. Assessments using this tool are conducted not for each trial as a whole, but for each outcome of a single trial [32]. This tool identifies the following five domains where bias may be introduced into the results: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in selection of the reported result [32]. Any disagreements between reviewers regarding the risk of bias were resolved through discussion. If an agreement could not be reached, the reviewers discussed the issue with a third reviewer.
2.7. Data Synthesis and Analytical Approach
A pairwise meta-analysis was conducted using a random-effects model to estimate the pooled effect size for each outcome combination. Given the limited number of included studies, we prioritized the use of a traditional pairwise meta-analysis to enhance the interpretability and reproducibility of the results. An intention-to-treat analysis was used to conduct the meta-analysis utilizing the Review Manager Version 5.4 software (RevMan 5). The heterogeneity among the studies included in the meta-analysis was assessed using the estimated value of τ2, which corresponds to the between-study variance, and the Chi-squared test. Additionally, graphical assessments were made using horizontal lines in the forest plots for each outcome. The I2 statistic was used to examine heterogeneity, with values ≥75% indicating considerable heterogeneity, as defined in the Cochrane Handbook for Systematic Reviews of Interventions [24]. Risk ratios (RRs) were used for dichotomous data, whereas mean differences (MDs) were used for continuous data, accompanied with their corresponding 95% confidence intervals (CIs). At least two studies were required as a criterion for conducting a meta-analysis. When the number of studies was less than two, the results were narratively described in accordance with the study protocol. We did not test for publication bias using funnel plots and Egger tests in the pairwise meta-analyses owing to an insufficient number of studies. Sensitivity analysis was performed if the studies had a high risk of bias.
2.8. Assessment of the Certainty of the Evidence
The certainty of evidence regarding the effectiveness of the IoT on outcomes for pregnant and postpartum women was assessed using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach [33]. This system assesses the certainty of evidence and grades it into four categories: high, moderate, low, and very low [33]. The certainty of evidence may be downgraded if there is an increased risk of bias in the studies, inconsistency of results across studies, indirectness of evidence, imprecision of estimates, or concerns about publication bias [34]. In accordance with the protocol, a table title “Summary of findings” was generated using GRADEPro [35] (https://www.gradepro.org/product, accessed on 30 April 2025) for the primary outcome results (Table S3).
3. Results
3.1. Search Results
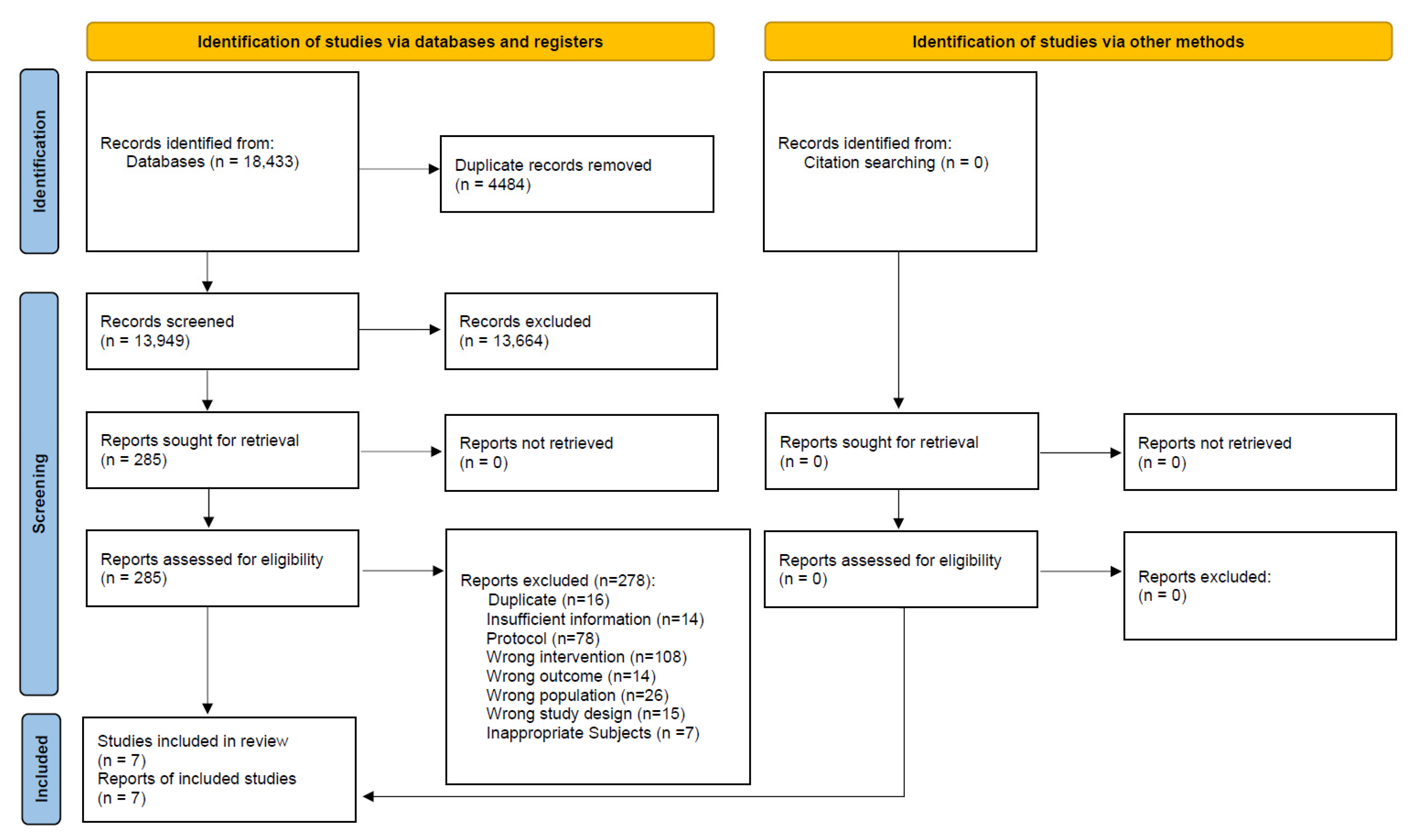
A total of 18,433 records were identified from all targeted electronic databases and other resources, excluding 4484 duplicate records. Moreover, 278 reports were excluded during full-text screening: 141 for ineligible population or intervention, 15 for ineligible study design, 78 study protocols, 16 duplicates, 14 for ineligible outcomes, and 14 for insufficient information. Finally, seven studies were included in this review. The study selection process is described in the flow diagram (Figure 1).
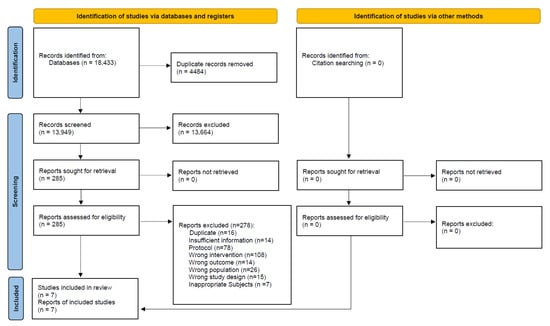
Figure 1.
Flow diagram of the search results and study selection for pregnant and postpartum women.
3.2. Characteristics of the Included Studies
All seven studies included in this review were individual RCTs, half of which were pilot RCTs. These studies were conducted in the United States, Australia, South Korea, Taiwan, Spain, Singapore, and Belgium. Overall, 1638 pregnant and postpartum women participated in the studies. Of the seven included studies, half targeted women with gestational diabetes [15,36,37] and the other half targeted women with obesity [38,39,40,41]. All interventions included activity trackers or wearable devices to monitor physical activity and/or body weight in real time. In addition, all interventions incorporated feedback and counseling provided to the study participants. The characteristics of the included studies are detailed in Table 1 and Tables S4–S8 in the Supplementary Materials. In the included studies, no significant missing data and outliers were identified for key primary and secondary outcomes. Ethics approval was obtained from the relevant institutional review boards in all seven trials; however, the IRB numbers were not listed in five studies.

Table 1.
Characteristics of the included studies.
3.3. Overall Risk of Bias Assessment of the Included Studies
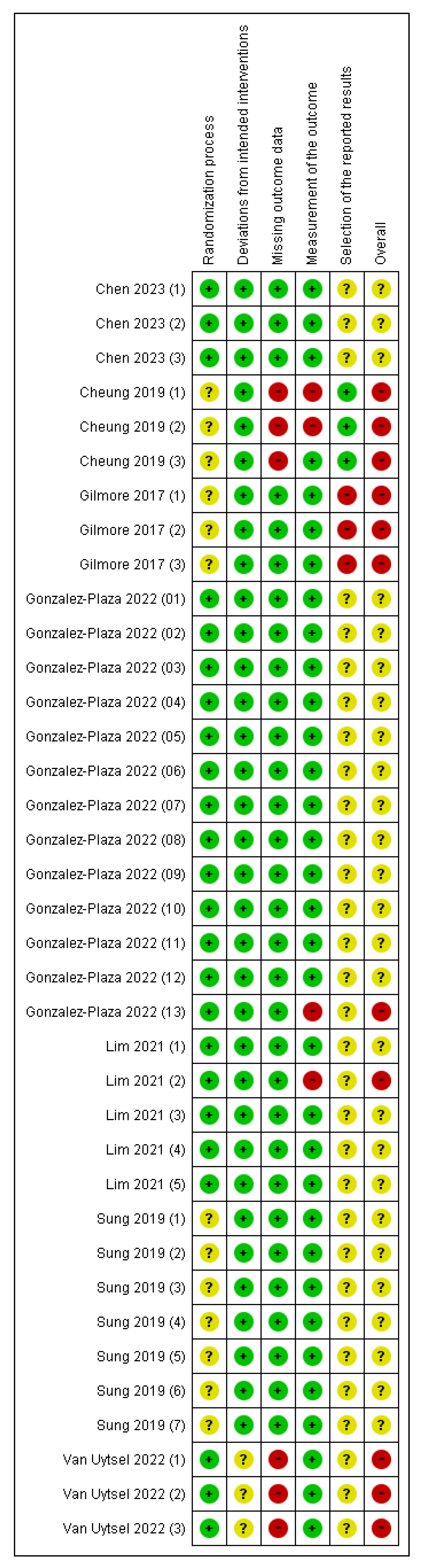
The most frequently identified biases across outcomes were selection bias (risk of bias arising from the randomization process) and reporting bias (risk of bias in the selection of the reported result) (Figure 2 and Figure S1). Most outcomes regarding reporting bias were unclear. Therefore, the “overall” risk of all outcomes was either unknown or high. The blinding of participants and assessors was not feasible due to the nature of the interventions. However, deviations from the intended interventions were unlikely due to the implementation context of the trials. Furthermore, most outcomes assessed using the risk of bias tool relied on objective data, indicating that the potential impact of the lack of blinding on bias was minimal. The studies conducted in Australia [36] and Belgium [41] had high dropout rates, the reasons for which were unclear, leading to a high risk of bias in missing outcome data (Figure 2). In the study conducted in Spain [40], some participants were excluded due to strict COVID-19 lockdown measures; however, the reasons were documented and unrelated to the intervention, resulting in a low risk of bias in missing outcome data. Despite the use of RCTs, the overall risk of bias was high, particularly due to the attrition and outcome reporting domains.
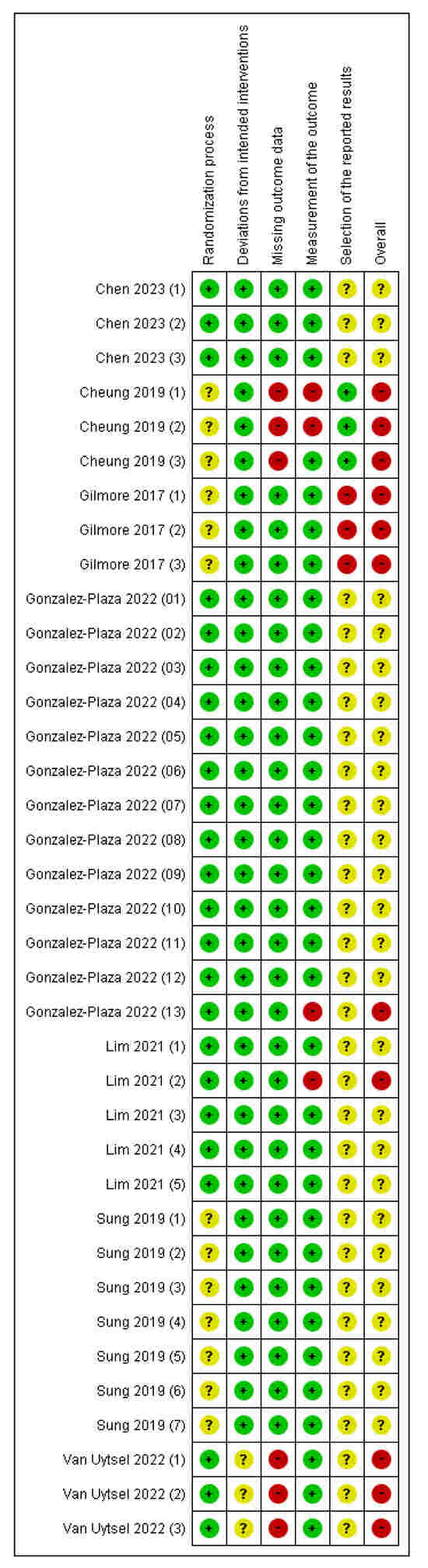
Figure 2.
Risk of bias summary [15,36,37,38,39,40,41]. Red with a - symbol: high risk of bias, yellow with a ? symbol: unclear risk of bias, and green with a + symbol: low risk of bias. Chen 2023(1): Gestational weight gain (kg) first trimester, Chen 2023(2): Gestational weight gain (kg) second trimester, Chen 2023(3): Gestational weight gain (kg) third trimester, Cheung 2019(1): Weight change between final evaluation and 10–12 weeks post-partum (kg), Cheung 2019(2): Total weekly activity time (minutes) at final evaluation, Cheung 2019(3): 7-day pedometer step count, Gilmore 2017(1): Change in weight, Gilmore 2017(2): Body fat percentage, Gilmore 2017(3): Waist circumference, Gonzalez-Plaza 2022(1): Unplanned cesarean, Gonzalez-Plaza 2022(2): Gestational weight gain (kg), Gonzalez-Plaza 2022(3): Gestational weight gain–weekly weight gain (kg), Gonzalez-Plaza 2022(4): Composite pregnancy morbidity, Gonzalez-Plaza 2022(5): Miscarriage ≤22 weeks, Gonzalez-Plaza 2022(6): Gestational diabetes, Gonzalez-Plaza 2022(7): Preeclampsia or gestational hypertension, Gonzalez-Plaza 2022(8): Preterm labor ≤37 weeks, Gonzalez-Plaza 2022(9): Birthweight ≤2500 g, Gonzalez-Plaza 2022(10): Perinatal death, Gonzalez-Plaza 2022(11): Early neonatal death, Gonzalez-Plaza 2022(12): Antepartum stillbirth, Gonzalez-Plaza 2022(13): Physical activity (moderate to high), Lim 2021(1): Absolute difference in weight (kg) from first trimester, Lim 2021(2): Waist circumference (cm), Lim 2021(3): Systolic blood pressure (mm Hg), Lim 2021(4): Fasting OGTT test (mmol/L), Lim 2021(5): 2 h OGTT (mmol/L), Sung 2019(1): Cesarean section, Sung 2019(2): Weight gain during study period (kg), Sung 2019(3): Body fat percentage at postpartum, Sung 2019(4): Systolic blood pressure (mm Hg), Sung 2019(5): Fasting glucose, mg/dL, Sung 2019(6): HOMA-IR, Sung 2019(7): Diabetes at 4–12 weeks postpartum, Van Uytsel 2022(1): Weight retention (kg) change after intervention (6 months postpartum), Van Uytsel 2022(2): Body fat percentage change after intervention (6 months postpartum), Van Uytsel 2022(3): Waist circumference (cm) change after intervention (6 months postpartum).
3.4. Effects of IoT Interventions on Improving Pregnant and Postpartum Women’s Health Outcomes
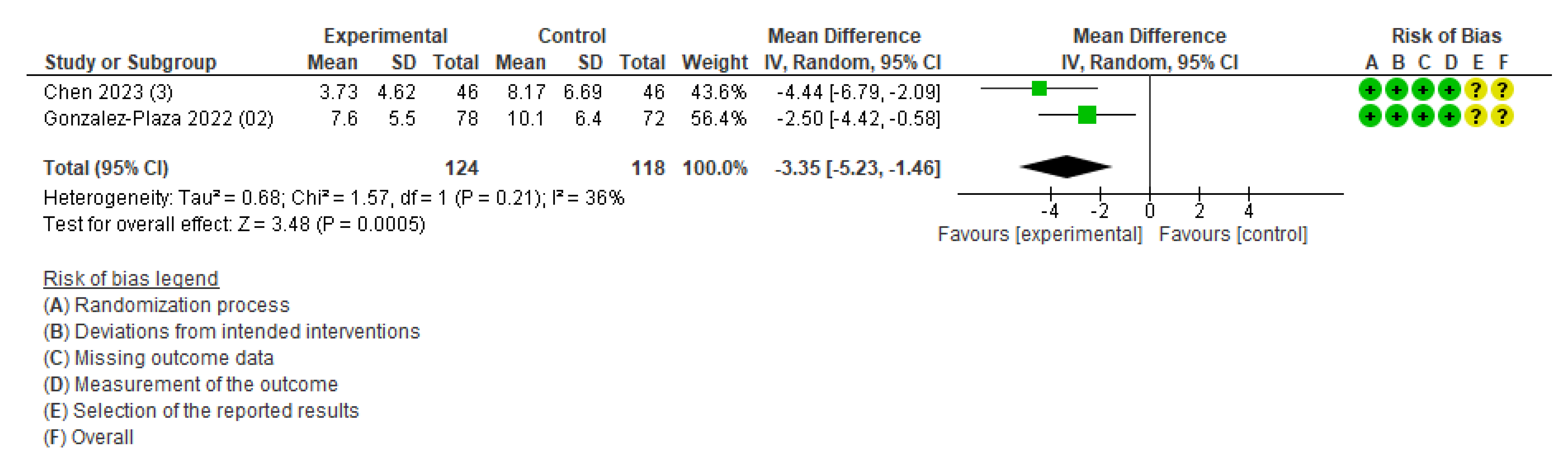
The primary outcomes of maternal health (cesarean section, pregnancy complications, and postpartum diabetes) and neonatal health (low birth weight, perinatal death, neonatal death, and stillbirth) were not available for the meta-analysis owing to an insufficient number of studies. The meta-analysis was performed for three outcomes: weight change (Figure S2), gestational weight gain (Figure 3), and body fat (Figure 4). Table S3 demonstrates the certainty of evidence for the effectiveness of the IoT on the outcomes, which was assessed using the GRADE approach. The certainty was rated as moderate for GWG and low to very low for all other outcomes.
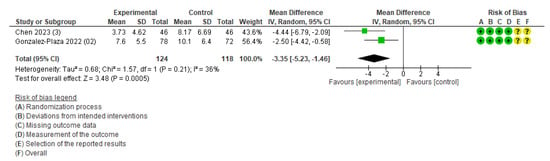
Figure 3.
Meta-analysis for the effect of IoT interventions vs. no IoT intervention on gestational weight gain (kg) [38,40]. Yellow with a ? symbol: unclear risk of bias, and green with a + symbol: low risk of bias.
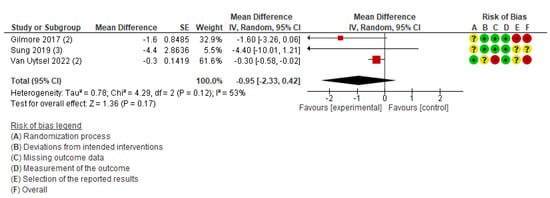
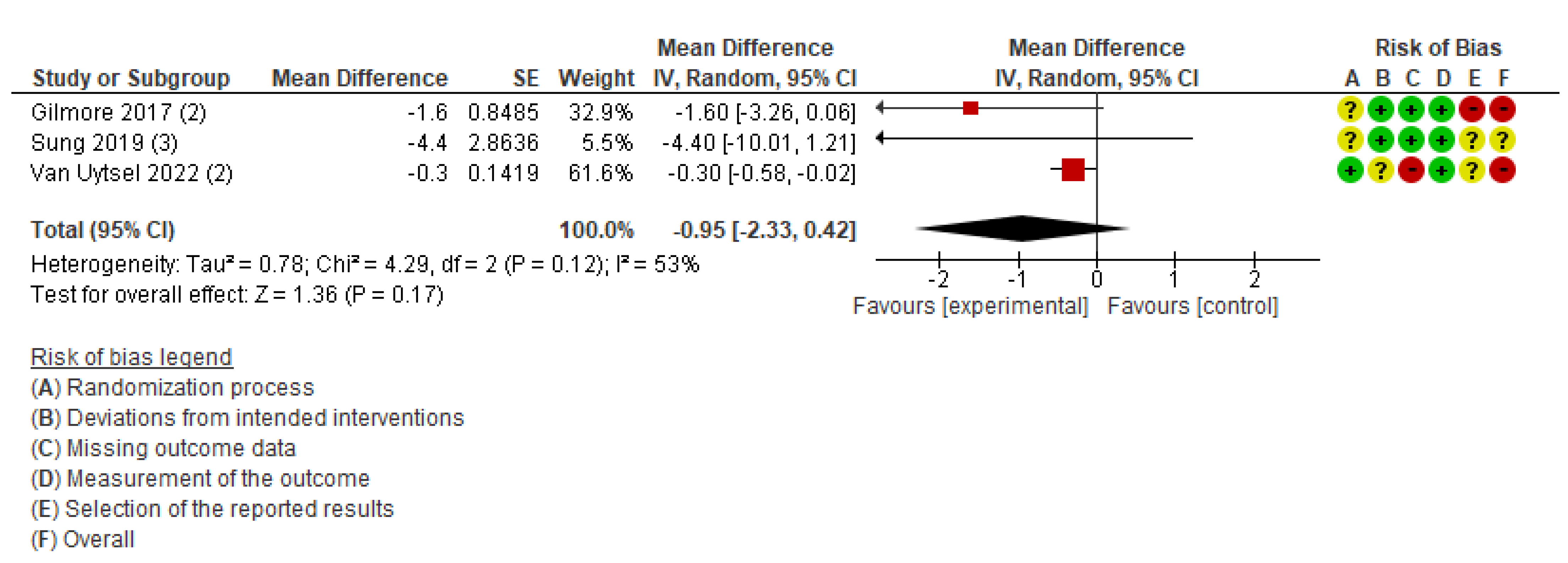
Figure 4.
Meta-analysis for the effect of IoT interventions vs. no IoT intervention on fat change (%) of postpartum women [37,39,41]. Red with a - symbol: high risk of bias, yellow with a ? symbol: unclear risk of bias, and green with a + symbol: low risk of bias.
3.4.1. Maternal Health Outcomes
One study of pregnant women with a BMI of ≥30 kg/m2 reported unplanned cesarean sections, with no difference between the intervention and control groups (RR, 1.38; 95% CI: 0.60 to 3.19; 150 pregnant women) [40]. No differences were observed in the prevalence of composite pregnancy morbidity (RR, 0.85; 95% CI: 0.52 to 1.37; 150 pregnant women), preeclampsia or gestational hypertension (RR, 0.62; 95% CI: 0.23 to 1.64; 150 pregnant women), gestational diabetes (RR, 0.77; 95% CI: 0.35 to 1.67), miscarriage ≤22 weeks (RR, 4.62; 95% CI: 0.23 to 94.64; 150 pregnant women), and preterm labor ≤37 weeks (RR, 1.29; 95% CI: 0.43 to 3.89; 150 pregnant women) between the intervention and control groups [40].
Similarly, a study of women with gestational diabetes in South Korea reported no difference in the prevalence of diabetes at postpartum between the intervention and control groups (RR, 0.91; 95% CI: 0.16–5.30; 21 pregnant women) [37].
Although maternal clinical outcomes such as cesarean section, gestational hypertension, and postpartum diabetes were reported, none of the results reached statistical significance. However, these important clinical outcomes were assessed in a small number of studies, limiting the feasibility of conducting a meta-analysis and reducing the generalizability of the findings.
3.4.2. Neonatal Health Outcomes
Regarding neonatal health outcomes, the study conducted in Spain reported no difference in the prevalence of birthweight ≤2500 g (RR, 2.31; 95% CI: 0.46 to 11.52; 150 pregnant women), perinatal death (RR, 0.46; 95% CI: 0.04 to 4.98), early neonatal death (RR, 0.92; 95% CI: 0.06 to 14.49), and antepartum stillbirth (RR, 0.31; 95% CI: 0.01 to 7.44) between the intervention and control groups [40]. Owing to the limited number of studies and wide CIs, no firm conclusions could be drawn regarding the neonatal benefits of IoT-based interventions.
3.4.3. Maintaining a Healthy Weight or Other Indicators
We conducted the meta-analysis to synthesize the intervention effect of the outcome of mean weight change at postpartum. This outcome included three studies [36,39,41]. The pooled estimates showed no difference between the intervention and control groups (MD, −0.25; 95% CI: −0.62 to 0.12, I2 = 0%, three studies, 1550 women, very low certainty of evidence) (Figure S2). According to the GRADE assessment, the certainty of evidence for this outcome was very low. In addition, as two of the three studies were pilot trials with small sample sizes, the analysis may have been underpowered to detect a statistically significant difference.
Other weight-related outcomes included gestational weight gain and optimal weight at four months postpartum. The outcome of gestational weight gain included two studies [38,40]. The study conducted in Taiwan was an RCT of pregnant women with a BMI ≥ 25 kg/m2, and participants in the intervention group were required to achieve 8500 steps per day and wear the smartband Mi Band 5 (WAT) [38]. Another study conducted in Spain also targeted pregnant women with obesity (BMI > 30 kg/m2), and participants in the intervention group were advised to walk 10,000 steps per day and wear a smartband (Mi Band 2) synchronized with an application offering health counseling and midwifery support [40]. The pooled estimates showed that gestational weight gain was 3.35 kg lower in the intervention compared to that in the control group (MD, −3.35; 95% CI: −5.23 to −1.46; I2 = 36%; two studies; 242 women; moderate certainty of evidence) (Figure 3). The outcome of the number of women achieving optimal weight at four months postpartum included only one study [40], and the intervention group did not significantly differ from the control group.
This outcome included three studies conducted in the USA [39], South Korea [37], and Belgium [41]. The participants in these studies were postpartum women with a BMI of 25–40 kg/m2 [39], women with excessive gestational weight gain [41], and women diagnosed with gestational diabetes mellitus [37]. The percentage of body fat was measured postpartum in all three studies. Using a fixed-effects model for meta-analysis, the results indicated moderate heterogeneity (MD, −0.35; 95% CI: −0.62 to −0.07; I2 = 53%). Therefore, a sensitivity analysis was conducted using a random-effects model, which showed no statistically significant difference between the intervention and comparison groups (MD, −0.95; 95% CI: −2.33 to 0.42; I2 = 53%; three studies; 1,511 women; very low certainty of evidence) (Figure 4). The difference in results between the fixed-effects and random-effects models may reflect methodological heterogeneity, such as variations in the types of devices used (e.g., accelerometers, wearable activity trackers), study settings, and participant characteristics.
One study conducted in Belgium [41] reported the outcome of waist circumference and found no difference between the intervention and control groups (RR, −0.10; 95% CI: −0.57 to 0.37; 1450 women).
3.4.4. Physical Activity
Three studies reported physical activity [36,40]. However, a meta-analysis could not be conducted since each study used different ways of measuring physical activity. The findings for physical activity were as follows: the 7-day pedometer step count (MD, 6600.00; 95% CI: −4945.03 to 18,145.03; 60 women) [36], total weekly activity time (min) at final evaluation (MD, 67.00; 95% CI: −32.95 to 166.95; 60 women) [36], and physical activity (moderate to high) (RR, 1.25; 95% CI: 0.97 to 1.63; 150 pregnant women) [40]. The lack of standardization in physical activity measurement tools (e.g., self-reports vs. accelerometers) restricted comparability across studies.
4. Discussion
4.1. Principal Findings
In this systematic review, we evaluated the effectiveness of IoT interventions on the health of pregnant and postpartum women. The meta-analysis indicated that IoT interventions might help reduce gestational weight gain among obese pregnant women; however, this conclusion was based on only two RCTs involving a combined sample of 242 women. The limited number of studies and relatively small sample size indicate that the evidence is preliminary and should be interpreted with caution. Moreover, the meta-analysis revealed moderate heterogeneity (I2 = 36 %), suggesting that differences in study design or interventions may have influenced the estimated effect.
Therefore, while the potential effect of IoT interventions is clinically promising, the certainty of evidence remains moderate, and confirmation in larger, well-designed trials is warranted.
Two studies [38,40] that focused on preventing excessive gestational weight gain in obese pregnant women aimed to manage weight gain within the guidelines set by the Institute of Medicine [42]. The IoT-based interventions led to a 3.35 kg reduction in gestational weight gain compared to the control groups. Higher pre-pregnancy BMI and excessive gestational weight gain are associated with an increased risk of adverse maternal and neonatal outcomes [43,44], including gestational hypertension, gestational diabetes, and large-for-gestational-age infants [44]. Systematic reviews and meta-analyses on gestational weight gain among women with obesity have reported that restricting gestational weight gain reduces the risk of LGA [45,46], lowers the likelihood of cesarean delivery [45,46], and may also decrease the incidence of hypertensive disorders such as preeclampsia [46]. Therefore, the reduction in gestational weight gain observed among obese pregnant women through IoT-based interventions may have clinically important implications.
The interventions employed behavior-change strategies based on Bandura’s Social Cognitive Theory (SCT) [47,48], focusing on both diet and physical activity. In the study conducted in Taiwan, nurses supported pregnant women’s health behaviors through personalized SMS messages [38], and in the study conducted in Spain, midwives provided individualized health information and responded to questions via a mobile application [40]. From an IoT perspective, both studies utilized wearable activity monitors (smartbands) and mobile applications to track physical activity [38,40]. In health promotion practices, components of SCT are widely used and have been suggested to positively influence health outcomes and intervention effectiveness [49]. Within SCT, the belief in self-efficacy plays a crucial role, as enhancing self-efficacy directly or indirectly leads to behavioral change [48]. Self-monitoring and self-management are key factors in increasing self-efficacy, and the IoT interventions facilitate this process by enabling individuals to track their physical activity and manage their health more effectively. Differences in professional support (nurses versus midwives), cultural context, and intervention intensity across these two trials may partly explain the moderate heterogeneity observed in our meta-analysis. These contextual variations highlight the need for caution when generalizing the findings and underscore the importance of future research with more standardized interventions. Although the included interventions ranged from simple step counters to more complex coaching programs, none of the trials delivered a wearable-only intervention; all combined wearable devices with some form of counseling or coaching. Therefore, subgroup analyses comparing wearable-only versus wearable-plus-coaching interventions were not feasible, and the conceptual heterogeneity of the interventions should be kept in mind when interpreting the pooled results.
A Cochrane review published in 2015 indicated that interventions targeting diet, exercise, or both during pregnancy may mitigate the risk of excessive gestational weight gain [50]. While the Cochrane review did not conduct a meta-analysis based on BMI classification [50], women with high BMI values in early pregnancy tend to have lower activity levels and are more prone to increased gestational weight gain [51]. The current study suggests that IoT interventions utilizing smartbands may contribute to increased physical activity levels and reduced gestational weight gain in pregnant women with obesity. However, given the modest sample size, limited number of studies, and moderate heterogeneity in the meta-analysis, the magnitude and generalizability of this effect remain uncertain.
Only a limited number of studies reported key clinical outcomes, such as cesarean section, gestational diabetes, and neonatal morbidity; existing evidence is insufficient to draw firm conclusions regarding the clinical effectiveness of IoT interventions. This gap underscores the need for future high-quality studies that standardize the reporting of clinically important maternal and neonatal outcomes.
The results of the present meta-analysis did not show any significant effect of IoT interventions on postpartum weight reduction. This outcome encompassed three studies [36,39,41]. As an intervention, a wearable activity monitor and a mobile application were used to provide dietary and physical activity interventions [36,39,41]. Combined dietary and exercise interventions have been considered optimal strategies for promoting postpartum weight loss [52]. However, previous studies have reported inconsistent findings regarding the success of postpartum weight reduction [53]. In our meta-analysis, the lack of significant improvement in postpartum weight may be attributed to several contextual and methodological factors. One possible reason is the relatively short duration of interventions in the included studies, which ranged from four to six months. This period may have been insufficient to observe substantial behavioral changes and the resulting impact on body weight [41]. Moreover, two studies identified high dropout rates and low adherence to activity tracker usage as key challenges [36,41]. Possible reasons included postpartum lifestyle changes and technical difficulties. A study conducted in Australia reported that primiparous women and South Asian women were significantly more likely to not complete the study [36]. Additionally, two of the three included studies were pilot trials [36,39], and their small sample sizes may have resulted in insufficient statistical power, potentially obscuring the true effects of the intervention.
Postpartum body fat is a crucial outcome since a high body fat percentage is associated with increased health risks, even if the BMI is within the normal range [54,55].The interventions targeted women with postpartum overweight or obesity [39], those with excessive gestational weight gain [41], and those with gestational diabetes [37], utilizing interventions involving Bluetooth-connected activity trackers and scales [39,41] or monitoring system devices, including Bluetooth-connected glucometers and accelerometers for detecting physical activity levels [37]. When the meta-analysis was performed using a fixed-effects model according to the protocol, women in the IoT intervention group experienced a slight reduction in body fat percentage. However, after switching to a random-effects model to account for heterogeneity in intervention methods and study populations, the statistical significance disappeared, and the robustness of the effect was not demonstrated [37,39,41]. One possible reason for the lack of significant differences between the intervention and comparison groups is the short duration of the intervention. Postpartum women who are overweight or obese tend to experience slower reductions in body weight and fat percentage compared to those with normal weight, suggesting that longer-term interventions may be necessary.
Additionally, two of the three studies were pilot trials [37,39], and their small sample sizes may have limited the statistical power to detect significant effects. The inconsistency between the fixed effects and random effects results suggests that the pooled effect is sensitive to assumptions about between-study variance. Potential sources of heterogeneity include differences in device type (e.g., Bluetooth-connected scales versus comprehensive monitoring systems), variation in participant populations (postpartum women with overweight/obesity, excessive gestational weight gain, or gestational diabetes), and the length of follow-up across trials. Moreover, the total number of participants contributing to the body fat outcome was small, implying that the analyses may have been underpowered to detect modest but clinically meaningful effects.
Furthermore, waist circumference is an important indicator as it is associated with the incidence and mortality of future cardiometabolic diseases [56,57]. Regarding postpartum women, changes in weight and waist circumference in those who developed GDM have been suggested to influence cardiometabolic risk factors even in the first year postpartum [58]. However, due to difficulties in comparing results across studies, a meta-analysis could not be conducted.
A meta-analysis on physical activity could not be performed due to heterogeneity in measurement methods across the included studies. The lack of standardization in physical activity measurement tools (e.g., self-report vs. accelerometers, varying definitions of moderate activity) limits cross-study comparability and highlights the need for harmonized metrics in IoT-based intervention studies.
In the current review, the application of RoB 2.0 revealed that many of the included trials were rated as having “some concerns” or “high risk” due to the lack of participant blinding, high attrition bias, and incomplete outcome reporting. Moreover, most studies were small pilot trials with short follow-up periods; therefore, the GRADE assessments rated the certainty of evidence as low to moderate for gestational weight gain and as low certainty for postpartum outcomes, such as weight retention and body fat percentage. These methodological limitations may decrease the confidence in the observed effects and indicate the need for larger, high-quality trials. Several of the included trials exhibited a high risk of bias or “some concerns” in the domains of randomization and selective outcome reporting. Inadequate randomization can introduce baseline imbalances, and selective reporting may distort effect estimates, both of which threaten the internal validity of the findings. For example, Cheung et al. 2019 and Van Uytsel et al. 2022 had high dropout rates leading to missing outcome data [36,41], while Chen et al. 2023 had unclear allocation concealment [38]; these methodological issues raise concerns about bias. We had planned to conduct sensitivity analyses excluding high-risk trials, but only two studies contributed to the gestational weight-gain meta-analysis, and the postpartum analyses were based on three small pilot trials; therefore, conducting a meta-analysis excluding these studies was not feasible. Consequently, the pooled results should be interpreted with caution, and future research should prioritize robust randomization procedures and complete reporting of all study details.
4.2. Role of IoT Interventions in Behavior Change Strategies
In health promotion practices, the components of SCT are widely utilized and are considered to have a positive impact on health outcomes and intervention effectiveness [49]. SCT identifies key determinants of behavior change, including knowledge about health risks and the benefits of health practices, self-efficacy, outcome expectations, health goals and planning, and environmental factors (facilitators and barriers). Among these, the belief in self-efficacy plays a particularly crucial role [48]. By leveraging IoT technologies such as wearable activity monitors (smartbands) and mobile applications, patients can gain health-related knowledge, visually monitor their physical activity, enhance their sense of control, set and achieve goals, and ultimately increase their health awareness [38,40]. Interventions targeting weight loss in adults with obesity often involve dietary and physical activity modifications [59]. Goal setting and self-monitoring using IoT technology are considered effective behavioral change techniques for individuals with obesity [60].
However, individuals with obesity often exhibit low adherence to interventions [61], and in studies targeting postpartum women—whose lifestyles shift due to childcare and returning to work—discontinuation due to forgetting or losing wearable devices is a common challenge [39,51]. Although there are cost considerations and physical limitations, wearable devices that do not need to be removed during daily activities or can be inconspicuously worn may be more suitable for study participants given their potential to enhance adherence and comfort.
Technical issues [36], decreased usage of IoT devices and applications over long periods [15], and cultural and social challenges [36] have also been reported. While IoT devices can function as facilitators within environmental factors, they may also act as barriers. Thus, when designing intervention methods, it is essential to consider measures to reduce barriers, such as by providing human support.
In this review, behavioral and lifestyle modifications were defined as secondary outcomes; however, changes such as increased physical activity, dietary improvements, and regular self-monitoring serve as important mediators of the clinical effects of IoT interventions. These behavioral modifications represent key mechanisms through which improvements in maternal and neonatal outcomes are achieved and are essential for understanding the pathways through which the interventions exert their effects.
4.3. Future Research Utilizing IoT Technology
Postpartum women experience significant lifestyle changes due to childbirth. Since RCTs using IoT technology during pregnancy have demonstrated intervention effectiveness, it is necessary to consider long-term interventions starting from pregnancy rather than from the postpartum period. Pregnancy is a time when individuals can more easily receive social support, such as support from professionals and pregnancy communities [62], making it easier to establish an environment that promotes healthy behaviors.
The studies included in this review used IoT technology primarily to track physical activity. However, future studies should also explore the possibility of integrating physiological data such as blood pressure, heart rate, and sleep, as well as physical activity levels. IoT technology is already being used for remote monitoring of hypertensive patients [63]. By utilizing multiple IoT devices to collect and analyze these data, IoT-based interventions could be adapted and developed for high-risk pregnant women, such as those with gestational hypertension. Monitoring high-risk pregnant women using IoT technologies such as wearable devices requires close integration with healthcare services [64]. Previous studies have reported that adopting a cloud-based support model enables efficient processing and storage of large volumes of data, as well as effective communication with applications, thereby addressing various aspects of utilizing data effectively [64]. This approach has the potential to contribute to continuous monitoring, early detection of abnormalities, and improved personalized management.
While this systematic review and meta-analysis focused on evaluating the effectiveness of IoT interventions in improving maternal health outcomes, future research should also consider the cost-effectiveness, scalability, and real-world implementation of these interventions. Understanding the financial feasibility and integration of IoT technologies into existing healthcare systems will be essential for their broader adoption and long-term sustainability.
From an ethical perspective, studies utilizing IoT technology must consider the possibility of a digital divide even in high-income countries. IoT interventions assume access to smartphones and wearable devices; however, these technologies may not be equally available across all socioeconomic groups. A large-scale national survey conducted among Japanese women revealed that nearly 80% of respondents had never used IoT or applications [65]. The digital divide is typically categorized into a first-order divide, which represents inequalities in physical access to digital resources, and a second-order divide, which pertains to disparities in usage [66]. While infrastructure and economic development are believed to have reduced access inequalities in developed environments [66], wearable devices may still contribute to a first-order digital divide even in high-income countries.
A study conducted in the United States found that 95.3% (4686/4915) of individuals who do not use wearable devices stated that they would use them if provided for free [67], indicating the presence of economic barriers. Additionally, prior literature on the ethical issues arising from research using IoT devices has pointed out that the widespread use of IoT devices may raise concerns regarding privacy and data security [68]. As a recommendation, it has been suggested that specific protocols should be developed to ensure both proper consent and data protection [68].
As mentioned earlier, in RCTs utilizing IoT technology, issues such as dropout rates and forgetting to wear the devices have been identified as challenges for conducting long-term research. Future studies will require interventions that address these challenges in IoT-based research. Moreover, theory-based interventions have been implemented in medical interventions [69,70] and in studies utilizing IoT technology. Theories such as SCT [38,40] and the Transtheoretical Model of Behavior Change [41] have been used to promote behavioral change, though only in a limited number of studies. It is necessary to develop interventions that can mitigate inhibitory factors as a whole and conduct evaluations that will contribute to future research.
4.4. Strengths and Limitations of This Review
A key strength of this review is that it is one of the most comprehensive and up-to-date reviews and meta-analyses evaluating the effectiveness of various forms of IoT in enhancing the health outcomes of pregnant and postpartum women. Interventions utilizing IoT contributed to the mitigation of gestational weight gain in pregnant women with obesity. With the increasing prevalence of maternal obesity and gestational diabetes as a public health concern [71,72], IoT-based interventions may have potential utility as a public health strategy to improve maternal and child health.
However, this study has some limitations. First, the synthesis through meta-analysis for primary outcomes was challenging owing to the scarcity of outcomes encompassing two or more studies. Additionally, many were pilot studies with small sample sizes, and some had high dropout rates, which reduced the certainty of evidence. Due to the small number of studies included and the moderate heterogeneity, this meta-analysis may not have sufficient statistical power to detect clinically meaningful differences. Another limitation is that in interventions using IoT, blinding of participants and intervention providers is impossible, which may lead to performance bias. However, the outcomes used in the meta-analysis were based on objective data in most studies, making them less susceptible to bias even if blinding was not implemented. Furthermore, this review was limited to high-income countries, which restricted the generalizability of the findings to low- and middle-income countries. Therefore, future systematic reviews should include studies conducted in low- and middle-income countries to evaluate the effectiveness of IoT-based interventions in settings where the adoption of IoT technologies and healthcare systems differ. Finally, the inclusion of interventions beyond IoT utilization, such as lifestyle coaching sessions and mobile healthcare services, alongside data collection and monitoring through sensors in wearable devices, causes difficulty in attributing the effects solely to the use of IoT. Nevertheless, a commonality across all included studies was that only the intervention groups used IoT devices to self-monitor their physical activity.
5. Conclusions
This review identified a moderate reduction in gestational weight gain, indicating that IoT-based interventions can be a promising tool for improving maternal weight related outcomes. Evidence from previous studies suggests that restricting gestational weight gain in obese pregnant women may reduce the likelihood of gestational diabetes, preeclampsia, and large-for-gestational-age births, contributing to better maternal and neonatal health outcomes. However, clinical outcomes such as cesarean section or neonatal morbidity were inconsistently reported and showed no significant effects, limiting the clinical generalizability of the current findings. To enhance their effectiveness, IoT-based interventions should be designed using behavior change theories and incorporate continuous monitoring of physical activity and physiological data, along with personalized, real-time feedback. Additionally, the long-term effects on maternal and neonatal outcomes should be evaluated using rigorous study designs, large sample sizes, and extended follow-up periods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13172103/s1, Table S1: PRISMA 2020 checklist; Table S2: Search strategy; Table S3: Summary of findings; Table S4: Additional characteristics of the included studies; Table S5: Body fat change; Table S6: Gestational weight gain; Table S7: Weight change in postpartum women; Table S8: Summary of outcomes reported in included studies, categorized as primary clinical outcomes and behavioral/intermediate outcomes; Figure S1: Risk of bias graph; Figure S2: Meta-analysis of the effect of IoT interventions vs. no IoT intervention on the mean change in weight (kg) of postpartum women. Reference [73] is cited in the supplementary materials.
Author Contributions
Conceptualization, N.Y., D.Y., E.S., and E.O.; methodology, E.N., D.Y., and E.O.; formal analysis, E.N. and E.O.; data curation, E.N., N.Y., K.S., M.O.R., K.d.S.L., C.G.M., M.N., P.P.T., R.S., D.S., and A.N.; writing—original draft preparation, E.N.; writing—review and editing, E.N., N.Y., K.S., M.O.R., K.d.S.L., C.G.M., M.N., P.P.T., R.S., D.S., A.N., D.Y., E.S., and E.O.; supervision, D.Y., E.S., and E.O.; project administration, E.N. and N.Y.; funding acquisition, D.Y., E.S., and E.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Japan Agency for Medical Research and Development (grant number: 22rea522103h0001) and JST, PRESTO Grant Number JPMJPR21RC, Japan.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available upon request from the corresponding author.
Acknowledgments
We would like to thank Hitomi Hamada, who was a member of Global Health Nursing at St. Luke’s International University, for supporting this study in the screening process.
Conflicts of Interest
Katharina da Silva Lopes serves as a scientific consultant at NEZU Biotech GmbH, Heidelberg, Germany. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IoT | Internet of Things |
| SCT | Social Cognitive Theory |
| BMI | body mass index |
| GA | gestational age |
| GDM | gestational diabetes mellitus |
| GWG | gestational weight gain |
| HbA1C | glycated hemoglobin |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| OGTT | oral glucose tolerance test |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128.9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Cheng, Y.; Li, T.; Fan, Y.; Zhang, Q.; Cheng, H. Prediction of Gestational Diabetes Mellitus by Different Obesity Indices. BMC Pregnancy Childbirth 2022, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Ben-Haroush, A.; Chen, R.; Rosenn, B.; Hod, M.; Langer, O. Diurnal Glycemic Profile in Obese and Normal Weight Nondiabetic Pregnant Women. Am. J. Obstet. Gynecol. 2004, 191, 949–953. [Google Scholar] [CrossRef]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; de Onis, M.; Ezzati, M.; Grantham-McGregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef]
- Han, Z.; Mulla, S.; Beyene, J.; Liao, G.; McDonald, S.D. Maternal Underweight and the Risk of Preterm Birth and Low Birth Weight: A Systematic Review and Meta-Analyses. Int. J. Epidemiol. 2011, 40, 65–101. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Care in Diabetes—2023 Abridged for Primary Care Providers. Clin. Diabetes 2022, 41, 4–31. [Google Scholar] [CrossRef]
- Anothaisintawee, T.; Reutrakul, S.; Van Cauter, E.; Thakkinstian, A. Sleep Disturbances Compared to Traditional Risk Factors for Diabetes Development: Systematic Review and Meta-Analysis. Sleep Med. Rev. 2016, 30, 11–24. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Cui, D.; Li, C.; Ma, R.C.W.; Li, J.; Yang, X. Effects of Lifestyle Intervention on Long-Term Risk of Diabetes in Women with Prior Gestational Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Obes. Rev. 2021, 22, e13122. [Google Scholar] [CrossRef]
- Taousani, E.; Papaioannou, K.-G.; Mintziori, G.; Grammatikopoulou, M.G.; Antonakou, A.; Tzitiridou-Chatzopoulou, M.; Veneti, S.; Goulis, D.G. Lifestyle Behaviors and Gestational Diabetes Mellitus: A Narrative Review. Endocrines 2025, 6, 6. [Google Scholar] [CrossRef]
- Kawasaki, M.; Mito, A.; Waguri, M.; Sato, Y.; Abe, E.; Shimada, M.; Fukuda, S.; Sasaki, Y.; Fujikawa, K.; Sugiyama, T.; et al. Protocol for an Interventional Study to Reduce Postpartum Weight Retention in Obese Mothers Using the Internet of Things and a Mobile Application: A Randomized Controlled Trial (SpringMom). BMC Pregnancy Childbirth 2021, 21, 582. [Google Scholar] [CrossRef]
- Luo, J.; Mao, A.; Zeng, Z. Sensor-Based Smart Clothing for Women’s Menopause Transition Monitoring. Sensors 2020, 20, 1093. [Google Scholar] [CrossRef] [PubMed]
- Sarhaddi, F.; Azimi, I.; Labbaf, S.; Niela-Vilén, H.; Dutt, N.; Axelin, A.; Liljeberg, P.; Rahmani, A.M. Long-Term IoT-Based Maternal Monitoring: System Design and Evaluation. Sensors 2021, 21, 2281. [Google Scholar] [CrossRef]
- Tsirmpas, C.; Kouris, I.; Anastasiou, A.; Giokas, K.; Iliopoulou, D.; Koutsouris, D. An Internet of Things Platform Architecture for Supporting Ambient Assisted Living Environments. Technol. Health Care 2017, 25, 391–401. [Google Scholar] [CrossRef]
- Bonato, P. Wearable Sensors and Systems. IEEE Eng. Med. Biol. Mag. 2010, 29, 25–36. [Google Scholar] [CrossRef]
- Lim, K.; Chan, S.Y.; Lim, S.L.; Tai, B.C.; Tsai, C.; Wong, S.R.; Ang, S.M.; Yew, T.W.; Tai, E.S.; Yong, E.L. A Smartphone App to Restore Optimal Weight (SPAROW) in Women with Recent Gestational Diabetes Mellitus: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e22147. [Google Scholar] [CrossRef]
- Minschart, C.; Maes, T.; De Block, C.; Van Pottelbergh, I.; Myngheer, N.; Abrams, P.; Vinck, W.; Leuridan, L.; Mathieu, C.; Billen, J.; et al. Mobile-Based Lifestyle Intervention in Women with Glucose Intolerance after Gestational Diabetes Mellitus (MELINDA), A Multicenter Randomized Controlled Trial: Methodology and Design. J. Clin. Med. 2020, 9, 2635. [Google Scholar] [CrossRef] [PubMed]
- Grym, K.; Niela-Vilén, H.; Ekholm, E.; Hamari, L.; Azimi, I.; Rahmani, A.; Liljeberg, P.; Löyttyniemi, E.; Axelin, A. Feasibility of Smart Wristbands for Continuous Monitoring during Pregnancy and One Month after Birth. BMC Pregnancy Childbirth 2019, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Saarikko, J.; Niela-Vilen, H.; Ekholm, E.; Hamari, L.; Azimi, I.; Liljeberg, P.; Rahmani, A.M.; Löyttyniemi, E.; Axelin, A. Continuous 7-Month Internet of Things-Based Monitoring of Health Parameters of Pregnant and Postpartum Women: Prospective Observational Feasibility Study. JMIR Form. Res. 2020, 4, e12417. [Google Scholar] [CrossRef]
- Alim, A.; Imtiaz, M.H. Wearable Sensors for the Monitoring of Maternal Health—A Systematic Review. Sensors 2023, 23, 2411. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kashem, M.A.; Islam, M.M.; Sahidullah, M.; Mumu, S.H.; Uddin, J.; Aray, D.G.; de la Torre Diez, I.; Ashraf, I.; Samad, M.A. Internet of Things in Pregnancy Care Coordination and Management: A Systematic Review. Sensors 2023, 23, 9367. [Google Scholar] [CrossRef]
- Bertini, A.; Gárate, B.; Pardo, F.; Pelicand, J.; Sobrevia, L.; Torres, R.; Chabert, S.; Salas, R. Impact of Remote Monitoring Technologies for Assisting Patients with Gestational Diabetes Mellitus: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 819697. [Google Scholar] [CrossRef]
- Huhn, S.; Axt, M.; Gunga, H.C.; Maggioni, M.A.; Munga, S.; Obor, D.; Sié, A.; Boudo, V.; Bunker, A.; Sauerborn, R.; et al. The Impact of Wearable Technologies in Health Research: Scoping Review. JMIR Mhealth Uhealth 2022, 10, e34384. [Google Scholar] [CrossRef]
- Yamaji, N.; Nitamizu, A.; Nishimura, E.; Suzuki, D.; Sasayama, K.; Rahman, M.O.; Saito, E.; Yoneoka, D.; Ota, E. Effectiveness of the Internet of Things for Improving Working-Aged Women’s Health in High-Income Countries: Protocol for a Systematic Review and Network Meta-Analysis. JMIR Res. Protoc. 2023, 12, e45178. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- World Bank. World Bank Country and Lending Groups. 2023. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 14 February 2023).
- WHO Global Observatory for eHealth. mHealth: New Horizons for Health through Mobile Technologies: Second Global Survey on eHealth; World Health Organization: Geneva, Switzerland, 2011; Available online: https://iris.who.int/handle/10665/44607 (accessed on 4 August 2025).
- Higgins, J.P.; Lasserson, T.; Chandler, J.; Tovey, D.; Churchill, R. Methodological Expectations of Cochrane Intervention Reviews; Cochrane: London, UK, 2016. [Google Scholar]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2022. [Google Scholar]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H.; et al. GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- GRADEpro GDT. GRADEpro Guideline Development Tool; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2025. [Google Scholar]
- Cheung, N.W.; Blumenthal, C.; Smith, B.J.; Hogan, R.; Thiagalingam, A.; Redfern, J.; Barry, T.; Cinnadaio, N.; Chow, C.K. A Pilot Randomised Controlled Trial of a Text Messaging Intervention with Customisation Using Linked Data from Wireless Wearable Activity Monitors to Improve Risk Factors Following Gestational Diabetes. Nutrients 2019, 11, 996. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Lee, D.Y.; Min, K.P.; Park, C.Y. Peripartum Management of Gestational Diabetes Using a Digital Health Care Service: A Pilot, Randomized Controlled Study. Clin. Ther. 2019, 41, 2426–2434. [Google Scholar] [CrossRef]
- Chen, H.H.; Lee, C.F.; Huang, J.P.; Hsiung, Y.; Chi, L.K. Effectiveness of a Nurse-Led mHealth App to Prevent Excessive Gestational Weight Gain among Overweight and Obese Women: A Randomized Controlled Trial. J. Nurs. Scholarsh. 2023, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, L.A.; Klempel, M.C.; Martin, C.K.; Myers, C.A.; Burton, J.H.; Sutton, E.F.; Redman, L.M. Personalized Mobile Health Intervention for Health and Weight Loss in Postpartum Women Receiving Women, Infants, and Children Benefit: A Randomized Controlled Pilot Study. J. Womens Health 2017, 26, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Plaza, E.; Bellart, J.; Arranz, A.; Lujan-Barroso, L.; Crespo Mirasol, E.; Seguranyes, G. Effectiveness of a Step Counter Smartband and Midwife Counseling Intervention on Gestational Weight Gain and Physical Activity in Pregnant Women with Obesity (Pas and Pes Study): Randomized Controlled Trial. JMIR Mhealth Uhealth 2022, 10, e28886. [Google Scholar] [CrossRef]
- Van Uytsel, H.; Bijlholt, M.; Devlieger, R.; Ameye, L.; Jochems, L.; van Holsbeke, C.; Schreurs, A.; Catry, V.; Bogaerts, A. Effect of the E-Health Supported INTER-ACT Lifestyle Intervention on Postpartum Weight Retention and Body Composition, and Associations with Lifestyle Behavior: A Randomized Controlled Trial. Prev. Med. 2022, 164, 107321. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Yaktine, A.L.; Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines (Eds.) Weight Gain during Pregnancy: Reexamining the Guidelines; The National Academies Press: Washington, DC, USA, 2009. [Google Scholar] [CrossRef]
- Choi, H.; Lim, J.Y.; Lim, N.K.; Ryu, H.M.; Kwak, D.W.; Chung, J.H.; Park, H.J.; Park, H.Y. Impact of Pre-Pregnancy Body Mass Index and Gestational Weight Gain on the Risk of Maternal and Infant Pregnancy Complications in Korean Women. Int. J. Obes. 2022, 46, 59–67. [Google Scholar] [CrossRef]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of Maternal Body Mass Index and Gestational Weight Gain on Pregnancy Complications: An Individual Participant Data Meta-Analysis of European, North American and Australian Cohorts. BJOG 2019, 126, 984–995. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-Analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Mustafa, H.J.; Seif, K.; Javinani, A.; Aghajani, F.; Orlinsky, R.; Alvarez, M.V.; Ryan, A.; Crimmins, S. Gestational Weight Gain below instead of within the Guidelines per Class of Maternal Obesity: A Systematic Review and Meta-Analysis of Obstetrical and Neonatal Outcomes. Am. J. Obstet. Gynecol. MFM 2022, 4, 100682. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Health Promotion from the Perspective of Social Cognitive Theory. Psychol. Health 1998, 13, 623–649. [Google Scholar] [CrossRef]
- Bandura, A. Health Promotion by Social Cognitive Means. Health Educ. Behav. 2004, 31, 143–164. [Google Scholar] [CrossRef]
- Islam, K.F.; Awal, A.; Mazumder, H.; Munni, U.R.; Majumder, K.; Afroz, K.; Tabassum, M.N.; Hossain, M.M. Social Cognitive Theory-Based Health Promotion in Primary Care Practice: A Scoping Review. Heliyon 2023, 9, e14889. [Google Scholar] [CrossRef]
- Muktabhant, B.; Lawrie, T.A.; Lumbiganon, P.; Laopaiboon, M. Diet or Exercise, or Both, for Preventing Excessive Weight Gain in Pregnancy. Cochrane Database Syst. Rev. 2015, 6, CD007145. [Google Scholar] [CrossRef]
- Darvall, J.N.; Wang, A.; Nazeem, M.N.; Harrison, C.L.; Clarke, L.; Mendoza, C.; Parker, A.; Harrap, B.; Teale, G.; Story, D.; et al. A Pedometer-Guided Physical Activity Intervention for Obese Pregnant Women (the Fit MUM Study): Randomized Feasibility Study. JMIR Mhealth Uhealth 2020, 8, e15112. [Google Scholar] [CrossRef]
- Amorim Adegboye, A.R.; Linne, Y.M. Diet or Exercise, or Both, for Weight Reduction in Women after Childbirth. Cochrane Database Syst. Rev. 2013, 7, CD005627. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; Flynn, A.C.; Relph, S.A.; O’Keeffe, M.; Poston, L. Lifestyle Interventions in Overweight and Obese Pregnant or Postpartum Women for Postpartum Weight Management: A Systematic Review of the Literature. Nutrients 2018, 10, 1704. [Google Scholar] [CrossRef] [PubMed]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced Body Composition Assessment: From Body Mass Index to Body Composition Profiling. J. Investig. Med. 2018, 66, 887–895. [Google Scholar] [CrossRef]
- Madden, A.M.; Smith, S. Body Composition and Morphological Assessment of Nutritional Status in Adults: A Review of Anthropometric Variables. J. Hum. Nutr. Diet. 2016, 29, 7–25. [Google Scholar] [CrossRef]
- Jacobs, E.J.; Newton, C.C.; Wang, Y.; Patel, A.V.; McCullough, M.L.; Campbell, P.T.; Thun, M.J.; Gapstur, S.M. Waist Circumference and All-Cause Mortality in a Large US Cohort. Arch. Intern. Med. 2010, 170, 1293–1301. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.M.; Rosner, B.A.; Zera, C.A.; Seely, E.W. Association between Changes in Postpartum Weight and Waist Circumference and Changes in Cardiometabolic Risk Factors among Women with Recent Gestational Diabetes. Prev. Chronic Dis. 2019, 16, E47. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long-Term Maintenance of Weight Loss with Non-Surgical Interventions in Obese Adults: Systematic Review and Meta-Analyses of Randomised Controlled Trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective Techniques in Healthy Eating and Physical Activity Interventions: A Meta-Regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight Loss Intervention Adherence and Factors Promoting Adherence: A Meta-Analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef]
- Renbarger, K.M.; Place, J.M.; Schreiner, M. The Influence of Four Constructs of Social Support on Pregnancy Experiences in Group Prenatal Care. Womens Health Rep. (New Rochelle) 2021, 2, 154–162. [Google Scholar] [CrossRef]
- Persell, S.D.; Peprah, Y.A.; Lipiszko, D.; Lee, J.Y.; Li, J.J.; Ciolino, J.D.; Karmali, K.N.; Sato, H. Effect of Home Blood Pressure Monitoring via a Smartphone Hypertension Coaching Application or Tracking Application on Adults with Uncontrolled Hypertension: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200255. [Google Scholar] [CrossRef]
- Waleed, M.; Kamal, T.; Um, T.-W.; Hafeez, A.; Habib, B.; Skouby, K.E. Unlocking Insights in IoT-Based Patient Monitoring: Methods for Encompassing Large-Data Challenges. Sensors 2023, 23, 6760. [Google Scholar] [CrossRef] [PubMed]
- Sasayama, K.; Nishimura, E.; Yamaji, N.; Ota, E.; Tachimori, H.; Igarashi, A.; Arata, N.; Yoneoka, D.; Saito, E. Current Use and Discrepancies in the Adoption of Health-Related Internet of Things and Apps among Working Women in Japan: Large-Scale, Internet-Based, Cross-Sectional Survey. JMIR Public Health Surveill. 2024, 10, e51537. [Google Scholar] [CrossRef] [PubMed]
- Elena-Bucea, A.; Cruz-Jesus, F.; Oliveira, T.; Coelho, P.S. Assessing the Role of Age, Education, Gender and Income on the Digital Divide: Evidence for the European Union. Inf. Syst. Front. 2021, 23, 1007–1021. [Google Scholar] [CrossRef]
- Venn, R.A.; Khurshid, S.; Grayson, M.; Ashburner, J.M.; Al-Alusi, M.A.; Chang, Y.; Foulkes, A.; Ellinor, P.T.; McManus, D.D.; Singer, D.E.; et al. Characteristics and Attitudes of Wearable Device Users and Nonusers in a Large Health Care System. J. Am. Heart Assoc. 2024, 13, e032126. [Google Scholar] [CrossRef]
- Scheibner, J.; Jobin, A.; Vayena, E. Ethical Issues with Using Internet of Things Devices in Citizen Science Research: A Scoping Review. Front. Environ. Sci. 2021, 9, 629649. [Google Scholar] [CrossRef]
- Bonner, C.; Fajardo, M.A.; Doust, J.; McCaffery, K.; Trevena, L. Implementing Cardiovascular Disease Prevention Guidelines to Translate Evidence-Based Medicine and Shared Decision Making into General Practice: Theory-Based Intervention Development, Qualitative Piloting and Quantitative Feasibility. Implement. Sci. 2019, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Presseau, J.; Hawthorne, G.; Sniehotta, F.F.; Steen, N.; Francis, J.J.; Johnston, M.; Mackintosh, J.; Grimshaw, J.M.; Kaner, E.; Elovainio, M.; et al. Improving Diabetes Care through Examining, Advising, and Prescribing (IDEA): Protocol for a Theory-Based Cluster Randomised Controlled Trial of a Multiple Behaviour Change Intervention Aimed at Primary Healthcare Professionals. Implement. Sci. 2014, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global Causes of Maternal Death: A WHO Systematic Analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tang, K.; Magee, L.A.; von Dadelszen, P.; Ekeroma, A.; Li, X.; Zhang, E.; Bhutta, Z.A. A Global View of Hypertensive Disorders and Diabetes Mellitus during Pregnancy. Nat. Rev. Endocrinol. 2022, 18, 760–775. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).