Abstract
Background/Objectives: Adult vaccination remains suboptimal, particularly among older adults and individuals with chronic conditions. Hospitals represent a strategic setting for improving vaccination coverage among these high-risk populations. This systematic review and meta-analysis evaluated hospital-based interventions aimed at enhancing vaccine uptake in adults aged ≥60 years or 18–64 years with at-risk medical conditions. Methods: We conducted a systematic review and meta-analysis following PRISMA and MOOSE guidelines. Searches in PubMed, EMBASE, and Scopus identified studies published in the last 10 years evaluating hospital-based interventions reporting vaccination uptake. The risk of bias was assessed using validated tools (NOS, RoB 2, ROBINS-I, QI-MQCS). A meta-analysis was conducted for categories with ≥3 eligible studies reporting pre- and post-intervention vaccination coverage in the same population. Results: We included 44 studies. Multi-component strategies (n = 21) showed the most consistent results (e.g., pneumococcal uptake from 2.2% to 43.4%, p < 0.001). Reminder-based interventions (n = 4) achieved influenza coverage increases from 31.0% to 68.0% and a COVID-19 booster uptake boost of +38% after SMS reminders. Educational strategies (n = 11) varied in effectiveness, with one study reporting influenza coverage rising from 1.6% to 12.2% (+662.5%, OR 8.86, p < 0.01). Standing order protocols increased pneumococcal vaccination from 10% to 60% in high-risk adults. Hospital-based catch-up programs improved DTaP-IPV uptake from 56.2% to 80.8% (p < 0.001). For patient education, the pooled OR was 2.11 (95% CI: 1.96–2.27; p < 0.001, I2 = 97.2%) under a fixed-effects model, and 2.47 (95% CI: 1.53–3.98; p < 0.001) under a random-effects model. For multi-component strategies, the OR was 2.39 (95% CI: 2.33–2.44; p < 0.001, I2 = 98.0%) with fixed effects, and 3.12 (95% CI: 2.49–3.92; p < 0.001) with random effects. No publication bias was detected. Conclusions: Hospital-based interventions, particularly those using multi-component approaches, effectively improve vaccine coverage in older and high-risk adults. Embedding vaccination into routine hospital care offers a scalable opportunity to reduce disparities and enhance population-level protection. Future policies should prioritize the institutional integration of such strategies to support healthy aging and vaccine equity.
1. Introduction
Vaccination stands as a fundamental pillar of preventive medicine, offering one of the most effective and cost-efficient means to reduce the burden of infectious diseases across the lifespan [1,2]. While childhood immunization programs have achieved considerable success, adult vaccination has historically received less focus, resulting in relevant gaps in protection [3]. This is particularly concerning for older adults (aged ≥60 years) and individuals with chronic health conditions or who are immunocompromised. These populations face higher risks of infection and severe complications due to factors such as immunosenescence [4], a high prevalence of multi-morbidity [5], and altered immune responses. Consequently, they are at increased risk for complications from vaccine-preventable diseases, making vaccination not only reduces morbidity and mortality but also prevents disability, loss of autonomy, and functional decline, outcomes particularly relevant in the context of healthy aging [6,7]. Low vaccination coverage in these groups not only leads to increased morbidity and mortality, but also places a significant burden on healthcare systems. Preventable infections among older adults and medically vulnerable individuals often result in prolonged hospital stays, the increased use of intensive care services, antimicrobial resistance, and higher rates of readmission [8,9]. These outcomes not only compromise individual health and functional independence but also generate substantial direct and indirect healthcare costs [10]. Furthermore, unvaccinated individuals may contribute to nosocomial transmission of infectious diseases, posing additional risks to other hospitalized patients and healthcare workers [11]. Improving vaccination coverage in these populations is therefore essential not only for individual protection, but also for enhancing the resilience and sustainability of healthcare delivery systems.
Ongoing demographic transitions further amplify the urgency of optimizing adult immunization strategies. By 2050, the global population of adults aged 65 years and older is expected to exceed 1.5 billion, more than doubling compared to 2019 [12]. At the same time, global life expectancy has increased markedly—from 61.7 years in 1980 to 72.6 years in 2019 and is projected to reach 77.1 years by 2050 [12,13]. As life expectancy rises, so does the prevalence of age-related and chronic diseases, reinforcing the need for effective, scalable preventive interventions such as vaccination. Thus, there is a need for effective prevention strategies to support healthy aging [14].
Despite clear recommendations from health authorities, adult vaccination coverage consistently falls short of public health goals [15,16]. For instance, in the United States, during the 2023–2024 season, only 43.6% of adults aged ≥18 years received the influenza vaccine, with coverage reaching 69.7% among those aged ≥65 years [17]. In the European Union and European Economic Area, the median vaccination coverage among older adults was 45.7% in the 2023–2024 season, down from 59% in 2020–2021, indicating a declining trend [18]. Notably, only a few countries, such as Denmark, have met the 75% coverage target for older adults [18]. In many low-, lower-middle-, and upper-middle-income countries, coverage remains low; for instance, in South Africa, an upper-middle-income country, only 5% of privately insured individuals were vaccinated in 2015 [19]. Pneumococcal vaccine uptake is notably low among at-risk adults under 65 and often inadequate even in those ≥65 years [20]. Similarly, coverage for herpes zoster and needed tetanus boosters is frequently insufficient [20].
The coronavirus disease 2019 (COVID-19) pandemic further strained immunization systems and potentially exacerbated existing challenges and disparities [21,22,23]. Factors contributing to under-vaccination are multi-faceted: limited patient awareness, provider-related factors such as lack of strong recommendations or time constraints, fragmented vaccine delivery systems causing missed opportunities, access barriers including cost and convenience, and vaccine hesitancy often fueled by misinformation [24]. Hospitals emerge as uniquely strategic environments to address these challenges and improve adult immunization coverage [25]. As high-contact settings are frequently utilized by older adults and those with chronic conditions, hospitalization, whether through inpatient admission or emergency department visits, presents an opportunity to identify undervaccinated individuals and provide targeted preventive interventions [25]. Importantly, hospitalized individuals may be more receptive to vaccination due to a heightened awareness of their health status or recent experiences with acute illness [26]. Moreover, hospitals often have access to diagnostic and administrative data, which can support patient identification and tracking of vaccine eligibility and delivery [27]. This context shifts the view of the hospital from solely a site for acute treatment to a vital node within the public health infrastructure capable of delivering crucial preventive care. Leveraging the existing hospital infrastructure, including clinical staff, electronic health records (EHRs), and established patient pathways, can facilitate the integration of vaccination into routine clinical practice. Several systematic and narrative reviews have previously examined interventions to improve adult vaccination coverage [28], often focusing on primary care [3] or community-based settings [29]. However, evidence regarding hospital-based strategies remains limited and fragmented. This fragmentation is largely due to the heterogeneity in study designs (e.g., randomized trials, cohort studies, and quality improvement projects), settings (e.g., inpatient wards, outpatient clinics, and tertiary care centers), and populations (e.g., older adults, immunocompromised individuals, and patients with different chronic diseases). These methodological and contextual differences have limited the generalizability of findings and made comparisons across studies challenging. This systematic review and meta-analysis aims to evaluate the effectiveness of recent hospital-based interventions designed to enhance vaccine coverage among adults aged ≥60 years or between 18 and 64 with one or more high-risk medical conditions. By synthesizing current evidence on various strategies implemented within hospital settings, this report seeks to provide actionable insights to inform clinical and organizational practices, ultimately promoting vaccine equity and improving disease prevention in these vulnerable populations.
2. Materials and Methods
This systematic review and meta-analysis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and followed the PRISMA 2020 (Preferred Reporting Items for Systematic reviews and Meta-Analyses) and MOOSE (Meta-analysis of Observational Studies in Epidemiology) guidelines for reporting [30]. The study protocol was developed a priori, shared within the research team, and registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number: CRD420251031996).
2.1. Literature Search Strategy
A comprehensive literature search was performed using PubMed/MEDLINE, Embase, and Scopus. The search strategy combined Medical Subject Headings (MeSH) and free-text terms related to hospital settings and vaccination coverage, including: (“hospital*” OR “acute care” OR “inpatient” OR “outpatient”) AND (“vaccination coverage” OR “vaccine uptake” OR “immunization adherence”). The search string used for PubMed is provided in Supplementary Table S1. Search strategies for other databases were adapted based on this string to accommodate the specific syntax and indexing of each database. Boolean operators (AND/OR) were applied to refine the search. The final search was conducted on 13 March 2025. Additional studies were identified by screening the reference lists of included articles and by consulting domain experts.
2.2. Inclusion and Exclusion Criteria
Study selection followed the PICOS (Population, Intervention, Comparison, Outcomes and Study) framework (Supplementary Table S3): Population (adults aged ≥60 years or between 18 and 64 with one or more high-risk medical conditions, including oncological diseases, immunosuppressive states, diabetes, cardiovascular or other chronic illnesses), Intervention (hospital-based interventions to improve uptake of recommended vaccines), Comparison (no intervention or usual care), Outcome (vaccine uptake rate), and S (Peer-reviewed experimental and observational studies).
In this review, effectiveness was operationally defined as any measurable change in vaccine uptake rate among eligible adult populations following a hospital-based intervention. Vaccine uptake was expressed as the proportion of eligible individuals who received at least one recommended vaccine dose, either reported as a percentage change (pre-post), odds ratio (OR), relative risk (RR), or absolute difference between control and intervention groups. We included studies with various follow-up periods as long as vaccination uptake was clearly reported post-intervention. This decision reflected the variability in study designs and allowed for the inclusion of both short-term and longer-term evaluations of hospital-based interventions.
Due to heterogeneity across vaccine types and schedules, adherence to complete multi-dose schedules was not required unless explicitly stated in the study. For multi-dose vaccine schedules, we accepted studies that reported the administration of at least the first dose, unless full schedule adherence was specifically evaluated by the study authors. Given the variability in how vaccine completion was reported, we did not require full schedule completion as a criterion for inclusion. When available, information on dose adherence was extracted and reported descriptively.
Eligible studies were peer-reviewed original articles published in English in the last 10 years. To be included, articles needed to be interventions (clinical, behavioral, or structural) to increase any vaccine uptake for adult inpatients and must have measured vaccination uptake as an outcome.
Exclusion criteria included non-original works (e.g., conference abstracts, editorials, books, systematic or narrative reviews, commentaries, expert opinions), ongoing clinical trials, studies not reporting vaccine adherence data specifically for individuals ≥60 years or high-risk patients aged 18–64, studies conducted in non-hospital settings, and publications in languages other than English. Studies published in languages other than English were excluded to ensure accurate comprehension and critical appraisal by all members of the review team. Given the complexity of intervention designs and outcome measures, the ability to thoroughly understand the methodology and interpret the results was prioritized to maintain the quality of data extraction.
2.3. Study Selection and Data Extraction
Study selection was performed in two stages: initial screening of titles and abstracts, followed by full-text review of potentially eligible articles. Two reviewers independently assessed all records, resolving disagreements through discussion or consultation with a third senior reviewer. Data extraction was carried out using a predefined and pilot-tested Excel spreadsheet. Extracted variables included: first author, year, study design, country, hospital setting, participants’ characteristics (age, gender, and clinical condition), vaccine type, intervention strategy, sample size, number vaccinated (pre and post intervention, if applicable), and outcome measures. Outcome indicators were reported as described in the original studies, including absolute vaccine uptake rates, percentage changes, and relative measures (when available). Data extraction was conducted in duplicate; discrepancies were resolved by discussion.
2.4. Data Synthesis
The study selection process followed PRISMA 2020 guidelines and was documented using a flow diagram. Reasons for exclusion at the full-text stage were recorded. Extracted data were synthesized in tabular form and summarized narratively. Results from statistical analyses were reported using appropriate tables and figures. The included studies were grouped and analyzed based on the type of hospital-based intervention strategy employed to improve vaccine uptake. These categories included: reminders (addressed to patients or staff), education (targeting patients and/or staff), standing order protocols (SOPs), multi-component strategies, clinician prompts, and hospital-based catch-up strategies. In addition, subgroup characteristics such as vaccine type, participant age, and geographic region were summarized to account for clinical and contextual heterogeneity across studies.
2.5. Statistical Analysis
A meta-analysis was conducted to evaluate the impact of hospital-based interventions on vaccination uptake by comparing pre- and post-intervention coverage data. Interventions were categorized into seven predefined groups, and a separate meta-analysis was performed for each category that included at least three independent studies. This threshold was met by only two intervention categories, for which meta-analyses were subsequently carried out. For each study, the number of individuals vaccinated before and after the intervention and the corresponding sample sizes were extracted. Only studies with a pre-post design within the same patient population were included; studies using intervention-control or other non-pre/post comparative designs were excluded.
The analysis was performed using ProMeta® 3 (Internovi, Cesena, Italy). The selected effect size was the odds ratio (OR), calculated from binary data (number of vaccinated individuals and total sample size) in two matched groups (pre- and post-intervention). A correlation coefficient of 0.5 between pre- and post-intervention measures was assumed for all included studies, and a sensitivity analysis was performed by repeating the meta-analysis with correlation values of 0.3 and 0.7. The consistency of results across these values supported the robustness of the overall findings.
A second sensitivity analysis was also conducted within each intervention category, limiting the analysis to studies evaluating the same vaccine (e.g., influenza only, pneumococcal only), provided that at least three data points were available for that specific antigen. Meta-analyses were conducted using both fixed-effect and random-effects models, depending on the level of between-study heterogeneity. Heterogeneity was assessed using the I2 statistic and interpreted as follows: low (<25%), moderate (25–50%), substantial (50–75%), and considerable (>75%). Publication bias was evaluated through visual inspection of funnel plot asymmetry and formally tested using Egger’s test (p < 0.10 considered indicative of potential bias).
2.6. Assessment of Study Quality
The risk of bias in the included studies was independently assessed by two reviewers using validated critical appraisal tools, depending on the study design. Discrepancies were resolved by consensus or by a third reviewer when necessary. Observational studies were assessed using an adapted version of the Newcastle–Ottawa Scale (NOS) [31]. The NOS evaluates three domains: selection of participants (0–4 points), comparability of study groups based on control for confounding factors (0–2 points), and outcome or exposure assessment (0–3 points), for a total score ranging from 0 to 9. Studies scoring ≥8 were considered high quality. Non-randomized interventional studies were assessed using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool, adapted as appropriate to the context of this review [32]. Based on the ROBINS-I framework, studies were judged as having a low, moderate, serious, or critical risk of bias. Randomized controlled trials (RCTs) were evaluated using the Cochrane Risk of Bias 2 (RoB 2) tool [33]. This tool assesses 5 domains: the randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported result. Each study was categorized as having low risk of bias, some concerns, or high risk of bias. Quality Improvement (QI) studies were identified among the included articles and further appraised using the 16-domain Quality Improvement Minimum Quality Criteria Set (QI-MQCS), specifically developed for critical appraisal of QI studies [34].
3. Results
3.1. Literature Search
A total of 5382 records were identified through database searches in PubMed/MEDLINE (n = 1129), Scopus (n = 2546), and EMBASE (n = 1707). Three [35,36,37] additional articles were included based on reference screening and expert consultation. After removal of duplicates (n = 2081), 3301 records were screened based on titles and abstracts. Of these, 109 articles were selected for full-text review. Following full-text assessment, 37 articles were excluded because they were non-original works or conference abstracts, 16 focused on a different population, 1 was a duplicate, 8 investigated a different intervention or outcome, and 6 had no full text available. As a result, 44 articles [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] were included in the current systematic review. The study selection process is illustrated in Figure 1.
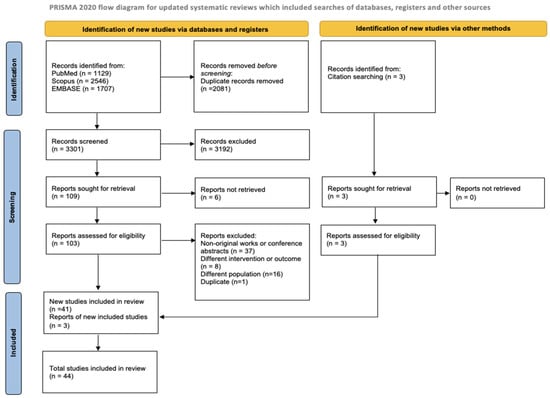
Figure 1.
PRISMA study search flow diagram.
3.2. Descriptive Characteristics of Included Studies
As shown in Table 1, the majority of included studies (n = 36) [35,37,38,39,41,42,43,44,45,46,47,48,50,53,54,55,58,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78] were conducted in high-income countries, while a smaller number (n = 7) [36,49,51,52,56,57,59] originated from upper-middle-income settings, and only 1 [40] from a lower-middle-income country. Country income classification was based on the World Bank 2024–2025 income categories [79]. Taiwan, not individually listed by the World Bank, was considered part of China and thus classified as an upper-middle-income setting.

Table 1.
Characteristics of the included studies.
3.3. Geographic Distribution of Studies
As illustrated in the geographic map (Figure 2), the included studies were conducted across a diverse range of geographic settings. The majority originated from the United States, 14 studies [35,37,38,41,42,47,53,55,62,65,69,70,71,74], followed by 6 studies in France [39,43,67,68,72,76], 3 studies in Singapore [58,73,78], 2 studies in the United Kingdom [44,66], Turkey [49,56], Spain [50,60], and Australia [54,63]. Other countries represented by a single study included China [52], Germany [48], Mexico [51], Belgium [46], Brazil [36], Nepal [40], Hong Kong [45], Taiwan (China) [57], Ireland [61], Canada [64], Georgia [52], Italy [77], and France and Monaco [75]. This geographic diversity allowed for a broad perspective on vaccination strategies.
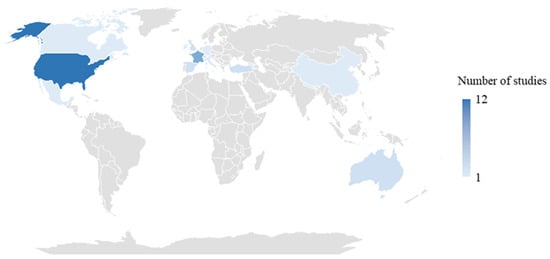
Figure 2.
Geographical distribution of included studies.
3.4. Vaccine Subgrouping
Among the studies included in this review, several investigated more than one vaccine. Overall, the most frequently assessed was the influenza vaccine, examined in 25 studies [35,36,37,38,43,44,46,48,50,51,53,55,58,59,60,61,64,67,68,72,73,75,76,77,78], followed by pneumococcal vaccines in 17 studies [37,43,44,45,46,48,49,51,53,55,64,67,68,69,72,75,77], hepatitis B (HBV) in 10 [36,38,43,44,46,48,51,55,62,68], PCV13 in 9 [35,38,41,42,56,63,70,73,74], and PPSV23 in 9 studies [35,38,40,41,42,61,70,73,74]. The herpes zoster (HZ) vaccine was investigated in five studies [35,51,55,71,74] and hepatitis A and COVID-19 vaccines in four studies [47,52,54,66]. Human papillomavirus (HPV) [44,51,55], tetanus, and Tdap [55,68,74] vaccines were each addressed in three studies, while diphtheria–tetanus–pertussis (DTP) [43,67] studies and meningococcal vaccines [44,64] were each assessed in two studies. A single study investigated each of the following: tuberculosis (TB) [38], DTaP-IPV [39], measles-mumps-rubella (MMR) [44], varicella [44], diphtheria [48], pneumonia [36], COVID-19 (booster) [57], Hemophilus influenzae type b (Hib) [64], and pneumococcal (PCV20) [65].
3.5. Participant Age Summary
Age data were extracted when available as means or ranges. One study included individuals <40 years [54], three studies [44,46,55] focused on the 40–49 age group, five [36,48,51,68,78] on 50–59, three [43,49,76] on 60–69, and four [39,58,75,77] on 70–79. Additional studies reporting only age thresholds (e.g., ≥65 years) or no age data were excluded from the age-specific summary.
3.6. Distribution of Studies Based on Pandemic Period
The majority of included studies (n = 32) referred to the pre-pandemic period, five studies [36,38,43,56,67] concerned the pandemic period (some [36,38,56,67] included selected patients from previous years as well) while only seven studies [47,49,52,54,57,59,77] referred to the post-pandemic period. Focusing on the type of vaccine in the pandemic period studies, four studies related to influenza [36,38,43,67] and pneumococcal [38,43,56,67], three related to HBV vaccines [36,38,43], two to DTP [43,67], two to DT [43,67], and one to TB [38]. The types of interventions implemented were multi-component strategies (n = 2) [56,67] like EMR alerts, posters, patient/provider education, and dedicated vaccine units, patient education (n = 1) [43], patients/staff reminders (n = 2) [36,38] like EHR-integrated vaccination checklist for IBD patients at each clinic visit.
Each hospital-based intervention strategy type is presented in a dedicated section below, synthesizing evidence across studies for that specific intervention category, as summarized in Table 2.

Table 2.
Summary of vaccination outcomes before and after intervention, including effect size and relative increase.
3.7. Patient/Staff Reminders
Of the included studies, four studies [36,38,41,57], three cohort studies (COHs) [38,41,57], and one randomized controlled trial (RCT) [36] assessed reminder-based strategies aimed at patients and healthcare professionals to improve vaccination coverage. The studies, published between 2019 [41] and 2024 [57], were conducted in the United States [38,41], Brazil [36], and Taiwan [57], and took place in outpatient clinics and veterans’ healthcare facilities. Participants included adults with chronic conditions, hospitalized older adults, and individuals ≥65 years scheduled for COVID-19 booster vaccination. Mean age ranged from 40.64 (13.93) [38] to 72.7 years [41], with female representation between 54% [38] and 96.3% [41]. The interventions involved EHR prompts, discharge instruction note reminders, and personalized SMS messages. The vaccines promoted included influenza, Pneumococcal Conjugate Vaccine 13-valent (PCV13), Pneumococcal Polysaccharide Vaccine 23-valent (PPSV23), hepatitis B, tuberculosis, and COVID-19 boosters. Sample sizes ranged from 139 [36] to 3500 [57]. Post-intervention increases were notable: influenza uptake +119% [38], and COVID-19 booster coverage reached 38% among those who received SMS reminders (compared to a 4% national increase) [57]. Two studies [38,57] reported statistically significant improvements, while two [36,41] others reported statistically significant improvements for only some types of vaccines.
3.8. Patient/Staff Education
A total of 11 [43,45,48,49,50,51,59,61,65,72,78] studies investigated educational interventions targeting either patients or healthcare staff. These studies were published between 2015 [45] and 2025 [59] and employed a range of study designs, including six COH [48,49,59,61,72,78], two RCTs [43,45], two [50,65] quasi-experimental studies (QESs), and one [51] cross-sectional study (CSS). The interventions took place in various hospital contexts: four [45,48,51,72] studies were conducted in outpatient clinics, three [43,61,78] in tertiary/university hospitals, and only one [49] had its setting a multicenter or national study, general inpatient wards, specialty program or unit, and veterans’/military facility. Target populations included transplant candidates, older adults with chronic diseases, and immunosuppressed patients. Mean age ranged from 48.5 [72] to 65.15 (±9.2) [59] years, and the percentage of female participants varied widely, from 14.1% [59] to 94.5% [51]. The most frequently targeted vaccines were influenza, pneumococcal, hepatitis B, and Diphtheria, Tetanus, Pertussis (DTP).
Educational strategies included pre-transplant consultations, structured patient education programs, and information materials. Sample sizes ranged from 39 [43] to 7834 [59] participants. All included studies reported positive changes between pre- and post-intervention periods, with no instances of decreased uptake post-intervention. For example, influenza vaccination coverage increased from 1.6% to 12.1%, corresponding to a relative increase of +662.5% [59], and pneumococcal vaccination uptake rose from 16.1% to 85.7%, with a relative increase of +432.3% [72]. 4 articles [49,59,61,78] report the OR, with OR as high as 9.01 [61] (95% CI 4.40–18.42) for influenza vaccine uptake. Statistical significance was observed in 7 [43,45,49,59,61,72,78] out of 10 studies that reported p-values.
3.9. Standing Order Protocols (SOPs)
Only one study [74] assessed the implementation of standing order protocols (SOPs), which empower nurses or pharmacists to administer vaccines independently of direct physician orders. The study was conducted in the United States (Minnesota) in 2020 and included one QES design. The setting was limited to outpatient clinics, and participants were adults with chronic health conditions such as heart disease or renal failure. Although detailed demographics were often lacking, interventions led to increases in vaccination rates across multiple antigens. For example, in patients aged 19–64, pneumococcal vaccine coverage rose from 10% to 23% in one site, and in another site improved from 24% to 60%. Relative increases ranged from −14.3% to 2400.0%. Negative values indicate a post-intervention decrease in vaccination uptake compared to baseline, observed in only 2 out of 13 measurements. The study reported statistical significance.
3.10. Multi-Component Strategies
Multi-component interventions were the most frequently studied approach, featured in 21 articles [35,37,40,44,47,52,53,56,58,62,63,64,66,67,69,70,71,73,75,76,77] published between 2015 [37] and 2024 [52,56,63,77]. Study designs included 12 QESs [35,37,44,52,53,58,62,63,64,67,70,71], 3 COHs [56,66,69], 3 [40,47,73] quality improvement projects (QIs), and 1 case–control study (CCS) [76], CSS [77], and RCT [75]. Research was conducted across 11 countries, most notably in the USA, the United Kingdom, Singapore, and France.
Interventions were carried out in various settings, the most representative: seven [47,56,64,66,67,73,77] in a tertiary/university hospital, six [35,37,62,69,70,71] in outpatient clinics, and four [40,53,63,75] in general inpatient wards. Target populations included hospitalized older adults, patients with HIV, and those with chronic illnesses. Age ranged from 48 [44] to 82.4 (±6.9) [63] years, and female representation varied from 8% [58] to 83.5% [35]. The most commonly promoted vaccines were pneumococcal (n = 11), influenza (n = 9), COVID-19 (n = 3), and hepatitis B (n = 2). Strategies typically involved combinations of provider education, EHR alerts, discharge checklists, vaccine passports, and patient outreach. Sample sizes ranged from 28 [64] to 29,530 [56] individuals. Out of 21 studies, only 1 [35] reported a negative relative increase, while all others showed positive changes, with the highest reported increase reaching +1872.7% [63]; OR were reported by only 4 [52,58,70,75] studies, while 1 [76] study reported RR. 12 [35,40,44,52,53,56,58,62,63,70,71,76] of 14 studies reported statistically significant results.
3.11. Clinician Prompt
Only two studies [42,46] evaluated clinician prompts, typically embedded within the EHR. These included one COH [42] and one RCT [46] conducted between 2017 [43] and 2018 [42] in the USA [42] and Belgium [46].
Settings included outpatient clinics and veterans/military facilities. Participants were immunocompromised adults, including HIV-positive veterans and patients with inflammatory bowel disease. The mean age was around 44 years; data on sex distribution were around 47% female. Vaccines promoted included pneumococcal, PCV13, PPSV23, influenza, hepatitis B, and tetanus. Interventions involved automatic alerts to providers, supplemented by reminder letters to patients. Sample sizes ranged from 99 [42] to 505 [46]. One study [42] showed a significant increase in pneumococcal vaccination from 0% to 38.4% within 180 days. In the second study, significant differences in vaccination uptake were observed between the control and intervention groups: influenza (10% vs. 36%), pneumococcal (23% vs. 62%), hepatitis B (5% vs. 27%), and tetanus (2% vs. 33%).
3.12. Hospital-Based Catch-Up Strategy
A total of five studies [39,54,55,60,68] implemented catch-up vaccination strategies during hospitalization, aimed at patients with incomplete vaccine histories. These studies, conducted from 2020 [39] to 2023 [54], included three cohort studies [54,55,68] and two [39,60] randomized trials across four countries. Participants included elderly inpatients and individuals with severe mental illness or chronic gastrointestinal conditions. Mean age ranged from 36.5 [54] to 81.4 [39] years, and female representation ranged between 36% [68] and 65.8% [39]. Vaccines addressed included influenza, pneumococcal, and hepatitis A/B. Interventions typically included real-time eligibility assessments and vaccine offers during hospitalization. Sample sizes ranged from 142 [54] to 524 [60]. One study [39] reported a relative increase of 43.8% in the intervention group (vs. 6.3% in the control group). One study [55] stood out by reporting predominantly negative relative changes across several vaccines, including –29.5% for influenza, –5.0% for pneumococcal, –34.0% for HZ, –37.8% for HAV, and –8.7% for HBV, with only modest increases for Tdap (+2.1%) and HPV (+34.9%). This contrasts with the overall trend of positive post-intervention changes observed in the majority of studies. One study reported OR up to 2.48 [60] (95% CI 1.6–3.8).
3.13. Meta-Analysis
In our meta-analysis, both patient education interventions and multi-component strategies showed substantial effectiveness across different vaccine types, despite the high heterogeneity observed among the included studies.
For patient education interventions, six studies [43,48,50,51,72,78] encompassing 15 vaccine uptake outcomes were analyzed. The fixed effects model (FEM) yielded an effect size (ES) of 2.11 (95% CI: 1.96–2.27, p < 0.001) based on a total sample of 7449 participants, although significant heterogeneity was detected (I2 = 97.21%, p < 0.001). When using the random effects model (REM), the ES increased to 2.47 (95% CI: 1.53–3.98, p < 0.001). There was no indication of publication bias, as confirmed by the funnel plot and Egger’s test (intercept: 2.31, p = 0.524). These results are presented in Figure 3 (a: Forest plot; b: Funnel plot) and S4 (a: Forest plot; b: Funnel plot).
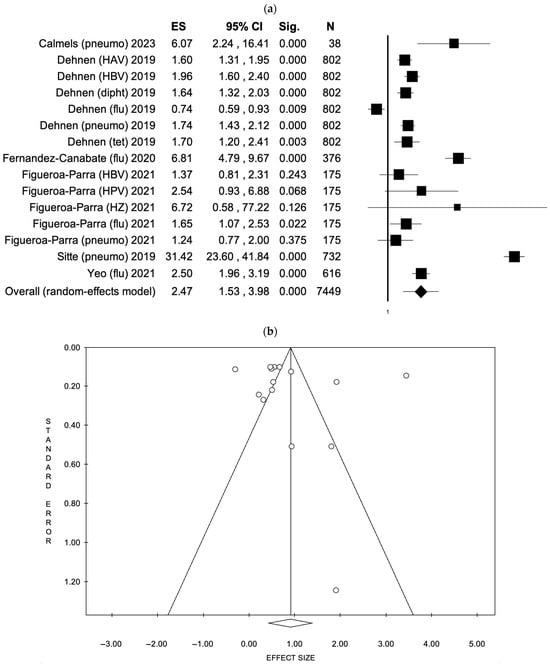
Figure 3.
(a) Forest plot and (b) funnel plot of the random effect model assessing patient education interventions [43,48,50,51,72,78].
For multi-component strategies, 17 studies [35,37,40,44,47,52,53,56,58,62,63,64,67,69,71,73,76] comprising 33 vaccine uptake outcomes were evaluated. The FEM reported an ES of 2.39 (95% CI: 2.33–2.44, p < 0.001), with considerable heterogeneity (I2 = 98.01%, p < 0.001), based on a total of 91,800 participants. Under the REM, the ES increased to 3.12 (95% CI: 2.49–3.92, p < 0.001). As with the previous analysis, no evidence of publication bias was found (Egger’s test intercept: 2.36, p = 0.133). The results are illustrated in Figure 4 (a: forest plot; b: funnel plot) and S5(a: forest plot; b: funnel plot).
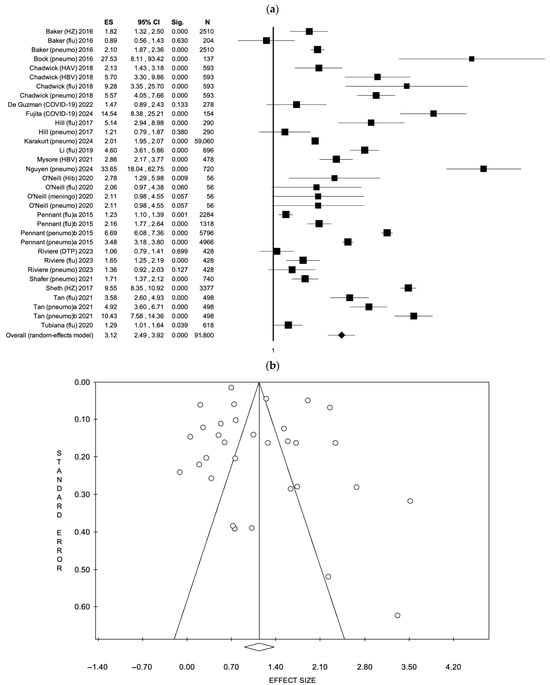
Figure 4.
(a) Forest plot and (b) funnel plot of the random effect model assessing multi-component strategies [35,37,40,44,47,52,53,56,58,62,63,64,67,69,71,73,76].
3.14. Sensitivity Analysis
To assess the robustness of the assumed correlation between pre- and post-intervention data, a sensitivity analysis was conducted using correlation coefficients of 0.3 and 0.7 in addition to the primary value of 0.5. The results across all models remained consistent, with no meaningful variation in the direction or magnitude of the effect sizes. Given the comparability of outcomes across these assumptions, only the estimates based on the 0.5 correlation coefficient are reported in the main analysis.
We conducted a sensitivity analysis stratified by vaccine type. For patient education interventions, analysis restricted to studies using the influenza vaccine included 4 studies [48,50,51,78] with a total of 1969 participants. The effect sizes (ORs) ranged from 0.74 to 6.81 across the studies (Figure 5a). The FEM yielded an ES of 1.74 (95% CI: 1.51–2.00, p < 0.001), although heterogeneity remained high (I2 = 97.55%, p < 0.001). The REM indicated an ES of 2.13 (95% CI: 0.83–5.43, p = 0.114). For pneumococcal vaccines, four studies [43,48,51,72] with a combined sample of 732 participants showed an ES of 3.93 (95% CI: 3.37–4.58, p < 0.001) under the FEM. The REM reported an ES of 4.49 (95% CI: 0.78–25.89, p = 0.092). Pneumococcal vaccination showed a wider range of ORs (1.24 to 31.42), driven by a single outlier study, with a visually higher median (Figure 5a).
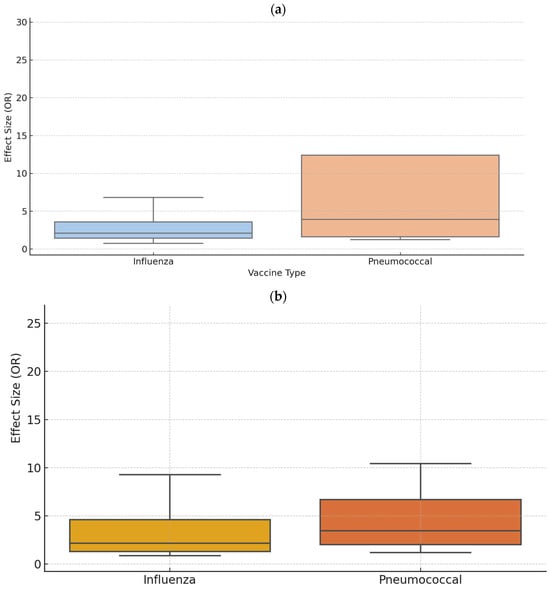
Figure 5.
Distribution of effect sizes (odds ratios) for (a) patient education and (b) multi-component hospital-based strategies by vaccine type: sensitivity analysis comparing influenza and pneumococcal vaccines.
Regarding multi-component strategies, eight studies [35,37,44,53,58,64,73,76] focused on influenza vaccination (n = 6557) reported an ES of 1.76 (95% CI: 1.62–1.91, p < 0.001) under the FEM, with significant heterogeneity (I2 = 94.87%, p < 0.001). The REM indicated an ES of 2.42 (95% CI: 1.59–3.69, p < 0.001). For studies using pneumococcal vaccines (11 studies, n = 76,292) [35,37,40,44,53,56,63,64,67,69,73], the FEM produced an ES of 2.35 (95% CI: 2.29–2.41, p < 0.001), with pronounced heterogeneity (I2 = 98.65%, p < 0.001). The REM showed a higher ES of 3.91 (95% CI: 2.77–5.53, p < 0.001). Influenza-related studies showed a more compact distribution (median 2.2), while pneumococcal studies demonstrated a broader range and higher variability, including outlier values exceeding OR > 25 (Figure 5b).
3.15. Quality Assessment
The quality assessment of the 44 studies was evaluated using four different checklists, based on the type of study:
- The NOS checklist was used to qualitatively assess the risk of bias in 17 [38,41,42,48,49,54,55,56,57,59,66,68,69,72,76,77,78] of the studies included in the review, all of which were observational studies. Among the 17 articles where it was applied, 14 [38,41,42,54,55,56,57,59,66,68,69,72,77,78] scored ≥ 8/9 points, 3 [48,49,54] received a total score of ≤ 7/9 (Supplementary Table S4).
- The QI-MQCS was applied to 12 articles [35,37,40,47,51,58,61,62,64,70,71,73]. Among them, 10 [37,40,51,58,61,62,64,70,71,73] scored ≥ 14/16, whereas 2 articles [35,47] received a score of ≤ 13/16 (Supplementary Table S5).
- The RoB 2 tool was used to qualitatively assess the risk of bias in 7 [36,39,43,45,46,60,75] of the studies included in this review. Three studies had low risk of bias [43,45,60], two showed some concerns [39,75], and two were rated as high risk of bias [36,46] (Supplementary Table S6).
- Among the eight [44,50,52,53,63,65,67,74] non-randomized studies assessed with ROBINS-I, three had a moderate risk of bias [50,52,63], four had a serious risk [44,53,67,74], and one had a critical risk of bias [65] (Supplementary Table S7).
In Table 3, all studies were reported with the specific checklist used and the score was associated with a color that graphically represents the risk of bias. Overall, 27 studies [37,38,40,41,42,43,45,51,55,56,57,58,59,60,61,62,64,66,68,69,70,71,72,73,76,77,78] had a low risk of bias, 3 had a moderate risk of bias [50,52,63], 4 had a serious risk of bias, 8 studies had a high risk of bias [35,36,46,47,48,49,54,65], and 2 studies [39,75] had some concerns.

Table 3.
Risk of bias assessment of the included studies, according to the methodological checklist used in each study (QI-MQCS, NOS, RoB 2 tool, or ROBINS-I).
4. Discussion
The findings from this systematic review with meta-analysis indicate that, as a whole, hospital-based interventions can significantly improve vaccination uptake among older adults and high-risk populations. Across the 44 included studies, a wide range of strategies, such as patient and staff education, electronic reminders, standing order protocols, and multi-component interventions, were associated with increased coverage for vaccines, including influenza, pneumococcal, hepatitis B, tetanus, and COVID-19. However, the review also found that the effectiveness of interventions varied considerably. While most studies reported positive changes in uptake, particularly those lacking control groups, showed minimal or even negative changes. Notably, several studies reported absolute increases in uptake exceeding 30%. Hospital-based interventions, if adequately structured and integrated, can address several barriers that typically hinder vaccine uptake in community settings.
This meta-analysis also provided quantitative evidence supporting the efficacy of both patient education interventions and multi-component strategies in enhancing vaccine uptake across diverse populations and vaccine types. Notably, multi-component interventions exhibited greater pooled effect sizes compared to education-only strategies, suggesting a potential cumulative or synergistic effect when multiple implementation components are deployed concurrently.
Patient education interventions were associated with significant improvements in vaccine adherence, particularly in the context of pneumococcal vaccination. However, the high heterogeneity observed (I2 > 97%) and the broad confidence intervals under the random effects model underscore the methodological and contextual variability across studies. The limited precision in effect size estimates, especially for influenza-focused education interventions, may reflect variability in intervention design, delivery modalities, population literacy levels, and baseline vaccine hesitancy.
Multi-component strategies yielded higher and more consistent effect sizes, with a particularly notable impact observed in studies targeting pneumococcal vaccination. These findings align with implementation science literature, which emphasizes that interventions addressing multiple barriers, such as structural access, provider recommendation, behavioral prompts, and informational deficits, are more likely to produce meaningful changes in preventive health behaviors. The significantly higher ES observed in the REM further supports the robustness of these approaches, despite substantial between-study variability (I2 > 98%).
These results align with findings from other systematic reviews on interventions in primary care [80,81] and community settings [82], which demonstrate that multifactorial approaches are the most effective. However, our review highlights the added value of the hospital setting, where the integration of organizational and technological strategies enables reaching high-risk populations often excluded from traditional vaccination pathways.
This review provides an important and timely contribution to the existing literature by offering an up-to-date synthesis of hospital-based strategies to improve adult vaccination uptake, including during the COVID-19 era. To our knowledge, this is the first systematic review and meta-analysis to assess hospital-based interventions for adult vaccination that includes COVID-19 vaccines. Several studies addressed COVID-19 immunization using strategies such as bedside catch-up programs, SMS reminders, and multi-component campaigns. For instance, Fujita et al. [52] implemented bedside COVID-19 vaccination for hospitalized adults, leading to an increase in vaccination coverage from 21.0% to 79.0%, with a significant post-intervention odds ratio of 3.92 (95% CI: 2.24–6.87, p < 0.001). Similarly, Hooper et al. [54] reported that offering COVID-19 vaccines to psychiatric inpatients resulted in 28.3% of eligible individuals being vaccinated during hospitalization, a marked improvement compared to general population rates at the time. Lee et al. [57] demonstrated the effectiveness of SMS-based reminders: a 38% relative increase in booster uptake was observed following message delivery, significantly outperforming the national average increase of only 4%. Finally, De Guzman et al. [47] showed that even a relatively simple intervention, such as a multi-phase campaign using inpatient pamphlets and staff alerts, resulted in a 43.9% relative increase in inpatient COVID-19 vaccine coverage.
However, during the COVID period, fear and anxiety about the coronavirus may have also played a role in the results and delays in some vaccinations. Calmels et al. [43] underlined that for several patients, the vaccinations recommended were delayed due to the COVID vaccination campaign. For the same reasons, since vaccines could only be given 2 weeks after a SARS-CoV2 vaccination, not all of them were injected on the day of the consultation. Furthermore, Riviere et al. [67] stated that delaying the training in the third center could have modified the reassessment of seasonal influenza VC because the influenza epidemic and the national vaccination campaign would have been completed long before the evaluation.
Among the hospital-based strategies analyzed, multi-component interventions consistently emerged as the most effective and scalable approach to improving vaccine uptake in high-risk adult populations. These interventions, which combine several elements, such as staff education, EHR prompts, structured discharge planning, and patient-targeted communication, were not only the most frequently implemented (n = 21) [34,37,41,44,49,51,54,56,60,61,62,64,65,66,68,69,70,72,74,75,76], but also yielded the largest and most consistent improvements in coverage across diverse settings and vaccine types. For instance, a comprehensive pneumococcal campaign conducted in Turkey [56] led to a 74.4% relative increase in vaccination coverage (from 15.0% to 26.2%, p < 0.001), while a bundled intervention in Singapore [73] raised influenza uptake from 63.0% to 86.0% (+36.5%). Studies using multi-faceted models also reported broader impacts, such as fewer vaccine refusals, improved documentation, and higher clinician engagement—underscoring their systemic value. These results are consistent with previous studies [82,83] focused on different populations. A recent systematic review and meta-analysis [84] found that multi-component interventions generally exhibited excellent effectiveness in vaccination (54.3% increase, 95% CI: 40.5 to 69.6%), with the combination of dialog, incentive and reminder/recall proving more effective than other multi-component interventions.
Educational interventions have traditionally been the most widely used approach to improve vaccination rates; nonetheless, their actual effectiveness remains controversial [83,85]. In our study, patient and provider education, though widely employed, demonstrated mixed results. While some studies showed substantial improvements, such as a 232.9% increase in influenza vaccination among kidney transplant candidates following intensive counseling [43], others yielded minimal or statistically non-significant changes [50,65]. This variability may reflect differences in the format, duration, and intensity of educational content. Previous evidence suggests that education alone is insufficient to drive behavior change in complex health systems. A review [86] of educational interventions for adult vaccination concluded that standalone education modestly improves knowledge (10% relative increase) but rarely translates into meaningful uptake unless combined with active follow-up or vaccine access. Dubé et al. [85] conducted a systematic review of 15 prior reviews and concluded that the evidence supporting educational interventions was generally limited and inconsistent. Similarly, a comprehensive review published in 2000 [87] found that reminder-based strategies targeting either healthcare providers or patients were effective in improving vaccination coverage, whereas education-only interventions did not yield significant benefits. These findings suggest that while education on vaccine safety [88] and benefits is essential, it is rarely sufficient alone to meaningfully increase vaccine uptake. Education remains essential but should be embedded within broader structural or behavioral frameworks. Further research is warranted to clarify which patient populations are most likely to benefit from traditional educational approaches, such as those already inclined to accept vaccination, and in which groups, such as vaccine-hesitant individuals, such interventions may be less effective or even counterproductive.
Reminder-based strategies (e.g., EHR alerts, SMS messages) were effective, particularly when personalized and timely. Prior literature confirms these effects, showing that patient reminders significantly increase vaccination rates, particularly for adult influenza, with a reported RR of 1.29 (95% CI: 1.17–1.43) [89]. Notably, reminders perform best when they target both patients and providers and are coupled with real-time decision support.
SOPs empower nurses and pharmacists to administer vaccines without direct physician orders, streamlining workflows and reducing missed opportunities. In our review, SOPS produced modest increases (e.g., PPSV23 from 24% to 60% in one site), yet these gains are consistent with previous evidence. By standardizing and streamlining vaccine administration, SOPs help integrate vaccination into routine care processes in outpatient settings, reducing dependence on individual clinician orders and thereby minimizing missed opportunities. Their effectiveness appears greatest when supported by staff training, leadership buy-in, and integration with EHR systems [90,91,92].
Catch-up interventions during hospitalization or follow-up visits demonstrated high potential, especially for patients with complex needs or limited outpatient access. In one included study, DTaP-IPV uptake rose from 56.2% to 80.8% among hospitalized older adults (p < 0.001 [39]. This is in line with the “opportunistic immunization” in acute care settings to close protection gaps [93]. However, practical barriers, including vaccine availability, staff workload, and competing clinical priorities, must be addressed to ensure effectiveness.
4.1. Organizational and Public Health Implications
Organizationally, incorporating vaccination into routine hospital care demands careful planning but is feasible. Common implementation barriers include a lack of standardized procedures, insufficient training, physician shortages [94], and workflow disruptions [95]. These can be mitigated through well-defined SOPS, integration into electronic systems, and assigning responsibility to dedicated personnel (e.g., vaccination nurses or pharmacists). Involving staff early in the design process improves ownership and acceptability. Several studies underscore the importance of a multidisciplinary approach, engaging clinicians, nurses, pharmacists, and IT personnel to ensure smooth operationalization.
Implementing standardized protocols, electronic reminders, and catch-up vaccination programs should be considered a priority in hospital policies, as these strategies have the potential to reduce health disparities and substantially improve vaccination coverage among the most vulnerable adults.
From a public health perspective, expanding vaccination beyond traditional outpatient settings is essential to increase coverage and equity. Reaching broader segments of the population requires bringing vaccines closer to where people already receive care or services. In this context, hospitals, as well as schools [96] and community pharmacies [97], play a strategic role in capturing individuals who may otherwise remain unvaccinated due to logistical, socioeconomic, or informational barriers. Offering vaccines during hospital stays is particularly impactful for older adults and people with chronic conditions.
Sustained implementation of hospital-based vaccination initiatives can yield long-term benefits, including reduced morbidity, fewer readmissions, and improved patient awareness. Ensuring that patients are discharged with updated immunization records and clear follow-up instructions supports continued coverage. On a systems level, scaling these models can significantly improve national vaccination coverage, particularly among high-priority groups.
Policymakers and public health authorities play a crucial role in sustaining and scaling these interventions by embedding them into national immunization plans, establishing supportive legislation, and aligning hospital incentives with vaccination targets. Frontline practitioners, including nurses, primary care physicians, and pharmacists, are equally vital for translating policy into action and ensuring the continuity of care after discharge. Collaborative engagement between policy and practice is essential to optimize uptake and reduce structural barriers.
In summary, these findings reinforce the value of educational and multi-component interventions as effective strategies to promote vaccine uptake. Patient education alone can yield significant benefits, particularly when adapted to specific sociodemographic and clinical contexts. However, the greater efficacy observed with multi-component approaches highlights the importance of integrated, system-level strategies in vaccination promotion. Future research should aim to elucidate the optimal combination and sequencing of intervention components, with particular attention to cost-effectiveness, scalability, and contextual adaptability in real-world public health settings.
4.2. Limitations
This systematic review and meta-analysis has several limitations. First, a substantial proportion of included studies were observational and thus subject to selection bias, confounding, and lack of randomization. Although the risk of bias was systematically assessed using validated tools, the overall methodological quality varied, with some studies demonstrating moderate to serious risk of bias. Publication bias cannot be ruled out, as studies with positive findings are more likely to be published than those reporting null or negative results. In addition, the exclusion of the gray literature (such as technical reports, theses, or conference proceedings not published in peer-reviewed journals) may have further amplified this bias, thereby reducing the overall representativeness of the available evidence.
Most interventions were conducted in high-income countries, potentially limiting applicability to low- and middle-income settings. Furthermore, variations in vaccine types and reporting metrics (e.g., absolute vs. relative increases) complicate cross-study comparisons.
Moreover, only peer-reviewed articles published in English were included. The restriction to English-language publications was applied to ensure accurate comprehension and critical appraisal of the studies by all members of the review team, given the methodological complexity of the interventions assessed. However, this choice may have introduced language bias and potentially excluded relevant data from non-English-speaking countries. Future updates may consider including non-English studies and the gray literature sources, provided appropriate translation and quality appraisal mechanisms are available.
Data extraction was conducted using standardized protocols and independently cross-checked by multiple reviewers; however, extraction errors cannot be entirely ruled out, particularly in cases of ambiguous or incomplete reporting. The future use of automated tools may help enhance the accuracy and consistency of data extraction in such contexts.
Among the various intervention categories identified, meta-analysis was possible only for patient education and multi-component strategies. This was primarily due to the limited number of studies per category, missing data (e.g., total sample size or baseline adherence), or substantial heterogeneity in study design. As a result, the generalizability of our findings to other intervention types remains limited. Further standardized studies are needed to enable broader quantitative synthesis.
Further research is needed to assess the long-term sustainability and cost-effectiveness of these interventions, as well as their impact on specific subgroups, such as individuals with multiple comorbidities or those from socioeconomically disadvantaged backgrounds. In addition, studies evaluating the scalability of these approaches in low- and middle-income countries would be valuable to inform global policy.
5. Conclusions
Hospital-based interventions represent an effective and underutilized opportunity to increase adult vaccination coverage, particularly among older adults and high-risk populations. The meta-analysis demonstrated that multi-component strategies and structured patient education interventions are effective in significantly increasing vaccine uptake. Hospitals offer a critical point of contact to reach individuals who may otherwise face structural, informational, or behavioral barriers to vaccination. In the context of an aging population and ongoing risks from vaccine-preventable diseases, institutionalizing adult vaccination within hospital settings is both evidence-based and important for advancing immunization equity and public health resilience.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare13141667/s1, Table S1: Literature search strategy (PubMed); Table S2: Literature search strategy (Scopus and Embase); Table S3: Inclusion/exclusion criteria based on PICOS (Population, Intervention, Comparison, Outcome, and Study Design); Table S4: Risk of Bias checklist NOS [38,41,42,48,49,54,55,56,57,59,66,68,69,72,76,77,78]; Table S5: Risk of Bias checklist QI-MQCS [35,37,40,47,51,58,61,62,64,70,71,73]; Table S6: Risk of Bias checklist RoB 2 tool [36,39,41,45,46,60,75]; Table S7: Risk of Bias checklist ROBINS-I tool [44,50,52,53,63,65,67,74]; Figure S1: (a) forest plot and (b) funnel plot of the fixed effect model assessing patient education interventions [43,48,50,51,72,78]; Figure S2: (a) forest plot and (b) funnel plot of the fixed effect model assessing multi-component strategies [35,37,40,44,47,52,53,56,58,62,63,64,67,69,71,73,76].
Author Contributions
Conceptualization, F.P. and C.S.; methodology, F.P., S.B., A.C.D., R.C. (Rita Cuciniello), and A.P.; software, F.P., S.B., and R.C. (Rita Cuciniello); validation, F.P. and S.B.; formal analysis, F.P., A.P., and A.C.D.; investigation, R.C. (Rita Cuciniello) and R.C. (Rosaria Calabretta); resources A.P. and R.C. (Rosaria Calabretta); data curation, F.P., A.P., and S.B.; writing—original draft preparation, F.P., A.P., S.B., R.C. (Rita Cuciniello), and A.C.D.; writing—review and editing, F.P., A.P., and S.B.; visualization, R.C. (Rosaria Calabretta), and S.B.; supervision, C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are presented in the current manuscript (text, tables, figures).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doherty, M.; Buchy, P.; Standaert, B.; Giaquinto, C.; Prado-Cohrs, D. Vaccine impact: Benefits for human health. Vaccine 2016, 34, 6707–6714. [Google Scholar] [CrossRef] [PubMed]
- Kayser, V.; Ramzan, I. Vaccines and vaccination: History and emerging issues. Hum. Vaccines Immunother. 2021, 17, 5255–5268. [Google Scholar] [CrossRef]
- Wheeler, S.G.; Beste, L.A.; Overland, M.K.; Wander, P.L. Interventions in primary care to increase uptake of adult vaccines: A systematic review. J. Public Health 2025, 47, fdaf008. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Smetana, J.; Chlibek, R.; Shaw, J.; Splino, M.; Prymula, R. Influenza vaccination in the elderly. Hum. Vaccines Immunother. 2018, 14, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Gianfredi, V.; Nucci, D.; Pennisi, F.; Maggi, S.; Veronese, N.; Soysal, P. Aging, and healthy aging: The public health approach. Aging Clin. Exp. Res. 2025, 37, 125. [Google Scholar] [CrossRef]
- Pennisi, F.; Ricciardi, G.E.; von Wagner, C.; Smith, L.; Kaushal, A.; Lyratzopoulos, G.; Merriel, S.W.D.; Hamilton, W.; Abel, G.; Valderas, J.M.; et al. Impact of Self-Reported Long-Term Mental Health Morbidity on Help-Seeking and Diagnostic Testing for Bowel-Related Cancer Symptoms: A Vignette Study. Cancer Med. 2024, 13, e70426. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Crispino, P.; Buono, P.; Giordano, V.; Trama, U.; Iodice, V.; Leoncini, L.; Giordano, M. Efficacy and Safety of Vaccinations in Geriatric Patients: A Literature Review. Vaccines 2023, 11, 1412. [Google Scholar] [CrossRef]
- Doherty, M.T.; Aris, E.; Servotte, N.; Beck, E. Capturing the value of vaccination: Impact of vaccine-preventable disease on hospitalization. Aging Clin. Exp. Res. 2022, 34, 1551–1561. [Google Scholar] [CrossRef]
- Brassel, S.; Neri, M.; Schirrmacher, H.; Steuten, L. The Value of Vaccines in Maintaining Health System Capacity in England. Value Health 2023, 26, 1067–1072. [Google Scholar] [CrossRef]
- Baxi, R.; Mytton, O.T.; Abid, M.; Maduma-Butshe, A.; Iyer, S.; Ephraim, A.; Brown, K.E.; O’Moore, É. Outbreak report: Nosocomial transmission of measles through an unvaccinated healthcare worker-implications for public health. J. Public Health 2014, 36, 375–381. [Google Scholar] [CrossRef] [PubMed]
- United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights. 2019. Available online: https://www.un.org/en/desa/world-population-prospects-2019-highlights (accessed on 13 April 2025).
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.R.; Officer, A.; de Carvalho, I.A.; Sadana, R.; Pot, A.M.; Michel, J.P. The World Report on ageing and health: A policy framework for healthy ageing. Lancet 2016, 387, 2145–2154. [Google Scholar] [CrossRef]
- World health statistics 2024. Monitoring Health for the SDGs, Sustainable Development Goals. Available online: https://iris.who.int/bitstream/handle/10665/376869/9789240094703-eng.pdf?sequence=1 (accessed on 13 April 2025).
- Del Riccio, M.; Guida, A.; Boudewijns, B.; Heemskerk, S.; van Summeren, J.; Schneeberger, C.; Stelma, F.; van der Velden, K.; Timen, A.; Caini, S. A Missed Opportunity? Exploring Changes in Influenza Vaccination Coverage During the COVID-19 Pandemic: Data From 12 Countries Worldwide. Influenza Other Respir. Viruses 2025, 19, e70057. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention; FluVaxView. Flu Vaccination Coverage, United States, 2023-24 Influenza Season. Available online: https://www.cdc.gov/fluvaxview/coverage-by-season/2023-2024.html (accessed on 13 April 2025).
- European Centre for Disease Prevention and Control. Survey Report on National Seasonal Influenza Vaccination Recommendations and Coverage Rates in EU/EEA Countries. Available online: https://www.ecdc.europa.eu/en/publications-data/survey-report-national-seasonal-influenza-vaccination-recommendations (accessed on 13 April 2025).
- Solanki, G.; Cornell, M.; Lalloo, R. Uptake and cost of influenza vaccines in a private health insured South African population. S. Afr. J. Infect. Dis. 2018, 33, 10.4102. [Google Scholar]
- Jaca, A.; Sishuba, M.; Jacobson Vann, J.C.; Wiysonge, C.S.; Ndwandwe, D. Interventions to improve vaccination uptake among adults. Cochrane Database Syst. Rev. 2021, 11, CD015057. [Google Scholar]
- Dagovetz, M.; Momchilov, K.; Blank, L.; Khorsandi, J.; Rizzo, A.; Khabbache, H.; Sitibondo, A.; Salgado, J.G.; Chirico, F.; Batra, K. Global COVID-19 Vaccination Challenges: Inequity of Access and Vaccine Hesitancy. J. Med. Surg. Public Health 2025, 6, 100197. [Google Scholar] [CrossRef]
- Signorelli, C.; De Ponti, E.; Mastrangelo, M.; Pennisi, F.; Cereda, D.; Corti, F.; Beretta, D.; Pelissero, G. The contribution of the private healthcare sector during the COVID-19 pandemic: The experience of the Lombardy Region in Northern Italy. Ann. Ig. 2024, 36, 250–255. [Google Scholar]
- Pennisi, F.; Odelli, S.; Borlini, S.; Morani, F.; Signorelli, C.; Renzi, C. Impact of the Covid pandemic on timely cancer diagnosis across European healthcare settings: A scoping review. Ann. Ig. 2024, 36, 194–214. [Google Scholar]
- Pennisi, F.; Genovese, C.; Gianfredi, V. Lessons from the COVID-19 Pandemic: Promoting Vaccination and Public Health Resilience, a Narrative Review. Vaccines 2024, 12, 891. [Google Scholar] [CrossRef]
- McFadden, K.; Seale, H. A review of hospital-based interventions to improve inpatient influenza vaccination uptake for high-risk adults. Vaccine 2021, 39, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Rouvière, J.; Merceron, A.; Launay, O.; Sotto, A.; on behalf of the Avnir Group. Patients’ Perception and Knowledge about Influenza and Pneumococcal Vaccination during the COVID-19 Pandemic: An Online Survey in Patients at Risk of Infections. Vaccines 2021, 9, 1372. [Google Scholar] [CrossRef] [PubMed]
- Chapman, L.; Kelly, M.; Piggott, R.S.; Carr, B.; Courtney, G.; McDonagh, R.; O’Connell, B.; Bannan, C.; Cunningham, C.; Briggs, R. An electronic medical record reminder is independently associated with improved opportunistic influenza vaccination rates for older inpatients. J. R. Coll. Physicians Edinb. 2023, 53, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Eiden, A.L.; Barratt, J.; Nyaku, M.K. Drivers of and barriers to routine adult vaccination: A systematic literature review. Hum. Vaccines Immunother. 2022, 18, 2127290. [Google Scholar] [CrossRef]
- Burson, R.C.; Buttenheim, A.M.; Armstrong, A.; Feemster, K.A. Community pharmacies as sites of adult vaccination: A systematic review. Hum. Vaccines Immunother. 2016, 12, 3146–3159. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 13 April 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Hempel, S.; Shekelle, P.G.; Liu, J.L.; Sherwood Danz, M.; Foy, R.; Lim, Y.W.; Motala, A.; Rubenstein, L.V. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): A tool for critical appraisal of quality improvement intervention publications. BMJ Qual. Saf. 2015, 24, 796–804. [Google Scholar] [CrossRef]
- Baker, D.W.; Brown, T.; Lee, J.Y.; Ozanich, A.; Liss, D.T.; Sandler, D.S.; Ruderman, E.M. A Multifaceted Intervention to Improve Influenza, Pneumococcal, and Herpes Zoster Vaccination among Patients with Rheumatoid Arthritis. J. Rheumatol. 2016, 43, 1030–1037. [Google Scholar] [CrossRef]
- Guerra, G.L.; Pedro, F.L.; Severo, M.D.; Guerra, G.L.; Ribeiro, T.A. Strategy to increase vaccination coverage in diabetic patients at a public tertiary university hospital: A randomized controlled trial. SAGE Open Med. 2023, 11, 20503121231161193. [Google Scholar] [CrossRef] [PubMed]
- Pennant, K.N.; Costa, J.J.; Fuhlbrigge, A.L.; Sax, P.E.; Szent-Gyorgyi, L.E.; Coblyn, J.; Desai, S.P. Improving Influenza and Pneumococcal Vaccination Rates in Ambulatory Specialty Practices. Open Forum Infect. Dis. 2015, 2, ofv119. [Google Scholar] [CrossRef] [PubMed]
- Bernasko, N.; Venkateswaran, N.; Coates, M.; Dalessio, S.; Williams, E.; Clarke, K. Improving outpatient care in adult inflammatory bowel disease: Effect of implementation of a reminder checklist in the electronic health records (IBD-ERS)—A pilot study. BMJ Open Qual. 2023, 12, e002008. [Google Scholar] [CrossRef]
- Blanchi, S.; Vaux, J.; Toqué, J.M.; Hery, L.; Laforest, S.; Piccoli, G.B.; Crochette, N. Impact of a Catch-Up Strategy of DT-IPV Vaccination during Hospitalization on Vaccination Coverage among People Over 65 Years of Age in France: The HOSPIVAC Study (Vaccination during Hospitalization). Vaccines 2020, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.; Chintamaneni, K.; Rein, L.; Frazer, T.; Kayastha, G.; Mackinney, T. Improving pneumococcal vaccination rates of medical inpatients in urban Nepal using quality improvement measures. BMJ Qual. Improv. Rep. 2016, 5, u212047.w4835. [Google Scholar] [CrossRef]
- Burka, A.T.; Fann, J.P.; Lamb, K.D.; Salvig, B.E.; Smith, T.L.; Wallace, J.L. Evaluation of a novel discharge reminder tool on pneumococcal vaccination in hospitalized elderly veterans. J. Am. Coll. Clin. Pharm. 2019, 2, 462–467. [Google Scholar] [CrossRef]
- Burns, C.M.; Banks, R.E.; Wilson, B.M.; Carter, R.R.; Jump, R.L.P.; Perez, F. A virtual clinic improves pneumococcal vaccination coverage among patients living with HIV at a Veterans Affairs Medical Center. AIDS Care 2018, 30, 146–149. [Google Scholar] [CrossRef]
- Calmels, A.; Heng, A.E.; Corbin, V.; Garrouste, C.; Greze, C.; Pereira, B.; Lesens, O. Vaccination coverage reinforced by an infectious disease consultation during pretransplant check-up in patients awaiting kidney transplantation: A randomized study. Infect. Dis. Now 2023, 53, 104718. [Google Scholar] [CrossRef]
- Chadwick, D.R.; Corbett, K.; Mann, S.; Teruzzi, B.; Horner, S. Evaluation of a vaccine passport to improve vaccine coverage in people living with HIV. Int. J. STD AIDS 2018, 29, 1190–1193. [Google Scholar] [CrossRef]
- Chan, S.S.; Leung, D.; Leung, A.Y.; Lam, C.; Hung, I.; Chu, D.; Chan, C.K.; Johnston, J.; Liu, S.H.; Liang, R.; et al. A nurse-delivered brief health education intervention to improve pneumococcal vaccination rate among older patients with chronic diseases: A cluster randomized controlled trial. Int. J. Nurs. Stud. 2015, 52, 317–324. [Google Scholar] [CrossRef]
- Coenen, S.; Weyts, E.; Jorissen, C.; De Munter, P.; Noman, M.; Ballet, V.; Vermeire, S.; Van Assche, G.; Ferrante, M. Effects of Education and Information on Vaccination Behavior in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 318–324. [Google Scholar] [CrossRef]
- De Guzman, E.; Boland, J.L.; Van De Rijn, J.; Suresh, M.; Li, F.; Makhoul, J.; Colon Rosa, G.; Rubin, A. Improving Inpatient COVID-19 Vaccination Rates among Adult Patients at a Tertiary Academic Medical Center. JCOM 2022, 29, 178–182. [Google Scholar] [CrossRef]
- Dehnen, D.; Herwig, A.; Herzer, K.; Weltermann, B. Improving the vaccination status of liver transplant patients: Effectiveness of personally addressing patients and written recommendations to family physicians after 3 years. Transpl. Infect. Dis. 2019, 21, e13140. [Google Scholar] [CrossRef] [PubMed]
- Ekin, T.; Kış, M.; Güngören, F.; Akhan, O.; Atıcı, A.; Kunak, A.Ü.; Mutlu, D.; Katkat, F.; Demir, M.; Saraç, İ.; et al. Awareness and Knowledge of Pneumococcal Vaccination in Cardiology Outpatient Clinics and the Impact of Physicians’ Recommendations on Vaccination Rates. Vaccines 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cañabate, E.; Martínez-Santana, V. Implementation of an anti-flu vaccination campaign in a hospital pharmacy service. Farm. Hosp. 2020, 44, 41–45. [Google Scholar] [PubMed]
- Figueroa-Parra, G.; Moreno-Salinas, A.; Santoyo-Fexas, L.; Gamboa-Alonso, C.M.; Carrizales-Luna, J.P.; De Jesus Hernandez-Galarza, I.; Galarza-Delgado, D.A.; Esquivel-Valerio, J.A. Vaccination in rheumatic disease patients: Results of a pilot quality improvement program. Rheumatology 2021, 59, 362–366. [Google Scholar] [CrossRef]
- Fujita, A.W.; Goolsby, T.A.; Powell, K.M.; Cartwright, E.J. Increased Vaccine Uptake Among Eligible Patients at a Veterans Affairs Hospital Through an Inpatient COVID-19 Vaccination Program, Atlanta, Georgia, 2021. Public Health Rep. 2024, 139, 94–101. [Google Scholar] [CrossRef]
- Hill, J.D.; Anderegg, S.V.; Couldry, R.J. Development of a Pharmacy Technician-Driven Program to Improve Vaccination Rates at an Academic Medical Center. Hosp. Pharm. 2017, 52, 617–622. [Google Scholar] [CrossRef]
- Hooper, K.; Hooper, M.; Nguyen, J.; Fukutomi, A. Are you vaccinated? COVID-19 vaccination rates and the effect of a vaccination program in a metropolitan mental health inpatient population in Australia. Australas. Psychiatry 2023, 31, 38–42. [Google Scholar] [CrossRef]
- Hussain, N.; Proctor, D.; Al-Bawardy, B. The Impact of Inflammatory Bowel Disease Clinic On-site Vaccination Services. Crohns Colitis 360 2021, 3, otab067. [Google Scholar] [CrossRef]
- Karakurt, Z.; Yalnız, E.; Altın, S.; Oruç, Ö.; Uslu, Ö.; Şimşek Veske, N.; Kılınç, O.; Kul, S.; Sayıner, A. Effectiveness of a Program to Raise Awareness About Pneumococcal Vaccination Among Physicians and Patients with Chronic Respiratory Diseases: A Multicenter Cohort Study. Thorac. Res. Pract. 2024, 25, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, B.H.; Lin, Y.H.; Wu, B.J.; Chen, T.J.; Chen, W.M.; Chen, Y.C. Enhancing COVID-19 booster vaccination among the elderly through text message reminders. Hum. Vaccines Immunother. 2024, 20, 2375665. [Google Scholar] [CrossRef]
- Li, A.; Chan, Y.H.; Liew, M.F.; Pandey, R.; Phua, J. Improving Influenza Vaccination Coverage Among Patients With COPD: A Pilot Project. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 2527–2533. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Song, Q.; Lin, L.; Li, T.; Zhang, P.; Zeng, Y.; Peng, Y.; Chen, Y.; Cai, S.; Chen, P. Impact of intensive health education on influenza vaccination and acute exacerbations in outpatients with chronic obstructive pulmonary disease: A real-world study. J. Glob. Health 2025, 15, 04047. [Google Scholar] [CrossRef]
- Muñoz-Miralles, R.; Bonvehí Nadeu, S.; Sant Masoliver, C.; Martín Gallego, A.; Gómez Del Canto, J.; Mendioroz Peña, J.; Bonet Esteve, A.M. Effectiveness of a brief intervention for acceptance of influenza vaccine in reluctant primary care patients. Gac. Sanit. 2022, 36, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Low, C.; O’Rourke, A.; Young, F.; Callanan, I.; Feeney, E.; Veale, D.J. A quality improvement intervention failed to significantly increase pneumococcal and influenza vaccination rates in immunosuppressed inflammatory arthritis patients. Clin. Rheumatol. 2020, 39, 747–754. [Google Scholar] [CrossRef]
- Mysore, P.; Khinkar, R.M.; McLaughlin, D.; Desai, S.; McMahon, G.M.; Ulbricht, C.; Mendu, M.L. Improving hepatitis B vaccination rates for advanced chronic kidney disease patients: A quality improvement initiative. Clin. Exp. Nephrol. 2021, 25, 501–508. [Google Scholar] [CrossRef]
- Nguyen, T.; Lam, P. Pharmacist impact on pneumococcal vaccination rates through incorporation of pharmacist-led opportunistic inpatient vaccination intervention. J. Pharm. Pract. Res. 2024, 54, 48–54. [Google Scholar] [CrossRef]
- O’Neill, N.E.; Baker, J.; Ward, R.; Johson, C.; Taggart, L.; Sholzberg, M. The development of a quality improvement project to improve infection prevention and management in patients with asplenia or hyposplenia. BMJ Open Qual. 2020, 9, e000770. [Google Scholar] [CrossRef]
- Pacheco, C.S.; Baxter, J.A.; Steigelman, D. Pneumococcal Perplexity: Improving Awareness of Updated Pneumococcal Vaccination Recommendations in Two Large Military Treatment Facilities. Mil. Med. 2024, 189, e1289–e1293. [Google Scholar] [CrossRef]
- Poulikakos, D.; Chinnadurai, R.; Anwar, S.; Ahmed, A.; Chukwu, C.; Moore, J.; Hayes, E.; Gorton, J.; Lewis, D.; Donne, R.; et al. Increasing Uptake of COVID-19 Vaccination and Reducing Health Inequalities in Patients on Renal Replacement Therapy-Experience from a Single Tertiary Centre. Vaccines 2022, 10, 939. [Google Scholar] [CrossRef]
- Rivière, P.; Penel, N.; Faure, K.; Marie, G.; Najem, A.; Rivière, M.K.; Panaget, S. Effect of medical staff training on vaccination coverage in outpatients with cancer: An interventional multicenter before-and-after study. Vaccine X 2023, 13, 100261. [Google Scholar] [CrossRef] [PubMed]
- Runyo, F.; Matignon, M.; Audureau, E.; Vindrios, W.; Boueilh, A.; Gomart, C.; Grimbert, P.; Gallien, S.; Melica, G. Infectious disease consultation is effective in boosting vaccine coverage in patients awaiting kidney transplantation: A French prospective study. Transpl. Infect. Dis. 2021, 23, e13607. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.; Kearns, C.; Carney, M.; Sagar, A. Leveraging Interdisciplinary Teams for Pre-Visit Planning to Improve Pneumococcal Immunization Rates Among Internal Medicine Subspecialty Practices. J. Prim. Care Community Health 2021, 12, 21501319211060986. [Google Scholar] [CrossRef]
- Sheth, H.S.; Grimes, V.D.; Rudge, D.; Ayers, B.; Moreland, L.W.; Fischer, G.S.; Aggarwal, R. Improving Pneumococcal Vaccination Rates in Rheumatology Patients by Using Best Practice Alerts in the Electronic Health Records. J. Rheumatol. 2021, 48, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Sheth, H.; Moreland, L.; Peterson, H.; Aggarwal, R. Improvement in Herpes Zoster Vaccination in Patients with Rheumatoid Arthritis: A Quality Improvement Project. J. Rheumatol. 2017, 44, 11–17. [Google Scholar] [CrossRef]
- Sitte, J.; Frentiu, E.; Baumann, C.; Rousseau, H.; May, T.; Bronowicki, J.P.; Peyrin-Biroulet, L.; Lopez, A. Vaccination for influenza and pneumococcus in patients with gastrointestinal cancer or inflammatory bowel disease: A prospective cohort study of methods for improving coverage. Aliment. Pharmacol. Ther. 2019, 49, 84–90. [Google Scholar] [CrossRef]
- Tan, H.Z.; Phang, C.C.; Wu, S.Y.; Sim, M.H.; Law, M.M.; Foo, M.W.Y.; Htay, H. Improving influenza and pneumococcal vaccination uptake among incident peritoneal dialysis patients: A quality improvement initiative. Int. Urol. Nephrol. 2021, 53, 2167–2175. [Google Scholar] [CrossRef]
- Tan, L.J.; VanOss, R.; Ofstead, C.L.; Wetzler, H.P. Maximizing the impact of, and sustaining standing orders protocols for adult immunization in outpatient clinics. Am. J. Infect. Control. 2020, 48, 290–296. [Google Scholar] [CrossRef]
- Tubiana, S.; Labarere, J.; Levraut, J.; Michelet, P.; de Vaux, F.J.; Doumenc, B.; Hausfater, P.; Choquet, C.; Plaisance, P.; Schmidt, J.; et al. Effectiveness of a Multifaceted Informational-Based and Text Message Reminders on Pneumococcal and Influenza Vaccinations in Hospital Emergency Departments: A Cluster-Randomized Controlled Trial. Vaccines 2021, 9, 962. [Google Scholar] [CrossRef]
- Tubiana, S.; Launay, O.; Galtier, F.; Tattevin, P.; Postil, D.; Vanhems, P.; Lenzi, N.; Verger, P.; Duval, X. Attitudes, knowledge, and willingness to be vaccinated against seasonal influenza among patients hospitalized with influenza-like-illness: Impact of diagnostic testing. Hum. Vaccines Immunother. 2020, 16, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Ragusa, F.S.; Titone, P.R.; Vernuccio, L.; Catanese, G.; Randazzo, M.A.; Palermo, M.; Di Bella, G.; Mansueto, P.; Dominguez, L.J.; et al. Management of the vaccination campaign in a population of frail older outpatients affected by cognitive or endocrinological conditions: A pilot study in Italy. Aging Clin. Exp. Res. 2024, 36, 179. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.T.; Song, J.; Ng, E.G.T.; Lee, N.L.B.I.; Kee, T.Y.S.; Tan, C.K.; Fan, P.Y.W.; Lee, P.H. Increasing influenza vaccination rates in solid organ transplant recipients in an outpatient transplant centre. Proc. Singap. Healthc. 2020, 29, 223–227. [Google Scholar] [CrossRef]
- Worldbank. World Bank Country Classification by Income Level for 2024–2025. Available online: https://blogs.worldbank.org/en/opendata/world-bank-country-classifications-by-income-level-for-2024-2025 (accessed on 18 April 2025).
- Smith, S.M.; Wallace, E.; O’Dowd, T.; Fortin, M. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst. Rev. 2016, 3, CD006560. [Google Scholar] [CrossRef]
- Carney, P.A.; Engstrom, M.B.; Barnes, C.; Ramalingam, N.; Dickinson, C.; Cox, C.; Ferrara, L.K.; Darden, P.M.; Fagnan, L.J.; Marino, M.; et al. Primary Care and Community-Based Partnerships to Enhance HPV Vaccine Delivery. J. Prim. Care Community Health 2024, 15, 21501319241231405. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.A.; Padhani, Z.A.; Salam, R.A.; Aliani, R.; Lassi, Z.S.; Das, J.K.; Bhutta, Z.A. Interventions to Improve Immunization Coverage Among Children and Adolescents: A Meta-analysis. Pediatrics 2022, 149, e2021053852D. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, C.; Wilson, R.; O’Leary, M.; Eckersberger, E.; Larson, H.J.; SAGE Working Group on Vaccine Hesitancy. Strategies for addressing vaccine hesitancy—A systematic review. Vaccine 2015, 33, 4180–4190. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Zhang, H.; Tan, H. Estimating the effects of interventions on increasing vaccination: Systematic review and meta-analysis. BMJ Glob. Health 2025, 10, e017142. [Google Scholar] [CrossRef]
- Dube, E.; Gagnon, D.; MacDonald, N.E.; SAGE Working Group on Vaccine Hesitancy. Strategies intended to address vaccine hesitancy: Review of published reviews. Vaccine 2015, 33, 4191–4203. [Google Scholar] [CrossRef]
- Labbé, S.; Bacon, S.L.; Wu, N.; Ribeiro, P.A.B.; Boucher, V.G.; Stojanovic, J.; Voisard, B.; Deslauriers, F.; Tremblay, N.; Hébert-Auger, L.; et al. Addressing vaccine hesitancy: A systematic review comparing the efficacy of motivational versus educational interventions on vaccination uptake. Transl. Behav. Med. 2025, 15, ibae069. [Google Scholar] [CrossRef]
- Briss, P.A.; Rodewald, L.E.; Hinman, A.R.; Shefer, A.M.; Strikas, R.A.; Bernier, R.R.; Carande-Kulis, V.G.; Yusuf, H.R.; Ndiaye, S.M.; Williams, S.M. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. Am. J. Prev. Med. 2000, 18, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; D’Amelio, A.C.; Cuciniello, R.; Borlini, S.; Mirzaian, L.; Ricciardi, G.E.; Minerva, M.; Gianfredi, V.; Signorelli, C. Post-Vaccination Anaphylaxis in Adults: A Systematic Review and Meta-Analysis. Vaccines 2025, 13, 37. [Google Scholar] [CrossRef]
- Jacobson Vann, J.C.; Jacobson, R.M.; Coyne-Beasley, T.; Asafu-Adjei, J.K.; Szilagyi, P.G. Patient reminder and recall interventions to improve immunization rates. Cochrane Database Syst. Rev. 2018, 1, CD003941. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Standards for Practice. Adult Vaccine Administration and Referral. Available online: https://www.cdc.gov/vaccines-adults/hcp/imz-standards/index.html (accessed on 18 April 2025).
- Community Preventive Services Task Force. Increasing Appropriate Vaccination: Standing Orders. Task Force Finding and Rationale Statement. Available online: https://www.thecommunityguide.org/sites/default/files/assets/Vaccination-Standing-Orders.pdf (accessed on 18 April 2025).
- Goebel, L.J.; Neitch, S.M.; Mufson, M.A. Standing orders in an ambulatory setting increases influenza vaccine usage in older people. J. Am. Geriatr. Soc. 2005, 53, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Elia, S.; Perrett, K.; Newall, F. Providing opportunistic immunisations for at-risk inpatients in a tertiary paediatric hospital. J. Spec. Pediatr. Nurs. 2017, 22, E127. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; Minerva, M.; Dalla Valle, Z.; Odone, A.; Signorelli, C. Genesis and prospects of the shortage of specialist physicians in Italy and indicators of the 39 schools of hygiene and preventive medicine. Acta Biomed. 2023, 94, e2023159. [Google Scholar]
- Signorelli, C.; Pennisi, F.; Lunetti, C.; Blandi, L.; Pellissero, G.; Fondazione Sanità Futura, W.G. Quality of hospital care and clinical outcomes: A comparison between the Lombardy Region and the Italian national data. Ann. Ig. 2024, 36, 234–249. [Google Scholar]
- Signorelli, C.; Pennisi, F.; D’Amelio, A.C.; Conversano, M.; Cinquetti, S.; Blandi, L.; Rezza, G. Vaccinating in Different Settings: Best Practices from Italian Regions. Vaccines 2025, 13, 16. [Google Scholar] [CrossRef]
- Pennisi, F.; Mastrangelo, M.; De Ponti, E.; Cuciniello, R.; Mandelli, A.; Vaia, F.; Signorelli, C. The role of pharmacies in the implementation of vaccination cover- age in Italy. Insights from the preliminary data of the Lombardy Region. Ann. Ig. 2024, 36, 363–369. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).