Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector
Abstract
1. Introduction
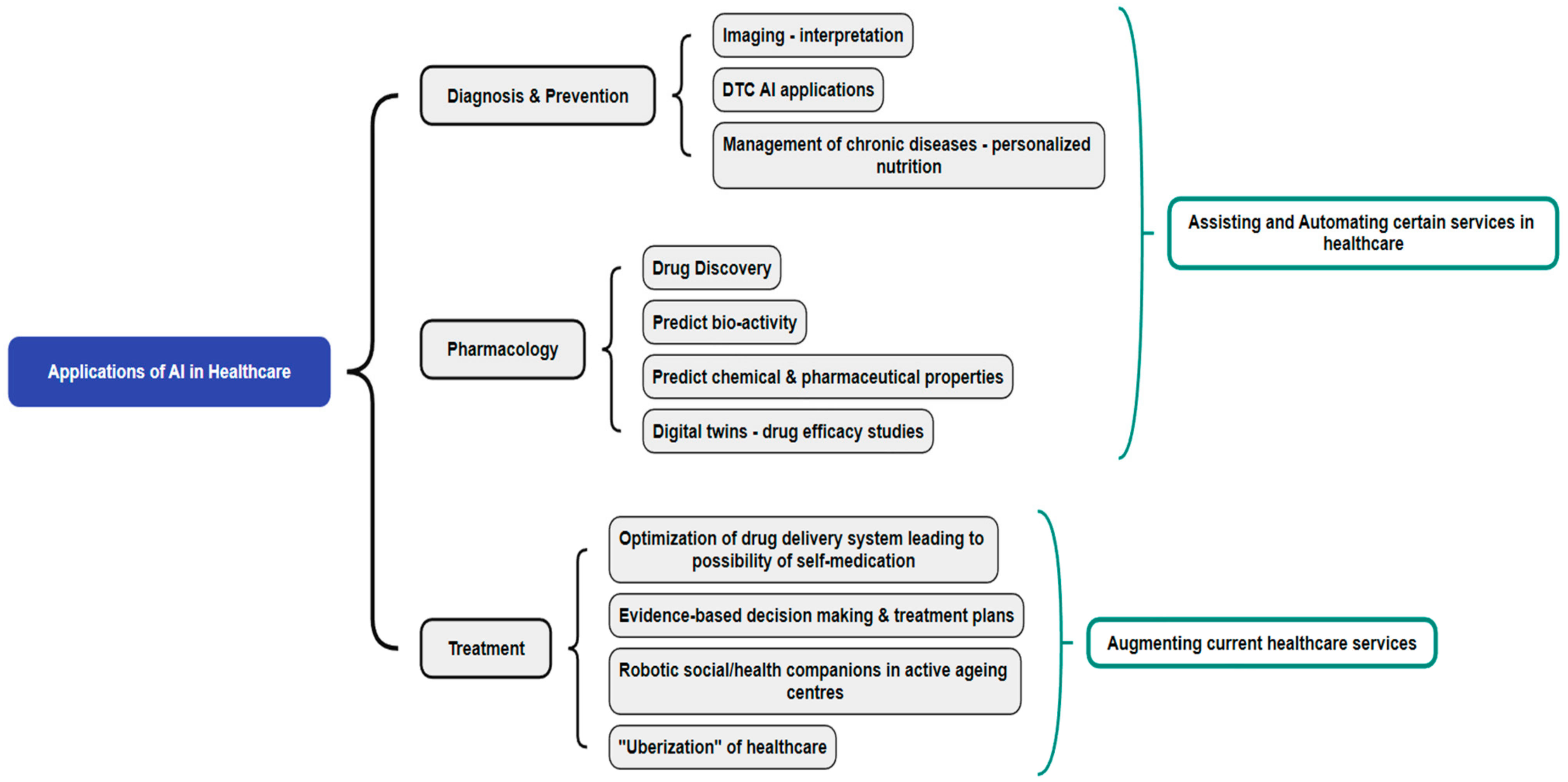
2. Role of AI in Healthcare Services
2.1. AI for Diagnosis and Prevention
2.2. AI in Pharmacology
2.3. AI for Treatment
3. Methodology
4. Existing Regulatory Landscape
4.1. United States of America (USA)
- Specific regulatory framework with the issuance of draft guidance on “Predetermined Change Control Plan”;
- Good machine learning practices;
- Patient-centric approach, including the transparency of devices to users;
- Methods for the elimination of ML algorithm bias and algorithm improvement;
- Real-world performance monitoring pilots.
- High relevance of available data to the clinical problem and current clinical practice;
- Consistency in data collection that does not deviate from the SaMD’s intended use;
- Planned modification pathway;
- Appropriate boundaries in the datasets used for training, tuning, and testing the AI algorithms;
- Transparency of the AI algorithms and their output for users [50].
4.2. United Kingdom (UK)
4.3. Europe
4.4. Australia
4.5. China
4.6. Brazil
4.7. Singapore
5. Limitations of This Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aung, Y.Y.M.; Wong, D.C.S.; Ting, D.S.W. The Promise of Artificial Intelligence: A Review of the Opportunities and Challenges of Artificial Intelligence in Healthcare. Br. Med. Bull. 2021, 139, 4–15. [Google Scholar] [CrossRef]
- WHO. Global Strategy on Human Resources for Health: Workforce 2030; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-151113-1. [Google Scholar]
- Howse, E.; Rychetnik, L.; Marks, L.; Wilson, A. What Does the Future Hold for Chronic Disease Prevention Research? Aust. N. Z. J. Public Health 2020, 44, 336–340. [Google Scholar] [CrossRef]
- Meskó, B.; Hetényi, G.; Győrffy, Z. Will Artificial Intelligence Solve the Human Resource Crisis in Healthcare? BMC Health Serv. Res. 2018, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Britannica, E. Artificial Intelligence (AI)|Definition, Examples, Types, Applications, Companies, & Facts. Available online: https://www.britannica.com/technology/artificial-intelligence (accessed on 5 February 2024).
- Google Cloud AI vs. Machine Learning: How Do They Differ? Available online: https://cloud.google.com/learn/artificial-intelligence-vs-machine-learning (accessed on 13 February 2024).
- Loh, H.W.; Ooi, C.P.; Seoni, S.; Barua, P.D.; Molinari, F.; Acharya, U.R. Application of Explainable Artificial Intelligence for Healthcare: A Systematic Review of the Last Decade (2011–2022). Comput. Methods Programs Biomed. 2022, 226, 107161. [Google Scholar] [CrossRef]
- Meskó, B.; Topol, E.J. The Imperative for Regulatory Oversight of Large Language Models (or Generative AI) in Healthcare. npj Digit. Med. 2023, 6, 120. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Allan, S.; Coghlan, S.; Cooper, P. A Governance Model for the Application of AI in Health Care. J. Am. Med. Inform. Assoc. 2020, 27, 491–497. [Google Scholar] [CrossRef]
- Monlezun, D.J. Chapter 2—AI+healthcare Systems: Efficiency and Equity. In The Thinking Healthcare System; Monlezun, D.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 37–67. ISBN 978-0-443-18906-7. [Google Scholar]
- NHS The Topol Review—NHS Health Education England. Available online: https://topol.hee.nhs.uk/ (accessed on 28 February 2023).
- Mori, Y.; Kudo, S.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann. Intern. Med. 2018, 169, 357. [Google Scholar] [CrossRef]
- Holme, Ø.; Aabakken, L. Making Colonoscopy Smarter With Standardized Computer-Aided Diagnosis. Ann. Intern. Med. 2018, 169, 409. [Google Scholar] [CrossRef] [PubMed]
- Babic, B.; Gerke, S.; Evgeniou, T.; Cohen, I.G. Direct-to-Consumer Medical Machine Learning and Artificial Intelligence Applications. Nat. Mach. Intell. 2021, 3, 283–287. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal Trial of an Autonomous AI-Based Diagnostic System for Detection of Diabetic Retinopathy in Primary Care Offices. npj Digit. Med. 2018, 1, 39. [Google Scholar] [CrossRef]
- Apple Take an ECG with the ECG App on Apple Watch. Available online: https://support.apple.com/en-us/HT208955 (accessed on 25 July 2023).
- Lu, M.; Yin, J.; Zhu, Q.; Lin, G.; Mou, M.; Liu, F.; Pan, Z.; You, N.; Lian, X.; Li, F.; et al. Artificial Intelligence in Pharmaceutical Sciences. Engineering, 2023; in press. [Google Scholar] [CrossRef]
- Ahneman, D.T.; Estrada, J.G.; Lin, S.; Dreher, S.D.; Doyle, A.G. Predicting Reaction Performance in C–N Cross-Coupling Using Machine Learning. Science 2018, 360, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Built in Merck. Available online: https://builtin.com/company/merck (accessed on 13 February 2024).
- Chen, Y.; Yang, O.; Sampat, C.; Bhalode, P.; Ramachandran, R.; Ierapetritou, M. Digital Twins in Pharmaceutical and Biopharmaceutical Manufacturing: A Literature Review. Processes 2020, 8, 1088. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Hu, Q. Emerging Self-Regulated Micro/Nano Drug Delivery Devices: A Step Forward towards Intelligent Diagnosis and Therapy. Nano Today 2021, 38, 101127. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, C. Potential Next-Generation Medications for Self-Administered Platforms. J. Control. Release 2022, 342, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Pieczynski, J.; Kuklo, P.; Grzybowski, A. The Role of Telemedicine, In-Home Testing and Artificial Intelligence to Alleviate an Increasingly Burdened Healthcare System: Diabetic Retinopathy. Ophthalmol. Ther. 2021, 10, 445–464. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.A.; Topol, E.J. With an Eye to AI and Autonomous Diagnosis. npj Digit. Med. 2018, 1, 40. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P. ChatGPT for Healthcare Services: An Emerging Stage for an Innovative Perspective. BenchCouncil Trans. Benchmarks Stand. Eval. 2023, 3, 100105. [Google Scholar] [CrossRef]
- Lee, C.-H.; Liu, C.-L.; Trappey, A.J.C.; Mo, J.P.T.; Desouza, K.C. Understanding Digital Transformation in Advanced Manufacturing and Engineering: A Bibliometric Analysis, Topic Modeling and Research Trend Discovery. Adv. Eng. Inform. 2021, 50, 101428. [Google Scholar] [CrossRef]
- Costa, A.; Martinez-Martin, E.; Cazorla, M.; Julian, V. PHAROS—PHysical Assistant RObot System. Sensors 2018, 18, 2633. [Google Scholar] [CrossRef]
- Clare & Me Answering the Most Frequent Questions and Our AI Mental Health Solution. Available online: https://www.clareandme.com/faq#faq-row-2 (accessed on 13 February 2024).
- David, P. ORA Raises $10 Million as Demand for Tele-Health in Asia Soars. Available online: https://www.forbes.com/sites/davidprosser/2023/05/16/ora-raises-10-million-as-demand-for-tele-health-in-asia-soars/?sh=67968e4e4a13 (accessed on 13 February 2024).
- Jourdan, A. AI Ambulances and Robot Doctors: China Seeks Digital Salve to Ease Hospital Strain. Reuters Publication London. 2018. Available online: https://www.reuters.com/article/idUSKBN1JO1V1/ (accessed on 8 February 2023).
- Tencent Bridging Gaps in Healthcare Industry with Technology. Available online: https://www.tencent.com/en-us/articles/2200933.html (accessed on 8 February 2023).
- Forkast How Baidu, Alibaba and Tencent Aim to Disrupt Chinese Health Care. Available online: https://forkast.news/baidu-alibaba-tencent-china-health-care-blo/ (accessed on 8 February 2023).
- MobiHealthNews a Look Back at Alphabet’s Moves in 2019. Available online: https://www.mobihealthnews.com/news/look-back-alphabets-moves-2019 (accessed on 8 February 2023).
- Ping an Healthcare Ecosystem. Available online: https://group.pingan.com/about_us/our_businesses/health-care-ecosystem.html (accessed on 22 November 2023).
- Shijia, O. Baidu to Offer AI-Powered Patient-Doctor Matching Service. Available online: https://global.chinadaily.com.cn/a/202107/14/WS60eea64ea310efa1bd661e69.html (accessed on 22 November 2023).
- Built in DispatchHealth Careers, Perks + Culture. Available online: https://frontend.builtin.com/3cb8c7ae696a9e1ec4537e9df7c387cdb10f8ef7/company/dispatchhealth (accessed on 13 February 2024).
- Built in DearDoc. Available online: https://builtin.com/company/deardoc (accessed on 13 February 2024).
- Matheny, M.; Israni, S.T.; Ahmed, M.; Whicher, D. Artificial Intelligence in Health Care: The Hope, the Hype, the Promise, the Peril; National Academy of Medicine: Washington, DC, USA, 2019. [Google Scholar]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Blumen, H.; Fitch, K.; Polkus, V. Comparison of Treatment Costs for Breast Cancer, by Tumor Stage and Type of Service. Am. Health Drug Benefits 2016, 9, 23–32. [Google Scholar]
- Markets and Markets Artificial Intelligence (AI) in Healthcare Market Size, Growth Report Analysis 2031. Available online: https://www.marketsandmarkets.com/Market-Reports/artificial-intelligence-healthcare-market-54679303.html (accessed on 27 November 2023).
- Essén, A.; Stern, A.D.; Haase, C.B.; Car, J.; Greaves, F.; Paparova, D.; Vandeput, S.; Wehrens, R.; Bates, D.W. Health App Policy: International Comparison of Nine Countries’ Approaches. npj Digit. Med. 2022, 5, 31. [Google Scholar] [CrossRef]
- Chatila, R.; Havens, J.C. The IEEE Global Initiative on Ethics of Autonomous and Intelligent Systems. In Robotics and Well-Being; Aldinhas Ferreira, M.I., Silva Sequeira, J., Singh Virk, G., Tokhi, M.O., E. Kadar, E., Eds.; Intelligent Systems, Control and Automation: Science and Engineering; Springer International Publishing: Cham, Switzerland, 2019; Volume 95, pp. 11–16. ISBN 978-3-030-12523-3. [Google Scholar]
- Jin, M.L.H.; Vogel, S.; Kitikiti, N.; Muthalagu, P.A. Artificial Intelligence in Healthcare: Landscape, Policies and Regulations in Asia-Pacific; NUS Initiative to Improve Health in Asia (NIHA) Report; Duke-NUS Medical School: Singapore, 2020; Available online: https://www.duke-nus.edu.sg/core/think-tank/news/publications/artificial-intelligence-in-healthcare-landscape-policies-and-regulations-in-asia-pacific (accessed on 27 February 2023).
- Tsang, L.; Kracov, D.A.; Mulryne, J.; Strom, L.; Perkins, N.; Dickinson, R.; Wallace, V.M.; Jones, B. The Impact of Artificial Intelligence on Medical Innovation in the European Union and United States. Intellect. Prop. Technol. Law J. 2017, 29, 3. [Google Scholar]
- Taeihagh, A. Governance of Artificial Intelligence. Policy Soc. 2021, 40, 137–157. [Google Scholar] [CrossRef]
- MOH; HSA. IHis MOH Artificial Intelligence in Healthcare Guidelines (AIHGle) 2021; MOH: Singapore, 2021. Available online: https://www.moh.gov.sg/licensing-and-regulation/artificial-intelligence-in-healthcare (accessed on 31 August 2023).
- Muehlematter, U.J.; Daniore, P.; Vokinger, K.N. Approval of Artificial Intelligence and Machine Learning-Based Medical Devices in the USA and Europe (2015–20): A Comparative Analysis. Lancet Digit. Health 2021, 3, e195–e203. [Google Scholar] [CrossRef]
- Harvey, H.B.; Gowda, V. How the FDA Regulates AI. Acad. Radiol. 2020, 27, 58–61. [Google Scholar] [CrossRef]
- FDA. Proposed Regulatory Framework for Modifications to Artificial Intelligence/Machine Learning-Based Software as a Medical Device 2019; FDA: Silver Spring, MD, USA, 2019. Available online: https://www.fda.gov/files/medical%20devices/published/US-FDA-Artificial-Intelligence-and-Machine-Learning-Discussion-Paper.pdf (accessed on 27 February 2023).
- Chhaya, V.; Khambholja, K. The SaMD Regulatory Landscape in the US and Europe. Regul. Focus 2021. Available online: https://www.raps.org/news-and-articles/news-articles/2021/8/the-samd-regulatory-landscape-in-the-us-and-eu-1 (accessed on 27 February 2023).
- FDA. Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan 2021; FDA: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device (accessed on 31 March 2023).
- Evidence Standards Framework (ESF) for Digital Health Technologies. Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (accessed on 24 February 2023).
- Darzi, A. The Regulation of Artificial Intelligence as a Medical Device; Regulatory Horizons Council: London, UK, 2022.
- European Commission. Ethics Guidelines for Trustworthy AI|Shaping Europe’s Digital Future. Available online: https://digital-strategy.ec.europa.eu/en/library/ethics-guidelines-trustworthy-ai (accessed on 24 February 2023).
- European Commission. Policy and Investment Recommendations for Trustworthy Artificial Intelligence|Shaping Europe’s Digital Future. Available online: https://digital-strategy.ec.europa.eu/en/library/policy-and-investment-recommendations-trustworthy-artificial-intelligence (accessed on 24 February 2023).
- European Commission. Communication on Fostering a European Approach to Artificial Intelligence|Shaping Europe’s Digital Future. Available online: https://digital-strategy.ec.europa.eu/en/library/communication-fostering-european-approach-artificial-intelligence (accessed on 24 February 2023).
- Meszaros, J.; Minari, J.; Huys, I. The Future Regulation of Artificial Intelligence Systems in Healthcare Services and Medical Research in the European Union. Front. Genet. 2022, 13, 927721. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rodríguez, N.; Del Ser, J.; Coeckelbergh, M.; López de Prado, M.; Herrera-Viedma, E.; Herrera, F. Connecting the Dots in Trustworthy Artificial Intelligence: From AI Principles, Ethics, and Key Requirements to Responsible AI Systems and Regulation. Inf. Fusion 2023, 99, 101896. [Google Scholar] [CrossRef]
- European Commission. The Act. Available online: https://artificialintelligenceact.eu/the-act/ (accessed on 27 February 2023).
- The EU Newsletter. The Artificial Intelligence Act. 2021. Available online: https://artificialintelligenceact.eu/ (accessed on 27 February 2023).
- Kenny, L.M.; Nevin, M.; Fitzpatrick, K. Ethics and Standards in the Use of Artificial Intelligence in Medicine on Behalf of the Royal Australian and New Zealand College of Radiologists. J. Med. Imaging Radiat. Oncol. 2021, 65, 486–494. [Google Scholar] [CrossRef] [PubMed]
- TGA. Regulatory Changes for Software Based Medical Devices; TGA: Woden, Australia, 2021. Available online: https://www.tga.gov.au/resources/resource/guidance/regulatory-changes-software-based-medical-devices (accessed on 31 March 2023).
- Song, X.; Hu, M.; Li, B.; Zhang, K.; Zhang, X.; Wang, L. Advancing Medical Device Regulatory Reforms for Innovation, Translation and Industry Development in China. J. Orthop. Transl. 2022, 37, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Yu, T. Three Guidelines Published Today, Propelling China to Be World Leader in Digital Health; China Med Device: Cambridge, MA, USA, 2022; Available online: https://chinameddevice.com/digital-health-nmpa-ai/ (accessed on 24 February 2023).
- da Conceição, L.H.M.; Perrone, C. The Brazilian Proposed Regulation of AI: Contextualization and Perspectives. MediaLaws 2022. Available online: https://www.medialaws.eu/the-brazilian-proposed-regulation-of-ai-contextualization-and-perspectives/ (accessed on 27 February 2023).
- Quathem, E.S. Anna Oberschelp de Meneses, Nicholas Shepherd, Kristof Van Brazil’s Senate Committee Publishes AI Report and Draft AI Law. Available online: https://www.insideprivacy.com/emerging-technologies/brazils-senate-committee-publishes-ai-report-and-draft-ai-law/ (accessed on 27 February 2023).
- NRF. National Artificial Intelligence Strategy; Smart Nation and Digital Government Office: Singapore, 2019. Available online: https://www.smartnation.gov.sg/files/publications/national-ai-strategy.pdf (accessed on 2 January 2024).
- PDPC. Singapore’s Approach to AI Governance. Available online: https://www.pdpc.gov.sg/Help-and-Resources/2020/01/Model-AI-Governance-Framework (accessed on 2 January 2024).
- Murray, L.E.; Barnes, M. Policy Brief: Proposal for a US-EU AI Code of Conduct 2023; CED: New York, NY, USA, 2023; Available online: https://www.ced.org/pdf/CED_Policy_Brief_US-EU_AI_Code_of_Conduct_FINAL.pdf (accessed on 4 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniappan, K.; Lin, E.Y.T.; Vogel, S. Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare 2024, 12, 562. https://doi.org/10.3390/healthcare12050562
Palaniappan K, Lin EYT, Vogel S. Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare. 2024; 12(5):562. https://doi.org/10.3390/healthcare12050562
Chicago/Turabian StylePalaniappan, Kavitha, Elaine Yan Ting Lin, and Silke Vogel. 2024. "Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector" Healthcare 12, no. 5: 562. https://doi.org/10.3390/healthcare12050562
APA StylePalaniappan, K., Lin, E. Y. T., & Vogel, S. (2024). Global Regulatory Frameworks for the Use of Artificial Intelligence (AI) in the Healthcare Services Sector. Healthcare, 12(5), 562. https://doi.org/10.3390/healthcare12050562




