Abstract
Background: Healthcare-associated infections are infections that patients acquire during hospitalization or while receiving healthcare in other facilities. They represent the most frequent negative outcome in healthcare, can be entirely prevented, and pose a burden in terms of financial and human costs. With the development of new AI and ML algorithms, hospitals could develop new and automated surveillance and prevention models for HAIs, leading to improved patient safety. The aim of this review is to systematically retrieve, collect, and summarize all available information on the application and impact of AI in HAI surveillance and/or prevention. Methods: We conducted a systematic review of the literature using PubMed and Scopus to find articles related to the implementation of artificial intelligence in the surveillance and/or prevention of HAIs. Results: We identified a total of 218 articles, of which only 35 were included in the review. Most studies were conducted in the US (n = 10, 28.6%) and China (n = 5; 14.3%) and were published between 2021 and 2023 (26 articles, 74.3%) with an increasing trend over time. Most focused on the development of ML algorithms for the identification/prevention of surgical site infections (n = 18; 51%), followed by HAIs in general (n = 9; 26%), hospital-acquired urinary tract infections (n = 5; 9%), and healthcare-associated pneumonia (n = 3; 9%). Only one study focused on the proper use of personal protective equipment (PPE) and included healthcare workers as the study population. Overall, the trend indicates that several AI/ML models can effectively assist clinicians in everyday decisions, by identifying HAIs early or preventing them through personalized risk factors with good performance. However, only a few studies have reported an actual implementation of these models, which proved highly successful. In one case, manual workload was reduced by nearly 85%, while another study observed a decrease in the local hospital’s HAI incidence from 1.31% to 0.58%. Conclusions: AI has significant potential to improve the prevention, diagnosis, and management of healthcare-associated infections, offering benefits such as increased accuracy, reduced workloads, and cost savings. Although some AI applications have already been tested and validated, adoption in healthcare is hindered by barriers such as high implementation costs, technological limitations, and resistance from healthcare workers. Overcoming these challenges could allow AI to be more widely and cost-effectively integrated, ultimately improving patient care and infection management.
1. Introduction
Previously known as “nosocomial infections”, healthcare-associated infections (HAIs) are defined by the World Health Organization (WHO) as infections that patients acquire during medical or surgical treatment in hospitals or while receiving healthcare in other facilities (e.g., long-term care, family medicine clinics, home care, and ambulatory care), which were not evident or incubating at the time of admission [1]. These infections are among the most common adverse outcomes in healthcare, despite being largely preventable, and are often used as a measure of healthcare quality [2]. HAIs endanger patient safety, causing unnecessary deaths, prolonged hospitalizations, long-term disabilities, and increased antibiotic resistance among bacteria. This, in turn, leads to significant extra costs and financial strain on both the healthcare system and the patients’ families [2]. Assessing the impact of HAIs is challenging due to a lack of high-quality data, but it is believed that 2.6 million HAI cases occur annually in the European Union and European Economic Area, resulting in roughly 2.5 million disability-adjusted life years (DALYs) [3]. In the United States, the US Center for Disease Control and Prevention (CDC) reports that nearly 1.7 million hospitalized patients contract HAIs each year while receiving treatment for other conditions, with over 98,000 of these cases—approximately 1 in 17—resulting in death [4]. These data place HAIs among the top ten leading causes of mortality in the country [4]. On a global scale, 1 in every 10 patients is affected by HAIs [1]. Out of every 100 patients, 7 in advanced countries and 10 in emerging countries may acquire an HAI, especially those admitted to intensive care units [1]. The economic impact of HAIs is considerable, with costs estimated at 28-to-45 billion USD per year in the United States [4], and around 7 billion EUR annually in Europe, though the latter figure may be significantly underestimated [5].
Given the significant economic and clinical burden that these infections impose on society, the goal should be to prevent avoidable infections by employing effective surveillance strategies for infection prevention and control. A 2014 study revealed that infection preventionists—professionals dedicated to ensuring healthcare workers and patients adhere to infection prevention protocols—spent on average about half of their work hours on surveillance-related activities [6]. There are still multiple limitations in HAI surveillance, as its accuracy heavily depends on the training of infection control practitioners and is prone to subjective interpretation and surveillance bias.
A report by the European Centre for Disease Prevention and Control (ECDC) on HAIs and antimicrobial use in European acute care hospitals during 2022–2023 highlights a varying degree of automation in HAI surveillance across Europe. The percentage of hospitals with any level of automated HAI surveillance ranged from 0 to 20% in the Balkan region, 20 to 40% in countries like Germany and Slovakia, and over 80% in Finland and up to 100% in Iceland [7]. Currently, the most frequently targeted HAIs for automated surveillance are Clostridium difficile infections and bloodstream infections, with the highest automation rates at 36.3% and 36.7%, respectively, while hospital-acquired pneumonia had the lowest rate at 30.4%. The feasibility of implementing automated surveillance was also assessed by determining whether key variables or data sources for automation were available in digital format, and if so whether they were structured and well defined. Key administrative data (e.g., admission and discharge dates) were most likely to be available in structured digital formats, while data on the use of invasive devices (e.g., mechanical ventilation) had the lowest availability, with only 57.6% of hospitals reporting such data in digital form [7].
The rise of artificial intelligence (AI) and all its subsets, especially machine learning (ML), has the potential to improve and even revolutionize healthcare, thanks to the medical and technological advancements in the last decades and the availability of data coming from the increasingly widespread electronic health records (HERs). Some of the already tested applications of AI in healthcare that have proven useful include the detection of clinical conditions in medical imaging and diagnostic services (providing a reduction in diagnostic errors). In neurology and neurosurgery, for example, AI can improve risk management and surgical decision making by predicting postoperative complications such as infections, bleeding, and neurological deficits, thereby enhancing patient safety [8]. Additionally, numerous AI models can forecast stroke risk and reduce stroke-related deaths and disability burdens by analyzing various health feature analyses into an ensemble machine (Neuro-Health Guardian) [9].
Other benefits include improving patient compliance and engagement, reducing the time professionals spend on administrative duties, aiding in the development of new drugs, and, during the COVID-19 outbreak, enabling early diagnosis, patient monitoring, and management through virtual patient care [10]. Artificial intelligence has the potential to enhance productivity and improve the quality of care in two main ways: information synthesis, as the amount and complexity of data (e.g., patient data from electronic health records, gene sequencing, or medical literature) is overwhelming for a human operator to handle alone; and enhancement of human performance, by helping clinicians track and analyze all available information [11].
The previously mentioned substantial economic and clinical burden of HAIs underscores the urgent need for effective infection prevention and control strategies. Monitoring HAIs is essential for developing, implementing, and sustaining effective infection prevention and control programs. It is precisely in this context that AI offers a promising tool.
This review aims to systematically retrieve, collect, and summarize all available information on the application and impact of AI in HAI surveillance over the past decade and assess where and how AI and its various subsets (especially ML) have been trained and implemented and evaluate their performance.
Advancements in AI are not only improving diagnostic and management capabilities in healthcare but also showing promise in addressing ongoing challenges, such as HAIs.
2. Materials and Methods
A systematic literature review was conducted according to the Supplementary Materials PRISMA 2020 guidelines. PubMed and Scopus were used to find articles related to the implementation of artificial intelligence in the surveillance and/or prevention of HAIs. The aim was to synthesize the main data published in the literature over the past ten years.
In the identification phase, the following combinations of keywords found within the titles and/or abstracts of the articles were used for PubMed: (ai OR artificial intelligence OR machine learning) AND healthcare-associated infections; (ai OR artificial intelligence) AND healthcare-associated infections; (ai OR artificial intelligence OR machine learning) AND nosocomial infections; (ai OR artificial intelligence) AND nosocomial infections; (ai OR artificial intelligence) AND surgical site infection; (ai OR artificial intelligence OR machine learning) AND hospital-acquired urinary tract infections; and (ai OR artificial intelligence) AND hospital-acquired pneumonia.
For Scopus, the following search strings were used: TITLE-ABS (ai) OR TITLE-ABS (artificial AND intelligence) AND TITLE-ABS (healthcare AND associated AND infections) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)); TITLE-ABS (ai) OR TITLE-ABS (artificial AND intelligence) AND TITLE-ABS (surgical AND site AND infections) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)); TITLE-ABS (ai) OR TITLE-ABS (artificial AND intelligence) AND TITLE-ABS (hospital AND acquired AND urinary AND tract AND infections) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)); and TITLE-ABS (ai) OR TITLE-ABS (artificial AND intelligence) AND TITLE-ABS (hospital AND acquired AND pneumonia) AND PUBYEAR > 2013 AND PUBYEAR < 2025 AND (LIMIT-TO (SUBJAREA, “MEDI”)) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)).
2.1. Criteria for Including/Excluding Studies
Articles in English, published in the last ten years (February 2014–February 2024), were included if the full text was available and they (1) presented the implementation of artificial intelligence or machine learning in the surveillance and/or prevention of HAIs; (2) reported original data.
2.2. Characteristics of Eligible Studies
A total of 218 articles were identified. Before the screening phase, duplicates were removed (n = 48). Subsequently, 170 articles were subjected to the screening phase: 116 articles were excluded as they were deemed irrelevant based on the title and/or abstract review, 15 were reviews, and 4 articles did not have the full-text version available. S.D.M. and M.C. were tasked with the assessment of the eligibility of the studies.
2.3. Quality Assessment and Risk of Bias
S.D.M. and M.C. independently assessed the abstracts and subsequently the full texts of the articles included in the review. The primary risk of bias was associated with the selection of the keywords for the search. No significant disagreements arose regarding the inclusion or exclusion of the articles and all studies included were considered of high quality and complete.
3. Results
A total of 35 articles were included in the systematic review. The flowchart in Figure 1 summarizes the flow of information through the different phases of the systematic review.
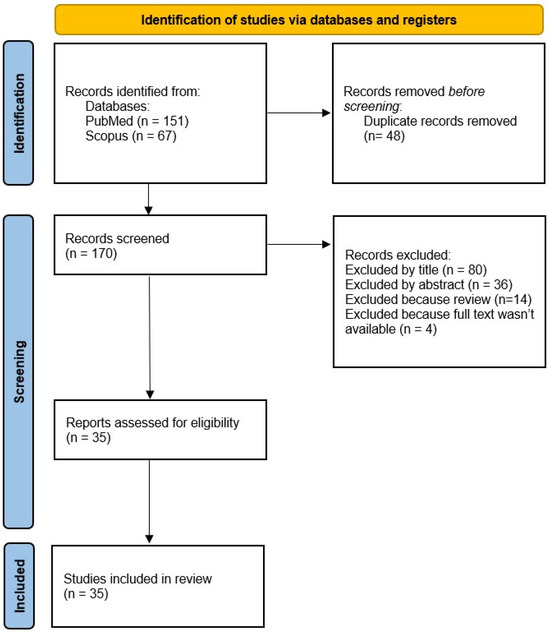
Figure 1.
PRISMA flow diagram.
3.1. Data Extraction
The collected data are summarized in Table 1. The essential information includes the study setting and population, with its objectives and conclusions (such as performance, e.g., the area under the receiver operator curve, area under the curve, accuracy, sensitivity, specificity, and other findings), the specific infection targeted for prevention or surveillance, as well as the methodology used for AI training (including sample size, study duration, analyzed variables, and dataset source).

Table 1.
Characteristics of the studies included in the review.
The articles included in the review were conducted across various countries. Most studies were from the USA [12,13,14,15,16,17,18,19,20,21] (n = 10, 28.6%), followed by China [22,23,24,25,26,27] with six studies (14.3%), and Denmark [28,29,30,31] with four studies (11.4%). Italy [32,33,34] and Canada [35,36,37] each contributed three studies. Brazil [38,39] and Japan [40,41] each provided two, and South Korea [42], Pakistan [43], Taiwan [44], the United Kingdom [45], and Spain [46] each had one study.
The included studies were published between 2017 and 2024, with the majority of them published between 2021 and 2023 (26 articles, 74.3%).
More than half of the articles (n = 22, 62.9%) did not specify the department where the study was conducted and gathered data from patients across the entire hospital. The departments most frequently involved in the studies were surgical units (n = 6, 17.1%), especially general surgery, orthopedics, and gynecology, as well as the intensive care unit (n = 4, 11.4%). Only two studies were conducted in pediatric departments, and one study focused on a psychiatric hospital. In most of the studies reviewed (n = 14, 40%), the data came from patients who had undergone some type of surgery (especially colon surgery, n = 4, 11.4%). In nine articles, however, the study population included all patients admitted to a particular hospital during a specific period, regardless of their conditions or departments. Interestingly, in only one instance, the study focused on the hospital staff to assess how proper handwashing and the procedures for putting on and taking off personal protective equipment affected the rate of hospital-acquired infections [27].
Most of the data used to train the AI and its different models came from internal sources within the hospital, such as electronic and paper health records, clinical or surgical notes, and lab results of the patient subjects of the study (n = 28, 80%), in some cases even using an AI model (natural language processing—NLP) to retrieve said data from clinical notes [12]. In the remaining cases, the data came from a combination of internal records and publicly available databases, or exclusively from the latter. These included resources like the MIMIC dataset, the eICU Collaborative Research Database, and the American College of Surgeons National Quality Improvement Program database [40].
3.2. Types of HAIs
To improve clarity and facilitate understanding, the studies and their outcomes have been categorized by HAI type: surgical site infections (SSIs), healthcare-associated pneumonia (HCAP), hospital-acquired urinary tract infections (HA-UTIs), and miscellaneous HAIs. Figure 2 displays the number of studies conducted for each infection type.
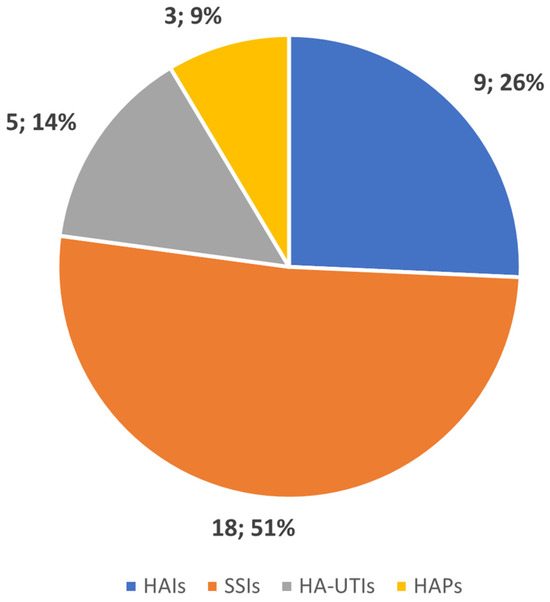
Figure 2.
Number of studies conducted by type of HAI.
3.2.1. Surgical Site Infections—SSIs
Surgical site infections are among the most common types of hospital-acquired infections, being the most frequent infection afflicting surgical patients, accounting for nearly 20% of all HAIs in European hospitals. SSIs are the costliest type of HAI, increasing the financial burden of surgery by leading to longer hospital stays, additional diagnostic tests, treatments, and frequently the need for further surgical procedures [47,48]. It is not surprising, then, that our review of the literature found that most studies involving AI, especially those using ML, were focused on developing predictive models for the early detection and prevention of SSIs (18 studies, 51% of the total articles reviewed).
SSIs following colorectal surgery received particular attention [9,18,38,39]. Sohn et al. [12] developed an automated Bayesian network system that uses risk factors from the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) and data extracted from clinical notes of surgical procedures using an NLP model. The system was able to identify SSIs with a Receiver Operating Characteristic (ROC) of 0.827, which increased to 0.892 when surgeons helped the AI to identify clinically meaningful SSIs.
Another team [30] developed a natural language processing (NLP) model able to read electronic health record chart notes and predict superficial surgical site infections in the postoperative period. By processing a vast amount of data—389,865 surgical cases, 3,983,864 unlabeled chart notes, and 1,231,656 labeled notes—the stand-alone ML model achieved a sensitivity of 0.604, a specificity of 0.996, a positive predictive value (PPV) of 0.763, and a negative predictive value (NPV) of 0.991. When a human-in-the-loop pipeline was introduced, some values improved, such as sensitivity and NPV (increasing to 0.854 and 0.997, respectively), while specificity and PPV decreased (specificity dropped to 0.987, PPV to 0.603). Despite this, the human-in-the-loop approach was still more cost-effective and less time-consuming than the manual curation. Da Silva et al. [38] also used a similar ML system, using text mining from operative and postoperative reports to predict the risk of infections, and for their identification achieving good results with the Stochastic Gradient Descent model, achieving an ROC-AUC of 79.7% for prediction and the logistic regression model reaching an ROC-AUC of 80.6% for detection. Cho et al. [42] developed and tested several ML models to detect SSIs and discovered that integrating a rule-based algorithm with a ML algorithm and using 19 variables extracted from 1652 surgical cases, instead of the original 29, significantly improved SSI surveillance after colon surgery, as the combination reduced the need for manual chart reviews (−83.9%) while maintaining a high sensitivity.
Three separate articles [14,23,26] shared the common goal of preventing SSIs in patients undergoing some type of spinal surgery. Wang and collaborators [23] developed and validated a supervised Naïve Bayes algorithm that predicted the risk of infections in patients who underwent minimally invasive transforaminal lumbar interbody fusion, using only readily available data from 705 patients, achieving an AUC of 0.78. Hopkins et al. [14] developed a Deep Neural Network algorithm able to predict the risk of SSIs in patients undergoing spinal surgery, with a PPV of 92.56%. The algorithm also identified the top five risk factors—congestive heart failure, chronic pulmonary failure, hemiplegia/paraplegia, multilevel fusion, and cerebrovascular disease—and, surprisingly, even some protective factors. Finally, Liu and contributors [26] conducted a retrospective analysis of data from 288 patients who underwent spinal surgery and developed the most effective predictive algorithm, the XGBoost model, which achieved an AUC of 0.926.
In a study using both administrative and electronic medical records data from 27,360 surgical admissions (including 16,561 total knee arthroplasties and 10,799 total hip arthroplasties), Wu et al. [37] developed nine XGBoost machine learning models to automate SSI detection, distinguishing between superficial/deep incisional and organ space infections. The top model showed an impressive performance, with an ROC area under the curve (AUC) of 0.906 and a Precision–Recall (PR) AUC of 0.637, highlighting how effective machine learning algorithms can be in automating the detection of complex SSIs. Similarly, Flores-Balado et al. [46] worked on preventing post-hip replacement infections by creating a multivariable algorithm that used NLP and extreme gradient boosting to screen orthopedic patients and identify key SSI markers. The model, which was tested on data from 7444 surgeries, performed exceptionally well, achieving a sensitivity of 99.18%, a specificity of 91%, and a negative predictive value of 99.98%. When this system was integrated into the hospital’s routine, it notably reduced the time spent on surveillance (from 975 person-hours to 63.5 person-hours) and cut down the volume of manual reviews by 88.95%.
Focusing on both deep and superficial SSIs, Rafaqat et al. [43] aimed to develop a machine learning model capable of predicting both the type of SSI, as deep infections require more intensive treatment and higher costs, and the timing of when these infections would develop. The best model for predicting the type of infection was the extreme gradient boosting (XGBoost) univariate model, which achieved an AUC of 0.84 and a positive predictive value of 0.94. For predicting the week in which the SSI would develop, five out of twelve models reached the highest accuracy, each with an AUC of 0.74.
Additionally, other studies have worked on developing different AI models and ML algorithms to identify, and in some cases, predict and prevent, surgical site infections, mainly by analyzing data from electronic health records [18,20,22,34,35,36].
3.2.2. Healthcare-Associated Pneumonia—HCAP
Epidemiological data show that healthcare-associated pneumonia (HCAP) is the most dangerous nosocomial infection, being the deadliest and the second most frequent type of HAI. HCAP is subdivided into hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP), with the latter making up the majority of HCAP cases [48]. Prevention is therefore essential to avoid higher mortality rates and the increased costs associated with the impact on quality of life.
Kuo et al. [44] focused on this very aspect, developing a machine learning model to predict HAP in schizophrenic patients under anti-psychotic drugs, using 11 predictive factors. Of the seven models tested, the random forest model delivered the best results, with an AUC of 0.994. The C5.0 forest tree model also performed well, with an AUC of 0.993. The algorithm also identified the six major risk factors for developing the infection in these patients: medication dosage, clozapine use, duration of medication, changes in neutrophil and leukocyte counts, and drug–drug interactions.
Another important aspect of nosocomial infections is the presence of antibiotic-resistant microorganisms, which are particularly difficult to treat. Given the challenges clinicians face in selecting appropriate antibiotic therapy for methicillin-resistant Staphylococcus aureus (MRSA) and its potential to easily spread to other patients, Hirano et al. [40] developed a machine learning algorithm designed to predict MRSA infections in patients on mechanical ventilation. Using data extracted from the MIMIC-IV database, the XGBoost model was able to predict MRSA screening positivity with an AUROC of 0.89, a sensitivity of 0.98, and a positive predictive value of 0.65; the values regarding specificity were, however, low, only reaching 0.47.
Sophonsri et al. [19] also addressed the challenge of pneumonia in ventilated patients, focusing on identifying risk factors associated with the development and mortality of HCAPs. Their goal was to use this information to improve patient treatment and enhance antibiotic stewardship. The machine learning model they developed was based on data from 457 patients, subdivided by infection type (non-ventilated hospital-acquired pneumonia, ventilated hospital-acquired pneumonia, and ventilator-associated pneumonia). The model identified key risk factors for both the development of VAP (alcohol use disorder, APACHE II score at diagnosis, positive cultures for ESBL-Enterobacterales, and the need for vasopressor therapy before infection) and mortality (recent hospitalization within the last 30 days, active malignancy, isolation of ceftriaxone-resistant pathogens, or Pseudomonas aeruginosa and vasopressor therapy). Also, the AUC ROC values for mortality prediction in nvHABP, vHABP, and VABP models were 0.80, 0.78, and 0.83, respectively.
3.2.3. Hospital-Acquired Urinary Tract Infections—HA-UTI
Nosocomial urinary tract infections are the most common type of HAIs, making up 40–60% of all cases, typically caused by the use of urinary catheters, either urethral or suprapubic. [48,49]. These infections are often complicated, sometimes leading to urosepsis, and diagnosing them correctly can be challenging [49].
Most of the articles reviewed focus on the early identification of risk factors for developing UTIs immediately upon hospital admission, with the ultimate goal of predicting an individual patient’s risk of acquiring a HA-UTI before it occurs [13,24,28,29,31]. Jakobsen et al. [29,31] conducted two studies focusing on the early detection of UTIs within 24 h of hospital admission. The 2023 study [29] used a Deep Neural Network model, achieving AUCs of 0.758 and 0.746 on full and reduced datasets, respectively, while the 2024 [31] study applied seven machine learning algorithms, particularly Bayesian Networks, with AUC values ranging from 0.720 to 0.746. In Denmark, Møller and collaborators [28] developed two predictive models for HA-UTIs within 48 h of admission using decision trees. They used both admission data and historical records from 301,932 patients, with the models achieving a good performance, with an ROC of 0.81 for the admission model and 0.74 for the 48 h model. Zachariah et al. [13] developed two predictive models, neural networks and decision trees, for assessing UTI risk at admission. While they achieved good sensitivity, specificity, and negative predictive values, their models had low positive predictive values, with 3.5% for the decision tree and 4.9% for the neural network. Finally, a study by Zhu et al. [24] in China developed a predictive system for UTIs in bedridden post-stroke patients, with the most effective model being an ensemble-learning model that achieved an AUROC of 82.2% in internal validation and 80.8% in external validation.
3.2.4. Hospital-Acquired Infections—HAIs
Different AI and ML models have been developed and validated to make the detection of HAIs more automated and efficient, with the goal of reducing costs in terms of time, quality of life, and resources. Some approaches have focused on early identification at the time of hospital or ICU admission [17], including the risk assessment of multi-resistant bacterial colonization [32]. In other cases, the main focus was on patient monitoring, aiming to develop algorithms that could automatically and correctly identify the infection, improving the surveillance performance [39].
When a patient is infected with an HAI-causing agent, the high likelihood of these microorganisms being multi-drug resistant underscores the critical importance of proper antibiotic management. Bolton et al.’s main objective was the improvement of antimicrobial stewardship by developing a machine learning model to optimize and personalize the transition from intravenous (IV) antibiotics, which carry a higher risk of catheter-related infections, to oral antibiotics. Using data from approximately 10,000 hospital stays from two distinct datasets, 10 key features were identified to help determine the appropriate timing for this switch. The most effective model predicted when a patient could theoretically transition from IV to oral antibiotics, achieving an AUROC of 0.80 [45].
If an infection is not identified early, it may lead to bacteremia, which can then progress to sepsis; in such cases, timely detection of sepsis is crucial [50]. Lind et al. developed two machine learning models for the early detection of high-risk bacteremia leading to sepsis in vulnerable patients, with the full decision support tool achieving an AUC of 0.85 and the clinical factor-specific tool an AUC of 0.72 [16]. In the study conducted by Li et al., a random forest model identified the key negative prognostic factors associated with mortality in hospitalized patients suffering from an invasive candida infection alongside bacteremia, using data from 246 cases [25].
Intravascular devices, due to their direct access to the bloodstream and potential for prolonged use, are a major source of healthcare-associated bloodstream infections, which account for a significant portion of HAIs and carry a 10-to-20% mortality rate, along with an economic cost of approximately 40,000 USD per survivor [48]. Montella et al. utilized logistic regression and multi-layer perceptron models to predict which neonates were at risk of developing central line-associated bloodstream infection (CLABSI), while also identifying various risk factors leading to infection [33]. When a catheter-related infection is detected, clinicians face a critical decision: either save the catheter with antibiotic therapy or replace it. To support this decision-making process, Walker et al. developed four predictive models using machine learning algorithms to evaluate the individual risk of future CVC reinfections, helping a clinician choose between catheter salvage and replacement in pediatric patients [15]. A study from China [27] took a different approach by focusing on healthcare workers instead of patients. Huang et al. created an AI-powered system with a camera and speaker to monitor and improve the use of personal protective equipment and hand hygiene. Healthcare workers performed their tasks in front of the camera, and the AI reviewed the footage to check for proper practices, together with a human review. If any mistake was detected, a speaker alerted the staff and provided immediate feedback and training. After introducing this system, hospital infections dropped significantly from 1.31% in 2019 to 0.38% in 2022, and correct PPE use among 163 staff members improved from 52.12% to 98.14%.
4. Discussion
The analysis clearly highlights the potential of artificial intelligence and machine learning as valuable emerging tools for preventing and identifying healthcare-associated infections. HAIs pose a significant challenge to healthcare systems globally, impacting mortality and patient quality of life, and incurring substantial economic costs [2]. The integration of AI-based solutions can significantly mitigate these issues, as AI models can analyze extensive healthcare data using advanced algorithms to extract valuable clinical insights, while continuously improving their accuracy through adaptive learning and feedback [51].
Our review identified 35 articles where AI and ML were applied, with some focused on the prevention and others on the early identification of HAIs. In almost all cases, these methods demonstrated a strong performance in terms of AUROC, sensitivity, and specificity. A high sensitivity in detecting HAIs is preferred, especially for high-risk patients like those in the ICU or with compromised immune systems, to initiate prompt treatment and prevent complications such as sepsis. On the other hand, a high specificity is crucial for effective resource management, avoiding unnecessary treatments, and preventing antibiotic resistance. It ensures only true infection cases are treated and reported, maintaining accuracy in hospital metrics and avoiding inflated HAI rates. The AUROC score helps hospitals balance these competing priorities by evaluating the performance of AI-driven surveillance systems. A high AUROC value (closer to 1.0) reflects a system’s ability to accurately distinguish between infected and non-infected patients, allowing hospitals to fine-tune AI models for an optimal balance between sensitivity and specificity based on clinical and administrative needs.
We recorded an important heterogeneity between the articles themselves: most of the studies came from the United States and China, SSIs received much more attention than other infections, and, in some cases, the populations studied could be considered very “niche”.
Despite this heterogeneity, this review underscores that AI and ML models can significantly enhance the early and precise identification of high-risk patients, leading to more effective targeting of infection prevention measures in healthcare settings, ultimately reducing both incidence rates and associated costs. Firstly, the use of AI allowed for more efficient and accurate surveillance of infections, allowing infections to be identified early and preventive measures to be implemented quickly. Alternatively, AI could predict the risk of HAIs by identifying personalized risk factors for patients, thereby preventing the problem at its source. The adoption of automated surveillance systems has allowed AI to significantly reduce the workload of doctors and healthcare professionals by quickly processing large volumes of data and providing detailed analysis, compared to previous methods of manual monitoring [52]. Only a couple of studies reported an implementation of the developed models; however, when effectively implemented within the hospital department, AI and ML models effectively reduced manual workload [46], in one case by almost 85% [42], and reduced the incidence of HAI by 1.31% at 0.58% [27]. Another positive result achieved is the improvement of current early warning scores, such as SAPS II, improving the accuracy in predicting the mortality of these patients [32].
Thanks to their wide adoption, electronic health records (EHRs) are highly valuable due to the vast amount of data they store, which is essential for training AI and ML models, as demonstrated by the fact that almost every single study used data deriving from there. EHRs are far superior to administrative data, such as International Classification of Diseases (ICD) codes, which can often be unreliable and fail to consider clinical context [53]. A significant challenge, however, is the presence of unstructured data within EHRs such as clinical signs and symptoms. One effective approach to address this issue is using NLP ML models to analyze free text, which has proven particularly useful for identifying surgical site infections (SSIs) [12,30]. Another problem concerns the availability of EHRs, whose adoption is widespread especially in industrialized Western countries, while in developing countries their use is still limited mainly due to high costs and inadequate infrastructure [54].
AI can support monitoring and training on the correct use of PPE and hand washing, as highlighted in the study by Huang et al. [27] and in a review on the impact of intelligent environments and robots in preventing infections. However, the current instrumental limitations of AI and the poor compliance of healthcare workers, who may oppose daily tracking, still represent a challenge [55].
There are, however, numerous challenges and problems that need to be overcome in the coming years to fully harness the power of AI. Some challenges are purely technical in nature, including limitations of artificial intelligence models, and the need for a continuous flow of high-quality, complete, valid, and standardized data. The more that diagnostic/therapeutic algorithms differ from each other, the less practical data sharing between hospitals becomes, and the less valid the results obtained are.
The effectiveness of AI is constrained by the challenge of acquiring large, high-quality, and diverse datasets, as its performance is only as strong as the data used for training. Data completeness is also vital; for instance, automated surveillance in outpatient hemodialysis centers missed many bloodstream infections due to the absence of blood culture data in the dialysis EHRs. [53]. This is not to mention that accurate medical record documentation is essential for quality patient care, as the quality of medical records is closely linked to patient outcomes, while inaccuracies can compromise patient safety and increase the risk of malpractice [56].
Furthermore, the integration of health data in different settings is also an issue, as large-scale data sharing between healthcare facilities is essential but remains unachieved. Incomplete post-discharge surveillance can severely underreport HAIs. Linking EHRs across multiple inpatient and outpatient settings in the future could facilitate interfacility surveillance, increasing the detection of HAIs in non-rehospitalized patients or those readmitted elsewhere [53]. AI systems seem proficient at integrating diverse health data, such as clinical records, laboratory results, lifestyle information, and environmental factors; this enables healthcare providers to design more personalized and comprehensive treatment plans, improving the precision and effectiveness of care delivery [57]. However, biases in the data could skew AI models, leading to inaccurate treatment plans.
The size of the dataset is crucial: validating AI with small datasets can limit its ability to accurately differentiate between normal and abnormal variations and adequately address confounders, hindering the performance [58]. Unbalanced datasets (a high number of healthy people compared to infected ones) can pose an obstacle, as the data tend to be biased toward classifying the dominant class. In most cases analyzed, this problem was overcome by using a different machine learning algorithm or by applying techniques such as oversampling or undersampling [20].
Security and privacy represent significant challenges in AI implementation. AI systems are vulnerable to cyber-attacks, which can lead to misuse or fraud, making the safeguarding of these algorithms crucial as AI adoption grows. [58]. In the European Union, the General Data Protection Regulation (GDPR) places strict requirements on data ownership and consent, ensuring that patients control their own data. Explicit patient consent is required for the use and sharing of data, with full transparency about who has access, and how it will be stored, used, and protected [59].
The economic aspect of implementing AI also cannot be ignored. Although the costs in terms of financial expenditure are extremely high [4,5], to our knowledge no studies have analyzed the cost/benefit ratio of implementing AI in combating HAIs. Furthermore, none of the studies in this review indicated the costs for developing the AI models or how much the hospitals saved, nor any estimation on the cost of developing and applying AI in healthcare. It was predicted that the implementation of AI for diagnosis and treatment in hospitals would, over ten years, lead to savings of 15.17 h/day and 122.83 h/day. Translated in economic terms, savings would amount to 17,881 USD and 289,634 USD per day per hospital, respectively [60]. In other studies, estimated savings ranged from 200 billion USD to 360 billion USD in the United States [61]. However, it is important to consider the financial availability of smaller hospitals, which may struggle to cover the initial cost.
The question of determining liability in the use of AI is also particularly complicated, as clinical staff are traditionally accountable for their own decisions. The integration of AI into decision making introduces questions of accountability in cases of negative outcomes, potentially implicating clinicians, software developers, vendors, healthcare institutions, or regulators. The element of causation is highly case-specific; however, the inherent opacity of AI systems can pose significant challenges for patients trying to establish causation. This issue is further complicated by the difficulty of clearly explaining the algorithmic details to patients, which is often impractical or unfeasible [62]. Legal and ethical concerns surrounding these issues remain unresolved, with healthcare professionals currently held liable for decisions made with AI, even when they have limited understanding or control over the technology. Conversely, if a professional disregards AI recommendations and there is a resulting poor outcome, it could be viewed as clinical negligence [58]. The potential for serious complications arising from AI-driven decisions may discourage physicians, as they may be reluctant to take responsibility for outcomes associated with technologies they do not fully comprehend.
Poor availability or commitment of healthcare workers, due to limited time, low technological literacy, and, in some cases, reluctance to understand or use AI, could also lead to a slow or lack of adoption [58].
Definitions of healthcare-associated infections (HAIs) mainly focus on bacterial infections for epidemiological research, but other types of infections, particularly in the medico–legal context, require a clear causal link to healthcare settings. This distinction could impact the development of AI algorithms, as an infection could be classified as an HAI or not [63].
Finally, we highlight a potential problem in the development of an AI model in infection prevention in the form of a publication bias favoring “positive” results, as all analyzed studies show a decline in hospital-acquired infection rates. Consequently, suboptimal outcomes from AI models may go unpublished, leading to a distorted view of AI’s effectiveness in this area.
5. Conclusions
While AI cannot completely replace clinicians yet, the value of AI technology cannot be ignored. The integration of AI and ML into healthcare offers significant opportunities to improve the prevention and identification of healthcare-associated infections. The potential benefits regarding prevention, surveillance, diagnostic and therapeutic accuracy, reduction in the workload of healthcare personnel, and economic savings have been well described and, in some cases, tested and validated in the field. Despite this theoretical and practical potential, the application of AI in the field of hospital infections and healthcare in general still faces numerous barriers that hinder the adoption of these advanced technologies. The hope is that, in the years to come, many of these limitations will be overcome, enabling the implementation of a system that, with minimal expense, can enhance patient care and infection management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare12191996/s1, Figure S1: PRISMA 2020 Checklist. The PRISMA 2020 statement comprises a 27-item checklist addressing the introduction, methods, results and discussion sections of a systematic review report. Ref. [64] in Supplementary materials cited
in the main text.
Author Contributions
Conceptualization, S.D.M. and S.D.; methodology, D.R. and M.B.; validation, Z.J. and D.A.; investigation, I.A.-H.; data curation, I.A.-H.; writing—original draft preparation, S.D.M.; writing—review and editing, M.C. and D.A.; visualization, D.A.; supervision, S.D.; project administration, S.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- World Health Organization. Health Care without Avoidable Infections: The Critical Role of Infection Prevention and Control; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. World Health Organization Report on the Burden of Endemic Health Care-Associated Infection Worldwide Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2011; Volume 3, pp. 1–34. [Google Scholar]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; Mckimm, J.; Abu Bakar, M. Infection and Drug Resistance Dovepress Health Care-Associated Infections-an Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- ter Meulen, V. Healthcare-Associated Infections: The View from EASAC; European Academies Science Advisory Council: Vienna, Austria, 2009; pp. 1–10. [Google Scholar]
- Stone, P.W.; Pogorzelska-Maziarz, M.; Herzig, C.; Weiner, L.; Furuya, Y.; Dick, A.; Larson, E. State of Infection Prevention in US Hospitals. AMJ Infect. Control 2014, 42, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Kärki, T.; Diamantis, P. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2022–2023; European Commission: Brussels, Belgium, 2022. [Google Scholar] [CrossRef]
- Arjmandnia, F.; Alimohammadi, E. The Value of Machine Learning Technology and Artificial Intelligence to Enhance Patient Safety in Spine Surgery: A Review. Patient Saf. Surg. 2024, 18, 11. [Google Scholar] [CrossRef]
- Islam, U.; Mehmood, G.; Al-Atawi, A.A.; Khan, F.; Alwageed, H.S.; Cascone, L. NeuroHealth Guardian: A Novel Hybrid Approach for Precision Brain Stroke Prediction and Healthcare Analytics. J. Neurosci. Methods 2024, 409, 110210. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Koski, E.; Murphy, J. AI in Healthcare. Stud. Health Technol. Inform. 2021, 284, 295–299. [Google Scholar] [CrossRef]
- Sohn, S.; Larson, D.W.; Habermann, E.B.; Naessens, J.M.; Alabbad, J.Y.; Liu, H. Detection of Clinically Important Colorectal Surgical Site Infection Using Bayesian Network. J. Surg. Res. 2017, 209, 168–173. [Google Scholar] [CrossRef]
- Zachariah, P.; Sanabria, E.; Liu, J.; Cohen, B.; Yao, D.; Larson, E. Novel Strategies for Predicting Healthcare-Associated Infections at Admission: Implications for Nursing Care. Nurs. Res. 2020, 69, 399–403. [Google Scholar] [CrossRef]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using Artificial Intelligence (AI) to Predict Postoperative Surgical Site Infection: A Retrospective Cohort of 4046 Posterior Spinal Fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef]
- Walker, L.W.; Nowalk, A.J.; Visweswaran, S. Predicting Outcomes in Central Venous Catheter Salvage in Pediatric Central Line-Associated Bloodstream Infection. J. Am. Med. Inform. Assoc. 2021, 28, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.L.; Mooney, S.J.; Carone, M.; Althouse, B.M.; Liu, C.; Evans, L.E.; Patel, K.; Vo, P.T.; Pergam, S.A.; Phipps, A.I. Development and Validation of a Machine Learning Model to Estimate Bacterial Sepsis Among Immunocompromised Recipients of Stem Cell Transplant. JAMA Netw. Open 2021, 4, e214514. [Google Scholar] [CrossRef] [PubMed]
- Caǧlayan, Ç.; Barnes, S.L.; Pineles, L.L.; Harris, A.D.; Klein, E.Y. A Data-Driven Framework for Identifying Intensive Care Unit Admissions Colonized With Multidrug-Resistant Organisms. Front. Public Health 2022, 10, 853757. [Google Scholar] [CrossRef] [PubMed]
- Kiser, A.C.; Eilbeck, K.; Bucher, B.T. Developing an LSTM Model to Identify Surgical Site Infections Using Electronic Healthcare Records. AMIA Jt. Summits Transl. Sci. Proc. 2023, 2023, 330–339. [Google Scholar] [PubMed]
- Sophonsri, A.; Lou, M.; Ny, P.; Minejima, E.; Nieberg, P.; Wong-Beringer, A. Machine Learning to Identify Risk Factors Associated with the Development of Ventilated Hospital-Acquired Pneumonia and Mortality: Implications for Antibiotic Therapy Selection. Front. Med. 2023, 10, 1268488. [Google Scholar] [CrossRef]
- Al Mamlook, R.E.; Wells, L.J.; Sawyer, R. Machine-Learning Models for Predicting Surgical Site Infections Using Patient Pre-Operative Risk and Surgical Procedure Factors. Am. J. Infect. Control 2023, 51, 544–550. [Google Scholar] [CrossRef]
- Chen, K.; Stem, J.; Guillem, J.G.; Gomez, S.M.; Kapadia, M.R. Predicting Ileus after Colorectal Surgery Using Machine Learning. J. Am. Coll. Surg. 2023, 236, S22. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z.; You, L.; Zhou, L.; Xu, J.; Chen, K. Artificial Intelligence-Based Multimodal Risk Assessment Model for Surgical Site Infection (AMRAMS): Development and Validation Study. JMIR Med. Inform. 2020, 8, e18186. [Google Scholar] [CrossRef]
- Wang, H.; Fan, T.; Yang, B.; Lin, Q.; Li, W.; Yang, M. Development and Internal Validation of Supervised Machine Learning Algorithms for Predicting the Risk of Surgical Site Infection Following Minimally Invasive Transforaminal Lumbar Interbody Fusion. Front. Med. 2021, 8, 771608. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Z.; Gu, Y.; Zheng, S.; Sun, X.; Cao, J.; Song, B.; Jin, J.; Liu, Y.; Wen, X.; et al. Prediction of Post-Stroke Urinary Tract Infection Risk in Immobile Patients Using Machine Learning: An Observational Cohort Study. J. Hosp. Infect. 2022, 122, 96–107. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Gao, Y.; Niu, X.; Li, J.; Tang, M.; Fu, C.; Qi, R.; Song, B.; Chen, H.; et al. Machine-Learning Based Prediction of Prognostic Risk Factors in Patients with Invasive Candidiasis Infection and Bacterial Bloodstream Infection: A Singled Centered Retrospective Study. BMC Infect. Dis. 2022, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Ying, H.; Liao, W.J.; Li, M.P.; Zhang, Y.; Luo, K.; Sun, B.L.; Liu, Z.L.; Liu, J.M. Using Preoperative and Intraoperative Factors to Predict the Risk of Surgical Site Infections After Lumbar Spinal Surgery: A Machine Learning–Based Study. World Neurosurg. 2022, 162, e553–e560. [Google Scholar] [CrossRef]
- Huang, T.; Ma, Y.; Li, S.; Ran, J.; Xu, Y.; Asakawa, T.; Lu, H. Effectiveness of an Artificial Intelligence-Based Training and Monitoring System in Prevention of Nosocomial Infections: A Pilot Study of Hospital-Based Data. Drug Discov. Ther. 2023, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.; Sørensen, M.; Hardahl, C. Prediction of Risk of Acquiring Urinary Tract Infection during Hospital Stay Based on Machine-Learning: A Retrospective Cohort Study. PLoS ONE 2021, 16, e0248636. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, R.S.; Nielsen, T.D.; Leutscher, P.; Koch, K. Clinical Explainable Machine Learning Models for Early Identification of Patients at Risk of Hospital-Acquired Urinary Tract Infection. J. Hosp. Infect. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Bonde, A.; Lorenzen, S.; Brixen, G.; Troelsen, A.; Sillesen, M. Assessing the Utility of Deep Neural Networks in Detecting Superficial Surgical Site Infections from Free Text Electronic Health Record Data. Front. Digit. Health 2023, 5, 1249835. [Google Scholar] [CrossRef]
- Jakobsen, R.S.; Nielsen, T.D.; Leutscher, P.; Koch, K. A Study on the Risk Stratification for Patients within 24 Hours of Admission for Risk of Hospital-Acquired Urinary Tract Infection Using Bayesian Network Models. Health Inform. J. 2024, 30, 14604582241234232. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Riela, P.M.; Gallo, G.; Mura, I.; Agodi, A. A Machine Learning Approach to Predict Healthcare-Associated Infections at Intensive Care Unit Admission: Findings from the SPIN-UTI Project. J. Hosp. Infect. 2021, 112, 77–86. [Google Scholar] [CrossRef]
- Montella, E.; Ferraro, A.; Sperlì, G.; Triassi, M.; Santini, S.; Improta, G. Predictive Analysis of Healthcare-Associated Blood Stream Infections in the Neonatal Intensive Care Unit Using Artificial Intelligence: A Single Center Study. Int. J. Environ. Res. Public Health 2022, 19, 2498. [Google Scholar] [CrossRef]
- Scala, A.; Loperto, I.; Triassi, M.; Improta, G. Risk Factors Analysis of Surgical Infection Using Artificial Intelligence: A Single Center Study. Int. J. Environ. Res. Public Health 2022, 19, 10021. [Google Scholar] [CrossRef]
- Petrosyan, Y.; Thavorn, K.; Smith, G.; Maclure, M.; Preston, R.; van Walravan, C.; Forster, A.J. Predicting Postoperative Surgical Site Infection with Administrative Data: A Random Forests Algorithm. BMC Med. Res. Methodol. 2021, 21, 179. [Google Scholar] [CrossRef]
- Rennert-May, E.; Leal, J.; MacDonald, M.K.; Cannon, K.; Smith, S.; Exner, D.; Larios, O.E.; Bush, K.; Chew, D. Validating Administrative Data to Identify Complex Surgical Site Infections Following Cardiac Implantable Electronic Device Implantation: A Comparison of Traditional Methods and Machine Learning. Antimicrob. Resist. Infect. Control 2022, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cheligeer, C.; Southern, D.A.; Martin, E.A.; Xu, Y.; Leal, J.; Ellison, J.; Bush, K.; Williamson, T.; Quan, H.; et al. Development of Machine Learning Models for the Detection of Surgical Site Infections Following Total Hip and Knee Arthroplasty: A Multicenter Cohort Study. Antimicrob. Resist. Infect. Control 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.A.; ten Caten, C.S.; dos Santos, R.P.; Fogliatto, F.S.; Hsuan, J. Predicting the Occurrence of Surgical Site Infections Using Text Mining and Machine Learning. PLoS ONE 2019, 14, e0226272. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.P.; Silva, D.; Menezes, A.; Lukasewicz, S.; Dalmora, C.H.; Carvalho, O.; Giacomazzi, J.; Golin, N.; Pozza, R.; Vaz, T.A. Automated Healthcare-Associated Infection Surveillance Using an Artificial Intelligence Algorithm. Infect. Prev. Pract. 2021, 3, 100167. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Shinmoto, K.; Okada, Y.; Suga, K.; Bombard, J.; Murahata, S.; Shrestha, M.; Ocheja, P.; Tanaka, A. Machine Learning Approach to Predict Positive Screening of Methicillin-Resistant Staphylococcus Aureus During Mechanical Ventilation Using Synthetic Dataset From MIMIC-IV Database. Front. Med. 2021, 8, 694520. [Google Scholar] [CrossRef]
- Ohno, Y.; Mazaki, J.; Udo, R.; Tago, T.; Kasahara, K.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y. Preliminary Evaluation of a Novel Artificial Intelligence-Based Prediction Model for Surgical Site Infection in Colon Cancer. Cancer Diagn. Progn. 2022, 2, 691–696. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, Z.; Chung, D.R.; Cho, B.H.; Chung, M.J.; Kim, J.H.; Jeong, J. Development of Machine Learning Models for the Surveillance of Colon Surgical Site Infections. J. Hosp. Infect. 2024, 146, 224–231. [Google Scholar] [CrossRef]
- Rafaqat, W.; Fatima, H.S.; Kumar, A.; Khan, S.; Khurram, M. Machine Learning Model for Assessment of Risk Factors and Postoperative Day for Superficial vs Deep/Organ-Space Surgical Site Infections. Surg. Innov. 2023, 30, 455–462. [Google Scholar] [CrossRef]
- Kuo, K.M.; Talley, P.C.; Huang, C.H.; Cheng, L.C. Predicting Hospital-Acquired Pneumonia among Schizophrenic Patients: A Machine Learning Approach. BMC Med. Inform. Decis. Mak. 2019, 19, 42. [Google Scholar] [CrossRef]
- Bolton, W.J.; Wilson, R.; Gilchrist, M.; Georgiou, P.; Holmes, A.; Rawson, T.M. Personalising Intravenous to Oral Antibiotic Switch Decision Making through Fair Interpretable Machine Learning. Nat. Commun. 2024, 15, 506. [Google Scholar] [CrossRef]
- Flores-Balado, Á.; Castresana Méndez, C.; Herrero González, A.; Mesón Gutierrez, R.; de las Casas Cámara, G.; Vila Cordero, B.; Arcos, J.; Pfang, B.; Martín-Ríos, M.D. Using Artificial Intelligence to Reduce Orthopedic Surgical Site Infection Surveillance Workload: Algorithm Design, Validation, and Implementation in 4 Spanish Hospitals. Am. J. Infect. Control 2023, 51, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Dickter, J.K. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Naber, K.G. Hospital-Acquired Urinary Tract Infections. J. Hosp. Infect. 2000, 46, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Gerlach, H. Recent Advances in Understanding and Managing Sepsis. F1000Res 2018, 7, 1570. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial Intelligence in Healthcare: Past, Present and Future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Van Mourik, M.S.M.; Perencevich, E.N.; Gastmeier, P.; Bonten, M.J.M. Designing Surveillance of Healthcare-Associated Infections in the Era of Automation and Reporting Mandates. Clin. Infect. Dis. 2018, 66, 970–976. [Google Scholar] [CrossRef]
- Sips, M.E.; Bonten, M.J.M.; Van Mourik, M.S.M. Automated Surveillance of Healthcare-Associated Infections: State of the Art. Curr. Opin. Infect. Dis. 2017, 30, 425–431. [Google Scholar] [CrossRef]
- Woldemariam, M.T.; Jimma, W. Adoption of Electronic Health Record Systems to Enhance the Quality of Healthcare in Low-Income Countries: A Systematic Review. BMJ Health Care Inform. 2023, 30, 8–10. [Google Scholar] [CrossRef]
- Piaggio, D.; Zarro, M.; Pagliara, S.; Andellini, M.; Almuhini, A.; Maccaro, A.; Pecchia, L. The Use of Smart Environments and Robots for Infection Prevention Control: A Systematic Literature Review. Am. J. Infect. Control 2023, 51, 1175–1181. [Google Scholar] [CrossRef]
- Albano, G.D.; Bertozzi, G.; Maglietta, F.; Montana, A.; Di Mizio, G.; Esposito, M.; Mazzeo, P.; D’Errico, S.; Salerno, M. Medical Records Quality as Prevention Tool for Healthcare-Associated Infections (HAIs) Related Litigation: A Case Series. Curr. Pharm. Biotechnol. 2019, 20, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology—Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Alnasser, B. A Review of Literature on the Economic Implications of Implementing Artificial Intelligence in Healthcare. Ehealth Telecommun. Syst. Netw. 2023, 12, 35–48. [Google Scholar] [CrossRef]
- Schönberger, D. Artificial Intelligence in Healthcare: A Critical Analysis of the Legal and Ethical Implications. Int. J. Law Inf. Technol. 2019, 27, 171–203. [Google Scholar] [CrossRef]
- Blandi, L.; Bolcato, V.; Meloni, A.; Bosone, D.; Odone, A. Healthcare-Associated-Infections: Preliminary Results from a Real-Time Reporting System of an Italian Neurologic Research Hospital. Ann. Ig. 2024, 36, 256–260. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).