Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review
Abstract
1. Introduction
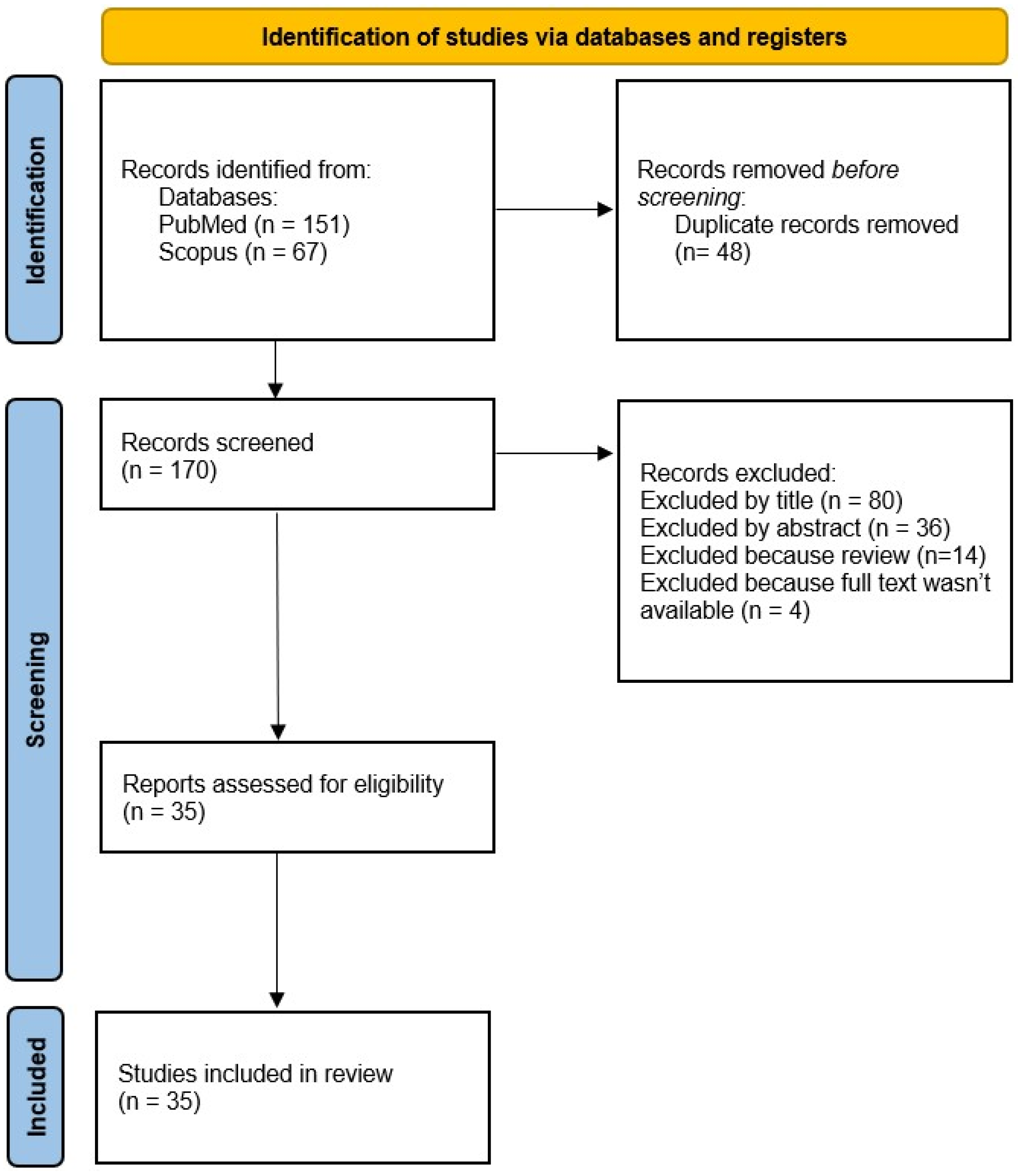
2. Materials and Methods
2.1. Criteria for Including/Excluding Studies
2.2. Characteristics of Eligible Studies
2.3. Quality Assessment and Risk of Bias
3. Results
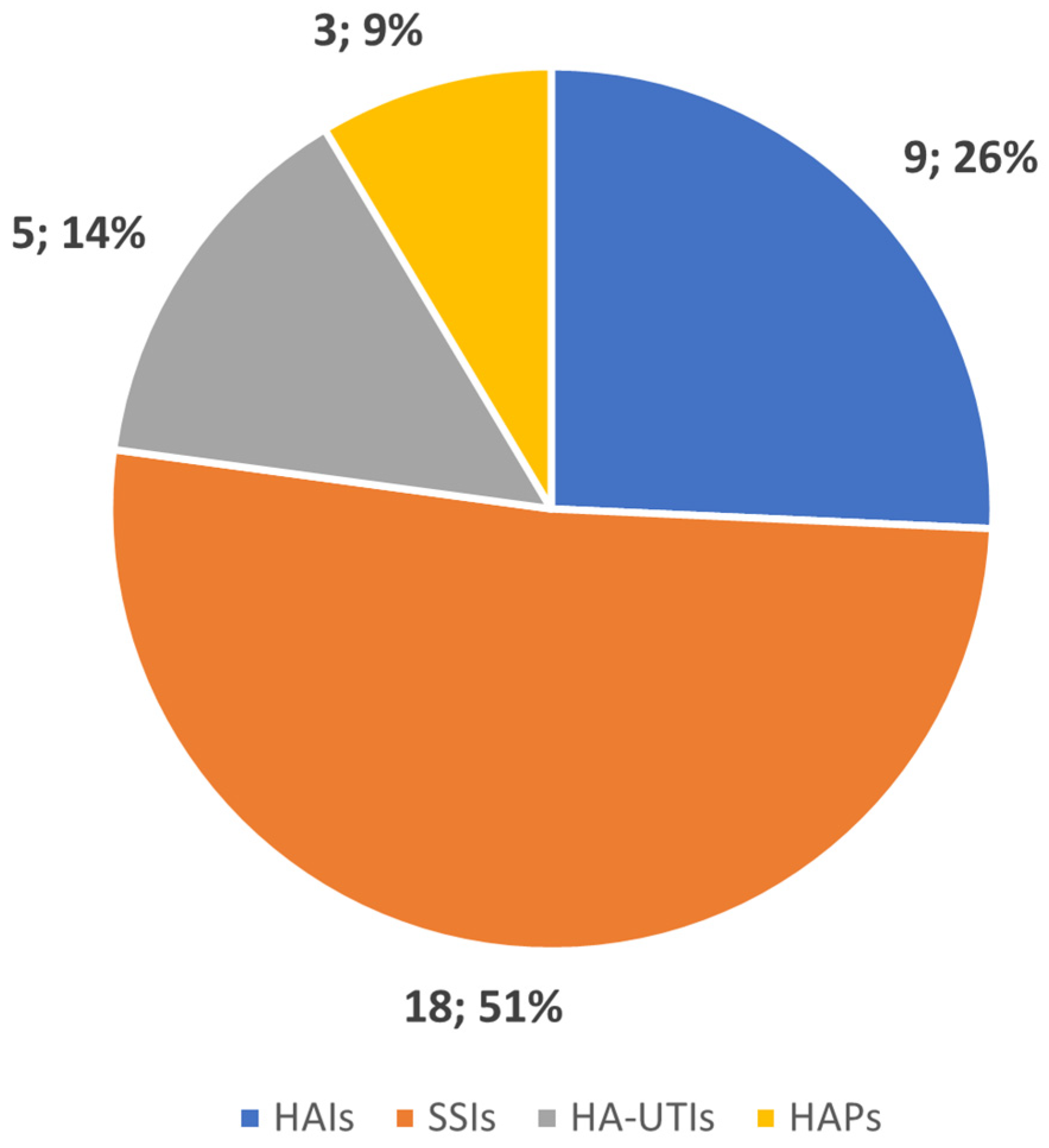
3.1. Data Extraction
3.2. Types of HAIs
3.2.1. Surgical Site Infections—SSIs
3.2.2. Healthcare-Associated Pneumonia—HCAP
3.2.3. Hospital-Acquired Urinary Tract Infections—HA-UTI
3.2.4. Hospital-Acquired Infections—HAIs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Health Care without Avoidable Infections: The Critical Role of Infection Prevention and Control; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- World Health Organization. World Health Organization Report on the Burden of Endemic Health Care-Associated Infection Worldwide Clean Care Is Safer Care; World Health Organization: Geneva, Switzerland, 2011; Volume 3, pp. 1–34. [Google Scholar]
- Cassini, A.; Plachouras, D.; Eckmanns, T.; Abu Sin, M.; Blank, H.P.; Ducomble, T.; Haller, S.; Harder, T.; Klingeberg, A.; Sixtensson, M.; et al. Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study. PLoS Med. 2016, 13, e1002150. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Sartelli, M.; Mckimm, J.; Abu Bakar, M. Infection and Drug Resistance Dovepress Health Care-Associated Infections-an Overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- ter Meulen, V. Healthcare-Associated Infections: The View from EASAC; European Academies Science Advisory Council: Vienna, Austria, 2009; pp. 1–10. [Google Scholar]
- Stone, P.W.; Pogorzelska-Maziarz, M.; Herzig, C.; Weiner, L.; Furuya, Y.; Dick, A.; Larson, E. State of Infection Prevention in US Hospitals. AMJ Infect. Control 2014, 42, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Kärki, T.; Diamantis, P. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2022–2023; European Commission: Brussels, Belgium, 2022. [Google Scholar] [CrossRef]
- Arjmandnia, F.; Alimohammadi, E. The Value of Machine Learning Technology and Artificial Intelligence to Enhance Patient Safety in Spine Surgery: A Review. Patient Saf. Surg. 2024, 18, 11. [Google Scholar] [CrossRef]
- Islam, U.; Mehmood, G.; Al-Atawi, A.A.; Khan, F.; Alwageed, H.S.; Cascone, L. NeuroHealth Guardian: A Novel Hybrid Approach for Precision Brain Stroke Prediction and Healthcare Analytics. J. Neurosci. Methods 2024, 409, 110210. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.; Nazer, K.; Al-Reedy, A.; Al-Shehri, S.; Al-Muhanna, A.; Subbarayalu, A.V.; Al Muhanna, D.; Al-Muhanna, F.A. A Review of the Role of Artificial Intelligence in Healthcare. J. Pers. Med. 2023, 13, 951. [Google Scholar] [CrossRef]
- Koski, E.; Murphy, J. AI in Healthcare. Stud. Health Technol. Inform. 2021, 284, 295–299. [Google Scholar] [CrossRef]
- Sohn, S.; Larson, D.W.; Habermann, E.B.; Naessens, J.M.; Alabbad, J.Y.; Liu, H. Detection of Clinically Important Colorectal Surgical Site Infection Using Bayesian Network. J. Surg. Res. 2017, 209, 168–173. [Google Scholar] [CrossRef]
- Zachariah, P.; Sanabria, E.; Liu, J.; Cohen, B.; Yao, D.; Larson, E. Novel Strategies for Predicting Healthcare-Associated Infections at Admission: Implications for Nursing Care. Nurs. Res. 2020, 69, 399–403. [Google Scholar] [CrossRef]
- Hopkins, B.S.; Mazmudar, A.; Driscoll, C.; Svet, M.; Goergen, J.; Kelsten, M.; Shlobin, N.A.; Kesavabhotla, K.; Smith, Z.A.; Dahdaleh, N.S. Using Artificial Intelligence (AI) to Predict Postoperative Surgical Site Infection: A Retrospective Cohort of 4046 Posterior Spinal Fusions. Clin. Neurol. Neurosurg. 2020, 192, 105718. [Google Scholar] [CrossRef]
- Walker, L.W.; Nowalk, A.J.; Visweswaran, S. Predicting Outcomes in Central Venous Catheter Salvage in Pediatric Central Line-Associated Bloodstream Infection. J. Am. Med. Inform. Assoc. 2021, 28, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.L.; Mooney, S.J.; Carone, M.; Althouse, B.M.; Liu, C.; Evans, L.E.; Patel, K.; Vo, P.T.; Pergam, S.A.; Phipps, A.I. Development and Validation of a Machine Learning Model to Estimate Bacterial Sepsis Among Immunocompromised Recipients of Stem Cell Transplant. JAMA Netw. Open 2021, 4, e214514. [Google Scholar] [CrossRef] [PubMed]
- Caǧlayan, Ç.; Barnes, S.L.; Pineles, L.L.; Harris, A.D.; Klein, E.Y. A Data-Driven Framework for Identifying Intensive Care Unit Admissions Colonized With Multidrug-Resistant Organisms. Front. Public Health 2022, 10, 853757. [Google Scholar] [CrossRef] [PubMed]
- Kiser, A.C.; Eilbeck, K.; Bucher, B.T. Developing an LSTM Model to Identify Surgical Site Infections Using Electronic Healthcare Records. AMIA Jt. Summits Transl. Sci. Proc. 2023, 2023, 330–339. [Google Scholar] [PubMed]
- Sophonsri, A.; Lou, M.; Ny, P.; Minejima, E.; Nieberg, P.; Wong-Beringer, A. Machine Learning to Identify Risk Factors Associated with the Development of Ventilated Hospital-Acquired Pneumonia and Mortality: Implications for Antibiotic Therapy Selection. Front. Med. 2023, 10, 1268488. [Google Scholar] [CrossRef]
- Al Mamlook, R.E.; Wells, L.J.; Sawyer, R. Machine-Learning Models for Predicting Surgical Site Infections Using Patient Pre-Operative Risk and Surgical Procedure Factors. Am. J. Infect. Control 2023, 51, 544–550. [Google Scholar] [CrossRef]
- Chen, K.; Stem, J.; Guillem, J.G.; Gomez, S.M.; Kapadia, M.R. Predicting Ileus after Colorectal Surgery Using Machine Learning. J. Am. Coll. Surg. 2023, 236, S22. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z.; You, L.; Zhou, L.; Xu, J.; Chen, K. Artificial Intelligence-Based Multimodal Risk Assessment Model for Surgical Site Infection (AMRAMS): Development and Validation Study. JMIR Med. Inform. 2020, 8, e18186. [Google Scholar] [CrossRef]
- Wang, H.; Fan, T.; Yang, B.; Lin, Q.; Li, W.; Yang, M. Development and Internal Validation of Supervised Machine Learning Algorithms for Predicting the Risk of Surgical Site Infection Following Minimally Invasive Transforaminal Lumbar Interbody Fusion. Front. Med. 2021, 8, 771608. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Z.; Gu, Y.; Zheng, S.; Sun, X.; Cao, J.; Song, B.; Jin, J.; Liu, Y.; Wen, X.; et al. Prediction of Post-Stroke Urinary Tract Infection Risk in Immobile Patients Using Machine Learning: An Observational Cohort Study. J. Hosp. Infect. 2022, 122, 96–107. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Gao, Y.; Niu, X.; Li, J.; Tang, M.; Fu, C.; Qi, R.; Song, B.; Chen, H.; et al. Machine-Learning Based Prediction of Prognostic Risk Factors in Patients with Invasive Candidiasis Infection and Bacterial Bloodstream Infection: A Singled Centered Retrospective Study. BMC Infect. Dis. 2022, 22, 150. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Ying, H.; Liao, W.J.; Li, M.P.; Zhang, Y.; Luo, K.; Sun, B.L.; Liu, Z.L.; Liu, J.M. Using Preoperative and Intraoperative Factors to Predict the Risk of Surgical Site Infections After Lumbar Spinal Surgery: A Machine Learning–Based Study. World Neurosurg. 2022, 162, e553–e560. [Google Scholar] [CrossRef]
- Huang, T.; Ma, Y.; Li, S.; Ran, J.; Xu, Y.; Asakawa, T.; Lu, H. Effectiveness of an Artificial Intelligence-Based Training and Monitoring System in Prevention of Nosocomial Infections: A Pilot Study of Hospital-Based Data. Drug Discov. Ther. 2023, 17, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.K.; Sørensen, M.; Hardahl, C. Prediction of Risk of Acquiring Urinary Tract Infection during Hospital Stay Based on Machine-Learning: A Retrospective Cohort Study. PLoS ONE 2021, 16, e0248636. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, R.S.; Nielsen, T.D.; Leutscher, P.; Koch, K. Clinical Explainable Machine Learning Models for Early Identification of Patients at Risk of Hospital-Acquired Urinary Tract Infection. J. Hosp. Infect. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Bonde, A.; Lorenzen, S.; Brixen, G.; Troelsen, A.; Sillesen, M. Assessing the Utility of Deep Neural Networks in Detecting Superficial Surgical Site Infections from Free Text Electronic Health Record Data. Front. Digit. Health 2023, 5, 1249835. [Google Scholar] [CrossRef]
- Jakobsen, R.S.; Nielsen, T.D.; Leutscher, P.; Koch, K. A Study on the Risk Stratification for Patients within 24 Hours of Admission for Risk of Hospital-Acquired Urinary Tract Infection Using Bayesian Network Models. Health Inform. J. 2024, 30, 14604582241234232. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Riela, P.M.; Gallo, G.; Mura, I.; Agodi, A. A Machine Learning Approach to Predict Healthcare-Associated Infections at Intensive Care Unit Admission: Findings from the SPIN-UTI Project. J. Hosp. Infect. 2021, 112, 77–86. [Google Scholar] [CrossRef]
- Montella, E.; Ferraro, A.; Sperlì, G.; Triassi, M.; Santini, S.; Improta, G. Predictive Analysis of Healthcare-Associated Blood Stream Infections in the Neonatal Intensive Care Unit Using Artificial Intelligence: A Single Center Study. Int. J. Environ. Res. Public Health 2022, 19, 2498. [Google Scholar] [CrossRef]
- Scala, A.; Loperto, I.; Triassi, M.; Improta, G. Risk Factors Analysis of Surgical Infection Using Artificial Intelligence: A Single Center Study. Int. J. Environ. Res. Public Health 2022, 19, 10021. [Google Scholar] [CrossRef]
- Petrosyan, Y.; Thavorn, K.; Smith, G.; Maclure, M.; Preston, R.; van Walravan, C.; Forster, A.J. Predicting Postoperative Surgical Site Infection with Administrative Data: A Random Forests Algorithm. BMC Med. Res. Methodol. 2021, 21, 179. [Google Scholar] [CrossRef]
- Rennert-May, E.; Leal, J.; MacDonald, M.K.; Cannon, K.; Smith, S.; Exner, D.; Larios, O.E.; Bush, K.; Chew, D. Validating Administrative Data to Identify Complex Surgical Site Infections Following Cardiac Implantable Electronic Device Implantation: A Comparison of Traditional Methods and Machine Learning. Antimicrob. Resist. Infect. Control 2022, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cheligeer, C.; Southern, D.A.; Martin, E.A.; Xu, Y.; Leal, J.; Ellison, J.; Bush, K.; Williamson, T.; Quan, H.; et al. Development of Machine Learning Models for the Detection of Surgical Site Infections Following Total Hip and Knee Arthroplasty: A Multicenter Cohort Study. Antimicrob. Resist. Infect. Control 2023, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.A.; ten Caten, C.S.; dos Santos, R.P.; Fogliatto, F.S.; Hsuan, J. Predicting the Occurrence of Surgical Site Infections Using Text Mining and Machine Learning. PLoS ONE 2019, 14, e0226272. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.P.; Silva, D.; Menezes, A.; Lukasewicz, S.; Dalmora, C.H.; Carvalho, O.; Giacomazzi, J.; Golin, N.; Pozza, R.; Vaz, T.A. Automated Healthcare-Associated Infection Surveillance Using an Artificial Intelligence Algorithm. Infect. Prev. Pract. 2021, 3, 100167. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Shinmoto, K.; Okada, Y.; Suga, K.; Bombard, J.; Murahata, S.; Shrestha, M.; Ocheja, P.; Tanaka, A. Machine Learning Approach to Predict Positive Screening of Methicillin-Resistant Staphylococcus Aureus During Mechanical Ventilation Using Synthetic Dataset From MIMIC-IV Database. Front. Med. 2021, 8, 694520. [Google Scholar] [CrossRef]
- Ohno, Y.; Mazaki, J.; Udo, R.; Tago, T.; Kasahara, K.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y. Preliminary Evaluation of a Novel Artificial Intelligence-Based Prediction Model for Surgical Site Infection in Colon Cancer. Cancer Diagn. Progn. 2022, 2, 691–696. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kim, Z.; Chung, D.R.; Cho, B.H.; Chung, M.J.; Kim, J.H.; Jeong, J. Development of Machine Learning Models for the Surveillance of Colon Surgical Site Infections. J. Hosp. Infect. 2024, 146, 224–231. [Google Scholar] [CrossRef]
- Rafaqat, W.; Fatima, H.S.; Kumar, A.; Khan, S.; Khurram, M. Machine Learning Model for Assessment of Risk Factors and Postoperative Day for Superficial vs Deep/Organ-Space Surgical Site Infections. Surg. Innov. 2023, 30, 455–462. [Google Scholar] [CrossRef]
- Kuo, K.M.; Talley, P.C.; Huang, C.H.; Cheng, L.C. Predicting Hospital-Acquired Pneumonia among Schizophrenic Patients: A Machine Learning Approach. BMC Med. Inform. Decis. Mak. 2019, 19, 42. [Google Scholar] [CrossRef]
- Bolton, W.J.; Wilson, R.; Gilchrist, M.; Georgiou, P.; Holmes, A.; Rawson, T.M. Personalising Intravenous to Oral Antibiotic Switch Decision Making through Fair Interpretable Machine Learning. Nat. Commun. 2024, 15, 506. [Google Scholar] [CrossRef]
- Flores-Balado, Á.; Castresana Méndez, C.; Herrero González, A.; Mesón Gutierrez, R.; de las Casas Cámara, G.; Vila Cordero, B.; Arcos, J.; Pfang, B.; Martín-Ríos, M.D. Using Artificial Intelligence to Reduce Orthopedic Surgical Site Infection Surveillance Workload: Algorithm Design, Validation, and Implementation in 4 Spanish Hospitals. Am. J. Infect. Control 2023, 51, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Badia, J.M.; Casey, A.L.; Petrosillo, N.; Hudson, P.M.; Mitchell, S.A.; Crosby, C. Impact of Surgical Site Infection on Healthcare Costs and Patient Outcomes: A Systematic Review in Six European Countries. J. Hosp. Infect. 2017, 96, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Dickter, J.K. Nosocomial Infections: A History of Hospital-Acquired Infections. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Wagenlehner, F.M.E.; Naber, K.G. Hospital-Acquired Urinary Tract Infections. J. Hosp. Infect. 2000, 46, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Gerlach, H. Recent Advances in Understanding and Managing Sepsis. F1000Res 2018, 7, 1570. [Google Scholar] [CrossRef]
- Jiang, F.; Jiang, Y.; Zhi, H.; Dong, Y.; Li, H.; Ma, S.; Wang, Y.; Dong, Q.; Shen, H.; Wang, Y. Artificial Intelligence in Healthcare: Past, Present and Future. Stroke Vasc. Neurol. 2017, 2, 230–243. [Google Scholar] [CrossRef]
- Van Mourik, M.S.M.; Perencevich, E.N.; Gastmeier, P.; Bonten, M.J.M. Designing Surveillance of Healthcare-Associated Infections in the Era of Automation and Reporting Mandates. Clin. Infect. Dis. 2018, 66, 970–976. [Google Scholar] [CrossRef]
- Sips, M.E.; Bonten, M.J.M.; Van Mourik, M.S.M. Automated Surveillance of Healthcare-Associated Infections: State of the Art. Curr. Opin. Infect. Dis. 2017, 30, 425–431. [Google Scholar] [CrossRef]
- Woldemariam, M.T.; Jimma, W. Adoption of Electronic Health Record Systems to Enhance the Quality of Healthcare in Low-Income Countries: A Systematic Review. BMJ Health Care Inform. 2023, 30, 8–10. [Google Scholar] [CrossRef]
- Piaggio, D.; Zarro, M.; Pagliara, S.; Andellini, M.; Almuhini, A.; Maccaro, A.; Pecchia, L. The Use of Smart Environments and Robots for Infection Prevention Control: A Systematic Literature Review. Am. J. Infect. Control 2023, 51, 1175–1181. [Google Scholar] [CrossRef]
- Albano, G.D.; Bertozzi, G.; Maglietta, F.; Montana, A.; Di Mizio, G.; Esposito, M.; Mazzeo, P.; D’Errico, S.; Salerno, M. Medical Records Quality as Prevention Tool for Healthcare-Associated Infections (HAIs) Related Litigation: A Case Series. Curr. Pharm. Biotechnol. 2019, 20, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Maleki Varnosfaderani, S.; Forouzanfar, M. The Role of AI in Hospitals and Clinics: Transforming Healthcare in the 21st Century. Bioengineering 2024, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Spooner, B.; Isherwood, J.; Lane, M.; Orrock, E.; Dennison, A. A Systematic Review of the Barriers to the Implementation of Artificial Intelligence in Healthcare. Cureus 2023, 15, e46454. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.P.; Neri, E. Artificial Intelligence in Radiology—Ethical Considerations. Diagnostics 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- Khanna, N.N.; Maindarkar, M.A.; Viswanathan, V.; Fernandes, J.F.E.; Paul, S.; Bhagawati, M.; Ahluwalia, P.; Ruzsa, Z.; Sharma, A.; Kolluri, R.; et al. Economics of Artificial Intelligence in Healthcare: Diagnosis vs. Treatment. Healthcare 2022, 10, 2493. [Google Scholar] [CrossRef]
- Alnasser, B. A Review of Literature on the Economic Implications of Implementing Artificial Intelligence in Healthcare. Ehealth Telecommun. Syst. Netw. 2023, 12, 35–48. [Google Scholar] [CrossRef]
- Schönberger, D. Artificial Intelligence in Healthcare: A Critical Analysis of the Legal and Ethical Implications. Int. J. Law Inf. Technol. 2019, 27, 171–203. [Google Scholar] [CrossRef]
- Blandi, L.; Bolcato, V.; Meloni, A.; Bosone, D.; Odone, A. Healthcare-Associated-Infections: Preliminary Results from a Real-Time Reporting System of an Italian Neurologic Research Hospital. Ann. Ig. 2024, 36, 256–260. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Study | Year | Country | Study Setting | Studied Population | Infection Type | Aim of the Study | How the AI Was Trained | Results |
|---|---|---|---|---|---|---|---|---|
| Detection of Clinically Important Colorectal Surgical Site Infection using Bayesian Network [12] | 2017 | USA | General surgery | Patients that underwent colorectal surgery | Surgical site infection | Develop a Bayesian network automated detection system to identify surgical site infections after colorectal surgery | A Bayesian network was used to detect SSIs by utilizing risk factors from ACS-NSQIP data and keywords extracted from clinical notes through natural language processing(NLP) on data from 751 colorectal surgery cases. Two surgeons also provided the Bayesian network information on how to identify clinically meaningful SSIs (CM-SSIs) | The Bayesian network detected SSIs with an ROC of 0.827, which increased to 0.892 with surgeon-identified CM-SSI. |
| Novel Strategies for Predicting Healthcare-Associated Infections at Admission: Implications for Nursing Care [13] | 2020 | USA | Hospital | All patients | HA-UTI | Development of two machine learning models (neural networks and decision trees) to predict a patient’s risk of developing a UTI using data available on the first day of admission | Data from electronic health records of 897,344 hospitalized patients between 1 January 2009 and 31 December 2016 | The decision tree model had a higher sensitivity compared to the neural network (78.2% vs. 57.3%), but it had a lower specificity (64.2% vs. 81.4%). The positive predictive values were 3.5% for the decision tree model and 4.9% for the Deep Neural Network model, while the negative predictive values were 99.4% and 99.1%, respectively |
| Using artificial intelligence (AI) to predict postoperative surgical site infection: A retrospective cohort of 4046 posterior spinal fusions [14] | 2020 | USA | Neurological and orthopedic surgery | Patients that underwent posterior spinal fusion surgery | Surgical site infection | Develop a ML model for the prediction of SSIs | Data from 4046 patients between 1 January 2000 and 31 December 2015 | The Deep Neural Network model was able to predict postoperative SSIs with a PPV of 92.56% and an NPV of 98.45%, achieving a mean AUC of 0.775. It also helped identify risk factors and protective variables |
| Predicting outcomes in central venous catheter salvage in pediatric central line-associated bloodstream infection [15] | 2021 | USA | Pediatrics | Patients with central line-associated bloodstream infections (CLABSIs) | Healthcare-associated infections | Developing a machine learning approach to predict individual outcomes in central venous catheter (CVC) salvage | Data from electronic health records (patient demographics, diagnosis codes, medication records, infection history, laboratory data, all blood cultures, etc.) of 969 kids with CLABI between 2005 and 2018 | The models predicting infection recurrence had higher AUROCs at most time points compared to the models for CVC removal (0.83 and 0.77 vs. 0.66 and 0.76) |
| Development and Validation of a Machine Learning Model to Estimate Bacterial Sepsis Among Immunocompromised Recipients of Stem Cell Transplant [16] | 2021 | USA | Hospital | Patients that were recipients of allogeneic hematopoietic cell transplant | Healthcare-associated infections | Development of two automated systems using ML that utilize electronic health records to predict potential sepsis in patients undergoing allogeneic hematopoietic cell transplant | Data from electronic health records of 1943 patients who underwent transplants between 2010 and 2019 | The two systems (SHBSL and C-SHBSL) have sensitivities of 80% and 65.7%, respectively, and specificities of 72.8% and 66.9% in predicting the high risk of bacteremia for sepsis specifically in transplant patients |
| A Data-Driven Framework for Identifying Intensive Care Unit Admissions Colonized With Multidrug-Resistant Organisms [17] | 2022 | USA | ICU | All patients | HAI | Development of a data-driven framework to predict MRSA VRE and CRE colonization upon admission to ICU | Data from electronic healthcare records from University of Maryland‘s medical center of 3958 patients admitted to ICU from 2017 to 2018. in total 11 variables were included | The rate of colonization was 17,59% for MDRO, 13.03% for VRE, 1.45% for CRE, and 7.47% for MRSA. Sensitivity and specificity values with the best performing models, respectively were as follows: 80% and 66% for VRE with logistic regression, 73% and 77% for CRE with XGBoost, 76% and 59% for MRSA with random forest, and 82% and 83% for MDRO with random forest. It can be used as a clinical decision support tool for the identification of high-risk patients and the proper use of infection control measures |
| Developing an LSTM Model to Identify Surgical Site Infections using Electronic Healthcare Records [18] | 2023 | USA | Hospital | Patients that underwent surgery | Surgical site infection | Development of a long short-term memory (LSTM) model using electronic health record data to identify SSIs | Data from 9185 operative events from January 2016 to June 2021 | The best model had an AUROC of 0.905 |
| Machine learning to identify risk factors associated with the development of ventilated hospital-acquired pneumonia and mortality: implications for antibiotic therapy selection [19] | 2023 | USA | Hospital | Patients with pneumonia (non-ventilated, ventilated hospital-acquired, and ventilator-associated pneumonia) | Hospital-acquired pneumonia | Identify risk factors associated with the development and mortality of vHABP using ML | Data from inpatients who developed HAP (subdivided between vHABP, nvHABP, and VABP) between March 2014 and December 2019 (457 patients) | Identified the major risk factors for developing vHABP and the major risk for mortality. The AUC ROC of the nvHABP, vHABP, and VABP mortality models were 0.80, 0.78, and 0.83, respectively |
| Machine-learning models for predicting surgical site infections using patient pre-operative risk and surgical procedure factors [20] | 2023 | USA | Hospital | Patients that underwent surgery | Surgical site infection | Develop a ML model for the prediction of SSIs | Data from 2,882,526 patient records (sourced from the American College of Surgeons National Quality Improvement Program database) between 1 January 2013 and 31 December 2016 | The Deep Neural Network model provided the best predictive performance with an area under the curve of 0.8518, accuracy of 0.8518, precision of 0.8517, sensitivity of 0.8527, and an F1-score of 0.8518 |
| Predicting Surgical Site Infection after Colorectal Surgery Using Machine Learning [21] | 2023 | USA | Hospital | Patients who underwent colorectal surgery | Surgical site infection | Develop a ML model for the prediction of SSIs after colorectal surgery | Data from 275,152 patient records (sourced from the American College of Surgeons National Quality Improvement Program database) between 2012 and 2019 | The Deep Neural Network model demonstrated the best performance for predicting SSIs, achieving an AUROC of 0.769 (95% CI 0.762–0.777). With a specificity of 50%, the sensitivity was 82%, while at a specificity of 90%, the sensitivity was 36% |
| Artificial Intelligence-Based Multimodal Risk Assessment Model for Surgical Site Infection (AMRAMS): Development and Validation Study [22] | 2020 | China | General surgery, gynecology, orthopedics, and urology | Inpatients that underwent surgery during the hospital stay | Surgical site infection | Development of an AI-based risk assessment model for surgical site infections (AMRAS) | Clinical data from electronic medical records (patient demographics, routine blood examination, coagulation, liver and kidney function, plasma electrolytes, smoking status, marital status, emergency intervention, and anesthesia) collected from patients who underwent a single operation between 2014 and 2019 (21,611 patients) | The AMRAS model identifies high-risk patients better than other machine learning methods and the currently used NNIS risk index |
| Development and Internal Validation of Supervised Machine Learning Algorithms for Predicting the Risk of Surgical Site Infection Following Minimally Invasive Transforaminal Lumbar Interbody Fusion [23] | 2021 | China | Hospital | Patients who underwent minimally invasive transforaminal lumbar interbody fusion | Surgical site infection | Develop and validate supervised ML algorithms for predicting the risk of SSI following minimally invasive transforaminal lumbar interbody fusion (MIS-TLIF) | Clinical characteristics, surgery-related parameters, and routine laboratory tests of 705 patients between May 2012 and October 2019 | Naïve Bayes algorithm performed the best with an average AUC and ACC of 0.78 and 0.90 |
| Prediction of post-stroke urinary tract infection risk in immobile patients using machine learning: an observational cohort study [24] | 2022 | China | Hospital | Immobile stroke patients | HA-UTI | Develop predictive models for UTI risk identification for immobile stroke patients. | The derivation cohort used data from 3982 immobile stroke patients between 1 November 2015 and 30 June 2016. The external validation cohort used data of 3837 patients collected from 1 November 2016 to 30 July 2017 | The ensemble learning model had the best performance, with an AUROC during internal validation of 82.2% and of 80.8% during external validation and also had the highest sensitivity of 80.9% and 81.1% in both the internal and external validation sets |
| Machine-learning based prediction of prognostic risk factors in patients with invasive candidiasis infection and bacterial bloodstream infection: a singled centered retrospective study [25] | 2022 | China | ICU | Patients with invasive candida infection and bacterial bloodstream infection | HAI | Development of a machine learning algorithm for prognostic risk factors related to mortality in patients with candidiasis and bacteremia | Data from 246 hospitalized patients between 2013 and 2018 | The ML identified the ten most important risk factors. The random forest model had the best AUC of 0.919 |
| Using Preoperative and Intraoperative Factors to Predict the Risk of Surgical Site Infections After Lumbar Spinal Surgery: A Machine Learning Based Study [26] | 2022 | China | Hospital | Patients who underwent lumbar spinal surgery | Surgical site infection | Develop a ML model for the prediction of SSIs after lumbar spinal surgery | Data from 288 patients between December 2010 and December 2019 | The XG Boost model had the best prediction performance with an average AUC of 0.926 |
| Effectiveness of an artificial intelligence-based training and monitoring system in prevention of nosocomial infections: A pilot study of hospital-based data [27] | 2023 | China | Hospital | Hospital staff | HAI | Development of a camera/speaker system with integrated AI that monitors and provides training on the correct use of PPE and handwashing | Images taken from videos recorded directly by the system; subsequently, the “behaviors“ related to donning/removing PPE and handwashing were marked | After the introduction of the system, the accuracy of 163 operators increased from 52.15% to 98.14%. At the same time, the hospital infection rate decreased from 1.31% pre-AI to 0.58% in 2021 and 0.38% in 2022 |
| Prediction of risk of acquiring urinary tract infection during hospital stay based on machine-learning: A retrospective cohort study [28] | 2021 | Denmark | Hospital | All patients | HA-UTI | Develop two predictive models utilizing data from the initial hospital admission and the patient’s historical records to predict the development of UTI upon hospital admission and within the following 48 h | Data collected from 301,932 patients between January 2017 and May 2018. Variables included demographic information, laboratory results, antibiotic treatment data, past medical history (ICD-10 codes), and clinical data | Both models (decision trees) had high ROC indices on the validation dataset: 0.81 for the Entry Model and 0.74 for the HAI Model, indicating adequate sensitivity and specificity. Both models could play a crucial role in personalized UTI prevention strategies for hospitalized patients |
| Clinical explainable machine learning models for early identification of patients at risk of hospital-acquired urinary tract infection [29] | 2023 | Denmark | Hospital | All patients | HA-UTI | Develop an ML model to predict patients at risk of HA-UTI using available data from electronic health records collected at the time of hospital admission | Data from electronic health records of 138,560 hospital admissions from 1 January 2017 to 31 December 2018 | The Deep Neural Network model was the best-performing ML algorithm, with an AUC of 0.758 on a full dataset and an AUC of 0.746 on a reduced dataset. Within 24 h of admission, the ML model could identify patients at risk |
| Assessing the utility of deep neural networks in detecting superficial surgical site infections from free text electronic health record data [30] | 2023 | Denmark | Orthopedic surgery, general surgery, gynecology and obstetrics, urology, cardiothoracic surgery, ophthalmic surgery, plastic surgery, neurological surgery, otorhinolaryngology, oral and maxillofacial surgery, and vascular surgery | Patients who underwent surgery | Surgical site infection | Develop NLP on electronic health record chart notes to identify postoperative superficial surgical site infections | Deep Learning NLP models (stand-alone ML and a human-in-the-loop pipeline) were trained on data from 389,865 surgical cases between 1 January 2017 and 31 December 2021 | The performance of the SAM pipeline was superior to administrative data (sensitivity of 0.604, specificity of 0.996, positive predictive value of 0.763, and a negative predictive value of 0.991). The HITL pipeline had a sensitivity of 0.854, a specificity of 0.987, a PPV of 0.603, and an NPV of 0.997 |
| A study on the risk stratification for patients within 24 h of admission for risk of hospital-acquired urinary tract infection using Bayesian network models [31] | 2024 | Denmark | Hospital | All patients | HA-UTI | Using Bayesian Network-based machine learning models for risk stratification within 24 h of admission for HA-UTI to enable timely targeted preventive and therapeutic strategies | Information on 50 features (5 features in the reduced version selected based on expert-based knowledge) from 138,250 admissions from 1 January 2017 to 31 December 2018 | The reduced clinical BN model (only five predictive features) reached the highest AUC of 0.746. The seven full BN models reached AUC scores between 0.746 and 0.720 |
| A machine learning approach to predict healthcare-associated infections at intensive care unit admission: findings from the SPIN-UTI project [32] | 2021 | Italy | ICU | All patients | HAI | Development of a machine learning model that combines SAPS II with other patient data to predict the risk of ICU-associated infections at ICU admission | Data from the SPIN-UTI project (20,060 patients from 2006 to 2019). The algorithm combined SAPS II with other variables of the patients not already included in the SAPS II | AUC of 0.90 for the SAPS II + ML model |
| Predictive Analysis of Healthcare-Associated Blood Stream Infections in the Neonatal Intensive Care Unit Using Artificial Intelligence: A Single Center Study [33] | 2022 | Italy | Neonatal intensive care unit | Infants who stayed longer than two days in the level III NICU | HAI | Develop an AI model to predict whether a patient suffered from healthcare-associated bloodstream infection (HABSI) | Data (birth weight, gestational age, sex, length of stay, and duration of exposure to invasive devices) from 1203 neonates from 2016 to 2020 | The logistic regression and multi-layer perceptron models achieved the highest AUC, accuracy, and F1-macro score in predicting HABSI (0.6027, 0.9461, and 0.6439, respectively). They also identified the most important risk factors in the development of HABSI |
| Risk Factors Analysis of Surgical Infection Using Artificial Intelligence: A Single Center Study [34] | 2022 | Italy | Hospital | All patients (excluding those with diabetes and those undergoing corticosteroid therapy) | Surgical site infection | Develop a logistic regression model to analyze the impact of various factors on the risk of SSIs. An AI model has then been employed to predict the risk of infection | Data from electronic health records (gender, age, length of stay, admission type, department, number of antibiotics, presence of surgical site infection) from 4031 patients who underwent surgery between 2015 and 2019 | The K-Nearest-Neighbours model had the best performance, with a 94.9% accuracy and a 95.9% sensitivity and specificity |
| Predicting postoperative surgical site infection with administrative data: a random forests algorithm [35] | 2021 | Canada | Hospital | Patients that underwent surgery | Surgical site infection | Develop a ML model for the prediction of SSIs within 30 days after surgery | Administrative data from 14,351 patients who underwent surgery and enrolled in the National Surgical Quality Improvement Program from the Ottawa Hospital between 1 April 2010 and 31 March 2015 | The full model demonstrated the best results with an AUC of 0.91 (95% CI, 0.90–0.92) |
| Validating administrative data to identify complex surgical site infections following cardiac implantable electronic device implantation: a comparison of traditional methods and machine learning [36] | 2022 | Canada | Hospital | Patients that underwent de novo cardiac implantable electronic device (CIED) implantation or generator replacement | Surgical site infection | Develop a ML model for the identification of SSIs using administrative data | Data from 3536 CIED procedures between 1 January 2013 and 31 December 2019 | The ML model using administrative data achieved an AUC of 96.8% |
| Development of machine learning models for the detection of surgical site infections following total hip and knee arthroplasty: a multicenter cohort study [37] | 2023 | Canada | Hospital | Patients who underwent primary total elective hip (THA) or knee (TKA) arthroplasty | Surgical site infection | Develop a machine learning model to automate the process of SSI detection | Data from 22,059 patients (16,561 TKA and 10.799 THA) between 1 January 2013 and 31 August 2020 | The optimal model achieved an ROC AUC of 0.906, PR AUC of 0.637, F1 score of 0.79, and sensitivity of 83.9% |
| Predicting the occurrence of surgical site infections using text mining and machine learning [38] | 2019 | Brazil | Hospital | Patients that underwent surgery | Surgical site infection | Develop a ML model that uses text mining to predict and detect SSIs | Data from 15,479 surgical descriptions and 12,637 postoperative records | The best performance for predicting SSI was achieved with the Stochastic Gradient Descent method, which had an ROC-AUC of 79.7%. For detection, logistic regression yielded the best performance, with an ROC-AUC of 80.6% |
| Automated healthcare-associated infection surveillance using an artificial intelligence algorithm [39] | 2021 | Brazil | Hospital | All patients | HAI | Develop an AI model for monitoring HAIs | Data spanning 18 months from the electronic health records of 5105 patients | The final model (multi-layer perceptron neural network), achieved an AUROC of 90.27%, with a specificity of 78.86% and a sensitivity of 88.57%. It accurately identified 67 out of 73 patients with HAIs and correctly classified 4637 patients as non-infected |
| Machine Learning Approach to Predict Positive Screening of Methicillin-Resistant Staphylococcus aureus During Mechanical Ventilation Using Synthetic Dataset From MIMIC-IV Database [40] | 2021 | Japan | Hospital | Mechanically ventilated patients | Hospital-acquired pneumonia | Development of a machine learning model that predicts MRSA infection in patients with mechanical ventilation | Data from the MIMIC-IV database (809 mechanically ventilated patients screened for MRSA) | The XGBoost model demonstrated superior performance in predicting MRSA screening positivity, achieving an AUROC of 0.89, sensitivity of 0.98, specificity of 0.47, and a positive predictive value of 0.65. Risk factors identified included admission through the emergency department (ED), central catheter placement, prior quinolone use, haemodialysis, and admission to the Surgical Intensive Care Unit (SICU) |
| Preliminary Evaluation of a Novel Artificial Intelligence-based Prediction Model for Surgical Site Infection in Colon Cancer [41] | 2022 | Japan | General surgery | Patients that underwent surgery for stage II–III colon cancer | Surgical site infection | Development of an AI model to predict the development of surgical site infections in patients with stage II–III colon cancer using immunological and nutritional markers | Data from 730 patients who underwent surgery for stage II–III colon cancer between 2000 and 2018 | The accuracy of the AI model (area under the curve) is 0.73, comparable to previous risk prediction models using statistical analysis |
| Development of machine learning models for the surveillance of colon surgical site infections [42] | 2024 | South Korea | Hospital | Patients who underwent colorectal surgery | Surgical site infection | Develop ML models for the surveillance of SSIs for colon surgery | Data (two datasets with 26 and 33 variables) from 1652 surgical cases between January 2013 and December 2014 | The Deep Neural Network model with Recursive Feature Elimination, utilizing 29 variables from the second database, achieved the highest performance, with an AUC of 0.963 and a PPV of 21.1%. By integrating a rule-based algorithm with a machine learning algorithm and reducing the variables to 19, the PPV increased to 28.9%. This hybrid method also reduced the number of cases needing manual review by 83.9% compared to the conventional method |
| Machine Learning Model for Assessment of Risk Factors and Postoperative Day for Superficial vs. Deep/Organ-Space Surgical Site Infections [43] | 2023 | Pakistan | Hospital | Patients that underwent surgery | Surgical site infection | Develop an ML model to predict type (superficial vs. deep) and timing of SSIs | Data from 113 patients from January 2019 to December 2020 | The best model for predicting the type of SSI was the XGBoost univariate model, which achieved an AUC of 0.84, a PPV of 0.94, and an NPV of 1.57. For predicting the week of development of SSI, five models reached the same highest accuracy, with an AUC of 0.74 |
| Predicting hospital-acquired pneumonia among schizophrenic patients: a machine learning approach [44] | 2019 | Taiwan | Mental hospital | Schizophrenic patients under anti-psychotic drugs | Hospital-acquired pneumonia | Develop a ML model for predicting hospital-acquired pneumonia among schizophrenic patients | Data (gender, age, clozapine use, drug–drug interaction, dosage, duration of medication, coughing, change in leukocyte count, change in neutrophil count, change in blood sugar level, and change in body weight) from medical records of 185 schizophrenic inpatients between 2013 and 2018 | The random forest model gave the best results, with an AUC of 0.994, a sensitivity of 1000, and a specificity of 0.831. The study also identified the six most important risk factors for pneumonia among patients with schizophrenia |
| Personalising intravenous to oral antibiotic switch decision making through fair interpretable machine learning [45] | 2024 | UK | ICU | All patients | HAI | Develop a ML model to predict when a patient could switch from IV to oral antibiotics | Data from 10,362 unique ICU stays (8694 from the MIMIC dataset and 1668 from eICU); 10 clinical features were selected based on the UK antimicrobial IVOS criteria | The best model achieved a mean AUROC of 0.80 and could detect when an individual patient could switch from IV to oral antibiotics |
| Using artificial intelligence to reduce orthopedic surgical site infection surveillance workload: Algorithm design, validation, and implementation in 4 Spanish hospitals [46] | 2023 | Spain | Orthopedic surgery | Patients undergoing hip replacement surgery | Surgical site infection | Develop an AI model to predict SSIs in patients undergoing hip replacement surgery | Data from electronic health records of 6741 patients (7444 surgeries) between January 2014 and May 2024 | The AI model (natural language processing and extreme gradient boosting) demonstrated a sensitivity of 99.18% and a specificity of 91%, with a negative predictive value of 99.98% and an AUC of 0.989. It has been integrated as a screening tool for postoperative patients, significantly reducing the volume of clinical records that need manual evaluation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radaelli, D.; Di Maria, S.; Jakovski, Z.; Alempijevic, D.; Al-Habash, I.; Concato, M.; Bolcato, M.; D’Errico, S. Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review. Healthcare 2024, 12, 1996. https://doi.org/10.3390/healthcare12191996
Radaelli D, Di Maria S, Jakovski Z, Alempijevic D, Al-Habash I, Concato M, Bolcato M, D’Errico S. Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review. Healthcare. 2024; 12(19):1996. https://doi.org/10.3390/healthcare12191996
Chicago/Turabian StyleRadaelli, Davide, Stefano Di Maria, Zlatko Jakovski, Djordje Alempijevic, Ibrahim Al-Habash, Monica Concato, Matteo Bolcato, and Stefano D’Errico. 2024. "Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review" Healthcare 12, no. 19: 1996. https://doi.org/10.3390/healthcare12191996
APA StyleRadaelli, D., Di Maria, S., Jakovski, Z., Alempijevic, D., Al-Habash, I., Concato, M., Bolcato, M., & D’Errico, S. (2024). Advancing Patient Safety: The Future of Artificial Intelligence in Mitigating Healthcare-Associated Infections: A Systematic Review. Healthcare, 12(19), 1996. https://doi.org/10.3390/healthcare12191996






