Abstract
Background: Vitamin B12 is one of the most important B-Vitamins that the human body needs on a daily basis, the lack of which can precipitate several neurological issues. Objectives: This systematic aimed to investigate the neurological implications of Vitamin B12 deficiency and the effects when B12 levels were corrected in susceptible individuals. Methods: The databases PubMed-MEDLINE, Web of Science, Cochrane, and Scopus were all searched using pertinent keywords, reference searches, and citation searches. The terms used to access the database were “Cognition”, “Dietary patterns”, “Neurology”, “Nutritional profile”, and “Vitamin B12”. Results: Vitamin B12 was shown to noticeably improve cognition and other neurological parameters in the short term in older adults and the short-to-medium term in children; however, there was no perceived increase/improvement when the Vitamin was administered in the longer term, either alone or in conjunction with other similar nutritional interventions. Conclusion: Vitamin B12’s role in the improvement of neurological functions over a long-term period remains somewhat inconclusive to date, as the majority of our selected control trials did not display much correlation between the two factors. However, Vitamin B12 did improve cognition levels in both children and older adults over a short course of administration.
1. Introduction
The sophisticated molecular molecule called B12 is water soluble. It is essential for the normal development of people, animals, and even a few microorganisms [1]. It must be obtained through food because the human body is unable to produce enough of it. It has a complex structure and a metallic particle in addition to cobalt. It comes in a variety of forms. However, cobalamin and cyano cobalamin are the two most common forms [2]. It is created by microorganisms in livestock and cows. In cows, it travels from the rumen and other organs to the muscle. Humans ingest cow’s meat, which contains this Vitamin [3]. Dairy goods and eggs are extra nutrient-rich foods. Strict vegetarians who experience B12 deficiency must take supplements enriched with B12 [4]. B12 can be eliminated from the body because it is water soluble. Furthermore, it cannot be stored in fatty acids or adipose cells. A severe B12 deficiency has been linked in some cases [5,6] to neuropathy, pernicious anaemia, ileal resections, and other gastrointestinal complications. Pernicious anaemia is rarely reported in people who are dietary B12 deficient, with the exception of small infants who are solely breastfed or severe vegetarians [5,7].
Other kinds of B12 include methylcobalamin, deoxyadenosylcobalamin, hydroxocobalamin, and cyanocobalamin. Methyl cobalamin, which is also present in dietary supplements, is the most active form of cobalamin discovered in human circulation. This form must be converted by the organism into either methyl cobalamin or 5-deoxyandenosylcobalamin in order to be absorbed [8]. Cobalamin is taken through the ileum’s cubilin receptor. Its complex structure is made up of megalin, cubilin, and amnion-related transmembrane protein (AMN) [9]. Its 460 kDa molecular weight and location in the proximal tubule make it interesting. At a pH of 5.4, which is acidic, absorption happens. Three cobalamin-binding proteins (ascobalo-philins), one carrier protein (haptocorrin), and the R protein are associated with absorption in adult granulocytes and monocytes of progenitor cells. Additionally, it has been discovered in exocrine epithelial cells that secrete saliva, bile, gastric acid, and breast milk [9].
People of all ages, financial statuses, races, and sexes are susceptible to deficiency. It is the most common dietary deficiency in the United States [1]. Early identification and therapy are essential to prevent neurological issues, poor outcomes, and premature mortality [10]. Pernicious anaemia is the most common source of B12 deficiency. An intrinsic factor required for gastrointestinal absorption of Vitamin B12 from the diet is absent in this auto-immune disease. However, women, people over 60, and those who have auto-immune illnesses such as Addison’s disease and vitiligo are more likely to have the disorder [11]. The illness known as autoimmune gastrectomy (AG) happens when the body produces antibodies against healthy stomach cells that are usually produced against viruses and bacteria. Healthy stomach cells that generate acidic fluids are impacted by autoimmune AG. The intrinsic component that causes pernicious anaemia is also affected by these antibodies.
These days, people of all ages have psychiatric problems, acute anxiety, and sadness. Although these people are given expensive psychiatric medications, opioids, or benzodiazepines, in many instances, there is also the presence of an underlying moderate-to-severe level of Vitamin B12 deficiency [3]. Hence, through this systematic review and meta-analysis, we aimed to shed light upon the neurological implications of diet in cases in which the individual is suffering from a Vitamin B12 deficiency, and if the introduction of this Vitamin into the diet can help to ameliorate some of the symptoms that accompany the neurological ailment. We aimed to analyze the effect of Vitamin B12 in the human diet and its correlation with the neurological health of an individual.
2. Materials and Methods
2.1. Protocol Employed
This systematic review was performed as per the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) (Figure 1) strategy and rules from the Cochrane group, and using the book Orderly Reviews in Healthcare: Meta-Examination [12], with the PROSPERO registration number being CRD42023387063.
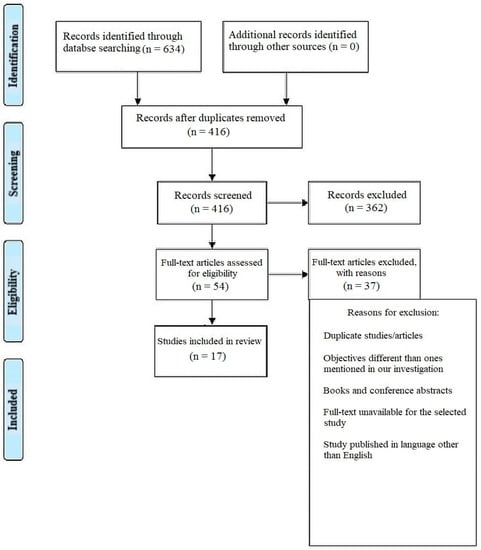
Figure 1.
Representation of selection of articles through PRISMA framework.
2.2. Review Hypotheses
Through this systematic review, our primary objective was to review studies that were published in the neurological literature and discussed the effects of Vitamin B12 deficiency on the neurological parameters of individuals, such as cognition, overall health and/or any pre-existing neurological/systemic disorder.
2.3. Study Selection Process
There were a total of 634 documents discovered after extensive search of the online journals, and 416 of the papers were selected initially. Following that, 362 similar/duplicate articles were eliminated, which resulted in 54 separate papers being available. The abstracts and titles of submissions were then reviewed, and a further 37 papers were eliminated. Finally, 17 documents that met the requisite inclusion and exclusion criteria were chosen; these primarily included in vitro studies, literature reviews and comparative assessments.
2.4. Inclusion Criterion
Articles that contained relevant data for our review objectives were selected for full-text screening. Studies that reported clinical trials, in vitro studies, randomised/non-randomised studies, systematic/literature reviews containing substantial sample volume and detailed case reports were considered for inclusion in our review. We also monitored studies that possessed higher methodological quality.
2.5. Exclusion Criteria
The following were excluded from the scope of our systematic review: incomplete data, seminar presentations, scholarly articles, placebo-controlled studies, and opinion articles.
Since the literature available on this topic was quite scant in volume, we did not limit our search in terms of the time period in which the studies were published, i.e., we took into account all the papers that were published within the context of our topic (where the number of papers itself was found to be quite sparse in number). Additionally, literature reviews and cases published in languages other than English were excluded.
2.6. Search Strategy
Using relevant keywords, reference searches, and citation searches, the databases PubMed-MEDLINE, Web of Science, Cochrane, and Scopus were all searched. “Cognition”, “Dietary patterns”, “Neurology”, “Nutritional profile” and “Vitamin B12” were the search terms used to access the database.
2.7. Data Selection and Coding
Two independent reviewers located the relevant papers by using the right keywords in various databases and online search tools. The chosen articles were compared, and a third reviewer was brought in if there was a dispute.
After choosing the articles, the same two reviewers independently extracted the following data: author, year of publication, country, kind of publication, study topic, population demographics (n, age), outcome measure(s), relevant result(s), and conclusion(s). The data were compared and any differences were discussed with the third reviewer.
2.8. Risk of Bias Assessment
The AMSTAR-2 technique [13] was used to evaluate the risk of bias in the studies we chose (Table 1). AMSTAR 2 joins a number of other instruments that have been released for this purpose, as a critical evaluation tool for systematic reviews. As seen in Table 2 below, it is a 16-point checklist. Two instruments that have drawn a lot of attention served as the foundation for the creation of the original AMSTAR tool. The original AMSTAR was duplicated in two newly produced instruments. The AMSTAR items identify the domains specified in the Cochrane risk of bias instruments for systematic reviews. In each case, these indicate an agreement that was achieved after input from more than 30 methodology experts.

Table 1.
AMSTAR-2 16-point checklist of risk of bias assessment in studies selected for the systematic review.

Table 2.
Description and outcomes as observed in the studies selected for the systematic review.
2.9. Statistical Analysis
After selecting data on the sample size, variables analyzed, and various elements of the investigations, the data were then entered into the Revman 5 programme for meta-analysis. Forest plots illustrating the risk ratio (RR), and risk difference (RD) for different study methodologies were obtained as part of the meta-analysis for our study, as shown in Figure 2, Figure 3, Figure 4 and Figure 5.
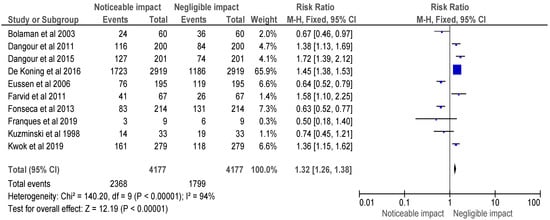
Figure 2.
Risk ratio of selected randomised control trials represented on a forest plot after their meta-analysis, in which the noticeable impact of Vitamin B12 interventions compared to their negative to negligible impact was assessed (where the blue dot represents the study with the most weight and the rhombus represents the total weight of the forest plot) [15,16,17,18,19,20,21,22,26,27].
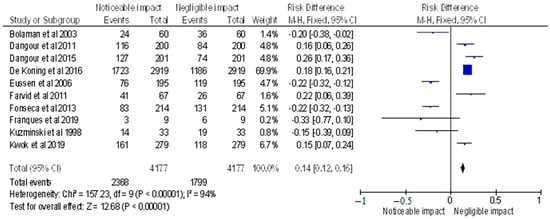
Figure 3.
Risk difference of selected randomised control trials represented on a forest plot after their meta-analysis, in which the noticeable impact of Vitamin B12 interventions compared to their negative to negligible impact was assessed (where the blue dot represents the study with the most weight and the rhombus represents the total weight of the forest plot) [15,16,17,18,19,20,21,22,26,27].
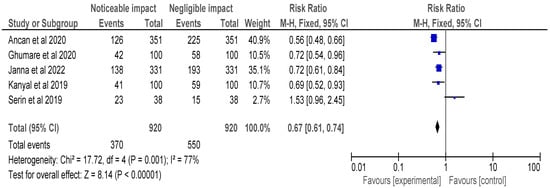
Figure 4.
Risk ratio of selected cross-sectional, cohort and retrospective investigations represented on a forest plot after their meta-analysis, in which the noticeable impact of Vitamin B12 interventions compared to their negative to negligible impact was assessed (where the blue dot represents the study with the most weight and the rhombus represents the total weight of the forest plot) [14,23,24,25,30].
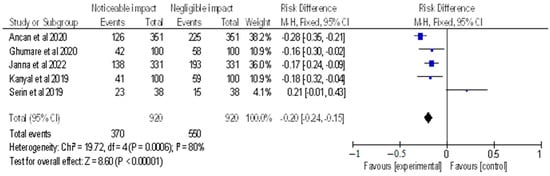
Figure 5.
Risk difference of selected cross-sectional, cohort and retrospective investigations represented on a forest plot after their meta-analysis, in which the noticeable impact of Vitamin B12 interventions compared to their negative to negligible impact was assessed (where the blue dot represents the study with the most weight and the rhombus represents the total weight of the forest plot) [14,23,24,25,30].
3. Results
The AMSTAR-2 [14] checklist is displayed in Table 1. The study design, methodology employed, description and outcome are mentioned in Table 2. The results of the meta-analysis are provided in Figure 2, Figure 3, Figure 4 and Figure 5.
The meta-analysis of the included selected randomised control trials is represented in Figure 2, and a forest plot was used to represent the RR of the studies, which was found to be 1.32 [1.26, 1.38]. The analysis revealed a noticeable impact of Vitamin B12 interventions, as compared to its negative to negligible impact. Heterogeneity was evaluated using Chi2 = 140.20, df = 9 (p < 0.00001); I2 = 94%, indicating significant heterogeneity among the studies. The test for overall effect was conducted using Z = 12.19 (p < 0.00001), indicating a statistically significant effect of Vitamin B12 interventions on neurological outcomes. These findings suggest that Vitamin B12 supplementation may have a beneficial impact on neurological function in individuals with a deficiency. However, further research is needed to determine the optimal dosing and duration of Vitamin B12 interventions in this population.
The meta-analysis included selected randomised control trials, and the forest plot depicted the RD of the studies (displayed in Figure 3), which was found to be 0.14 [0.12, 0.16]. The analysis revealed a noticeable impact of Vitamin B12 interventions, as compared to its negative to negligible impact. Heterogeneity was assessed using Chi2 = 157.23, df = 9 (p < 0.00001); I2 = 94%, indicating significant heterogeneity among the studies. The test for overall effect was conducted using Z = 12.68 (p < 0.00001), indicating a statistically significant effect of Vitamin B12 interventions on neurological outcomes. These findings suggest that Vitamin B12 supplementation may have a positive impact on neurological function in individuals with a deficiency. However, further research is required to determine the optimal dosage and duration of Vitamin B12 interventions in this population. The results of this meta-analysis provide evidence for the importance of adequate Vitamin B12 intake in maintaining neurological health.
Figure 4’s meta-analysis included selected cross-sectional, cohort, and retrospective investigations and the forest plot depicted the RR of the studies, which was found to be 0.67 [0.61, 0.74]. The analysis revealed a noticeable impact of Vitamin B12 interventions, as compared to their negative to negligible impact. Heterogeneity was assessed using Chi2 = 17.72, df = 4 (p = 0.001); I2 = 77%, indicating significant heterogeneity among the studies. The test for overall effect was conducted using Z = 8.14 (p < 0.00001), indicating a statistically significant effect of Vitamin B12 interventions on neurological outcomes. These findings suggest that Vitamin B12 supplementation may have a protective effect on neurological function in individuals with a deficiency. However, further research is needed to determine the optimal dosing and duration of Vitamin B12 interventions in this population. It is important to note that the studies included in this meta-analysis were observational in nature, and therefore, causality cannot be established. Overall, the results of this meta-analysis provide support for the importance of adequate Vitamin B12 intake in maintaining neurological health.
The meta-analysis (as shown in Figure 5) included selected cross-sectional, cohort, and retrospective investigations, and the forest plot depicted the RD of the studies, which was found to be −0.20 [−0.24, −0.15]. The analysis revealed a noticeable impact of Vitamin B12 interventions, as compared to their negative to negligible impact. Heterogeneity was assessed using Chi2 = 19.72, df = 4 (p = 0.0006); I2 = 80%, indicating significant heterogeneity among the studies. The test for overall effect was conducted using Z = 8.60 (p < 0.00001), indicating the statistically significant effect of Vitamin B12 interventions on neurological outcomes. These findings suggest that Vitamin B12 supplementation may have a beneficial impact on neurological function in individuals with a deficiency. However, further research is needed to determine the optimal dosing and duration of Vitamin B12 interventions in this population. The results of this meta-analysis provide evidence for the importance of adequate Vitamin B12 intake in maintaining neurological health. It is important to note that the studies included in this meta-analysis were observational in nature, and therefore, causality cannot be established.
4. Discussion
This systematic review and meta-analysis included a total of 17 studies and aimed to assess the neurological implications of Vitamin B12 deficiency in diet. The meta-analysis included selected randomised control trials and observational studies, which were analyzed using appropriate statistical methods.
The findings of the meta-analysis revealed a noticeable impact of Vitamin B12 interventions, as compared to its negative to negligible impact, on neurological outcomes in individuals with a deficiency. The meta-analysis showed that the risk ratio and risk difference for selected randomised control trials were 1.32 [1.26, 1.38] and 0.14 [0.12, 0.16], respectively, while for selected cross-sectional, cohort, and retrospective investigations, they were −0.20 [−0.24, −0.15] and 0.67 [0.61, 0.74], respectively. The heterogeneity across the studies was also assessed and found to be significant.
The significant heterogeneity in the studies suggests that the optimal dosing and duration of Vitamin B12 interventions in individuals with a deficiency require further investigation. However, the findings of this meta-analysis highlight the importance of adequate Vitamin B12 intake in the diet for maintaining neurological health. In summary, this systematic review and meta-analysis provide important insights into the role of Vitamin B12 in maintaining neurological health, particularly in individuals with a deficiency. The findings underscore the need for healthcare providers to ensure that individuals with a risk of Vitamin B12 deficiency receive appropriate dietary counselling and supplementation to prevent or alleviate neurological symptoms associated with a deficiency. The results of this study have important implications for public health policy and clinical practice, and provide valuable information for future research in this area.
Adults between the ages of 40 and 90 are more likely than younger adults to experience psychiatric issues as a result of B12 deficiency [31]. Cognitive changes such as memory loss, depression, delusions, hallucinations, and dementia are some of the psychiatric manifestations [32]. The causes include unstable neurotransmitter production, high homocysteine levels and elevated levels of methylmalonic acid (MMA) in B12 deficient individuals. If there is no other obvious cause of a psychiatric disorder, screening and B12 supplementation should be taken into consideration. The development of the foetus’ brain also depends critically on Vitamin B12 and folate. In addition, both are essential for infants’ myelination during the first two years of life and until puberty [33]. Depending on the part of the nervous system that is affected by B12 deficiency, a child may develop a variety of cognitive and intellectual issues. In order to prevent neurological disorders in the developing foetus, pregnant women who are deficient in both need to take supplements. Since elderly people have trouble absorbing this Vitamin from food sources, supplements can be used to make up for any deficiencies [34]. Supplements may be used to restore deficiencies that vegans may experience.
Older people frequently lack Vitamin B12, primarily due to malabsorption [24]. Cognitive impairment is linked to a high prevalence of Vitamin B12 status impairment. Such correlations might not, however, be causal. Additionally, although the estimated percentage of real reversible dementia in people with Vitamin B12 insufficiency is low [35], poor Vitamin B12 metabolism may be one of several variables that contribute to the onset of cognitive impairment and dementia and affect the disease’s course. Unknown factors that may contribute to this phenomenon include decreased methylation capacity, increased homocysteine concentrations and B Vitamins’ role in maintaining the blood–brain barrier’s integrity [36].
Since norms for cognitive performance [37] are rarely population specific and there are no age-specific reference data for neurologic function in older adults [38], interpretation is difficult. It is possible that study participants’ neurologic and cognitive function was not compromised at the start of the investigation. However, regardless of baseline function, the goal of our study was to find any neurologic and cognitive benefits of Vitamin B12 supplementation in older persons with moderate Vitamin B12 deficiency. The trial participants in our review were generally healthy, so it is possible that the findings do not apply to the entirety of the elderly population. Additionally, the course of treatment might have been too brief, and any effects of Vitamin B12 supplementation might not have been seen until years later or during follow-up [39]. To our knowledge, no relevant experiment using Vitamin B12 supplementation for a duration longer than two years has been carried out [39]. Direct links between nerve conduction and Vitamin B12 levels have been observed in observational studies [40], although this finding has not always been the case [40,41]. No prior randomised controlled trial has, to our knowledge, examined the effect of Vitamin B12 supplementation on neurologic function in elderly individuals. Prior studies examining the impact of Vitamin B12 supplementation on cognitive performance mostly failed to demonstrate any positive effects, although they were of inconsistent quality, small scale, and short duration [39,42]. Recently, there has been some indication of the benefit of taking numerous B Vitamins, particularly in subgroups of people with worse biochemical state when randomly assigned at baseline [43]. Our systematic study adds solid information on the impact of Vitamin B12 on cognitive performance as people age, and the results are in line with a recent meta-analysis that found no association between Vitamin B12 intake and cognitive ageing [44].
Our study included a higher percentage of randomised clinical trials, which could be said to be a major drawback, alongside the fact that the overall number of investigations that we selected for assessment and subsequent meta-analysis might be deemed to be less than ideal. The lack of studies assessing the long-term effects (>2 years for example) of Vitamin B12 on the neurological parameters of individuals can be identified as another limitation of this review, but the fact that no such current investigation could be found in the literature warrants further research in this field so that a unified consensus can be reached with respect to the effect of Vitamin B12 in alleviating neurological decline in susceptible individuals.
Despite the valuable insights provided by this systematic review and meta-analysis, there are some limitations to consider. Firstly, the studies included in the meta-analysis were heterogeneous in terms of study design, participant characteristics, dosages, and the duration of Vitamin B12 interventions. Secondly, the studies included were conducted in different populations with varying risk factors for Vitamin B12 deficiency. This could have introduced bias into the findings of the meta-analysis. Thirdly, the studies were conducted in different countries and settings, which could have influenced the results due to variations in dietary and lifestyle factors. Fourthly, the quality of the studies included in the meta-analysis varied, which could have affected the robustness of the findings. Lastly, the meta-analysis did not assess potential publication bias, which could have influenced the findings. Therefore, caution should be taken when interpreting the results of this meta-analysis, and further research is needed to address these limitations and provide more conclusive evidence regarding the role of Vitamin B12 in neurological health.
5. Conclusions
Through this systematic review and subsequent meta-analysis, it was determined that the majority of the studies that were chosen did not find any advantages of daily Vitamin B12 supplementation over a long period of time (around 1 year or more) on neurologic or cognitive function in older people with moderate Vitamin B12 deficiency who were asymptomatic, nonanemic, and without anaemia. These findings are directly applicable to modern clinical practice, which has identified low Vitamin B12 status as a risk factor for neurologic and cognitive impairments, particularly in older adults. Further, the current study’s findings raise concerns about the usefulness of screening for mild Vitamin B12 deficiency in the absence of anaemia and signs of neurologic or cognitive impairment, and point to the need for stricter definitions of Vitamin B12 deficiency.
Author Contributions
Conceptualization, R.B., M.A., R.A., A.M.A. and N.H.A.; methodology, R.B., M.A. and R.A.; software, R.B., M.A. and R.A.; validation, R.B., M.A. and R.A.; formal analysis, R.B., M.A. and R.A.; investigation, R.B., M.A. and R.A.; resources, R.B. and M.A.; data curation, R.B., M.A. and R.A.; writing—original draft preparation, R.B., M.A., R.A., A.M.A. and N.H.A.; writing—review and editing, R.B., M.A., R.A., A.M.A. and N.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Allen, L.H. Folate and Vitamin B12 status in the Americas. Nutr. Rev. 2004, 62, S29–S33. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.F.; Madsen, M.; Storm, T.; Moestrup, S.K.; Andersen, G.R. Structural basis for receptor recognition of Vitamin-B 12–intrinsic factor complexes. Nature 2010, 464, 445. [Google Scholar] [CrossRef] [PubMed]
- Selhub, J.; Jacques, P.F.; Dallal, G.; Choumenkovitch, S.; Rogers, G. The use of blood concentrations of Vitamins and their respective functional indicators to define folate and Vitamin B12 status. Food Nutr. Bull. 2008, 29 (Suppl. S1), S67–S73. [Google Scholar] [CrossRef]
- Black, M.M. Effects of Vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29 (Suppl. S1), S126–S131. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, P.C.; van Staveren, W.A.; Vergote, F.J.; Dingjan, P.G.; Van Den Berg, H.; Hautvast, J. Increased risk of Vitamin B-12 and iron deficiency in infants on macrobiotic diets. Am. J. Clin. Nutr. 1989, 50, 818–824. [Google Scholar] [CrossRef]
- Kozyraki, R.; Fyfe, J.; Kristiansen, M.; Gerdes, C.; Jacobsen, C.; Cui, S.; Christensen, E.I.; Aminoff, M.; De La Chapelle, A.; Krahe, R.; et al. The intrinsic factor–Vitamin B 12 receptor, cubilin, is a high-affinity apolipoprotein AI receptor facilitating endocytosis of high-density lipoprotein. Nat. Med. 1999, 5, 656. [Google Scholar] [CrossRef]
- Fyfe, J.C.; Madsen, M.; Højrup, P.; Christensen, E.I.; Tanner, S.M.; de la Chapelle AMoestrup, S.K. The functional cobalamin (Vitamin B12)–intrinsic factor receptor is a novel complex of cubilin and amnionless. Blood 2004, 103, 1573–1579. [Google Scholar] [CrossRef]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.-R.; Jacobsen, C.; Nielsen, M.S.; Moestrup, S.K. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25 (OH) Vitamin D3. Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef]
- de Benoist, B. Conclusions of a WHO Technical Consultation on folate and Vitamin B12 deficiencies. Food Nutr. Bull. 2008, 29 (Suppl. S1), S238–S244. [Google Scholar] [CrossRef]
- de Jager, C.A. Critical levels of brain atrophy associated with homocysteine and cognitive decline. Neurobiol. Aging 2014, 35, S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA Reporting Guidelines for Meta-analyses and Systematic Reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Arıcan, P.; Bozkurt, O.; Cavusoglu, D.; Gencpınar, P.; Haspolat, S.; Duman, O.; Olgac Dundar, N. Various Neurological Symptoms with Vitamin B12 Deficiency and Posttreatment Evaluation. J. Pediatr. Neurosci. 2020, 15, 365–369. [Google Scholar] [CrossRef]
- Bolaman, Z.; Kadikoylu, G.; Yukselen, V.; Yavasoglu, I.; Barutca, S.; Senturk, T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: A single-center, prospective, randomized, open-label study. Clin. Ther. 2003, 25, 3124–3134. [Google Scholar] [CrossRef]
- Dangour, A.D.; Allen, E.; Clarke, R.; Elbourne, D.; Fasey, N.; Fletcher, A.E.; Letley, L.; Richards, M.; Whyte, K.; Mills, K.; et al. A randomised controlled trial investigating the effect of Vitamin B12 supplementation on neurological function in healthy older people: The Older People and Enhanced Neurological function (OPEN) study protocol [ISRCTN54195799]. Nutr. J. 2011, 10, 22. [Google Scholar] [CrossRef]
- Dangour, A.D.; Allen, E.; Clarke, R.; Elbourne, D.; Fletcher, A.E.; Letley, L.; Richards, M.; Whyte, K.; Uauy, R.; Mills, K. Effects of Vitamin B-12 supplementation on neurologic and cognitive function in older people: A randomized controlled trial. Am. J. Clin. Nutr. 2015, 102, 639–647. [Google Scholar] [CrossRef]
- de Koning, E.J.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Sohl, E.; Brouwer-Brolsma, E.M.; van Marwijk, H.W.; Enneman, A.W.; Swart, K.M.; van Dijk, S.C.; Ham, A.C.; et al. Effects of Two-Year Vitamin B12 and Folic Acid Supplementation on Depressive Symptoms and Quality of Life in Older Adults with Elevated Homocysteine Concentrations: Additional Results from the B-PROOF Study, an RCT. Nutrients 2016, 8, 748. [Google Scholar] [CrossRef]
- Eussen, S.J.; de Groot, L.C.; Joosten, L.W.; Bloo, R.J.; Clarke, R.; Ueland, P.M.; Schneede, J.; Blom, H.J.; Hoefnagels, W.H.; van Staveren, W.A. Effect of oral Vitamin B-12 with or without folic acid on cognitive function in older people with mild Vitamin B-12 deficiency: A randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 84, 361–370. [Google Scholar] [CrossRef]
- Farvid, M.S.; Homayouni, F.; Amiri, Z.; Adelmanesh, F. Improving neuropathy scores in type 2 diabetic patients using micronutrients supplementation. Diabetes Res. Clin. Pract. 2011, 93, 86–94. [Google Scholar] [CrossRef]
- Fonseca, V.A.; Lavery, L.A.; Thethi, T.K.; Daoud, Y.; DeSouza, C.; Ovalle, F.; Denham, D.S.; Bottiglieri, T.; Sheehan, P.; Rosenstock, J. Metanx in type 2 diabetes with peripheral neuropathy: A randomized trial. Am. J. Med. 2013, 126, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Franques, J.; Chiche, L.; De Paula, A.M.; Grapperon, A.M.; Attarian, S.; Pouget, J.; Mathis, S. Characteristics of patients with Vitamin B12-responsive neuropathy: A case series with systematic repeated electrophysiological assessment. Neurol. Res. 2019, 41, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Ghumare, S.S.; Chandanwale, A.S.; Jadhav, P.; Arya, S.; Rawat, S.S.; Sankar, S. A study of relationship between Vitamin b12 levels and neurological function in patients with cervical spondylotic myelopathy. Int. J. Orthop. Sci. 2020, 6, 611–617. [Google Scholar] [CrossRef]
- Warendorf, J.K.; van Doormaal, P.T.; Vrancken, A.F.; Verhoeven-Duif, N.M.; van Eijk, R.P.; van den Berg, L.H.; Notermans, N.C. Notermans Clinical relevance of testing for metabolic Vitamin B12 deficiency in patients with polyneuropathy. Nutr. Neurosci. 2022, 25, 2536–2546. [Google Scholar] [CrossRef]
- Lata Kanyal, M.T.; Mujawar, A. Status of Vitamin b12 in type 2 diabetes mellitus patients taking metformin based oral hypoglycemic agent-a cross sectional study. Indian J. Basic Appl. Med. Res. 2019, 1, 18–26. [Google Scholar]
- Kuzminski, A.M.; Del Giacco, E.J.; Allen, R.H.; Stabler, S.P.; Lindenbaum, J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood 1998, 92, 1191–1198. [Google Scholar] [CrossRef]
- Kwok, T.; Wu, Y.; Lee, J.; Lee, R.; Yung, C.Y.; Choi, G.; Lee, V.; Harrison, J.; Lam, L.; Mok, V. A randomized placebo-controlled trial of using B Vitamins to prevent cognitive decline in older mild cognitive impairment patients. Clin. Nutr. 2020, 39, 2399–2405. [Google Scholar] [CrossRef]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef]
- Nawaz, A.; Khattak, N.N.; Khan, M.S.; Nangyal, H.; Sabri, S.; Shakir, M. Deficiency of Vitamin B12 and its relation with neurological disorders: A critical review. J. Basic Appl. Zool. 2020, 81, 10. [Google Scholar] [CrossRef]
- Serin, H.M.; Arslan, E.A. Neurological symptoms of Vitamin B12 deficiency: Analysis of pediatric patients. Acta Clin. Croat. 2019, 58, 295–302. [Google Scholar] [CrossRef]
- Yuan, S.; Mason, A.M.; Carter, P.; Burgess, S.; Larsson, S.C. Homocysteine, B Vitamins, and cardiovascular disease: A Mendelian randomization study. BMC Med. 2021, 19, 97. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimers Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Reischl-Hajiabadi, A.T.; Garbade, S.F.; Feyh, P.; Weiss, K.H.; Mütze, U.; Kölker, S.; Hoffmann, G.F.; Gramer, G. Maternal Vitamin B12 Deficiency Detected by Newborn Screening—Evaluation of Causes and Characteristics. Nutrients 2022, 14, 3767. [Google Scholar] [CrossRef] [PubMed]
- Kurpad, A.V.; Ghosh, S.; Thomas, T.; Bandyopadhyay, S.; Goswami, R.; Gupta, A.; Gupta, P.; John, A.T.; Kapil, U.; Kulkarni, B.; et al. Perspective: When the cure might become the malady: The layering of multiple interventions with mandatory micronutrient fortification of foods in India. Am. J. Clin. Nutr. 2021, 114, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Campana, M.; Löhrs, L.; Strauß, J.; Münz, S.; Oviedo-Salcedo, T.; Fernando, P.; Maurus, I.; Raabe, F.; Moussiopoulou, J.; Eichhorn, P.; et al. Blood-brain-barrier dysfunction and folate and Vitamin B12 levels in first-episode psychosis. Eur. Arch. Psychiatry Clin. Neurosci. 2022; preprint. [Google Scholar] [CrossRef]
- Song, Y.; Quan, M.; Li, T.; Jia, J. Serum Homocysteine, Vitamin B 12, Folate, and Their Association with Mild Cognitive Impairment and Subtypes of Dementia. J. Alzheimers Dis. 2022, 681–691. [Google Scholar] [CrossRef]
- Kimura, J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice, 4th ed.; Oxford University Press: New York, NY, USA, 2013. [Google Scholar]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Olaso-Gonzalez, G.; Inzitari, M.; Bellelli, G.; Morandi, A.; Barcons, N.; Viña, J. Impact of supplementation with Vitamins B6, B12, and/or folic acid on the reduction of homocysteine levels in patients with mild cognitive impairment: A systematic review. IUBMB Life 2022, 74, 74–84. [Google Scholar] [CrossRef]
- Brenowitz, W.D.; Robbins, N.M.; Strotmeyer, E.S.; Yaffe, K. Associations of Lower Extremity Peripheral Nerve Impairment and Risk of Dementia in Black and White Older Adults. Neurology 2022, 98, e1837–e1845. [Google Scholar] [CrossRef]
- Acharya, M.; Jena, S.K. Reference values of dorsal sural sensory nerve action potential: A useful tool to diagnose peripheral neuropathy. Muller J. Med. Sci. Res. 2021, 12, 13–16. [Google Scholar]
- Balk, E.M.; Raman, G.; Tatsioni, A.; Chung, M.; Lau, J.; Rosenberg, I.H. Vitamin B6, B12, and folic acid supplementation and cognitive function: A systematic review of randomized trials. Arch. Intern. Med. 2007, 167, 21–30. [Google Scholar] [CrossRef]
- Macaron, T.; Giudici, K.V.; Bowman, G.L.; Sinclair, A.; Stephan, E.; Vellas, B.; de Souto Barreto, P. Associations of Omega-3 fatty acids with brain morphology and volume in cognitively healthy older adults: A narrative review. Ageing Res. Rev. 2021, 67, 101300. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B Vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).