The Role of the Prognostic Inflammatory and Nutritional Index (PINI) in the Evolution of Patients with Chronic Kidney Disease and Other Pathologies
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Sources
2.2. Inclusion and Exclusion Criteria
2.3. Date Abstraction
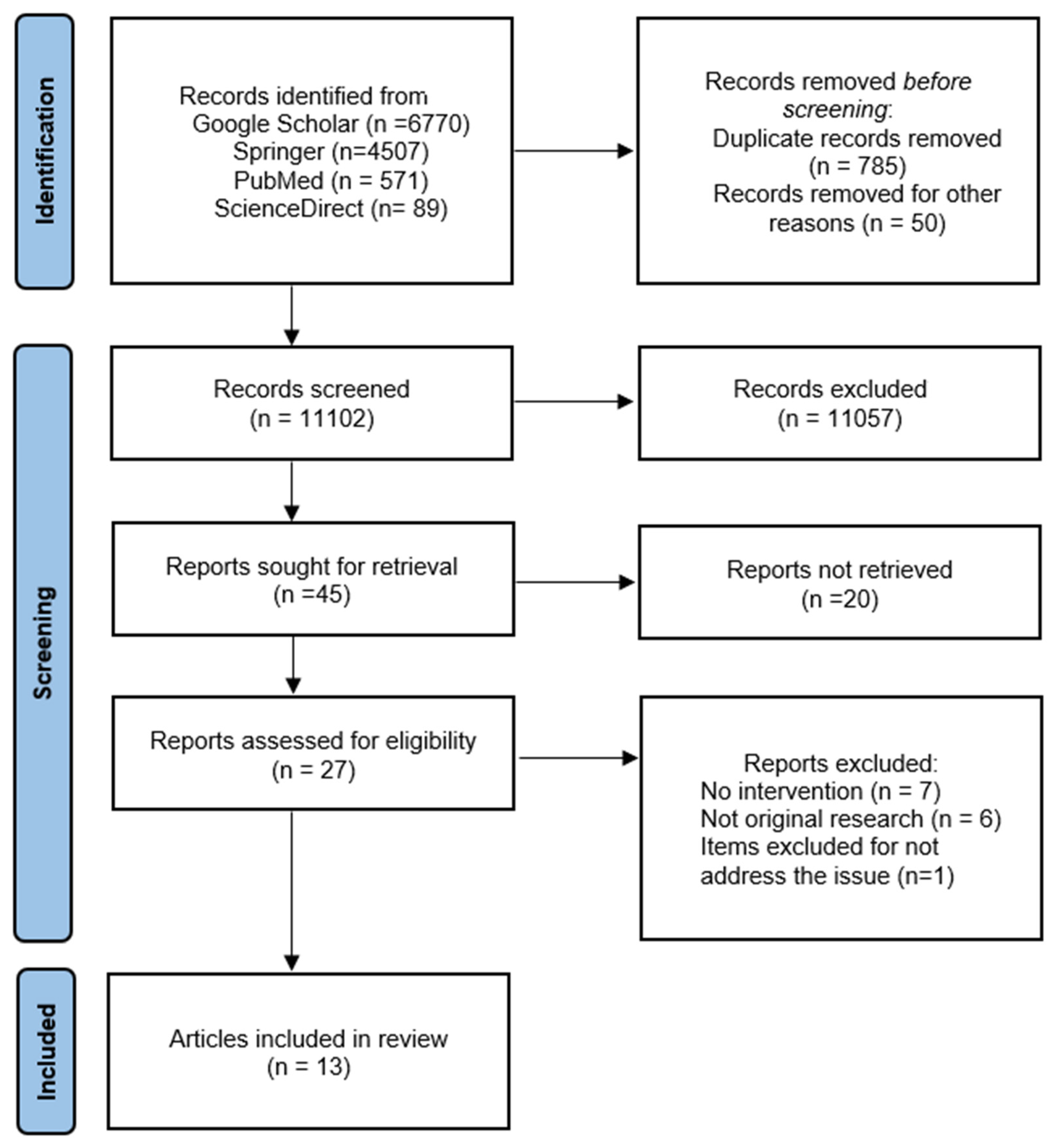
3. Results
3.1. Search Results
3.2. Study Characteristics
3.3. Association with Outcomes
3.3.1. The PINI Score in Intensive Care Unit (ICU)
3.3.2. The PINI Score in Hemodialysis (HD) Patients
3.3.3. The PINI Score in Solid and Haematological Malignancies
3.3.4. The PINI Score in Chronic Diseases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef] [PubMed]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and Inflammation in Cognitive Ageing and Alzheimer’s Disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Stumpf, F.; Keller, B.; Gressies, C.; Schuetz, P. Inflammation and Nutrition: Friend or Foe? Nutrients 2023, 15, 1159. [Google Scholar] [CrossRef]
- Perrone, F.; da-Silva-Filho, A.C.; Adorno, I.F.; Anabuki, N.T.; Leal, F.S.; Colombo, T.; da Silva, B.D.; Dock-Nascimento, D.B.; Damiao, A.; de Aguilar-Nascimento, J.E. Effects of preoperative feeding with a whey protein plus carbohydrate drink on the acute phase response and insulin resistance. A randomized trial. Nutr. J. 2011, 10, 66. [Google Scholar] [CrossRef]
- Costa, M.D.; Vieira de Melo, C.Y.; Amorim, A.C.; Cipriano Torres, D.D.O.; Dos Santos, A.C. Association Between Nutritional Status, Inflammatory Condition, and Prognostic Indexes with Postoperative Complications and Clinical Outcome of Patients with Gastrointestinal Neoplasia. Nutr. Cancer 2016, 68, 1108–1114. [Google Scholar] [CrossRef]
- Ingenbleek, Y.; Carpentier, Y.A. A prognostic inflammatory and nutritional index scoring critically ill patients. Int. J. Vitam. Nutr. Res. 1985, 55, 91–101. [Google Scholar]
- Dessi, M.; Noce, A.; Agnoli, A.; De Angelis, S.; Fuiano, L.; Tozzo, C.; Taccone-Gallucci, M.; Fuiano, G.; Federici, G. The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 811–815. [Google Scholar] [CrossRef]
- Gharsallah, H.; Hajjej, Z.; Naas, I.; Aouni, Z.; Stambouli, N.; Labbène, I.; Ferjani, M. Assessment of nutritional status and prognosis in surgical intensive care unit: The prognostic and inflammatory nutritional index (PINI). Int. J. Nutr. Food Sci. 2014, 3, 477–483. [Google Scholar] [CrossRef]
- Forget, P.; Echeverria, G.; Giglioli, S.; Bertrand, B.; Nikis, S.; Lechat, J.P.; De Kock, M. Biomarkers in immunonutrition programme, is there still a need for new ones? A brief review. Ecancermedicalscience 2015, 9, 546. [Google Scholar] [CrossRef]
- Wiese, D.; Lashner, B.; Seidner, D. Measurement of nutrition status in Crohn’s disease patients receiving infliximab therapy. Nutr. Clin. Pract. 2008, 23, 551–556. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Jenkins, R.O.; Goncharov, N.V. Serum Albumin in Health and Disease: Esterase, Antioxidant, Transporting and Signaling Properties. Int. J. Mol. Sci. 2021, 22, 10318. [Google Scholar] [CrossRef]
- De Simone, G.; di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zheng, S.; Li, M.; Zhang, M.; Sun, M.; Li, X.; Deng, A.; Cai, Y.; Zhang, H. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark. Med. 2020, 14, 827–837. [Google Scholar] [CrossRef]
- Lenartova, P.; Kopcekova, J.; Gazarova, M.; Mrazova, J.; Wyka, J. Biochemical parameters as monitoring markers of the inflammatory reaction by patients with chronic obstructive pulmonary disease (COPD). Rocz. Państwowego Zakładu Hig. 2017, 68, 185–190. [Google Scholar]
- Noh, E.; Moon, J.M.; Chun, B.J.; Cho, Y.S.; Ryu, S.; Kim, D. The clinical role of serum albumin in Organophospate poisoning. Basic Clin. Pharmacol. Toxicol. 2021, 128, 605–614. [Google Scholar] [CrossRef]
- Noh, S.; Kim, J.; Kim, G.; Park, C.; Jang, H.; Lee, M.; Lee, T. Recent Advances in CRP Biosensor Based on Electrical, Electrochemical and Optical Methods. Sensors 2021, 21, 3024. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-Reactive Protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef]
- Reynolds, T.M.; Stokes, A.; Russell, L. Assessment of a prognostic biochemical indicator of nutrition and inflammation for identification of pressure ulcer risk. J. Clin. Pathol. 2006, 59, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Schlossmacher, P.; Hasselmann, M.; Meyer, N.; Kara, F.; Delabranche, X.; Kummerlen, C.; Ingenbleek, Y. The prognostic value of nutritional and inflammatory indices in critically ill patients with acute respiratory failure. Clin. Chem. Lab. Med. 2002, 40, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Vehe, K.L.; Brown, R.O.; Kuhl, D.A.; Boucher, B.A.; Luther, R.W.; Kudsk, K.A. The prognostic inflammatory and nutritional index in traumatized patients receiving enteral nutrition support. J. Am. Coll. Nutr. 1991, 10, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Verove, C.; Maisonneuve, N.; El Azouzi, A.; Boldron, A.; Azar, R. Effect of the correction of metabolic acidosis on nutritional status in elderly patients with chronic renal failure. J. Ren. Nutr. 2002, 12, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Terrier, N.; Senecal, L.; Dupuy, A.M.; Jaussent, I.; Delcourt, C.; Leray, H.; Rafaelsen, S.; Bosc, J.Y.; Maurice, F.; Canaud, B.; et al. Association between novel indices of malnutrition-inflammation complex syndrome and cardiovascular disease in hemodialysis patients. Hemodial. Int. 2005, 9, 159–168. [Google Scholar] [CrossRef]
- Di Renzo, L.; Noce, A.; De Angelis, S.; Miani, N.; Di Daniele, N.; Tozzo, C.; De Lorenzo, A. Anti-inflammatory effects of combined treatment with acetyl salicylic acid and atorvastatin in haemodialysis patients affected by Normal Weight Obese syndrome. Pharmacol. Res. 2008, 57, 93–99. [Google Scholar] [CrossRef]
- Nelson, K.A.; Walsh, D. The cancer anorexia-cachexia syndrome: A survey of the Prognostic Inflammatory and Nutritional Index (PINI) in advanced disease. J. Pain Symptom Manag. 2002, 24, 424–428. [Google Scholar] [CrossRef]
- Dupire, S.; Wemeau, M.; Debarri, H.; Pascal, L.; Hivert, B.; Willekens, C.; Boyle, E.; Manier, S.; Beatrice, T.; Onraed, B.; et al. Prognostic value of PINI index in patients with multiple myeloma. Eur. J. Haematol. 2012, 88, 306–313. [Google Scholar] [CrossRef]
- Kirov, K.M.; Xu, H.P.; Crenn, P.; Goater, P.; Tzanis, D.; Bouhadiba, M.T.; Abdelhafidh, K.; Kirova, Y.M.; Bonvalot, S. Role of nutritional status in the early postoperative prognosis of patients operated for retroperitoneal liposarcoma (RLS): A single center experience. Eur. J. Surg. Oncol. 2019, 45, 261–267. [Google Scholar] [CrossRef]
- Sabharwal, S.; Wilson, H.; Reilly, P.; Gupte, C.M. Heterogeneity of the definition of elderly age in current orthopaedic research. SpringerPlus 2015, 4, 516. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Zhang, Z.; Pereira, S.L.; Luo, M.; Matheson, E.M. Evaluation of Blood Biomarkers Associated with Risk of Malnutrition in Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2017, 9, 829. [Google Scholar] [CrossRef]
- Ingenbleek, Y. Revisiting PINI Scoring in Light of Recent Biological Advances. Nutrients 2023, 15, 1846. [Google Scholar] [CrossRef]
- Taylor, A.K.; Cao, W.; Vora, K.P.; De La Cruz, J.; Shieh, W.J.; Zaki, S.R.; Katz, J.M.; Sambhara, S.; Gangappa, S. Protein energy malnutrition decreases immunity and increases susceptibility to influenza infection in mice. J. Infect. Dis. 2013, 207, 501–510. [Google Scholar] [CrossRef]
- Cederholm, T.; Bosaeus, I.; Barazzoni, R.; Bauer, J.; Van Gossum, A.; Klek, S.; Muscaritoli, M.; Nyulasi, I.; Ockenga, J.; Schneider, S.M.; et al. Diagnostic criteria for malnutrition—An ESPEN Consensus Statement. Clin. Nutr. 2015, 34, 335–340. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. S1), S1–S107. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Soeters, P.B.; Reijven, P.L.; van Bokhorst-de van der Schueren, M.A.E.; Schols, J.M.; Halfens, R.J.; Meijers, J.M.; van Gemert, W.G. A rational approach to nutritional assessment. Clin. Nutr. 2008, 27, 706–716. [Google Scholar] [CrossRef]
- Avesani, C.M.; Sabatino, A.; Guerra, A.; Rodrigues, J.; Carrero, J.J.; Rossi, G.M.; Garibotto, G.; Stenvinkel, P.; Fiaccadori, E.; Lindholm, B. A Comparative Analysis of Nutritional Assessment Using Global Leadership Initiative on Malnutrition Versus Subjective Global Assessment and Malnutrition Inflammation Score in Maintenance Hemodialysis Patients. J. Ren. Nutr. 2022, 32, 476–482. [Google Scholar] [CrossRef]
- Piccoli, G.B.; Cederholm, T.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Cuerda, C.; Cupisti, A.; Sabatino, A.; Schneider, S.; Torreggiani, M.; et al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—Implications for low protein intake and nutritional care: A critical review endorsed by ERN-ERA and ESPEN. Clin. Nutr. 2023, 42, 443–457. [Google Scholar] [CrossRef]
| Authors | Country/Location | Study Design | Setting | Follow Up | Sample Size | Mean Age (Years) | Type of Intervention | PINI | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| 1. Costa et al., 2016 [7] | Brazil | Prospective | Gastrointestinal neoplasia | 1 month | 29 | 60.6 ± 15.15 | Surgery Parenteral nutrition | PINI > 1 (62.1%) PINI < 1 (37.9%) | -PINI > 1 associated with surgical complication and death |
| 2. Dessi et al., 2009 [9] | Italy | Prospective | HD | 32 months | 121 | 59.64 ± 14.75 | NR | PINI < 1 (71.66%) PINI ≥ 1 (28.33%) | -PINI > 1 associated with higher risk of mortality and morbidity |
| 3. Gharsallah et al., 2014 [10] | Tunis | Prospective | ICU | 3 months | 20 | 56 ± 11 | Enteral nutrition Mechanical ventilation | PINI > 1 (100%) | -Mortality 35% -PINI correlated with organ failure but not with mortality |
| 4. Lenartova et al., 2017 [17] | Poland | Observational—Prospective | COPD | NR | 120 | NR | 60 patients with COPD 60 patients–control group (healthy individuals) | PINI + COPD > 1 PINI + Control Group < 1 | -PINI in COPD is significantly higher than in the control group |
| 5. Reynolds et al., 2006 [21] | UK | Prospective | ICU-pressure ulcers | NR | 158 | NR | NR | PINI > 1 | -PINI higher in patients whose ulcers are likely to worsen |
| 6. Schlossmacher et al., 2002 [22] | France | Prospective | ICU | 6 months | 83 | 63.9 ± 15 | -Mechanical ventilation -Ant biotherapy -Nutritional support | PINI > 1 Admission: >1 Extubating: >1 | -Declining PINI was associated with a reduction in the risk of death |
| 7. Vehe et al., 1991 [23] | USA | Prospective | ICU | 28 days | 15 | NR | Enteral nutrition | PINI > 1 | -PINI decreases significantly over time in critically ill traumatized patients |
| 8. Verove et al., 2002 [24] | France | Prospective | HD | 6 months | 18 | 73 ± 6 | -Sodium bicarbonate oral supplementation | PINI: <1 | -No significant change |
| 9. Terrier et al., 2005 [25] | France | Observational—Prospective | HD | NR | 177 | 67.73 | CVD+ CVD− | PINI < 1 (71%) PINI > 1 (49%) PINI < 1 (46%) PINI > 1 (11%) | -PINI strongly associated with prevalence of atherosclerosis after adjustment for age, gender, dialysis center |
| 10. Di Renzo et al., 2008 [26] | Italy | Prospective | HD | 8 weeks | 52 | 50 ± 11.41 | -Non-obese (NO) -Pre-obese (PO) -Acetyl salicylic 100 mg/day, -Atorvastatin 10 mg/day | -Combined treatment was effective in reducing inflammatory status | |
| 11. Nelson et al., 2002 [27] | USA | Prospective | Advanced cancer | NR | 50 | 64 | NR | PINI > 1 | -Significantly elevated PINI scores in patients with advanced cancer |
| 12. Dupire et al., 2012 [28] | France | Retrospective | Multiple myeloma | 70 months | 231 | 64 | NR | PINI > 1 (36.4%) PINI < 1 (63.6%) | −55% of the patients died -PINI > 4 shorter median -survival (24 ± 19 months) -PINI < 4 median survival (74 ± 5 months) -PINI useful to determine prognosis |
| 13. Kirov et al., 2019 [29] | France | Retrospective | Retroperitoneal liposarcoma | 7 months | 40 | 61 | Surgery Parenteral nutrition | PINI > 1 (50%) PINI < 1 (50%) | -PINI > 1 associated with longer hospitalization and postoperative complication |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordos, M.; Vlad, C.-E.; Hogas, S.-M.; Filip, R.; Geletu, G.; Bogdan, M.; Badescu, C.; Goriuc, A.; Foia, L.G. The Role of the Prognostic Inflammatory and Nutritional Index (PINI) in the Evolution of Patients with Chronic Kidney Disease and Other Pathologies. Healthcare 2023, 11, 1375. https://doi.org/10.3390/healthcare11101375
Cordos M, Vlad C-E, Hogas S-M, Filip R, Geletu G, Bogdan M, Badescu C, Goriuc A, Foia LG. The Role of the Prognostic Inflammatory and Nutritional Index (PINI) in the Evolution of Patients with Chronic Kidney Disease and Other Pathologies. Healthcare. 2023; 11(10):1375. https://doi.org/10.3390/healthcare11101375
Chicago/Turabian StyleCordos, Monica, Cristiana-Elena Vlad, Simona-Mihaela Hogas, Roxana Filip, Gabriela Geletu, Maria Bogdan, Codruta Badescu, Ancuta Goriuc, and Liliana Georgeta Foia. 2023. "The Role of the Prognostic Inflammatory and Nutritional Index (PINI) in the Evolution of Patients with Chronic Kidney Disease and Other Pathologies" Healthcare 11, no. 10: 1375. https://doi.org/10.3390/healthcare11101375
APA StyleCordos, M., Vlad, C.-E., Hogas, S.-M., Filip, R., Geletu, G., Bogdan, M., Badescu, C., Goriuc, A., & Foia, L. G. (2023). The Role of the Prognostic Inflammatory and Nutritional Index (PINI) in the Evolution of Patients with Chronic Kidney Disease and Other Pathologies. Healthcare, 11(10), 1375. https://doi.org/10.3390/healthcare11101375









