Abstract
Background: Hypertension in pregnancy causes significant maternal and fetal mortality and morbidity. A comprehensive assessment of the effectiveness of antihypertensive drugs for severe hypertension during pregnancy is needed to make informed decisions in clinical practice. This systematic review aimed to assess the efficacy and safety of antihypertensive drugs in severe hypertension during pregnancy. Methods: A systematic review using the electronic databases MEDLINE (PubMed) and Cochrane Library was performed until August 2021. The risk-of-bias 2 tool was used to assess the risk-of-bias in each study included. Meta-analysis was conducted to assess heterogeneity and to estimate the pooled effects size. Results: Seventeen studies fulfilled the inclusion criteria and 11 were included in the meta-analysis. Nifedipine was estimated to have a low risk in persistent hypertension compared to hydralazine (RR 0.40, 95% CI 0.23–0.71) and labetalol (RR 0.71, 95% CI 0.52–0.97). Dihydralazine was associated with a lower risk of persistent hypertension than ketanserin (RR 5.26, 95% CI 2.01–13.76). No difference was found in the risk of maternal hypotension, maternal and fetal outcomes, and adverse effects between antihypertensive drugs, except for dihydralazine, which was associated with more adverse effects than ketanserin. Conclusions: Several drugs can be used to treat severe hypertension in pregnancy, including oral/sublingual nifedipine, IV/oral labetalol, oral methyldopa, IV hydralazine, IV dihydralazine, IV ketanserin, IV nicardipine, IV urapidil, and IV diazoxide. In addition, nifedipine may be preferred as the first-line agent. There was no difference in the risk of maternal hypotension, maternal and fetal outcomes, and adverse effects between the drugs, except for adverse effects in IV dihydralazine and IV ketanserin.
1. Introduction
Hypertension is the most common cardiovascular disorder during pregnancy, which occurs in 5–10% of pregnancies, and causes poor mortality and morbidity for both mother and child [1,2]. In the early term of pregnancy, the blood pressure (BP) generally drops temporarily as an adaptation process, but then increases as the pregnancy progresses [3]. Hypertensive disorders in pregnancy increase the risks of preterm birth, placental abruption, fetal growth restriction, and other complications [4]. The risk of complication is related to the severity of BP elevation [4]. Complications caused by hypertension during pregnancy need to be taken seriously, especially severe hypertension [5]. Moreover, women with a history of hypertensive disorders in pregnancy have a risk of developing cardiovascular disorders later in life [6,7].
Severe hypertension during pregnancy is defined as a condition of systolic blood pressure (SBP) ≥ 160 or diastolic blood pressure (DBP) ≥ 110 mmHg [8,9]. This is an emergency situation that requires immediate antihypertensive medications to be administered to lower the BP [10,11,12]. Antihypertensive drugs are widely available and have been compared in clinical trials to assess their effectiveness in severe hypertension in pregnancy. Labetalol, hydralazine, or nifedipine are among the most commonly used antihypertensive drugs to manage severe hypertension in pregnancy [5,8,12,13]. Several international guidelines define BP targets in pregnancy differently. The American College of Obstetricians and Gynecologists (ACOG) recommends that antihypertensive medication be administered when the BP is ≥160/110 mmHg, whereas the National Institute for Health and Care Excellence (NICE) recommends antihypertensive medication to be initiated when the BP is ≥140/90 mmHg. On the other hand, the European Society of Cardiology (ESC) recommends that antihypertensives be administered when the BP is ≥150/95 mmHg [1,8,9].
The presence of adverse effects, such as a sudden drop in BP, should be avoided [14]. Women with hypertension in pregnancy are also at risk of preeclampsia [15]. Preeclampsia is defined as the new onset of high BP (persistent BP ≥ 140 mmHg systolic and/or ≥90 mmHg diastolic) that occurs after 20 weeks of gestation, accompanied by one of the following complications: new onset of proteinuria, thrombocytopenia, kidney disorders, liver disorders, or pulmonary edema [8]. Preeclampsia may increase the risk of maternal and fetal mortality and morbidity [16].
Hypertensive disorders are responsible for 14% of maternal deaths worldwide [17]. Maternal mortality is more likely to occur when the BP ≥ 160/110 mmHg [18]. Based on a retrospective study over 5 years (2011–2016), 57.3% of pregnant women with preeclampsia had severe hypertension called severe preeclampsia [19]. Risk factors for severe preeclampsia include multiple pregnancies, overweight, obesity, nulliparity, and diabetes [20]. Multiple pregnancies (pregnant with twins or triplets) and obesity are the strongest risk factors for severe preeclampsia, whereby women with multiple pregnancies or obesity have a fourfold risk of developing severe preeclampsia [20].
Recommendations to control the BP are one aspect of expectant management, instead of immediate delivery, especially for extremely preterm babies, in women with preeclampsia or severe hypertension in pregnancy [8]. However, delivery is recommended any time if the maternal or fetal condition shows deterioration, such as uncontrolled severe hypertension, stroke, haemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, etc. [8]. Expectant management is associated with higher gestational age, reduced fetal morbidity, and reduced length of intubation among neonates [21,22]. In addition, it is associated with lower cases of maternal intraventricular hemorrhage or hyaline membrane disease, and less requirement for maternal ventilation [21].
Uncontrolled hypertension causes 35.6% of women with severe preeclampsia to deliver the offspring immediately [23]. Premature delivery is common among these women, in addition to an increased risk of undergoing a cesarean section and having babies with low birth weight [2]. Hypertensive disorders in pregnancy and preeclampsia also increase the risk of preterm deaths [24]. Globally, premature birth causes 15% of mortality in infants [25]. Based on data from the World Health Organization (WHO), almost 99% of preterm deaths are caused by complications during pregnancy [26].
Effective treatment for severe hypertension in pregnancy is necessary to protect the mother and child from the risk of complications. Although several antihypertensive agents are used to treat severe hypertension, evidence on their effectiveness and safety profile is inconclusive. Therefore, this systematic review aimed to comprehensively assess the effectiveness and safety of antihypertensive agents for severe hypertension in pregnancy. The parameters used in this systematic review include the risk of persistent severe hypertension, risk of maternal hypotension, adverse effects, and maternal and fetal outcomes.
2. Material and Methods
2.1. Search Strategy
The systematic review was conducted following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A literature search was performed using the databases MEDLINE (PubMed) and Cochrane Library, up until August 2021. Severe hypertension was defined as a condition of SBP ≥ 160 and/or DBP ≥ 110 mmHg [8,9]. Search strategies included the use of the following terms: (“Hypertension, Pregnancy-Induced” [Mesh]) OR (“gestational hypertension” [tw] OR “Preeclampsia” [Mesh]) AND “Antihypertensive Agents” [Mesh]. The MEDLINE (PubMed) and Cochrane searches included only articles reporting randomized controlled trials (RCTs). The PRISMA checklist is provided in the Supplementary materials Table S1.
2.2. Study Selection
All search records from an electronic database were exported into the Mendeley Reference Manager and checked for duplicates. Screening was conducted by title and abstract, followed by selection based on a full-text article. The inclusion criteria used in the screening process included RCTs on antihypertensive medications in pregnancy and corresponding to the population, intervention, comparison, and outcome (PICO) analysis provided in Table 1. Articles excluded in the initial screening process were those reporting on studies involving animals and cells, studies conducted in the postpartum period, available in languages other than English, or when the full texts were inaccessible. Observational studies (cross-sectional, retrospective/prospective cohort, case–control designs, and non-intervention arms of RCTs), case series, case reports, and irrelevant studies were also excluded from this review. The PRISMA flowchart template for study selection is depicted in Figure 1.

Table 1.
PICO analysis.
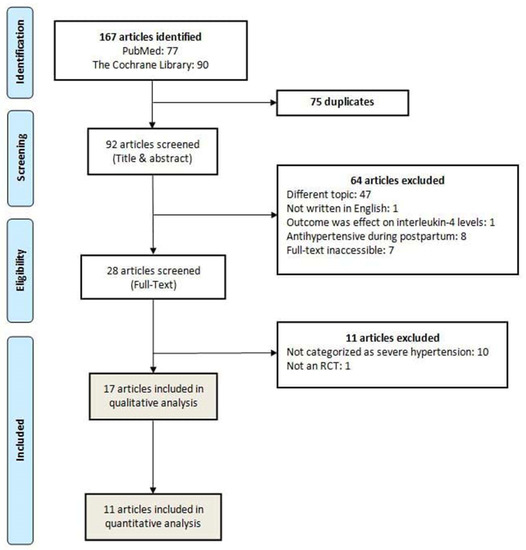
Figure 1.
PRISMA flowchart: literature search results.
2.3. Data Extraction
Data extraction was performed by manually entering the required information into the predetermined form of data extraction. The data extracted included author, year of publication, country, study design, definition of severe gestational hypertension or preeclampsia, number of participants, intervention arm, BP target, and observation period.
2.4. Risk-of-Bias and Quality Assessments
Each study was assessed for the quality and risk-of-bias using the Cochrane risk-of-bias 2 (RoB 2) tool for RCTs [27]. Five aspects were assessed for risk-of-bias, including bias arising from the randomization process, deviations from the intended intervention, missing outcome data, measurement of the outcome, and selection of the reported result [28]. The overall bias from each study was classified based on RoB 2 guidelines as high risk, some concerns, or low risk-of-bias [27].
2.5. Meta-Analysis
Cochrane Review Manager 5.4 (https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/ (accessed on 6 September 2021)) was used to perform statistical analyses for the meta-analysis. The analysis was attached in subgroups based on a comparison of antihypertensive medication. Meta-analysis was performed with a random-effect model to summarize all outcomes from the studies included. The effect size was presented as a relative risk (RR) with a 95% confidence interval (CI). For studies with significant RR, the risk difference (RD) was also calculated. Heterogeneity was analyzed using τ2, chi2, and I2.
3. Results
3.1. Study Characteristics
The initial search identified 167 articles in both databases. After removing 75 duplicates, 92 articles were screened by title and abstract, in which 64 articles were further excluded. In the full-text screening of 28 articles, 17 RCT articles met the inclusion criteria, consisting of 2312 women as participants [5,12,13,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. In three studies, 41 women were excluded for the following reasons: nine women decided to discontinue the intervention in one study [5]; 27 women were postnatal patients in one study [41]; and in one study, two were postpartum mothers, one woman was terminated due to early delivery before intervention was administered, one woman was incorrectly identified, and one woman was randomized twice [28]. One study with the most number of participants (894 women) compared interventions with three different antihypertensive agents: nifedipine, labetalol, and methyldopa [12]. Overall, the majority of women received the following medications: nifedipine (33.51%), labetalol (31.48%), methyldopa (13.25%), and hydralazine (10.83%). Other antihypertensive medications were prazosin, dihydralazine, diazoxide, ketanserin, nicardipine, and urapidil [30,31,37,38,39,41]. In addition, only 11 studies were included in the quantitative analyses because the other six studies presented different and incomparable outcome measures related to our inclusion criteria.
Each drug was administrated in various dosage forms, as seen in Table 2. There were nine different medication comparisons from all the studies, in which seven studies (580 women) compared nifedipine with labetalol [13,32,33,34,35,36,42], two studies (195 women) compared nifedipine with hydralazine [5,29], two studies (74 women) compared ketanserin with dihydralazine [30,31], one study (200 women) compared hydralazine with labetalol [40], one study (145 women) compared nifedipine with prazosin [38], one study (97 women) compared diazoxide with hydralazine [41], one study (60 women) compared nicardipine with labetalol [37], one study (26 women) compared urapidil with dihydralazine [39], and one study (894 women) compared three intervention arms: nifedipine, labetalol, and methyldopa [12]. Varying doses and durations of drug therapy were observed in each study.

Table 2.
Dosage forms of drugs administered.
The vast majority of studies included defined severe hypertension as SBP ≥ 160 mmHg and DBP ≥ 110 mmHg, but two studies used different definitions, whereby severe hypertension was defined as SBP ≥ 170 mmHg and DBP ≥ 110 mmHg [37,41]. Almost all studies defined the following BP targets of SBP ≤ 150 mmHg and DBP ≤ 100 mmHg. However, some studies had different BP targets, including three studies with SBP ≤ 140 mmHg and DBP ≤ 100 mHg [33,34,41], one study with a BP target of ≤160/110 mmHg [40], and one study with a BP target of a decrease of 20% arterial BP [37]. The characteristics of studies included are provided in Table 3, summary of results from the meta-analysis is provided in Table 4 and summary of interventions effects is provided in Table 5.

Table 3.
Characteristics of studies included.

Table 4.
Summary of meta-analysis comparing antihypertensive agents.

Table 5.
Summary of intervention effects.
3.2. Risk-of-Bias Assessment
According to the RoB 2 tool, five studies were identified as having low risk-of-bias, eight studies had some concern for risk-of-bias, and four studies had a high risk-of-bias. In detail, 23.53% had some concern for the randomization process and 52.94% had some concern at deviations from the intended interventions. A high risk-of-bias arose from deviations from the intended interventions in one study [30], from missing outcomes in three studies [30,33,34], and from selection bias in one study [12]. Figure 2 and Figure 3 depict the risk-of-bias assessments of the studies included.
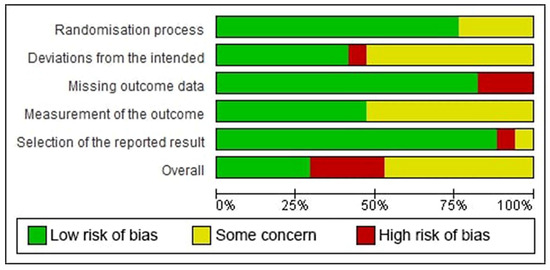
Figure 2.
Risk-of-bias graph: the authors’ judgments for each risk-of-bias item in the studies included, provided in percentage form.
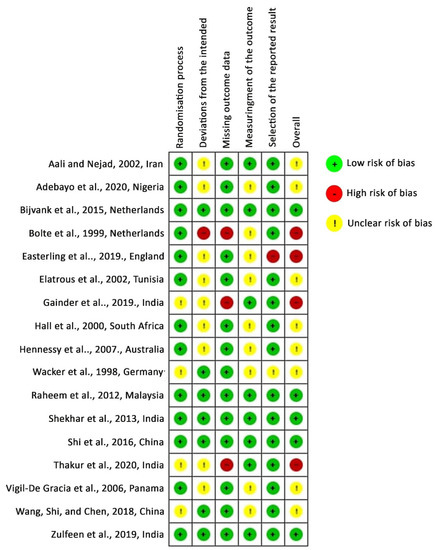
Figure 3.
Risk-of-bias assessment: authors’ judgments for risk-of-bias items in each study included. Studies are listed alphabetically by author name.
3.3. Effect of Interventions
3.3.1. Nifedipine versus Labetalol
Nifedipine had a significantly lower risk in persistent high BP than labetalol (RR 0.71, 95% CI 0.52–0.97, p = 0.03; five studies) (see Figure 4). However, when the RD was used as a summary statistic (RD –0.04, 95% CI –0.11 to 0.02; five studies), heterogeneity between the studies rose to 76% and sensitivity analysis became insignificant (p = 0.19). There was no significant difference between the two groups for maternal hypotension (RR 2.06, 95% CI 0.27–15.77, p = 0.49; seven studies). However, more hypotension occurred in the nifedipine group (see Figure 5). There was no significant difference for the incidence of adverse effects, but nifedipine was associated with more adverse effects. Similarly, there was no significant difference in maternal and fetal outcomes, including maternal death, neonatal death, eclampsia, pulmonary edema, renal failure, placental abruption, cesarean delivery, stillbirth, abnormal fetal heart rate, and the Appearance, Pulse, Grimace, Activity, Respiration (APGAR) score.
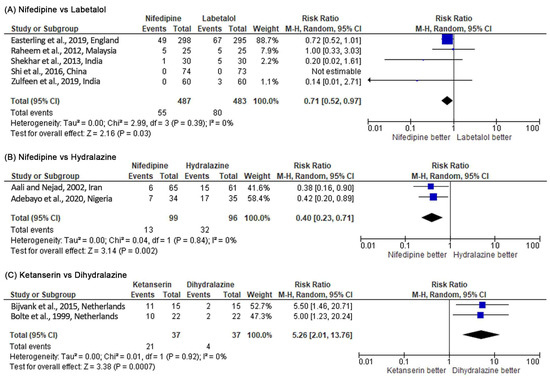
Figure 4.
Meta-analysis outcome 1: comparison of antihypertensive medication effectiveness in controlling blood pressure assessed by the risk of persistent severe hypertension incidence. The risk ratio (RR) was used to interpret the risk of persistent severe hypertension incidence after administering the drug. p-value in the overall effect shows the significance of the RR.
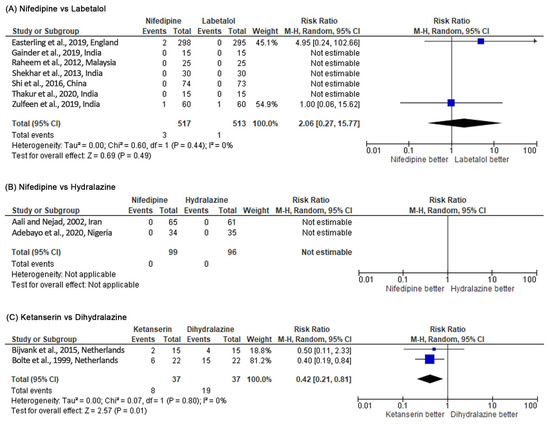
Figure 5.
Meta-analysis outcome 2: antihypertensive medication safety comparison assessed by the risk of maternal hypotension incidence. The risk ratio (RR) was used to interpret the risk of maternal hypotension incidence after administering the drug. p-value in the overall effect shows the significance of the RR.
3.3.2. Nicardipine versus Labetalol
Only one study compared nicardipine with labetalol. No significant difference was found in the nicardipine and labetalol groups for persistent high BP (RR 0.82, 95% CI 0.40–1.68, p = 0.59) and adverse effects (RR 1.07, 95% CI 0.76–1.57, p = 0.70). There was no incidence of maternal hypotension between the two groups.
3.3.3. Nifedipine versus Hydralazine
Nifedipine was more effective in controlling BP than hydralazine (RR 0.40, 95% CI 0.23–0.71, p = 0.002; two studies) (see Figure 4). There was no incidence of hypotension and maternal mortality in both study arms (two studies) (see Figure 5). There was no significant difference for the incidence of adverse effects, but nifedipine had a higher risk of headache. On the other hand, no significant difference was observed for maternal and fetal outcomes (cesarean delivery, perinatal death, stillbirth, neonatal intensive care unit (NICU) admission, and APGAR score) in both study arms.
3.3.4. Nifedipine versus Prazosin
Only one study compared nifedipine with prazosin. No significant difference was found between nifedipine and prazosin in controlling BP (RR 0.32, 95% CI 0.03–3.00, p = 0.32). Similarly, there was no significant difference in maternal and fetal outcomes (eclampsia, kidney disorders, HELLP syndrome, placenta abruption, pulmonary edema, maternal death, miscarriage, and NICU admission) between nifedipine and prazosin.
3.3.5. Ketanserin versus Dihydralazine
Dihydralazine was able to control high BP significantly better than ketanserin (RR 5.26, 95% CI 2.01–13.76, p = 0.0007; two studies) (see Figure 4). Based on the RR, dihydralazine was significantly associated with more risk of maternal hypotension (RR 0.42, 95% CI 0.21–0.81, p = 0.01) (see Figure 5). However, when the RD was used as a summary statistic, heterogeneity rose to 49% and sensitivity analysis became insignificant (RD –0.27, 95% CI –0.55 to 0.00, p = 0.05). Dihydralazine was significantly associated with more adverse effects than ketanserin (RR 0.38, 95% CI 0.23–0.64, p = 0.0002; two studies). There was no significant difference in maternal and fetal outcomes (eclampsia, HELLP syndrome, placenta abruption, cesarean delivery, and maternal and neonatal mortality) between these drugs.
3.3.6. Urapidil versus Dihydralazine
Only one study compared urapidil with dihydralazine. The outcomes of the study included adverse effects, eclampsia, cesarean delivery, abnormal fetal heart rate, and neonatal death. No significant difference was reported in maternal outcomes and neonatal deaths in both groups, and there was no incidence of adverse effects.
3.3.7. Hydralazine versus Labetalol
A comparison of effectiveness between hydralazine and labetalol was conducted in one study. The results indicated no difference in efficacy for controlling BP between the two groups (RR 1.00, 95% CI 0.30–3.35, p = 1.00) and no significant difference in the adverse effect incidence between hydralazine and labetalol. Adverse effects, such as palpitations, were significantly more common in the hydralazine group (RR 5.00, 95% CI 1.12–22.24, p = 0.03). There was no significant difference for maternal hypotension in both groups (RR 5.00; 95% CI 0.42–102.85, p = 0.30). In addition, no significant difference was noted in maternal and neonatal outcomes (eclampsia, pulmonary edema, cesarean delivery, neonatal hypotension, neonatal complications, abnormal fetal heart rate, NICU admission, HELLP Syndrome, and APGAR score).
3.3.8. Hydralazine versus Diazoxide
Only one study compared the effectiveness of hydralazine with diazoxide. There was no difference in the risk of persistent high BP between the two groups (RR 1.06, 95% CI 0.55–2.05, p = 0.85). Similarly, there was no significant difference between both groups in maternal and fetal outcomes (cesarean birth, APGAR score, hypoglycemia and neonatal respiratory distress syndrome, and perinatal death).
3.3.9. Methyldopa versus Nifedipine
A comparison of the effectiveness between methyldopa and nifedipine was reported by only one study. Nifedipine use was shown to be associated with a lower risk of persistent high BP (RR 1.43, 95% CI 1.03–1.99, p = 0.03). The risk of maternal hypotension was not significantly different between the two groups (RR 0.20, 95% CI 0.01–4.11, p = 0.30). However, nifedipine was associated with more adverse effects than methyldopa (RR 0.56, 95% CI 0.42–0.75, p = 0.0001). In addition, more infants in the nifedipine group were admitted to the NICU (RR 0.54, 95% CI 0.36–0.83, p = 0.005). There were no maternal deaths and no significant differences in adverse effects and maternal and fetal outcomes (placental abruption, cesarean delivery, neonatal death, stillbirth, abnormal fetal heart rate, and APGAR score) between these drugs.
3.3.10. Methyldopa versus Labetalol
Only one study compared the effectiveness of methyldopa and labetalol. The study found no significant difference between the two groups in controlling the BP (RR 1.04, 95% CI 0.78–1.39, p = 0.80). None of the mothers in either group experienced hypotension or death. Other maternal and fetal outcomes (placental abruption, cesarean delivery, neonatal death, stillbirth, abnormal fetal heart rate, NICU admission, and APGAR score) and adverse effects were found to be nonsignificant n both groups.
4. Discussion
The effectiveness of antihypertensives was assessed by the risk of persistent severe hypertension, whereas drug safety was assessed by maternal hypotension, maternal and fetal outcomes, and adverse effects. The outcomes between studies were compared in a meta-analysis. Of 17 studies included, we could not include six studies in the meta-analysis because the outcome measure data compatible with our inclusion criteria were not considered in those studies. Most comparative drug studies of severe hypertension treatment during pregnancy come from middle-income countries, although data were still sparse [11,44]. The definitive recommendation in these limited-resource countries is prominent because prioritization in health intervention, including BP-lowering drugs, can significantly reduce the health burden related to hypertension in pregnancy. Therefore, the use of effective antihypertensive agents to control BP was crucial [45].
The results of our meta-analysis estimated that oral nifedipine can significantly lower the risk of persistent high BP in pregnancy compared to intravenous (IV) hydralazine and IV labetalol, with no differences in the incidences of maternal hypotension and adverse effects, and on maternal and fetal outcomes. One of the studies comparing nifedipine and labetalol had a dominating number of participants that caused the study weight to become disproportionate [12]. The significance of RR in persistent high BP between nifedipine and labetalol should be viewed with caution because sensitivity and heterogeneity in the analysis become insignificant when the RD was used as a summary statistic. However, the therapeutic success rate favored oral nifedipine over IV hydralazine and IV labetalol. There was a significant difference in beneficial effects between oral nifedipine and IV hydralazine but not for IV labetalol. It was concluded that oral nifedipine was as efficacious as IV labetalol. Our study also showed that IV dihydralazine was significantly more effective than IV ketanserin in controlling BP during pregnancy. The results of our meta-analysis show that IV dihydralazine had more adverse effects than IV ketanserin. However, the use of IV dihydralazine did not show any difference in the risk of maternal hypotension, or maternal and fetal outcomes.
Several previous studies confirm our present findings. Duley et al. reported that nifedipine was significantly more effective in lowering the risk of persistent high BP during pregnancy than hydralazine, and that there was no difference between nifedipine, labetalol, and hydralazine in the risk of maternal hypotension, adverse effects, and on maternal and fetal outcomes [11]. Duley et al. also reported that IV dihydralazine was more effective in lowering the risk of persistent high BP than IV ketanserin. With regard to other outcomes, such as maternal hypotension, their results showed no difference in the risk of maternal hypotension between the two interventions (IV dihydralazine and IV ketanserin), but IV dihydralazine had more adverse effects than IV ketanserin, which is consistent with the results of our meta-analysis. Duley et al. used the random-effect model only if the heterogeneity was substantial (I2 > 30%, T2 > 0, or p < 0.1). Their study used the previous version of the RoB tool to assess the quality of studies; however, in our systematic review, we used the recent RoB tool (RoB 2.0). In addition, our systematic review contains recent studies with larger sample sizes and one of them was a multicenter study [12].
Another systematic review was conducted by Alavifard et al. using a network meta-analysis [44]. Similar to our findings, Alavifard et al. reported that oral nifedipine provides the highest therapeutic success rate for controlling BP in pregnancy compared to IV labetalol and IV hydralazine. A significant difference in effectiveness was observed when oral nifedipine was compared with IV hydralazine, but not with IV labetalol. Alavifard et al. also reported that there was no difference in the risk of adverse effects between oral nifedipine, IV labetalol, and IV hydralazine. Unfortunately, the risk of maternal hypotension was not assessed in Alavifard et al.’s study because of low event rates; therefore, meta-analysis for maternal hypotension could not be compared with our findings. However, a higher frequency of hypotension occurred with hydralazine use (hydralazine 7.6%, labetalol 1.7%, and nifedipine 0.6%) [44]. Regarding the number of participants in the effectiveness comparison between nifedipine and labetalol, Alavifard et al. had a smaller number of participants (490) than our study (970), which might cause differences in the size of the CI.
There were different findings from network meta-analysis by Sridharan et al. [46]. Their analysis showed no difference in effectiveness in controlling the BP in pregnancy for nifedipine compared to hydralazine (OR 2.1, 95% CI 0.9–5.2) and labetalol (OR 0.7, 95% CI 0.3–1.5). The wide estimate interval in the results of network meta-analysis caused unclear reliability. Sridharan et al. reported that adequate evidence was used to compare nifedipine and hydralazine against labetalol [46]. However, sufficient evidence regarding the comparison between nifedipine and hydralazine in pregnant women was still sparse; therefore, the recommendation was based on limited data.
This systematic review consists of recent studies and various intervention arms; therefore, the use of antihypertensive medication was more widely described. A secondary analysis was used in the meta-analysis using the RD to determine any significant effect of the RR. This systematic review was also based on PRISMA guidelines and the quality of the studies was assessed based on the recent Cochrane RoB 2 tool.
Inevitably, there were some limitations in this systematic review. First, only limited information was retrieved from some studies; hence, our meta-analysis had to exclude those studies. Second, this review included studies of various qualities. Finally, there were variations in the definition of outcome measures used in the studies included (e.g., severe hypertension and BP target). Therefore, we use broader inclusion criteria to standardize the different characteristics of each study. Despite these variations, most importantly, a clear definition was used for severe hypertension (SBP ≥ 160 mmHg or DBP ≥ 110 mmHg) and the studies included reported at least one outcome of the effectiveness or safety of antihypertensives. From the results of this study, we recommend that oral nifedipine be the first-choice drug in daily clinical practice as therapy for severe hypertension during pregnancy. For further research, a comprehensive economic evaluation related to preeclampsia therapy is also needed to generate more conclusive recommendation, due to current limited data about this topic [47].
5. Conclusions
Oral nifedipine may be considered the first-line antihypertensive agent for severe hypertension in pregnancy. The results of this review suggested that oral nifedipine had the highest therapeutic success rate in controlling hypertension during pregnancy compared to other common medications used (IV hydralazine and IV labetalol). Oral nifedipine was relatively safe and showed no difference in the risk of hypotension, maternal and fetal outcomes, and incidence of adverse effects compared to IV hydralazine and IV labetalol. However, there was not enough evidence that oral nifedipine was preferable to other antihypertensive medications for severe hypertension in pregnancy. IV dihydralazine could control high BP significantly better than IV ketanserin, with no difference in the risk of hypotension. However, IV dihydralazine was associated with more adverse effects than IV ketanserin. Adequately powered RCTs in the comparison between oral nifedipine and IV labetalol, or other agents such as diazoxide, urapidil, prazosin, and methyldopa, in the treatment of severe hypertension in pregnancy are required to guide a better clinical judgment on the most effective and safe drug. This is especially important in the setting of low- and middle-income countries, where access and drug of choice may be limited. Therefore, a conclusive recommendation can be crucial in the treatment of severe hypertension in pregnancy in clinical practice.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare10020325/s1, Table S1: PRISMA 2020 main checklist.
Author Contributions
Conceptualization, A.A. and N.Z.; methodology, A.A. and N.Z.; software, A.A.; validation, N.A.A.D., C.R. and N.Z.; formal analysis, A.A. and N.Z.; investigation, A.A. and N.Z.; resources, A.A., C.R. and N.Z.; data curation, A.A., N.A.A.D. and N.Z.; writing—original draft preparation, N.Z.; writing—review and editing, A.A., N.Z.; visualization, A.A., N.A.A.D. and N.Z.; supervision, C.R., N.A.A.D. and N.Z.; project administration, N.Z.; funding acquisition, N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported by a grant from Universitas Padjadjaran (Grant number: 1959/UN6.3.1/PT.00/2021).
Data Availability Statement
The data presented in this study are available in the manuscript and supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.N.; Obi, V.O.; Nwafor, J.I.; Onwe, B.I.; Onuchukwu, V.U.; Ugoji, D.-P.C.; Ibo, C.C. A comparative study of pregnancy outcome among women with preeclampsia and normotensive at the Alex Ekwueme Federal University Teaching Hospital Abakaliki, Nigeria. Int. J. Res. Med. Sci. 2019, 7, 3789–3794. [Google Scholar] [CrossRef]
- Shen, M.; Tan, H.; Zhou, S.; Smith, G.N.; Walker, M.C.; Wen, S.W. Trajectory of blood pressure change during pregnancy and the role of pre-gravid blood pressure: A functional data analysis approach. Sci. Rep. 2017, 7, 6227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [Green Version]
- Nwafor, J.I.; Adebayo, J.A.; Lawani, L.O.; Esike, C.O.; Olaleye, A.A.; Adiele, N.A. Efficacy of nifedipine versus hydralazine in the management of severe hypertension in pregnancy: A randomised controlled trial. Niger. Postgrad. Med. J. 2020, 27, 317–324. [Google Scholar] [CrossRef]
- Cusimano, M.; Pudwell, J.; Roddy, M.; Cho, C.-K.J.; Smith, G. The maternal health clinic: An initiative for cardiovascular risk identification in women with pregnancy-related complications. Am. J. Obstet. Gynecol. 2014, 210, 438.e1–438.e9. [Google Scholar] [CrossRef]
- McDonald, S.D.; Malinowski, A.; Zhou, Q.; Yusuf, S.; Devereaux, P. Cardiovascular sequelae of preeclampsia/eclampsia: A systematic review and meta-analyses. Am. Hear. J. 2008, 156, 918–930. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Clinical Management Guidelines for Obstetrician—Gynecologists Gestational Hypertension and Preeclampsia. ACOG Pract. Bull. No 222 2020, 135, e237–e260. [Google Scholar]
- Lowe, S.A.; Brown, M.A.; Dekker, G.A.; Gatt, S.; McLintock, C.K.; McMahon, L.P.; Mangos, G.; Moore, M.P.; Müller, P.; Paech, M.; et al. Guidelines for the management of hypertensive disorders of pregnancy 2008. Aust. N. Z. J. Obstet. Gynaecol. 2009, 49, 242–246. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Emergent therapy for acute-onset, severe hypertension during pregnancy and the postpartum period. ACOG Comm Opin No 767 2019, 133, e174–e180. [Google Scholar]
- Duley, L.; Meher, S.; Jones, L. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst. Rev. 2013, 7, 1–156. [Google Scholar] [CrossRef] [PubMed]
- Easterling, T.; Mundle, S.; Bracken, H.; Parvekar, S.; Mool, S.; Magee, L.A.; von Dadelszen, P.; Shochet, T.; Winikoff, B. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: An open-label, randomised controlled trial. Lancet 2019, 394, 1011–1021. [Google Scholar] [CrossRef] [Green Version]
- Zulfeen, M.; Tatapudi, R.; Sowjanya, R. IV labetalol and oral nifedipine in acute control of severe hypertension in pregnancy—A randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 236, 46–52. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia, 2011th ed.; WHO Press: Geneva, Switzerland, 2011. [Google Scholar]
- Albert, C.L.; Cho, L. Hypertension during Pregnancy. Curr. Hypertens. Rep. 2019, 22, 339–348. [Google Scholar] [CrossRef]
- Wilkerson, R.G.; Ogunbodede, A.C. Hypertensive Disorders of Pregnancy. Emerg. Med. Clin. N. Am. 2019, 37, 301–316. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, E323–E333. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; Das, R.; Sharma, N.; Das, A.; Deb, P.; Singh, K. Maternal and Perinatal Outcomes in Hypertensive Disorders of Pregnancy and Factors Influencing It: A Prospective Hospital-Based Study in Northeast India. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Tlaye, K.G.; Endalifer, M.L.; Getu, M.A.; Nigatu, A.G.; Kebede, E.T. A five-year trend in pre-eclampsia admission and factors associated with inpatient eclampsia: A retrospective study from a resource-limited hospital in northeast Ethiopia. BMJ Open 2021, 11, e040594. [Google Scholar] [CrossRef]
- Yang, Y.; Le Ray, I.; Zhu, J.; Zhang, J.; Hua, J.; Reilly, M. Preeclampsia Prevalence, Risk Factors, and Pregnancy Outcomes in Sweden and China. JAMA Netw. Open 2021, 4, e218401. [Google Scholar] [CrossRef]
- Churchill, D.; Duley, L.; Thornton, J.G.; Moussa, M.; Ali, H.S.; Walker, K.F. Interventionist versus expectant care for severe pre-eclampsia between 24 and 34 weeks’ gestation. Cochrane Database Syst. Rev. 2018, 2018, CD003106. [Google Scholar] [CrossRef]
- Cadoret, F.; Guerby, P.; Cavaignac-Vitalis, M.; Vayssiere, C.; Parant, O.; Vidal, F. Expectant management in HELLP syndrome: Predictive factors of disease evolution. J. Matern. Neonatal Med. 2020, 34, 4029–4034. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, J.I.; Ugoji, D.P.C.; Onwe, B.I.; Obi, V.O.; Obi, C.N.; Onuchukwu, V.J.U.; Ibo, C.C. Pregnancy outcomes among women with early-onset severe preeclampsia managed conservatively. Sahel Med. J. 2020, 23, 1. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Arvizu, M.; Rich-Edwards, J.W.; Wang, L.; Rosner, B.; Stuart, J.J.; Rexrode, K.M.; Chavarro, J.E. Hypertensive Disorders of Pregnancy and Subsequent Risk of Premature Mortality. J. Am. Coll. Cardiol. 2021, 77, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Oza, S.; Hogan, D.; Perin, J.; Rudan, I.; Lawn, J.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: An updated systematic analysis. Lancet 2015, 385, 430–440. [Google Scholar] [CrossRef]
- Hogan, D.; Chu, Y.; Liu, L.; Oza, S.; MCEE. MCEE-WHO Methods and Data Sources for Child Causes of Death 2000–2016; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Julian, P.T.; Higgins Savović, J.; Page, M.J.; Sterne, J.A. RoB 2 Guidance: Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2); Higgins, J.P., Savović, J., Page, M.J., Sterne, J.A., Eds.; Cochrane Methods Bias: London, UK, 2019. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aali, B.S.; Nejad, S.S. Nifedipine or hydralazine as a first-line agent to control hypertension in severe preeclampsia. Acta Obstet. Gynecol. Scand. 2002, 81, 25–30. [Google Scholar] [CrossRef]
- Bolte, A.C.; Van Eyck, J.; Kanhai, H.H.; Bruinse, H.W.; Van Geijn, H.P.; Dekker, G.A. Ketanserin versus dihydralazine in the management of severe early-onset preeclampsia: Maternal outcome. Am. J. Obstet. Gynecol. 1999, 180, 371–377. [Google Scholar] [CrossRef]
- Bijvank, S.W.N.; Visser, W.; Duvekot, J.J.; Steegers, E.A.; Edens, M.A.; Roofthooft, D.W.; Vulto, A.G.; Hanff, L.M. Ketanserin versus dihydralazine for the treatment of severe hypertension in early-onset preeclampsia: A double blind randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 189, 106–111. [Google Scholar] [CrossRef]
- Raheem, I.; Saaid, R.; Omar, S.; Tan, P. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: A randomised trial. BJOG Int. J. Obstet. Gynaecol. 2011, 119, 78–85. [Google Scholar] [CrossRef]
- Gainder, S.; Thakur, M.; Saha, S.; Prakash, M. To study the changes in fetal hemodynamics with intravenous labetalol or nifedipine in acute severe hypertension. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health 2019, 15, 12–15. [Google Scholar] [CrossRef]
- Thakur, M.; Gainder, S.; Saha, S.; Prakash, M. To study the changes in maternal hemodynamics with intravenous labetalol or nifedipine in acute severe hypertension. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health 2020, 21, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Sharma, C.; Thakur, S.; Verma, S. Oral Nifedipine or Intravenous Labetalol for Hypertensive Emergency in Pregnancy. Obstet. Gynecol. 2013, 122, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, D.; Chen, L. Lipid profile and cytokines in hypertension of pregnancy: A comparison of preeclampsia therapies. J. Clin. Hypertens. 2018, 20, 394–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elatrous, S.; Nouira, S.; Besbes, L.O.; Marghli, S.; Boussarssar, M.; Sakkouhi, M.; Abroug, F. Short-term treatment of severe hypertension of pregnancy: Prospective comparison of nicardipine and labetalol. Intensiv. Care Med. 2002, 28, 1281–1286. [Google Scholar] [CrossRef]
- Hall, D.R.; Odendaal, H.J.; Steyn, D.W.; Smith, M. Nifedipine or prazosin as a second agent to control early severe hypertension in pregnancy: A randomised controlled trial. BJOG Int. J. Obstet. Gynaecol. 2000, 107, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Wacker, J.; Werner, P.; Walter-Sack, I.; Bastert, G. Treatment of hypertension in patients with pre-eclampsia: A prospective parallel-group study comparing dihydralazine with urapidil. Nephrol. Dial. Transplant. 1998, 13, 318–325. [Google Scholar] [CrossRef] [Green Version]
- Gracia, P.V.-D.; Lasso, M.; Ruiz, E.; Vega-Malek, J.C.; de Mena, F.T.; López, J.C. Severe hypertension in pregnancy: Hydralazine or labetalol. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 128, 157–162. [Google Scholar] [CrossRef]
- Hennessy, A.; Thornton, C.E.; Makris, A.; Ogle, R.F.; Gillin, A.G.; Child, A.; Henderson-Smart, D.J. A randomised comparison of hydralazine and mini-bolus diazoxide for hypertensive emergencies in pregnancy: The PIVOT trial. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 279–285. [Google Scholar] [CrossRef]
- Shi, D.-D.; Yang, F.-Z.; Zhou, L.; Wang, N. Oral nifedipine vs. intravenous labetalol for treatment of pregnancy-induced severe pre-eclampsia. J. Clin. Pharm. Ther. 2016, 41, 657–661. [Google Scholar] [CrossRef]
- Davey, D.A.; MacGillivray, I. The classification and definition of the hypertensive disorders of pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 892–898. [Google Scholar] [CrossRef]
- Alavifard, S.; Chase, R.; Janoudi, G.; Chaumont, A.; Lanes, A.; Walker, M.; Gaudet, L. First-line antihypertensive treatment for severe hypertension in pregnancy: A systematic review and network meta-analysis. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health 2019, 18, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.N., Jr.; Tucker, J.M. Adverse Maternal Consequences Associated with Prolonged Acute-Onset Severe Systolic Hypertension during Pregnancy & Early Postpartum: Pitfalls in Practice & Lessons Learned. Open J. Obstet. Gynecol. 2021, 11, 626–635. [Google Scholar] [CrossRef]
- Sridharan, K.; Sequeira, R.P. Drugs for treating severe hypertension in pregnancy: A network meta-analysis and trial sequential analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 2018, 84, 1906–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakiyah, N.; On behalf of the IMPROvED Consortium; Postma, M.J.; Baker, P.N.; van Asselt, A.D.I. Pre-eclampsia Diagnosis and Treatment Options: A Review of Published Economic Assessments. Pharmacoeconomics 2015, 33, 1069–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).