Abstract
Ofatumumab is a monoclonal antibody that reduces the level of B cells that alter the progression of relapsing multiple sclerosis. Originally approved by the Food and Drug Administration (FDA) in August 2020, this meta-analysis determines the outcomes of four randomized controlled trials (RCTs) for endline outcomes of Gadolinium-enhancing T1 lesions on MRI scans reported as Cohen’s d and relapse rate reported as risk ratio. All four RCTs reported favorable findings of gadolinium-enhancing T1 lesions (Cohen’s d = −0.44, p < 0.00001). The relapse rate was reduced by 46% post ofatumumab administration (RR = 0.54, p < 0.00001). With 14 ongoing trials in this area, more data is required to consolidate our findings.
1. Introduction
Ofatumumab, a monoclonal antibody, works by reducing the level of B cells which contribute to the development and progression of MS [1]. Ofatumumab, a B-cell depleting medication delivered via subcutaneous injection [2], was approved by the FDA in August 2020 for adults with relapsing forms of MS, including clinically isolated syndrome, relapsing–remitting disease, and active secondary progressive MS [3]. In MS, B cells are posited to act via the antibody production and antigen presentation system to activate T cells; they are also a vital source of pro-inflammatory cytokines, which in unison orchestrate inflammatory infiltration in the central nervous system [4]. MS is an incurable disease that affects an estimated 2.8 million individuals across the world. While the exact mechanism with which ofatumumab works is not currently known, it is understood that the FAB portion of the drug inhibits the transmembrane phosphoprotein—CD20; this region is different compared to other anti-CD20 antibodies previously used for MS [1]. B-cell lysis associated with Ofatumumab correlates to completement-dependent cytotoxicity and antibody-dependent cell-mediated cytotoxicity [4]; this is unlike the mechanism seen in other treatments, including ocrelizumab and rituximab, which only bind to the large extracellular loops of the CD20 antigen; ofatumumab binds to both the small and large extracellular loops [4,5]. The key pharmacological properties of ofatumumab are, firstly, depleting the B cells, secondly, repleting the B cells, and thirdly, immunogenicity [1].
It is pertinent to consolidate existing therapeutic options to limit progression and disability associated with disease. With monoclonal antibodies or teriflunomide available as modalities to prevent relapsing MS, ofatumumab may be an emerging and viable therapy for patients considering self-administration once per month [6]. The applicability of ofatumumab has been evaluated in an online-based survey where 250 neurologists were questioned on their attitudes towards the therapy among other questions [7]. The key findings of the survey included 90% positive responses towards early use of the therapy [7]. Moreover, the neurologists believed that reduction of relapses, mode of administration and application intervals (i.e., daily use versus monthly use) are extremely imperative aspects of relapsing MS treatment.
It is all the more important to emphasize the importance of innovative relapsing MS therapies for treatment-naïve patients. Gadolinium-enhancing T1 lesions are critical in reflecting active disease and are critical for monitoring in MS. In general, relapses of disease are the defining feature of relapsing MS—the most prevalent MS phenotype [8]. While relapses are utilized in diagnoses, the value of relapse rates is seen with the high risk of association with incomplete remission, which can lead to residual disability [8,9]. Moreover, relapse frequency early in the course of MS has strong correlations with long-term disability [9]. The objectives of this report are to quantify, firstly, the outcomes of gadolinium-enhancing T1 lesions on MRI scan at treatment endline, and secondly, the relapse rate at treatment on usage of ofatumumab at endpoint. This meta-analysis will quantify the use of ofatumumab for relapsing MS using objective-based evidence from randomized controlled trials.
2. Methods
PubMed, Cochrane CENTRAL, Embase, and ClinicalTrials.Gov were systematically searched for randomized controlled trials (RCTs) with MeSH terms including “Ofatumumab” and “Relapsing Multiple Sclerosis”. The full keyword string for PubMed is as follows: Ofatumumab: “ofatumumab” [Supplementary Concept] OR “ofatumumab” [All Fields]; Relapsing: “recurrence” [MeSH Terms] OR “recurrence” [All Fields] OR “relapse” [All Fields] OR “relapses” [All Fields] OR “relapsing” [All Fields] OR “relapsed” [All Fields] OR “relapse” [All Fields] OR “relapsers” [All Fields]; Multiple Sclerosis: “multiple sclerosis” [MeSH Terms] OR (“multiple” [All Fields] AND “sclerosis” [All Fields]) OR “multiple sclerosis” [All Fields]. The databases were searched from inception until 5 September 2022. No language restrictions were applied; any non-English language study, if identified, was to be translated into English using Google Translate.
The inclusion criteria covered RCTs only enrolling adult patients aged 18 and above, of any gender, with definite diagnosis of relapsing MS as per the study-defined criteria. The participants were required to be intervened with ofatumumab, with placebo groups as comparators. Observational studies, case reports/series, systematic reviews/meta-analyses, brief reports, and letters to editors were excluded from this study.
In the screening phase, the titles and abstracts of shortlisted studies from the databases were screened independently by two reviewers (Z.S. and A.S.). In case of any disagreements, a third reviewer resolved any issues and reached a consensus (I.C.O). Figure 1 depicts the study selection process in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [10]. The data software EndNote X9 (Clarivate, London, UK) was used to omit any duplicates during the selection process and for storage of bibliographic entries. The kappa score was determined for inter-rater reliability as a measure of agreement between the two independent raters using the Statistical Package for Social Sciences (SPSS, v24).
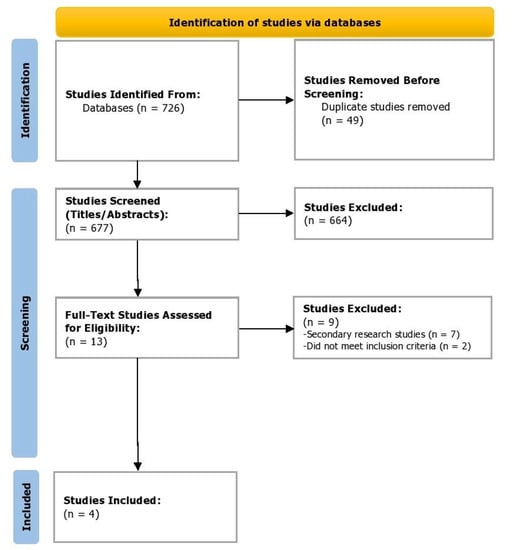
Figure 1.
PRISMA flowchart depicting the study selection process.
All quantitative data was collated into a data sheet by all authors for (i) mean number and standard deviation (SD) of Gd-enhancing T1 lesions per MRI scan, and (ii) proportion of participants presenting with relapse in intervention and placebo groups. The primary aim of this meta-analysis was to ascertain the effect size (standardized mean difference), reported as Cohen’s d, and comparing the differences in mean Gd-enhancing T1 lesions per MRI scan among intervention and control groups. The secondary aim was to determine the risk ratio of relapses in disease. Cohen’s d and risk ratio (RR) were computed applying 95% confidence intervals and were set to a significance level of less than 0.05. The findings were presented as forest plots along with the p-values. The I2 index was utilized to calculate the heterogeneity among the included studies. All statistical tests were conducted in Review Manager 5.4.1 (RevMan, Cochrane).
The included RCTs were assessed for quality using version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2). The RoB 2 tool assesses five domains comprised of the following: (1) bias arising from the randomization process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in the measurement of the outcome, and (5) bias in the selection of the reported result. The domain-based judgements were reported as (1) low risk of bias, (2) some concerns, and (3) high risk of bias. The results were reported as a traffic light plot of bias assessment and the weighted summary plot of overall domain-based type of bias.
3. Results
Of the 726 studies located, post appraisal by all authors, a total of four RCTs were selected for inclusion (Figure 1). We included two randomized, double-blind, double-dummy, parallel-group phase 3 trials (ASCLEPIOS I and II) [11] and two phase 2, randomized double-blind, placebo-controlled parallel-group trials (MIRROR Trial and APOLITOS Study), with a total of 2177 participants (Ofatumumab = 1153; Control = 1024) evaluating the efficacy of ofatumumab in patients with relapsing MS [12,13]. Gd-enhancing T1 lesions’ Cohen’s d had a medium effect direction in favor of ofatumumab as compared to control (Cohen’s d = −0.44, 95% CI = −0.56, −0.31; p < 0.00001, I2 = 31%) (Figure 2). On assessing the relapse rate among the intervention and control groups, it was ascertained that the risk of relapse was reduced by 46% among those intervened with ofatumumab compared to the control group (RR = 0.54, 95% CI = 0.46, 0.63; p < 0.00001, I2 = 0%). The data of these four trials are appended in Table 1 and Table 2.
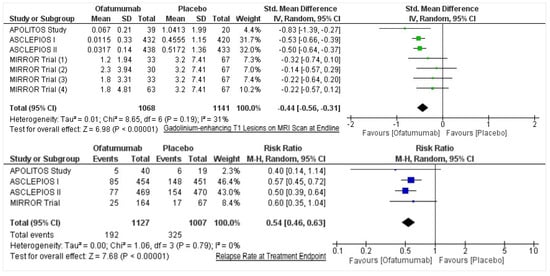
Figure 2.
Forest plots for gadolinium-enhancing T1 lesions on MRI scan at endline and relapse rate at treatment endpoint.

Table 1.
Study characteristics of the included trials (N = 4) in the meta-analysis.

Table 2.
Patient characteristics and outcomes of the included trials (N = 4) in the meta-analysis.
Currently, there are 14 ongoing clinical trials, of which six are in phase 3 and seven are in phase 4 of testing—with an enrollment of 5465 participants addressing the efficacy and safety profile for relapsing MS (Table 3).

Table 3.
Overview of ongoing clinical trials.
On noting the bias arising from the randomization process, only one trial had some concerns, whereas three trials had low concerns. On calibrating the biases arising due to deviations from the intended interventions, all four trials had low concerns. On assessing biases due to missing outcome data, all trials had low concerns, whereas assessment of bias in the measurement of the outcome resulted in one trial with some concerns and three with low concerns. For bias in the reported result, all four trials had low concerns. Overall, all four trials had low concerns (Figure 3).
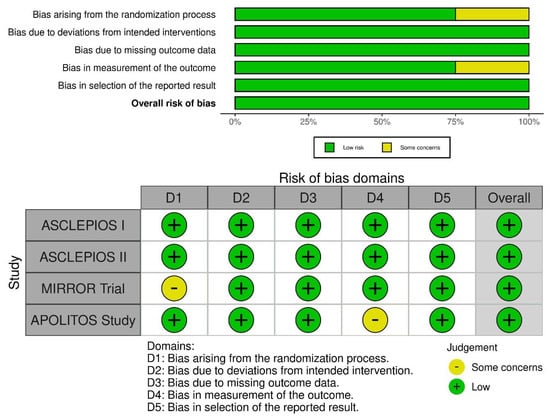
Figure 3.
Risk of bias assessment of included RCTs using the ROB-2 tool. The weighted summary plot depicts the overall type of bias encountered in all studies. The traffic light plot represents study-by-study bias assessment.
4. Discussion
On noting the efficacy of ofatumumab, the annualized relapse rate (ARR) for individuals with relapsing MS was decreased by a mean of 54.5% among both ASCLEPIOS I and II trials [11]. Moreover, the reduction in gadolinium-enhancing T1 lesions was noted at a relative rate of 97% and 94% in both trials, respectively (p < 0.001) [11]. In ASCLEPIOS I and II, the safety profile was similar to that displayed by teriflunomide—a drug which has shown 79% individuals with relapsing MS have remained free of disability progression, as compared to 80% with placebo; however, teriflunomide has not always been known to achieve statistically significant reduction in the risk of sustained disability progression [11]. The findings of this meta-analysis collate pooled evidence depicting the comparability of frequency of serious infections and neoplasms being comparable between treatment and control groups. In both ASCLPEIOS I and II, the infection-related adverse reactions have been 20.2% versus 15% among ofatumumab and teriflunomide/control groups [11]. The most commonly reported adverse events are headache, injection-site reaction, nasopharyngitis, urinary tract infection and upper respiratory tract infection [11].
The MIRROR trial explored minimally effective doses of ofatumumab to identify a potential treatment for relapsing MS. In the efficacy analysis, the treatment significantly reduced new GdE lesions by 65% as compared to placebo [12]. It ought to be noted that the endline assessment of MRI outcomes were included in this meta-analysis that provide more accurate efficacy measures. Currently approved anti-CD20 treatment for relapsing MS has shown either complete or near complete depletion of circulating B cells, although it is unclear if this is essential for high efficacy outcomes [12]. In the MIRROR trial, ofatumumab led to rapid-dose dependent B-cell depletion, where 60 mg dosage over 12 weeks provided maximum benefit; a higher dosage did not provide more robust treatment effects [12]. The tolerability and adverse event findings in the MIRROR trial were comparable and any symptomatology resolved within one day of onset [12].
Finally, the phase 2 APOLITOS study ascertained that ofatumumab reduced gadolinium-enhancing T1 lesions by 93.6% compared to placebo, and the findings were consistent across regions (Japan and Russia) [13]. The extension part had comparable benefits as well; however, the adverse events were determined to be lower with ofatumumab (69.8%) as compared to placebo (81%). Injection-related adverse reactions were the most common, and no opportunistic infections, deaths, or malignancies were reported. Safety findings were also consistent with pivotal trials [13].
5. Conclusions
The prospect of an effective subcutaneous B-cell targeted therapy, ofatumumab, increases the possibility of self-administration and improvement over available intravenous administration medications. However, while there is demonstrated convenience of usability and optimization of healthcare resources with this intervention, it remains to be seen whether the ongoing trials support the repletion of B cells achieved with ofatumumab, and whether the therapy will emerge with favorable safety and efficacy profiles. Our findings support the favorable effects of the administration of ofatumumab, subcutaneously in the controlled trial setting.
Author Contributions
H.M.T., M.T., and S.S., have contributed to the conception of the work, acquisition, analysis, interpretation of data for the work; drafted the initial study and revised portion, and final approval of the version to be published. A.S., Z.S., K.R.-V., and I.C.-O. have designed the study, interpreted the data, drafted the initial study, revised portion and final approval of the version to be published. Z.S. and I.C.-O. are co-guarantors of this study. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
All data utilized for the purpose of this study are available publicly and online.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kang, C.; Blair, H.A. Ofatumumab: A Review in Relapsing Forms of Multiple Sclerosis. Drugs 2021, 82, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Samjoo, I.A.; Klotz, L.; Giovannoni, G.; Drudge, C.; Haltner, A.; Worthington, E.; Zhao, M.; Brennan, R.; Häring, D.A.; Cameron, C. Simulated Treatment Comparison of Efficacy Outcomes for Ofatumumab in ASCLEPIOS I/II versus Ocrelizumab in OPERA I/II for the Treatment of Patients with Relapsing Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 66, 104031. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration KESIMPTA® (Ofatumumab) Injection, for Subcutaneous Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf (accessed on 24 August 2022).
- Yu, H.; Graham, G.; David, O.J.; Kahn, J.M.; Savelieva, M.; Pigeolet, E.; Das Gupta, A.; Pingili, R.; Willi, R.; Ramanathan, K. Population Pharmacokinetic–B Cell Modeling for Ofatumumab in Patients with Relapsing Multiple Sclerosis. CNS Drugs 2022, 36, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Lammens, A.; Schäfer, W.; Georges, G.; Schwaiger, M.; Mössner, E.; Hopfner, K.-P.; Umaña, P.; Niederfellner, G. Epitope Interactions of Monoclonal Antibodies Targeting CD20 and Their Relationship to Functional Properties. In Proceedings of the MAbs; Taylor & Francis: Oxfordshire, UK, 2013; Volume 5, pp. 22–33. [Google Scholar]
- Roach, C.A.; Cross, A.H. Anti-CD20 B Cell Treatment for Relapsing Multiple Sclerosis. Front. Neurol. 2021, 11, 595547. [Google Scholar] [CrossRef] [PubMed]
- Hemstedt, T.; Kugler, F. Ofatumumab as a Potential First-Line Therapy for Relapsing Multiple Sclerosis in Germany. Neurology 2021, 96, 4093. [Google Scholar]
- Kalincik, T. Multiple Sclerosis Relapses: Epidemiology, Outcomes and Management. A Systematic Review. Neuroepidemiology 2015, 44, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Derfuss, T.; Ontaneda, D.; Nicholas, J.; Meng, X.; Hawker, K. Relapse Rates in Patients with Multiple Sclerosis Treated with Fingolimod: Subgroup Analyses of Pooled Data from Three Phase 3 Trials. Mult. Scler. Relat. Disord. 2016, 8, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Cohen, J.A.; Comi, G.; Correale, J.; Coyle, P.K.; Cross, A.H.; De Seze, J.; Leppert, D.; Montalban, X. Ofatumumab versus Teriflunomide in Multiple Sclerosis. N. Engl. J. Med. 2020, 383, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, A.; Grove, R.A.; Austin, D.J.; Tolson, J.M.; VanMeter, S.A.; Lewis, E.W.; Derosier, F.J.; Lopez, M.C.; Kavanagh, S.T.; Miller, A.E. Subcutaneous Ofatumumab in Patients with Relapsing-Remitting Multiple Sclerosis: The MIRROR Study. Neurology 2018, 90, e1805–e1814. [Google Scholar] [CrossRef] [PubMed]
- Kira, J.; Nakahara, J.; Sazonov, D.V.; Kurosawa, T.; Tsumiyama, I.; Willi, R.; Zalesak, M.; Pingili, R.; Häring, D.A.; Ramanathan, K. Effect of Ofatumumab versus Placebo in Relapsing Multiple Sclerosis Patients from Japan and Russia: Phase 2 APOLITOS Study. Mult. Scler. J. 2022, 28, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).