Abstract
In this paper, we study the stability analysis of two within-host virus dynamics models with antibody immune response. We assume that the virus infects n classes of target cells. The second model considers two types of infected cells: (i) latently infected cells; and (ii) actively infected cells that produce the virus particles. For each model, we derive a biological threshold number . Using the method of Lyapunov function, we establish the global stability of the steady states of the models. The theoretical results are confirmed by numerical simulations.
1. Introduction
Recently, many mathematicians have proposed several mathematical models to describe the interaction between viruses (such as HIV, HCV, HBV, HTLV and CHIKV) and human target cells (see, e.g., [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36]). Mathematical models of human viruses can lead to the development of efficient antiviral drugs and to understand the interaction of the viruses with target cells [2]. Studying the stability analysis of the models is also important to understand the behavior of the virus. Immune response plays an important role in controlling the infection of several viruses. Cytotoxic T Lymphocyte (CTL) and antibodies are the two effector responses of the immune system. CTL cells attack and kill the infected cells. The B cell produces antibodies to neutralize the viruses. The antibody immune response is more effective than CTL immune response in some infection processes [37]. The basic virus dynamics model with antibody immune response has been presented in [29,30] as:
where S, I, V, and B are the concentrations of uninfected target cells, infected cells, virus particles and B cells, respectively. Parameters d and represent the death rate and birth rate constants of the uninfected cells, respectively. The uninfected cells become infected at rate , where b is rate constant of the virus-target incidence. The infected cells and free virus particles die at rates and , respectively. An actively infected cell produces an average number m of virus particles per unit time. The virus particles are attacked by the B cells at rate . The term represents the growth rate of B cells after encountering the virus. The B cells die at rate . In 2017, Wang and Liu [36] presented a mathematical model for in host virus infection by considering a constant production rate of the B cells in addition to their proliferation rate. Equation (4) has been modified as:
where is the production rate of the B cells.
The model in Equations (1)–(4) assumes that the virus infects only one category of target cells. There are several models for viral infections that have included two categories of target cells (see, e.g., [38,39,40,41,42,43,44,45,46,47]). It has been reported in [48] that HIV infects vital cells in the human immune system such as helper T cells (specifically CD4 T cells), macrophages, and dendritic cells. In the case of CHIKV infection, the CHIKV inoculates into the body via bites from infected mosquitoes and replicates in a variety of cells, such as skeletal muscle satellite cells, fibroblasts, macrophages, monocytes and other skin cells [49,50,51,52]. To model the virus dynamics with multiplecategories of target cells, Elaiw [53] proposed the following viral infection model:
where n is the number categories of target cells. This model was generalized by Xia et al. [54] by considering general nonlinear rates for viral infection and cell death. In [53,54], the antibody immune response has been neglected. Several mathematical models have been proposed which take the antibody immune response into account (see, e.g., [29,30,31,32,33,34,35]). However, these models have included one target cell population. Therefore, our aim in this paper is to introduce two virus infection models to describe the dynamics of the virus with n classes of target cells. The antibody immune response is considered where the population dynamics of the B cells is described by Equation (5). In the second model, we incorporate both latently and actively infected cells. We investigate the nonnegativity and boundedness of the solutions of the models. We establish the existence of the steady states and analyze their global stability. We construct Lyapunov function using the method of Korobeinikov [55].
2. Virus Dynamics Model
We consider a within-host virus dynamics model with n classes of uninfected target cells.
where represent the concentrations of the uninfected target cells and infected cells of class respectively.
2.1. Properties of Solutions
To show that the model in Equations (9)–(12) is biologically acceptable in the sense that no population goes negative or infinity, we establish the nonnegativity and boundedness of solutions of the model. Let and
2.2. Steady States
In this subsection, we show the existence of the steady states of the model in Equations (9)–(12). The basic reproduction number of the system in Equations (9)–(12) is defined as:
Lemma 1.
(i) If then the virus-free steady state is the only steady state for the system; and (ii) if then the system has a unique endemic steady state and where is the interior of
Proof.
Substituting Equations (13), (14) and (16) into Equation (15), we get
where and Equation (17) admits as a solution. Substituting in Equations (13),(14) and (16), we get the virus-free steady state where and The other possibility of Equation (17) is
where .
Let us define a function as:
Then, we get
Thus, if then and there exists such that Moreover, from Equations (13), (14) and (16), we obtain and . Then, exists when .
It follows that
2.3. Global Stability
In the following theorems, we establish the global stability of the two steady states of the system in Equations (9)–(12) by constructing suitable Lyapunov functions. Let us define
Clearly, for and .
The proofs of these theorems are given in the Appendix A.
Biologically, when , then each infected cell will produce less than one infected cell during its life at the beginning of the infection. The virus will be decreased and eliminated from the body. When , then, at the beginning of the infection, each infected cell will produce more than one infected cell during its life. The viruses will be increased and the infection becomes chronic.
3. Virus Model with Latency
In this section, we study the mathematical model of virus infection with n classes of uninfected target cells, taking into account the latently infected cells (such cells contain the viruses but are not producing it) and the actively infected cells (such cells are producing the viruses).
where and are the concentrations of latently infected and actively infected target cells of class respectively. A fraction of infected target cells is assumed to be latently infected cells and the remaining becomes actively infected cells, where The latently infected cells are transmitted to actively infected cells at rate and die at rate
3.1. Properties of Solutions
In the following, we establish the nonnegativity and boundedness of solutions of the model in Equations (19)–(23). Let
3.2. Steady States
In this subsection, we establish the existence of the steady states of the model in Equations (19)–(23). The basic reproduction number of the system in Equations (19)–(23) is defined as:
Lemma 2.
(i) If then the virus-free steady state is the only steady state for the system; and (ii) if then the system has a unique endemic steady state and
3.3. Global Stability
In this section, we use Lyapunov method to prove the global stability of the two steady states of the system in Equations (19)–(23).
The proofs of these theorems are given in the Appendix A.
4. Numerical Simulations
To illustrate our theoretical results, we perform numerical simulations for the systems in Equations (9)–(12) and Equations (19)–(23). We consider the case .
4.1. Simulations for Virus Dynamics Model
Using the values of the parameters given in Table 1, we show the dynamical behavior of the system states , , V and B, to confirm the theoretical results given in Theorems 1–2.
• Effect of and on the stability of steady states: To show the global stability results, we consider three different initial values as:
IV1: and
IV2: and
IV3: and
We consider two sets of the parameters and as follows:
Set (I): We choose and Using these data, we compute , then the system has one steady state . In Figure 1, we can see that the concentrations of the uninfected target cells and B cells return to their normal values and . On the other hand, the concentrations of infected target cells and virus particles are decaying and approaching zero for all the three initial values IV1–IV3. It means that is globally asymptotically stable and the virus will be cleared. This result confirms the result of Theorem 1.
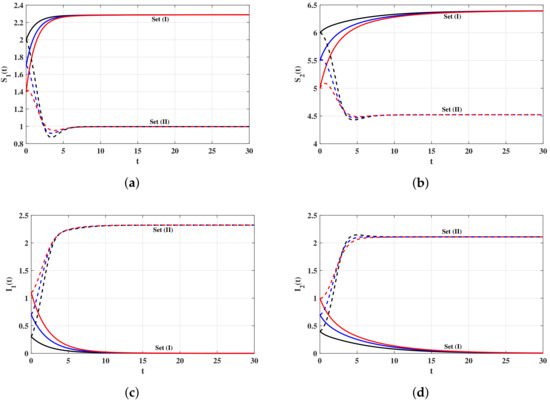
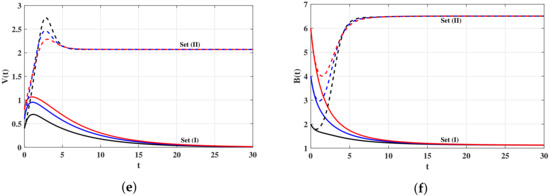
Set (II): We take and Then, we calculate The system has two steady states and . It is clear in Figure 1 that both the numerical results and the theoretical results of Theorem 2 are consistent. It is seen that the solutions of the system converge to the steady state for all the three initial values IV1–IV3.
4.2. Simulations for Virus Model with Latency
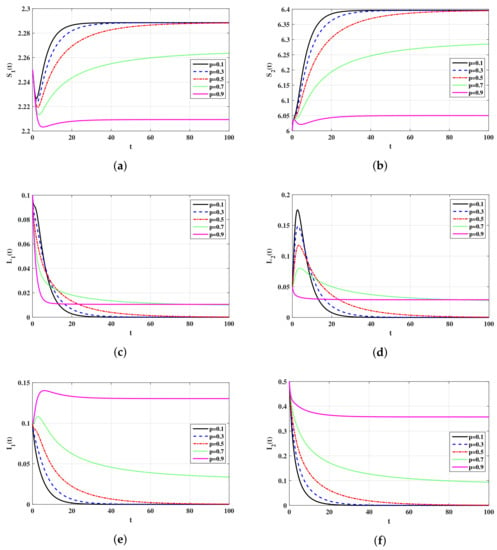
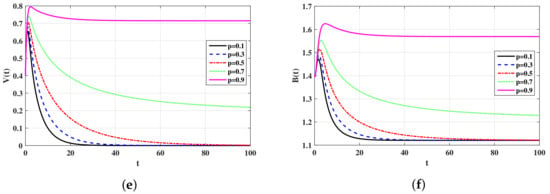
In this subsection, we show the numerical results for the system in Equations (19)–(23) with parameters values given in Table 2. The effect of parameters and on the qualitative behavior of the system is discussed below. We take The initial values are chosen as: ,, and In Table 3, we have calculated the values of the steady states and for different values of p. It is clearly seen that, as p is increased, is also increased. Let be the value of p, such that

Using the data given in Table 2, we obtain In Figure , we can see that, for the trajectory of the system will converge to and, for the trajectory will converge to This shows that, the factor plays the role of a controller which can used to stabilize the system around Biologically, the factor plays the role of an antiviral treatment which can be applied to eradicate the virus from the body.
5. Conclusions and Discussion
Most of the existing mathematical models of viral infection study the viral infection and production in one or two classes of target cells.However, HIV and CHIKV can infect three and five types of target cells, respectively. In this paper, we have studied two within-host virus dynamics models with antibody immune response and with n classes of target cells. In the second model, we have considered two types of infected cells, latently infected cells (such cells contain the viruses but are not producing it) and the actively infected cells (such cells are producing the viruses). We have shown that the solutions of each model are nonnegative and bounded, which ensure the well-posedness of the models. For each model, we have derived a biological threshold number (the basic reproduction number) which fully determines the existence and stability of the two steady states of the model. We have investigated the global stability of the steady states of the model by using Lyapunov method and LaSalle’s invariance principle. We have proven that: (i) if (), then the virus-free steady state () is globally asymptotically stable and the virus is predicted to be completely cleared from infected individuals; and (ii) if (, then the endemic steady state () is globally asymptotically stable and a chronic virus infection is attained. We have conducted numerical simulations and have shown that both the theoretical and numerical results are consistent. Our analysis extends some existing results in the literature. For example, the global stability was analyzed for a model with one target cell population [36].
The model in Equations (1)–(4) has three steady states virus-free steady state endemic steady state without antibody immune response and endemic steady state with antibody immune response . Moreover, the existence and stability of the steady states are determined by two threshold parameters, the basic reproduction number (which determines whether or not the disease will progress) and the antibody immune response activation number (which determines whether a persistent antibody immune response can be established ) [30], where
We note that the values of the parameters q, c and have no impact on the values of . Thus, the model in Equations (1)–(4) implies that the antibody immune response do not play a role in clearing the viruses but can play a significant role in reducing the infection progress.
Our models have two steady states and their existence and stability are determined by one threshold parameter . This is because of considering the production rate of the B cells . The basic reproduction number of the model in Equations (9)–(12) in the case of is given by:
We can see that depends on the parameter . Therefore, if the production rate of the B cells is increased such that , then is globally asymptotically stable. Thus, the model in Equations (9)–(12) implies that the antibody immune response can clear the virus from body.
In our proposed models, we have only considered one arm of the immune system which is based on the antibodies. CTL cells play a prominent role in achieving the best representation of the dynamics of some types of viruses. The virus dynamics model with n categories of target cells and CTL immune response can be given as:
In addition to this model, one can formulate a virus dynamics model with n categories of target cells and both antibodies and CTL immune response.
5.1. Effects of Latency on the Virus Dynamics
In this subsection, we show the effect of the presence of latently infected cells on the virus dynamics. Let us incorporate an antiviral treatment with drug efficacy u where . The virus dynamics model in Equations (9)–(12) under the effect of treatment is given by:
Since , then
Clearly, the presence of latently infected cells deceases the basic reproduction number of the system. Now we aim to determine the minimum drug efficacy that able to clear the viruses from the body. We determine and that make
to stabilize the system in Equations (31)–(39) around and , respectively. Now, we calculate and as:
Author Contributions
All authors contributed equally to the writing of this paper. All authors read and approved the final manuscript.
Acknowledgments
This article was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Conflicts of Interest
The authors declare te no conflict of interest.
Appendix A
Proof (Proof of Theorem A2).
Construct a Lyapunov function as:
Applying
we obtain
Using the endemic steady state conditions
we get
The relation between the geometrical mean and the arithmetical mean implies that
Therefore, if then and for all The solutions of system limit to the largest invariant subset of We have if and only if and It follows from LaSalle’s invariance principle that is globally asymptotically stable in
Proof (Proof of Theorem A4).
Construct a Lyapunov function as follows:
Applying
we obtain
Using the endemic steady state conditions
we get
and
Clearly, and if and only if and . It follows from LaSalle’s invariance principle, is globally asymptotically stable in
References
- Nowak, M.A.; Bangham, C.R.M. Population dynamics of immune responses to persistent viruses. Science 1996, 272, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Bonhoeffer, S.; May, R.M.; Shaw, G.M.; Nowak, M.A. Virus dynamics and drug therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 6971–6976. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; Raezah, A.A. Stability of general virus dynamics models with both cellular and viral infections and delays. Math. Meth. Appl. Sci. 2017, 40, 5863–5880. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Elnahary, E.K.; Raezah, A.A. Effect of cellular reservoirs and delays on the global dynamics of HIV. Adv. Differ. Equ. 2018, 2018, 85. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Stability of latent pathogen infection model with adaptive immunity and delays. J. Integr. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.K.; Chatterjee, A.N.; Greenhalgh, D.; Khan, Q.J.A. Long term dynamics in a mathematical model of HIV-1 infection with delay in different variants of the basic drug therapy model. Nonlinear Anal. Real World Appl. 2013, 14, 1621–1633. [Google Scholar] [CrossRef]
- Connell, M.C.; Yang, Y. Global stability of a diffusive virus dynamics model with general incidence function and time delay. Nonlinear Anal. Real World Appl. 2015, 25, 64–78. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L. Global stability of an HIV-1 model with distributed intracellular delays and a combination therapy. Math. Biosci. Eng. 2010, 7, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fu, S. Global stability of a virus dynamics model with intracellular delay and CTL immune response. Math. Meth. Appl. Sci. 2015, 38, 420–430. [Google Scholar] [CrossRef]
- Hattaf, K.; Yousfi, N.; Tridane, A. Mathematical analysis of a virus dynamics model with general incidence rate and cure rate. Nonlinear Anal. Real World Appl. 2012, 13, 1866–1872. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Raezah, A.A.; Hattaf, K. Stability of HIV-1 infection with saturated virus-target and infected-target incidences and CTL immune response. Int. J. Biomath. 2017, 15, 1750070. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Raezah, A.A.; Alofi, B.S. Dynamics of delayed pathogen infection models with pathogenic and cellular infections and immune impairment. AIP Adv. 2018, 8, 025323. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Raezah, A.; Alofi, A.S. Effect of humoral immunity on HIV-1 dynamics with virus-to-target and infected-to-target infections. AIP Adv. 2016, 6, 085204. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Raezah, A.; Alofi, A. Stability of a general delayed virus dynamics model with humoral immunity and cellular infection. AIP Adv. 2017, 7, 065210. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H.; Alofi, A.S. Stability of CTL immunity pathogen dynamics model with capsids and distributed delay. AIP Adv. 2017, 7, 125111. [Google Scholar] [CrossRef]
- Gibelli, L.; Elaiw, A.; Alghamdi, M.A.; Althiabi, A.M. Heterogeneous population dynamics of active particles: Progression, mutations, and selection dynamics. Math. Models Meth. Appl. Sci. 2017, 27, 617–640. [Google Scholar] [CrossRef]
- Huang, G.; Takeuchi, Y.; Ma, W. Lyapunov functionals for delay differential equations model of viral infections. SIAM J. Appl. Math. 2010, 70, 2693–2708. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Lu, X.; Liu, S. A delayed HIV-1 model with virus waning term. Math. Biosci. Eng. 2016, 13, 135–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhang, X.; Guo, Y.; Wang, H. Analysis of an HIV infection model with treatments and delayed immune response. Appl. Math. Model. 2016, 40, 3081–3089. [Google Scholar] [CrossRef]
- Wang, K.; Fan, A.; Torres, A. Global properties of an improved hepatitis B virus model. Nonlinear Anal. Real World Appl. 2010, 11, 3131–3138. [Google Scholar] [CrossRef]
- Manna, K. Dynamics of a diffusion-driven HBV infection model with capsids and time delay. Int. J. Biomath. 2017, 10, 1750062. [Google Scholar] [CrossRef]
- Peralta, R.; Vargas-De-Leon, C.; Miramontes, P. Global stability results in a SVIR epidemic model with immunity loss rate depending on the vaccine-age. Abstr. Appl. Anal. 2015, 2015, 341854. [Google Scholar] [CrossRef]
- Monica, C.; Pitchaimani, M. Analysis of stability and Hopf bifurcation for HIV-1 dynamics with PI and three intracellular delays. Nonlinear Anal. Real World Appl. 2016, 2, 55–69. [Google Scholar] [CrossRef]
- Neumann, A.U.; Lam, N.P.; Dahari, H.; Gretch, D.R.; Wiley, T.E.; Layden, T.J.; Perelson, A.S. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 1998, 282, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, M.Y.; Kirschner, D. Mathematical analysis of the global dynamics of a model for HTLV-I infection and ATL progression. Math. Biosci. 2002, 179, 207–217. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Son, X. Dynamical behavior of a delay virus dynamics model with CTL immune response. Nonlinear Anal. Real World Appl. 2010, 11, 1795–1809. [Google Scholar] [CrossRef]
- Shu, H.; Wang, L.; Watmough, J. Global stability of a nonlinear viral infection model with infinitely distributed intracellular delays and CTL imune responses. SIAM J. Appl. Math. 2013, 73, 1280–1302. [Google Scholar] [CrossRef]
- Wang, J.; Lang, J.; Zou, X. Analysis of an age structured HIV infection model with virus-to-cell infection and cell-to-cell transmission. Nonlinear Anal. Real World Appl. 2017, 34, 75–96. [Google Scholar] [CrossRef]
- Murase, A.; Sasaki, T.; Kajiwara, T. Stability analysis of pathogen-immune interaction dynamics. J. Math. Biol. 2005, 51, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zou, D. Global stability of in host viral models with humoral immunity and intracellular delays. J. Appl. Math. Mod. 2012, 36, 1313–1322. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Z.; Liao, F. Stability and Hopf bifurcation for a virus infection model with delayed humoral immunity response. J. Math. Anal. Appl. 2014, 411, 63–74. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Global stability of humoral immunity virus dynamics models with nonlinear infection rate and removal. Nonlinear Anal. Real World Appl. 2015, 26, 161–190. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Global properties of nonlinear humoral immunity viral infection models. Int. J. Biomath. 2015, 8, 1550058. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H. Stability of a general delay-distributed virus dynamics model with multi-staged infected progression and immune response. Math. Meth. Appl. Sci. 2017, 40, 699–719. [Google Scholar] [CrossRef]
- Elaiw, A.M.; AlShamrani, N.H.; Hattaf, K. Dynamical behaviors of a general humoral immunity viral infection model with distributed invasion and production. Int. J. Biomath. 2017, 10, 1750035. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X. Stability and Hopf bifurcation of a within-host chikungunya virus infection model with two delays. Math. Comput. Simul. 2017, 138, 31–48. [Google Scholar] [CrossRef]
- Deans, J.A.; Cohen, S. Immunology of malaria. Ann. Rev. Microbiol. 1983, 37, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Perelson, A.S.; Essunger, P.; Cao, Y.; Vesanen, M.; Hurley, A.; Saksela, K.; Markowitz, M.; Ho, D.D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997, 387, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolny, H.M.; Baron, M.J.; Gieschke, R.; Davies, B.E.; Jumbe, N.L.; Beauchemin, C.A.A. Exploring cell tropism as a possible contributor to influenza infection severity. PLoS ONE 2011, 5, e13811. [Google Scholar] [CrossRef] [PubMed]
- Bajaria, S.H.; Webb, G.; Cloyd, M.; Kirschner, D. Dynamics of naive and memory CD4 + T Lymphocytes in HIV-1 disease progression. J. Acquir. Immune Defic. Syndr. 2002, 30, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.J.H.; Nowak, M.A.; Blumberg, B.S. Analysis of a cellular model to account for the natural history of infection by the hepatitis B virus and its role in the development of primary hepatocellular carcinoma. J. Theor. Biol. 1992, 159, 215–240. [Google Scholar] [CrossRef]
- Payne, R.J.H.; Nowak, M.A.; Blumberg, B.S. A cellular model to explain the pathogenesis of infection by the hepatitis B virus. Math. Biosci. 1994, 123, 25–58. [Google Scholar] [CrossRef]
- Dahari, H.; Feliu, A.; Garcia-Retortillo, M.; Forns, X.; Neumann, A.U. Second hepatitis C compartment indicated by viral dynamics during liver transplantation. J. Hepatol. 2005, 42, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M.; Azoz, S.A. Global properties of a class of HIV infection models with Beddington-DeAngelis functional response. Math. Meth. Appl. Sci. 2013, 36, 383–394. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Hassanien, I.A.; Azoz, S.A. Global stability of HIV infection models with intracellular delays. J. Korean Math. Soc. 2012, 49, 779–794. [Google Scholar] [CrossRef]
- Elaiw, A.M. Global properties of a class of HIV models. Nonlinear Anal. Real World Appl. 2010, 11, 2253–2263. [Google Scholar] [CrossRef]
- Elaiw, A.M.; Almuallem, N.A. Global dynamics of delay-distributed HIV infection models with differential drug efficacy in cocirculating target cells. Math. Meth. Appl. Sci. 2016, 39, 4–31. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Donaghy, H.; Harman, A.N.; Kim, M.; Turville, S.G. Manipulation of dendritic cell function by viruses. Curr. Opin. Microbiol. 2010, 13, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Couderc, T.; Chretien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Lum, F.M.; Ng, L.F.P. Cellular and molecular mechanisms of chikungunya pathogenesis. Antivir. Res. 2015, 120, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Ozden, S.; Huerre, M.; Riviere, J.P.; Coffey, L.L.; Afonso, P.V.; Mouly, V. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE 2007, 2, e527. [Google Scholar] [CrossRef] [PubMed]
- Her, Z.; Malleret, B.; Chan, M.; Ong, E.K.; Wong, S.C.; Kwek, D.J. Active infection of human blood monocytes by Chikungunya virus triggers an innate immune response. J. Immunol. 2010, 184, 5903–5913. [Google Scholar] [CrossRef] [PubMed]
- Elaiw, A.M. Global properties of a class of virus infection models with multitarget cells. Nonlinear Dyn. 2012, 69, 423–435. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Tang, S.; Rong, L. Analysis of HIV models with multiple target cell populations and general nonlinear rates of viral infection and cell death. Math. Comput. Simul. 2016, 124, 87–103. [Google Scholar] [CrossRef]
- Korobeinikov, A. Global properties of basic virus dynamics models. Bull. Math. Biol. 2004, 66, 879–883. [Google Scholar] [CrossRef] [PubMed]
- LaSalle, J.P. Stability theory of ordinary differential equations. J. Differ. Equ. 1968, 4, 57–65. [Google Scholar] [CrossRef]
- LaSalle, J.P. The stability of dynamical system. In The Stability of Dynamical Systems; SIAM: Philadephia, PE, USA, 1976. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).