Abstract
Topological indices are often used to predict the physicochemical properties of molecules. The multiplicative sum Zagreb index is one of the multiplicative versions of the Zagreb indices, which belong to the class of most-examined topological indices. For a graph G with edge set , its multiplicative sum Zagreb index is defined as the product of the numbers , where is the sum of the degrees of the end vertices of . A chemical tree is a tree of maximum degree at most 4. In this research work, graphs possessing the maximum multiplicative sum Zagreb index are determined from the class of chemical trees with a given order and fixed number of segments. The values of the multiplicative sum Zagreb index of the obtained extremal trees are also obtained.
Keywords:
topological index; multiplicative sum Zagreb indices; chemical trees; segments; extremal problem MSC:
05C05; 05C07; 05C09; 05C35
1. Introduction
A characteristic of a graph that is preserved under graph isomorphism is commonly referred to as a graph invariant [1]. In chemical graph theory, graphical invariants that take numerical quantities are usually named topological invariants, or simply, topological indices. Zagreb indices, particularly the first and second Zagreb indices denoted by and , respectively, belong to well-examined categories of topological indices. Initially, they appeared in connection with the study molecules [2,3]. These indices can be defined as
where is an edge between the vertices u and v, while is the degree of the vertex x. Some information about the chemical applications of and can be found in [4,5]. These indices have also been the subject of extensive research into their relationship, comparison, and other mathematical properties [6,7,8,9,10,11,12,13,14]. Many existing facts of the Zagreb indices can be found in survey papers [15,16,17,18,19].
In 2010, Todeschini et al. [20] proposed to consider the multiplicative versions of topological indices. The multiplicative versions of and are defined [21] as follows:
Bozovic et al. [22] discussed extreme values of multiplicative Zagreb indices for chemical trees. Wang et al. [23] examined the extremum multiplicative Zagreb indices of trees with a specific number of vertices and maximum degree. Further mathematical features of these two multiplicative indices can be found in [21,24,25,26,27,28,29,30,31].
In 2012, Eliasi et al. [25] introduced a modification of the multiplicative first Zagreb index known as the multiplicative sum Zagreb index [32]. The mathematical formulation of the multiplicative sum Zagreb index is as follows:
In [25], it was shown that the path has the least -value across all connected graphs with the specified order. The trees achieving the second least -value were also determined in [25]. Xu and Das [31] described the extremal trees, unicyclic graphs and bicyclic graphs of a specified order with respect to . Azari and Iranmanesh [33] established bounds on for graph operations. Further mathematical features of can be found in [27,34].
The focus of this work is strictly on the mathematical structure of chemical graphs. (Such graphs have found applications in chemistry; see, for example, the recent article [35]). More precisely, in this study, graphs possessing the greatest -values are determined from the class of chemical trees with a given order and fixed number of segments. The -values of the obtained extremal trees are also obtained.
2. Preliminaries
In this section, some definitions as well as notations used in this paper are given. Undefined terminology from graph theory can be found in some standard books. The graphs under discussion here are simple, undirected, and finite. The degree of a vertex v is represented by . The distance between two vertices u and v is denoted by . A tree of maximum degree at most four is called a chemical tree. The notion ∣A∣ represents the cardinality of the set A. Consider a non-trivial path in a tree such that and . If , then every vertex of different from vertex and is called an internal vertex of . The path is referred to as an internal path if provided that all internal vertices have degree 2 (if exist). Furthermore, the path is an external path if one of the two numbers is equal to 1 and the other has a value greater than 2 provided that all internal vertices have degree 2 (if exist). A branching vertex in a tree is a vertex with a degree greater than 2. If S is an external path or an internal path in a tree G, then S is called a segment of the graph G. We call the path graph of order n also a segment of . Thus, a path graph has only one segment and there is no graph with exactly two segments. Let denote the class of all chemical trees with exactly n vertices and s segments, where . Let be the degree sequence of a graph G. Define . For a chemical tree G, we write (for the sake of simplicity) the degree sequence of G as
For example, if a chemical tree has the degree sequence then we write it as . Let be the set of neighbors of the vertex . In a graph G, let (or simply , when there is no confusion about G) be the set of edges of G with end vertices of degrees i and j. Certainly, . For , define .
3. Main Results
Before proceeding to our main results, we give some crucial lemmas that provide some useful information on obtaining the greatest possible value of the multiplicative sum Zagreb index for trees belonging to the class .
Lemma 1.
For a chemical tree with maximum multiplicative sum Zagreb index, the following statements are true:
- (a)
- If then ,
- (b)
- If then ,
- (c)
- If there is an internal path of the form in of length 1, then there is no internal path of the form in , with , having length larger than 1.
- (d)
- If a path , with , contains an internal vertex of degree four then .
- (e)
- The graph does not possess an internal path having a length of 1 and an internal path having a length larger than 2 simultaneously.
- (f)
- If has exactly two vertices of degree 3 then does not possess simultaneously the internal paths and both of length 1.
Proof.
Throughout this proof, whenever the degree notion is used, it represents the degree of a vertex in the graph .
- (a)
- If then there must be a vertex, say , of degree two laying on an external path of where , , and . Since , has another branching vertex, say (), which forms an internal path in the graph . Let be the neighbor of that lies on the path (the vertex may coincide with ).Now, we consider a tree that can be found in the class and is obtained from by the following operation:There exists a positive real number such that these two graphs satisfy the following:Since and , Equation (1) yields , a contradiction.
- (b)
- Assume that the hypothesis holds but the conclusion does not hold; that is, suppose that the inequality ∣ holds. Let v denotes the only branching vertex in (as ) with two distinct external paths and , each one having length of at least 2. Now, a new tree is constructed from using the following operation:This operation emphasizes that . There is such thatSince , Equation (2) gives , a contradiction.
- (c)
- Assume contrarily that possesses an internal path of the form of length larger than 1, such that . Suppose that and , where and . Then, a tree is considered that is obtained using the following operation:There is a number , such thatThe following possible cases are discussed next:: .In this case, . Thus, and consequently, Equation (3) givesa contradiction.: Either and or and .In this case, . Thus, and consequently, Equation (3) givesa contradiction again.In both possible cases, we arrive ata contradiction because of our contrary assumption that possess an internal path of the form of length larger than 1, such that .
- (d)
- Contrarily, assume that . Then, there exists , such that and . Let be a path with an internal vertex of degree 4, such that . Without loss of generality, suppose that is the only internal vertex on ; otherwise, we may consider a subpath of containing exactly one internal vertex of degree 4, such that . The following cases are to be discussed here::If , then we assume that is a neighbor of not lying on the path ; otherwise, we assume that is a neighbor of lying on the path. Whether z lies on or not, in either of the two cases, we define a new graph as follows:Note that the tree belongs to the collection . Whether z lies on or not, in either of the two cases, there exists a positive real number , such thatwhich is a contradiction.: .In this case, we consider a tree obtained using the operation described as follows:Whether z lies on or not, in either of the two cases, there exists a positive real number , such thatNote that there are the following three possibilities concerning the degrees of the vertices and :
- –
- and ,
- –
- and ,
- –
- .
In every case, Equation (4) givesa contradiction. - (e)
- Although the proof of this part is slightly different from that of part (c), we provide its proof here for the sake of completeness. Assume contrarily that simultaneously possesses an internal path of length and an internal path of length 1, where . Then, a tree is considered that is obtained using the following operation:There is a number , such thatSince and , Equation (5) providesa contradiction.
- (f)
- Assume contrarily that possesses simultaneously the internal paths and both of length 1. Let , such that , the distance is minimum, and . Note that y and u lie on the unique path. Let be the neighbor of y lying on the path. By part (c), the degree of is 4.First, we discuss the case when . Consider a tree that is obtained using the following operation:Then, we obtain , a contradiction.Next, consider the case when . Then, has a neighbor, say , of degree 2 lying on the path. Let be the neighbor of lying on the path. The vertices and u may be the same. By part (e), . Now, consider a tree that is obtained using the following operation:Certainly, . Now, defineThen, and , which yields a contradiction again.
□
If we consider the class for , then we note that consists of exactly one element for every . For with , we have the next result, which follows from Lemma 1(b).
Corollary 1.
The graph constructed by attaching pendent vertices to a single pendent vertex of the path possesses uniquely the greatest multiplicative sum Zagreb index in for every with . The mentioned greatest value is .
Because of Corollary 1, in the rest of the current section, we focus on the case when for the class . To prove our next lemma, we need the following existing result:
Lemma 2
([36]). For every graph , the following statements holds:
- (a)
- The equation holds if and only if , , , for some positive integer t.
- (b)
- The equation holds if and only if , , , for some positive integer t.
- (c)
- The equation holds if and only if , , for some positive integer t.
Lemma 3.
Let be a chemical tree with maximum multiplicative sum Zagreb index and t be a positive integer. Then,
Proof.
Throughout this proof, whenever the degree notion is used, it represents the degree of a vertex in the graph . First, we show that . We suppose on the contrary that the inequality holds. Take , such that . If all these three vertices are on one path, then (without loss of generality) suppose that q lies on the unique path in . In either of the two cases, suppose that is the set of neighbors of r with the condition that is located on path ( may coincide with q). Now, a chemical tree is obtained in the collection using the following operation:
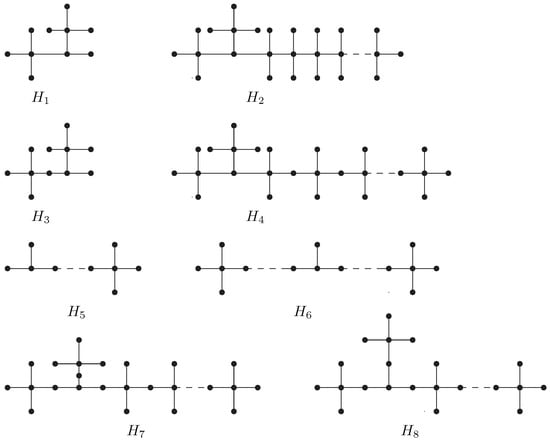
For the case when all the three vertices are on one path, see Figure 1.

Figure 1.
The transformation applied on the graph to obtain a new graph in Lemma 3.
Note that there is a real number , such that
Since , inequality (6) yields
a contradiction. Hence, the inequality holds, which together with Lemma 2 gives the required result. □
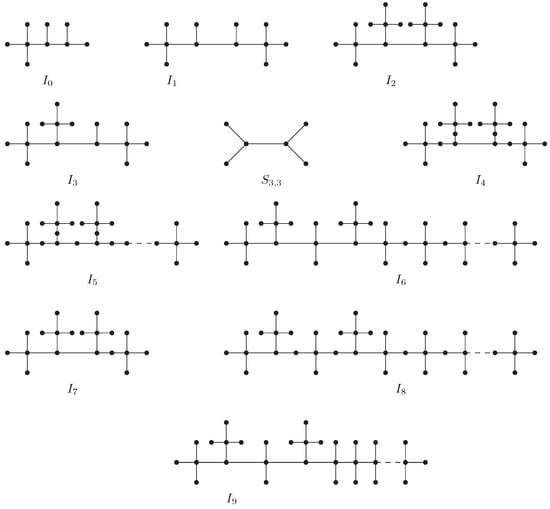
We now define three subclasses of as follows when and for some integer :
- .
- consists of those members of that obey and and .
- consists of those members of that obey and .
Three examples , , and , one from each of the classes , , and , respectively, are depicted in Figure 2.
Figure 2.
Three examples , , and , one from each of the classes , , and , respectively.
Theorem 1.
Let be a chemical tree with maximum multiplicative sum Zagreb index such that and for some integer . Then .
Proof.
By Lemma 3, it holds that . If then and we are done. If then Lemma 1(a) confirms that . Now, the conclusion is obtained from Lemma 1(e). □
Next, we define five subclasses of as follows when and for some integer :
Theorem 2.
Let be a chemical tree with maximum multiplicative sum Zagreb index such that and for some integer . Then .
Proof.
By Lemma 3, it holds that . If then Lemma 1(a) implies that . If , then by the parts (a), (d), and (e) of Lemma 1, we have . If (i) and , or (ii) and , or (iii) and , then by the parts (a), (c), (d), and (e) of Lemma 1, we conclude that , or , or , respectively. □
We next define six subclasses of as follows when and for some integer :
Theorem 3.
Let be a chemical tree with the greatest multiplicative sum Zagreb index such that and for some integer . Then .
Proof.
By Lemma 3, it holds that . If then Lemma 1(a) implies that . If (i) and , or (ii) and , or (iii) and , or (iv) and , or (v) and , then by Lemma 3, we conclude that , or , or , or , or , respectively. □
Theorem 4.
Let be a chemical tree with maximum multiplicative sum Zagreb index such that for some integer . Then,
Proof.
By Lemma 3, we have
The following cases arise:
:
By using the above degree sequence of , we have . Thus, in this case, consists of vertices of degree 4 and 1 only. Consequently, we have and . Hence,
Therefore,
: .
Note, in this case, that . By keeping in mind Lemma 1, we obtain
Therefore,
: .
Note, in the present case, that . Bearing in mind Lemma 1, we obtain
Therefore,
□
Theorem 5.
Let be the chemical tree with maximum multiplicative sum Zagreb index such that for some integer . Then,
Proof.
By Lemma 3, we have
: .
In the present case, it holds that . We discuss two possible subcases of the present case as follows:
: .
Note, in the present subcase, that . Since in the consider case, if then Equation (7) yields
and hence . Thus, is the graph constructed by attaching two new pendent vertices to a pendent vertex of the star . Similarly, if , then Equation (7) gives
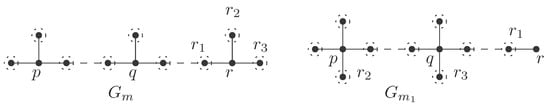
and hence . Thus, by Lemma 1(e), is the graph depicted in Figure 3. Hence, if , then
Therefore,
: .
Note, in the present subcase, that . Recall also that in the present case. By Lemma 1(d), every neighbor of the unique vertex of degree 3 in is of degree 4; for example, see in Figure 3. Hence,
Therefore,
: .
Observe, in the current case, that . In the following, we discuss two subcases according to whether or .
: .
Observe that . If , then and hence by Equation (7), we have
If then, by Lemma 1, is the graph depicted in Figure 3. If then, again by Lemma 1, is the graph constructed from by inserting a vertex of degree 2 on its unique internal path of length 1. Thus, all possible non-zero values of are given as follows:
Therefore,
: .
Observe, in the current subcase, that and .
: and
Note that
that is, . Hence, by Lemma 1, every internal path of the form (in ) has length 2, and its unique vertex of degree 3 is adjacent to vertex/vertices of degree 4; for example, consider a graph constructed from the graph (shown in Figure 3) by inserting a vertex of degree 2 on each of the internal path(s) of the form . Hence, the possible non-zero values of of are given as follows:
Therefore,
: and .
Observe, in the current subcase, that . Note that
that is, . Hence, by Lemma 1, internal path(s) of the form in has/have length 2; for example, consider a graph constructed from the graph (shown in Figure 3) by inserting a vertex of degree 2 on each of the internal path(s) of the form . Hence, the possible non-zero values of are given as follows:
Therefore,
: .
In the following, we discuss subcases for the current case.
: .
Observe, in the present subcase, that . If then , and Equation (7) yields
Thus, by Lemma 1, is of the form given in Figure 3.
If then , and Equation (7) gives
Thus, by Lemma 1, is a graph constructed from (given in Figure 3) by inserting at least one vertex of degree 2 on each of two internal paths of length 1.
Hence, for , the possible non-zero values of are given as
Therefore,
: .
Since , we have
Thus, by Lemma 1, every internal path of has a length of at least 2; for example, consider a graph constructed from (given in Figure 3) by inserting at least one vertex of degree 2 on some internal path(s). Hence, all possible non-zero values of are as follows:
Therefore,
Consequently, the proof is now completed. □
Theorem 6.
Let be a chemical tree with maximum multiplicative sum Zagreb index such that for some integer . Then
Proof.
By Lemma 3, it holds that
: .
Certainly, in the current case, . The following subcases are further discussed:
: .
Here, and hence . Now, Equation (8) gives
Because of Lemma 1, is isomorphic to , , , , or when is equal to , , , , or , respectively. Hence, all possible non-zero values of are as follows:
Therefore,
: .
Clearly, . By Lemma 1, all possible non-zero values of in (for example, see shown in Figure 4) are as follows:
Therefore,
: .
In this case, we have . The following subcases are further considered:
: .
Note, in the current subcase, that . By Lemma 1, the graph is constructed by inserting vertices of degree 2 on the internal path of the graph shown in Figure 4. Hence, all possible non-zero values of are mentioned below:
Therefore,
: and .
Note here that and hence belongs to the set
Now, Equation (8) gives
Because of Lemma 1, is isomorphic to a graph constructed from , , , or by inserting one vertex of degree 2 on each of the internal path(s) of the form . Hence, all possible non-zero values of are as follows:
Therefore,
: and .
By Lemma 1, is isomorphic to a graph constructed from , , , or by inserting at least one vertex of degree 2 on each its internal path(s). Hence, all possible non-zero values of are as follows:
Therefore,
: and .
Note, in the current subcase, that and . (If then we obtain , a contradiction.) Also, note that
Thus, by Lemma 1, all possible non-zero values of are as follows (for example, may be a graph constructed from (shown in Figure 4) by inserting a vertex of degree 2 on each of its internal path(s) of the form ):
Therefore,
: and .
In the current case, observe that . Also, note that
that is, . Thus, by Lemma 1, all possible non-zero values of are as follows (for example, may be a graph constructed from (shown in Figure 4) by inserting one vertex of degree 2 on each of its internal path(s) of the form ):
Therefore,
: and .
Observe, in the current subcase, that
Thus, by Lemma 1, all possible non-zero values of are as follows (for example, may be a graph constructed from (shown in Figure 4) by inserting at least one vertex of degree 2 on each of its internal paths:
Therefore,
□
4. Concluding Remarks
In this paper, we have determined graphs possessing the greatest possible values of the multiplicative sum Zagreb (MSZ) index over the class of chemical trees with a given number of segments and fixed order. We have also calculated the values of the MSZ index of the obtained extremal trees. Possible future work toward the study of the maximum MSZ index for chemical trees includes characterizing graphs with the greatest MSZ index from the set of chemical trees with a given order and some additional graph invariants (for example, the number of pendent vertices, matching number, etc.).
Author Contributions
Methodology, A.A., S.N. and A.M.; validation, A.A., S.N., A.M., N.I. and T.S.H.; writing—original draft, A.A., S.N. and A.M.; writing—review & editing, N.I. and T.S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gross, J.L.; Yellen, J. Graph Theory and Its Applications, 2nd ed.; Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Gutman, I.; Rušić, B.; Trinajstić, N.; Wilcox, C.F. Graph theory and molecular orbitals. XII. Acyclic polyenes. J. Chem. Phys. 1975, 62, 3399–3405. [Google Scholar] [CrossRef]
- Gutman, I.; Trinajstić, N. Graph theory and molecular orbitals. Total-π electron of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Balaban, A.; Motoc, I.; Bonchev, D.; Mekenyan, O. Steric Effects in Drug Design; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1983; Volume 114. [Google Scholar]
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Buyantogtokh, L.; Horoldagva, B.; Das, K.C. On reduced second Zagreb index. J. Comb. Optim. 2020, 39, 776–791. [Google Scholar] [CrossRef]
- Das, K.C. Maximizing the sum of the squares of the degrees of a graph. Discret. Math. 2004, 285, 57–66. [Google Scholar] [CrossRef]
- Deng, H. A unified approach to the extremal Zagreb indices for trees, unicyclic graphs and bicyclic graphs. MATCH Commun. Math. Comput. Chem. 2007, 57, 597–616. [Google Scholar]
- Furtula, B.; Gutman, I.; Ediz, S. On difference of Zagreb indices. Discret. Appl. Math. 2004, 178, 83–88. [Google Scholar] [CrossRef]
- Horoldagva, B.; Buyantogtokh, L.; Das, K.C. On general reduced second Zagreb index of graphs. Hacet. Math. Stat. 2019, 48, 1046–1056. [Google Scholar] [CrossRef]
- Horoldagva, B.; Das, K.C.; Selenge, T.A. Complete characterization of graphs for direct comparing Zagreb indices. Discret. Appl. Math. 2016, 215, 146–154. [Google Scholar] [CrossRef]
- Selenge, T.A.; Horoldagva, B.; Das, K.C. Direct comparison of the variable Zagreb indices of cyclic graphs. MATCH Commun. Math. Comput. Chem. 2017, 78, 351–360. [Google Scholar]
- Wang, H.; Yuan, S. On the sum of squares of degrees and products of adjacent degrees. Discret. Math. 2016, 339, 212–1220. [Google Scholar] [CrossRef]
- Xu, K. The Zagreb indices of graphs with a given clique number. Appl. Math. Lett. 2011, 24, 1026–1030. [Google Scholar] [CrossRef]
- Borovićanin, B.; Das, K.C.; Furtula, B.; Gutman, I. Bounds for Zagreb indices. MATCH Commun. Math. Comput. Chem. 2017, 78, 17–100. [Google Scholar]
- Das, K.C.; Gutman, I. Some properties of the second Zagreb index. MATCH Commun. Math. Comput. Chem. 2004, 52, 103–112. [Google Scholar]
- Gutman, I.; Das, K.C. The first Zagreb index 30 years after. MATCH Commun. Math. Comput. Chem. 2004, 50, 83–92. [Google Scholar]
- Gutman, I.; Milovanović, E.; Milovanović, I. Beyond the Zagreb indices. AKCE Int. J. Graphs Comb. 2020, 17, 74–85. [Google Scholar] [CrossRef]
- Liu, B.; You, Z. A survey on comparing Zagreb indices. MATCH Commun. Math. Comput. Chem. 2011, 65, 581–593. [Google Scholar]
- Todeschini, R.; Consonni, V. New local vertex invariants and molecular descriptors based on functions of the vertex degrees. MATCH Commun. Math. Comput. Chem. 2010, 64, 359–372. [Google Scholar]
- Gutman, I. Multiplicative Zagreb indices of trees. Bull. Soc. Math. Banja Luka. 2011, 18, 17–23. [Google Scholar]
- Bozovic, V.; Vukicevic, Z.K.; Popivoda, G. Chemical trees with extreme values of a few types of multiplicative Zagreb indices. MATCH Commun. Math. Comput. Chem. 2016, 76, 207–220. [Google Scholar]
- Wang, S.; Wang, C.; Chen, L.; Liu, J.B. On extremal multiplicative Zagreb indices of trees with given number of vertices of maximum degree. Discret. Appl. Math. 2017, 227, 66–173. [Google Scholar] [CrossRef]
- Alfuraidan, M.R.; Balachandran, S.; Vetrík, T. General multiplicative Zagreb indices of unicyclic graphs. Carpath. J. Math. 2021, 37, 1–11. [Google Scholar] [CrossRef]
- Eliasi, M.; Iranmanesh, A.; Gutman, I. Multiplicative versions of first Zagreb index. MATCH Commun. Math. Comput. Chem. 2012, 68, 217–230. [Google Scholar]
- Eliasi, M. A simple approach to order the multiplicative Zagreb indices of connected graphs. Trans. Comb. 2012, 1, 17–24. [Google Scholar]
- Horoldagva, B.; Xu, C.; Buyantogtokh, L.; Dorjsembe, S. Extremal graphs with respect to the multiplicative sum Zagreb index. MATCH Commun. Math. Comput. Chem. 2020, 84, 773–786. [Google Scholar]
- Liu, J.; Zhang, Q. Sharp upper bounds on multiplicative Zagreb indices. MATCH Commun. Math. Comput. Chem. 2012, 68, 231–240. [Google Scholar]
- Vetrík, T.; Balachandran, S. General multiplicative Zagreb indices of trees. Discret. Appl. Math. 2018, 247, 341–351. [Google Scholar] [CrossRef]
- Xu, K.; Hua, H. A unified approach to extremal multiplicative Zagreb indices for trees, unicyclic and bicyclic graphs. MATCH Commun. Math. Comput. Chem. 2012, 68, 241–256. [Google Scholar]
- Xu, K.; Das, K.C. Trees, unicyclic, and bicyclic graphs extremal with respect to multiplicative sum Zagreb index. MATCH Commun. Math. Comput. Chem. 2012, 68, 257–272. [Google Scholar]
- Eliasi, M.A. Ghalavand, On trees and the multiplicative sum Zagreb index. Commun. Comb. Optim. 2016, 1, 137–148. [Google Scholar]
- Azari, M.; Iranmanesh, A. Some inequalities for the multiplicative sum Zagreb index of graph operations. J. Math. Inequal 2015, 9, 727–738. [Google Scholar] [CrossRef]
- Kazemi, R. Note on the multiplicative Zagreb indices. Discret. Appl. Math. 2016, 198, 147–154. [Google Scholar] [CrossRef]
- Desmecht, D.; Dubois, V. Correlation of the molecular cross-sectional area of organic monofunctional compounds with topological descriptors. J. Chem. Inf. Model. 2024. [Google Scholar] [CrossRef] [PubMed]
- Noureen, S.; Bhatti, A.A.; Ali, A. On the extremal Zagreb indices of n-vertex chemical trees with fixed number of segments or branching. MATCH Commun. Math. Comput. Chem. 2020, 84, 513–514. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).