Abstract
Releasing Wolbachia-infected mosquitoes to suppress or replace wild vector mosquitoes has been carried out in 24 countries worldwide, showing great promise in controlling mosquitoes and mosquito-borne diseases. To face the instability of Wolbachia infection in different environments during the area-wide application, we should consider the overlapping of two Wolbachia strains. In this case, bidirectional cytoplasmic incompatibility occurs, which results in mating partners infected with exclusive Wolbachia strains producing inviable offspring. To determine the better Wolbachia candidate for release, we develop an ordinary differential equation model to study the global dynamics for competition between two Wolbachia strains. Our theoretical results on the sharp estimate of stable curves completely determine the fate of the two Wolbachia strains, which help choose appropriate strains for release.
Keywords:
mosquito-borne diseases; stable curve; planar system; stability analysis; complete or incomplete cytoplasmic incompatibility MSC:
92B05; 37N25; 34D23; 92D30
1. Introduction
With an estimated 700,000 deaths annually, vector-borne infections cause an overwhelming disease burden on humans [1]. Dengue fever is a mosquito-borne disease transmitted through bites of female Aedes mosquitoes, including Aedes aegypti and Aedes albopictus mosquitoes. More than half of the world’s population is at risk of dengue [2,3]. The most direct method to combat dengue vectors is spraying insecticides to kill them, which has only short-term effects due to the appearance of insecticide resistance [4]. Meanwhile, the development of dengue vaccines is still at a difficult stage because of antibody-dependent enhancement (ADE) among the four serotypes of dengue viruses [5,6]. Several biological control methods have been implemented to deal with this situation, one of which involves the endosymbiotic bacteria Wolbachia.
Wolbachia are common and widespread intracellular bacteria of arthropods, which exist in more than 66% of all insect species [7], including some species of mosquitoes. Wolbachia were first identified in 1924 by Hertig and Wolbach [8]. In 1952, scientists found that Wolbachia in Culex autogenicus [9] caused unidirectional cytoplasmic incompatibility (CI for short): when Wolbachia-infected males mate with uninfected females, their offspring are not viable. Since then, Wolbachia-infected males that cause CI have been proposed as an innovative way to decrease vector populations. However, Aedes aegypti does not carry Wolbachia, and although Aedes albopictus naturally carries two kinds of Wolbachia, it does not induce CI. This situation halted the progress of the use of Wolbachia to combat dengue fever until 2005, by embryonic microinjection. The authors in [10] successfully established the first Wolbachia strain (wRi) that causes CI in Aedes aegypti. Later in 2006 [11], they also established wAlbB in Aedes albopictus by transferring Wolbachia from Drosophila simulans. In addition to inducing CI, both wAlbB in Aedes aegypti and wRi in Aedes albopictus are completely maternally transmitted, that is, the offspring of Wolbachia-infected females are all infected [10,11]. Furthermore, both of them can block the replication of dengue viruses in mosquitoes. Hence, Wolbachia-infected males that induce CI can be regarded as a flying and resistance-free insecticide, and Wolbachia-infected females can block the transmission of dengue viruses between humans and mosquitoes.
Two Wolbachia release strategies have been proposed based on these observations. One is to only release Wolbachia-infected males to sterilize and suppress wild female mosquitoes; this is named population suppression. The other is usually termed population replacement, in which both Wolbachia-infected females and Wolbachia-infected males are released to replace the wild mosquito populations with Wolbachia-infected ones, so they have reduced or no capacity to transmit dengue viruses. The first field trial of population suppression was carried out from 2015 to 2017 in Guangzhou, China, which suppressed more than 90% of the wild-type Aedes albopictus field populations [12]. The first population replacement was implemented in 2009 in Cairns, Australia [13,14,15]. Since then, the Wolbachia infection frequency in mosquito populations has been kept high enough to make the release sites dengue-free areas. The success of population suppression and population replacement makes the Wolbachia release method a promising method to control mosquito and mosquito-borne diseases [16]. Nowadays, Wolbachia release has been carried out worldwide, including in Yogyakarta, Indonesia (2014) with nearly 100% Wolbachia coverage rate, and 77% reduction in dengue incidence, in Kuala Lampur, Malaysia (2013) with 80% Wolbachia coverage rate, and 40% reduction in dengue incidence, and in Niteroi, Brazil (2014) with 40–80% Wolbachia coverage rate, and 69% reduction in dengue incidence.
As a biologically safe and environmentally friendly method, mathematical models aiming to analyze the interactive dynamics of the released and wild mosquitoes have been developed. We refer to [12,17,18,19,20] for models on population suppression, and [21,22,23,24] for models on population replacement, to cite a few. However, all the above models only include a single Wolbachia strain, which induces unidirectional CI. In this paper, we focus on the case of bidirectional CI, which results in the mating partners infected with mutually exclusive Wolbachia strains producing inviable offspring [25,26,27]. For example, in the host Aedes albopictus, the authors in [28] found bidirectional CI for crosses between ARwP and SR lines in Aedes albopictus. The egg hatch rate of the reciprocal crosses between ARwP and SR mosquitoes are 0, while almost 90% eggs hatch successfully from crosses ARwP × ARwP and SR × SR. Bidirectional CI has also been found in the species Culex quinquefasciatus carrying wPip and wAlbA [29], in the species Porcellio dilatatus carrying wPet and wDil [30], and in Aedes aegypti carrying three strains wAlbA, wAlbB, and wMel [31].
Another motivation driving us to study bidirectional CI is that for the area-wide application of Wolbachia release, we should take the overlapping of two Wolbachia strains into consideration, which could induce bidirectional CI. This consideration is based on mounting evidence showing that temperature and possibly other environmental and ecological conditions impact the maternal transmission rate and the CI intensity, and hence impact the potential of Wolbachia mosquitoes to invade populations and persist [32,33,34,35]. For example, in [32], the authors checked three Wolbachia strains, wMel, wMelPop-CLA, and wAlbB. They found that under cyclical temperatures of 26–37 °C, only wAlbB shows complete CI, while both wMel and wMelPop-CLA show incomplete CI. Regarding the maternal transmission efficiency, both wMel and wMelPop-CLA completely lose their maternal transmission ability when the maintenance temperature for offspring is 26–37 °C. Therefore, if the released Wolbachia mosquitoes lose their reproductive advantage to replace wild mosquitoes, another new Wolbachia strain should be supplemented to achieve the replacement.
2. Model Development
To model this, we denote the two mutually exclusive Wolbachia strains as w-A and w-B. Then only both parents harboring the same Wolbachia strains can produce viable offspring; otherwise, there will be no offspring due to complete CI. Let , , and be the numbers of w-A females, w-A males, w-B females, and w-B males at time t, respectively.
Denote the natural birth rate of w-A mosquitoes by and that of w-B mosquitoes by . Let be the proportion of unhatched eggs produced from the incompatible cross if the father carries with w-B. Therefore, quantifies the intensity of CI of the incompatible mating between the w-A female and the w-B male [36,37]. Similarly, let be the CI intensity if the father is infected with w-A. Denote the density-dependent death rates for w-A mosquitoes and w-B mosquitoes by and , respectively. With the assumption of random mating behavior [38], the probability of mating with is , and the probability mating with is . If we assume that the birth ratio of female to male is 1:1 [39], then the growth of is
To investigate the density-dependent effect of all mosquitoes on w-A, we denote the total number of mosquitoes as
Thus, we obtain the equation for
where denotes the density-dependent decay. Similarly, regarding , and , we have the following equations:
To help track the above equations, we refer the readers to Table 1 for the derivation of the first terms in Equations (1)–(4). The green and pink blocks are for the female offspring of w-A and w-B mosquitoes, respectively. The yellow blocks represent the loss due to CI.
The assumption of equal sex determination for offspring [39] allows us to assume Let
Applying the same process to the second equation in System (5), we can rescale System (5) as
Let and , respectively, be the relative birth and death rates of w-A females to w-B females. By replacing u, v and s by x, y and t, System (8) becomes
Remark 1.
The rescaling (6) not only reduces the number of parameters from six to four but also makes the w-B mosquitoes stabilize at 1 without the interference of w-A mosquitoes. In such a situation, the value of is not the absolute number of w-A mosquitoes. Instead, it estimates the proportion of w-A mosquitoes among all mosquitoes, which has been frequently used to record the Wolbachia infection frequency when studying the Wolbachia spread dynamics in mosquito populations [12,22,23,24,36,37,38,40,41,42].
The rest of this paper is organized as follows. In Section 3.1, we analyze the dynamics of System (9) with complete CI, that is, = = 1. In this case, System (9) admits four equilibria, and we offer a relatively sharp estimation of the stable curve of the only saddle. Section 3.2 is devoted to the case of incomplete CI, which has been frequently reported in Wolbachia hosts. The entire classification of the global dynamics of System (9) with incomplete CI is offered. Finally, in Section 4, we provide a survey on the theoretical results and numerical simulations for the competition outcome between two Wolbachia strains. Table 2 provides the glossary of notation for model development.

Table 2.
The glossary of notation.
3. Results
3.1. Complete CI with
Complete CI has been repeatedly observed. For example, the experiment for wMelPop in Aedes aegypti in [43] shows that no eggs hatched from more than 2500 embryos obtained from the incompatible mating. The Wolbachia strain wAlbB in Culex quinquefasciatus [29], and wPip, wAlbA and wAlbB in Aedes albopictus [44] also manifest complete CI. In this section, we study the dynamics of System (9) when both w-A and w-B induce complete CI, that is, . In this case, System (9) becomes
By defining
we have as a trivial equilibrium. The remediation (11) will be maintained in the following discussion without further mention. Relying on the technique of linearization in the classical context of nonlinear dynamics [45], we count the equilibria of system (10) and analyze their stability in the following theorem.
Theorem 1.
System (10) has four equilibria in the first quadrant, , , , and . The origin is a source, and are sinks, and is a saddle.
Proof.
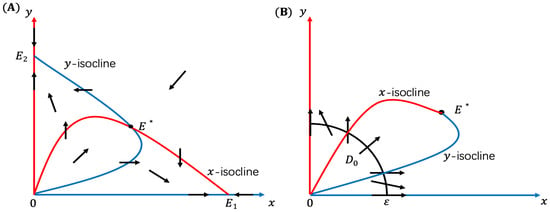
The calculation to get the existence of the equilibria is trivial, and we omit it here. With the help of the x-isocline and the y-isocline , in Figure 1A we plot the direction field of System (10). Furthermore, panel B locates the neighborhood of such that any solution of System (10) initiated from will eventually exit from . Hence, there exists an and
such that if , then any solutions of System (10) initiated from will eventually exit from , proving the instability of .

Figure 1.
Panel (A) is for the direction field of System (10), and panel (B) spots an unstable neighborhood of .
Regarding the local asymptotic stability of and , we calculate the Jacobian matrix of (10) at as
At , the matrix is
with eigenvalues and , implying that is a sink. At , the matrix takes the form
Hence, again, is a sink.
By (12), the Jacobian matrix at is
It is easy to calculate the characteristic polynomial of in (13) as
which has positive discriminant, and hence the roots of are real. Since , we have that is a saddle. This completes the proof. □
The stable curve of , denoted by , plays a key role in characterizing the global dynamics of System (10): solutions initiated above tend to , and otherwise to . The basins of attraction of and , denoted by and , are then defined by
The x-isocline and the y-isocline intersect at and , which decompose the first quadrant into four basic regions:
To locate , we check the direction field of System (10), and find that and . Hence must lie in and separately. See Figure 2 below.
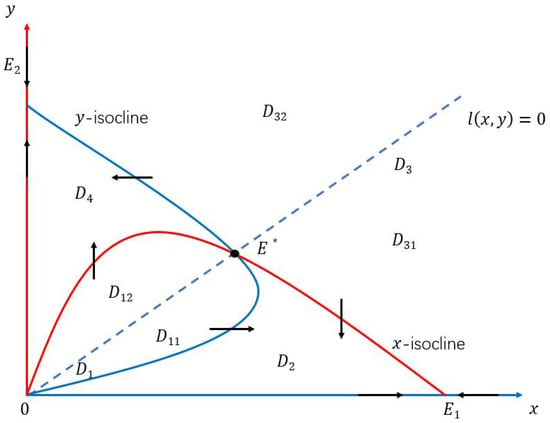
Figure 2.
State domain decomposition by the x-isocline , the y-isocline , and the straight line . The stable curve coincides with when lies entirely in and when , and lies entirely in and when .
It is easy to see that solutions lying in or satisfy
Hence, the stable curve can be expressed as a smooth and strictly increasing function . In , note that if tends toward in backward time, then the -limit set of the stable curve must be . Thus, the curve is also the heteroclinic orbit connecting and . In , both and are negative, so we are still concerned with -limit set of . Denote
In addition, we claim that both and are infinity. Otherwise, since there is no equilibrium in , either or must be infinity. If , then by substituting and into (10) we get as . On the other hand, because , we can infer as , which yields a contradiction. Continuing in this fashion, we can also derive .
In summary, in the first quadrant , the stable curve of is given by
where the function h is smooth. Furthermore, it satisfies
such that the portion of for defines the heteroclinic orbit connecting the trivial equilibrium and , and the portion of for defines the other branch of the stable curve. Since it is usually a formidable task to compute the exact form of , we get around this difficulty by finding sharp estimates of in the following theorem.
Theorem 2.
Define
and then at and , and is sandwiched between and . When , the three functions are identical, and the curve coincides with the straight line
When , we have
When , we have
Proof.
To locate , we divide into
and
Similarly, we denote the subregion of below as , and the subregion above as (see Figure 2).
When , we claim that the straight line coincides with . In fact, on the straight line , we have and . For defined in (15), we have
showing that consists of solutions of (10). Hence the curve coincides with the line , and the three functions , h, and are identical. Moreover, we expect that the direction vector of must be an eigenvector of defined in (13). Indeed, by direct calculation we find
It follows from (19) that the direction vector of is an eigenvector of if and only if .
For the case with , equality (18) implies that does not coincide with the straight line anymore. For an arbitrary solution of (10) with , we have . To locate , we compute the from
When , since and in , it follows from (20) that
Combined with (22), (21) gives , which implies that the solution is concave-down in . Now we assume that if the stable curve has a segment lying in , then there are with , such that both and stay on , and stays below for . This contradicts the fact that is concave-down in . Then, we can conclude that the heteroclinic orbit connecting and must lie entirely in .
To find an upper bound of in , we introduce
By visiting (10), we find
Since for all , we see that
Thus decreases monotonically with t in , leading to , and therefore
By substituting and into the last inequality, we get
Heretofore we have verified the first part of (16).
For the second part of (16), we use the properties of the eigenvectors of to analyze the behavior of in . It follows from (19) that the direction vector of is not an eigenvector of for , which implies that is not tangential to the stable curve at . Hence the stable curve lies in when with x being sufficiently close to . Actually, the stable curve will always stay in for all . To see this, we recall (18) and find that all trajectories initiated at with move into when t increases from . Hence, the stable curve cannot meet from the domain when x increases, or equivalently, when t decreases. As in , increases strictly along the stable curve in this domain, giving . By the same calculation of deriving (25), we obtain the estimate
and complete the proof of (16).
Next, we consider the case . Since and in , it follows from (20) that
Now (21) gives , resulting in the solution that is concave-up in . If the stable curve has a segment lying in , then there are with , such that both and stay on , and stays above for . This yields a contradiction because the secant line cannot be located below the concave-up curve over . Therefore, we find that the heteroclinic orbit connecting and must lie entirely in in this case.
Since for all , it follows from (24) that increases along the heteroclinic orbit. Hence , and with the same process yielding (25) we derive
Again, the direction vector of is not an eigenvector of for ; thus, the stable curve lies in when with x being sufficiently close to . Therefore, (18) gives that all trajectories initiated at with must move into when t increases from , which implies that the stable curve cannot meet from the domain as t decreases. This ensures that the stable curve stays in for all . As in , decreases strictly along the stable curve in this domain, giving , and
Mathematically, by finding two exclusive regions and in (14), Theorems 1 and 2 offer a complete characterization of the global dynamics between two Wolbachia strains with complete CI. Biologically, these two theorems indicate that once the parameter values and the initial population sizes lie in , then w-A strain outcompetes the w-B strain eventually. Otherwise, the w-B strain washes out the w-A strain.
3.2. System (9) with Incomplete CI
Complete CI makes eggs produced from incompatible matings that do not hatch. However, incomplete CI is much more common in Wolbachia hosts. For example, the authors in [11] scored 2972 eggs from the incompatible matings for the wRi in Aedes albopictus; 422 of them survived from CI and hatched. The Wolbachia strain cifB in Anopheles gambiae [46], and wPip in Culex pipiens [47] also fail to induce complete CI. To explore the effect of incomplete CI on the global dynamics of Wolbachia, in this subsection, we study System (9) with . It is trivial that is an equilibrium of System (9). Any other equilibria of System (9) satisfy
and
which admits two boundary equilibria and . It follows from (28) and (29) that any interior equilibrium point satisfies
which leads to
To unload the notation burden, we let , , and . Then, the interior equilibrium reads as
When , we have and . If , we define
and if , we define
Then lies in the first quadrant when either
or
holds.
To analyze the stability of the equilibria, we calculate the Jacobian matrix of (9) as
Next, we count the number of equilibria of System (9) and analyze the corresponding stability in three different cases in terms of the comparison between the magnitude of and , namely, , and .
Similar to the proof of Theorem 1, we can prove that is unstable. Substituting into (32), we have
with eigenvalues and , implying that is a sink. At , the Jacobian matrix takes the form
Since , is also a sink.
The Jacobian matrix at takes the form
Direct calculation yields , and hence, is a saddle.
Case II. . In such a situation, we have three subcases. The first one is when , and System (9) has defined in (31) lying in the first quadrant. The origin is a source. It follows from (33) and (34) that both and are sinks since and . At with , we have
Tedious but simple calculation reaches , and hence is a saddle if .
If , then and . By analyzing the direction field (see Figure 3A) of System (9), we find that for any small , any solution of System (9) initiated from will eventually exit from , implying the instability of .
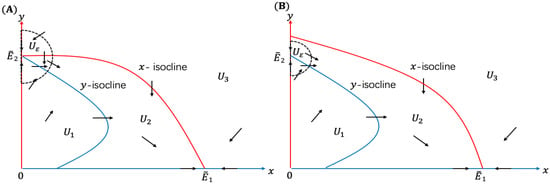
Figure 3.
The direction field of System (9) with . Panel (A) is for , and panel (B) is for .
To investigate the stability of , we denote
By graphing the direction field in these subregions, we find that is the basin of attraction of . Furthermore, solutions of System (9) initiated from or will enter in the finite time, and then tends to as . These observations prove that is a sink, which is globally asymptotically stable.
If , then , and stays in the second quadrant. In this case, following the same procedure as Case II, with the help of the direction field plotted in Figure 3B, we can prove that is globally asymptotically stable, and is a saddle. It is obvious that when , is a source.
Case III. Following the lines in Case II, we can count the number of equilibria and analyze their corresponding stability, and we omit it here. To make the conclusions concise, we summarize the above analysis in Table 3.

Table 3.
Condition for the existence and stability of equilibria of System (9).
Biologically, what we are concerned about most is the outcome of the competition between two Wolbachia strains, that is, which strain will outcompete the other one. Mathematically, the fate of each strain is completely determined by whether or not the initial population size lies in the basin of attraction of or , denoted by or , respectively. The introduction of the incomplete CI makes the characterization of and much more complex than the case of complete CI. However, the summary in Table 3 leads to the following results.
Theorem 3.
(1) If , , then , and .
(2) If , , then , and .
To make the characterization of and complete, we still have three cases to consider:
For these three cases, the outcome of the competition between two Wolbachia-strains is uncertain, which depends on the position of the initial population size . If , then w-A wins. If , then w-B wins, as shown in Theorem 3. The separatrix of and is exactly the stable curve of the saddle , denoted by . Next, we offer a relatively sharp estimation of . Based on the location of , we have
For cases in (35), the x-isocline and the y-isocline intersect at and , which decompose the first quadrant into four basic regions:
To locate , we check the direction field of System (10), and find that and . Hence, must lie in and separately. See Figure 4 below.
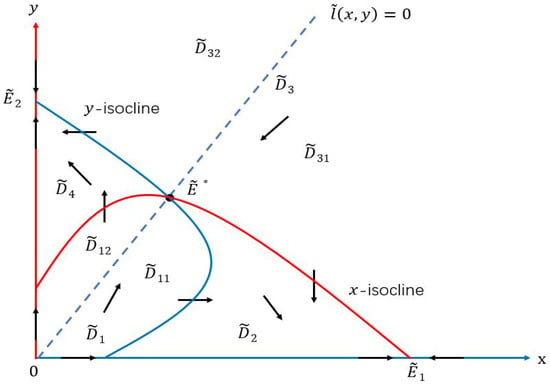
Figure 4.
State domain decomposition by the x-isocline , the y-isocline , and the straight line . The stable curve coincides with when , lies entirely in and when , and lies entirely in and when .
Similar to the proof of Theorem 2, we have the following.
Theorem 4.
Define
and then at and , and is sandwiched between and . When , the three functions are identical, and the curve coincides with the straight line
When , we have
When , we have
Proof.
The region can be further divided into
and
Similarly, the region is divided into and by (see Figure 4).
When , we have
showing that is a special solution of (9). Hence, coincides with the line . Meanwhile, the direct calculation yields
proving that is an eigenvector of if and only if .
If , then with (36) we get
When , since and in , we have
With the help of (40) and (41), we have proven that , and hence the solution of (9) is concave-down in which is impossible. Therefore, we have proven that the heteroclinic orbit connecting and must lie entirely in .
To get an estimation of the upper bound of , we revisit (23) to get
Therefore, decreases monotonically with t in , and then
By substituting and into the last inequality, we get
verifying the first part of (37).
Regarding the second part of (37), we notice that the direction vector of is not an eigenvector of for , and hence is not tangential to the stable curve at . This implies that the stable curve lies in when with x being sufficiently close to . Actually, the stable curve will always stay in for all . To see this, we recall (39) and find that all trajectories initiated at with move into when t increases from . Hence, cannot meet from the domain when x increases, or equivalently, when t decreases. As in , increases strictly along in this domain, giving . By the same calculation of deriving (42), we obtain the estimate
completing the proof of (37). The proof of (38) for the case is similar, and we omit it here. This completes the proof. □
4. Conclusions and Discussion
As one of the most rapidly spreading mosquito-borne infectious diseases, dengue threatens the health of more than half of the global population. In Guangdong Province, China, there was an unprecedented outbreak of dengue in 2014, which reported more cases than the total number in the last decade. Currently, there are no safe and effective specific drugs and vaccines for dengue. Thus, the primary method of prevention and control is to eliminate dengue vectors, including Aedes albopictus and Aedes aegypti. The direct method to kill them is spraying insecticides, which is unsustainable because of insecticide resistance, high cost, and environmental pollution. In recent years, scientists have discovered a novel microbial pesticide involving the maternally transmitted endosymbiotic bacteria Wolbachia. By inducing cytoplasmic incompatibility (CI), Wolbachia-infected males that cause CI have been proposed as an ovicide. Furthermore, Wolbachia can block dengue replication in mosquitoes, implying that Wolbachia vaccinates female mosquitoes. With these mechanisms, the Wolbachia release has been implemented in field experiments in 24 countries worldwide. Wolbachia release has been carried out in two different strategies, including population suppression and population replacement. Population suppression requires the inundative releases of Wolbachia males to guarantee the effective incompatible matings between wild females and released males. In contrast, population replacement is an inoculated release strategy, which releases both Wolbachia males and females to drive the pathogen-blocking trait into the population.
Although various mathematical models for population suppression and population replacement have been developed [17,18,19,21,40,41,42,48,49], the model involving bidirectional CI is rarely studied in the previous study. As important as unidirectional CI, bidirectional CI has been frequently reported [28,29,30,31]. In addition, the transmission rate and CI intensity are affected by temperature and possibly other environmental conditions [32], which can induce the overlapping of different Wolbachia strains in population replacement strategy. Motivated by these considerations, we developed Model (9) to study the global dynamics for competition between two Wolbachia strains with bidirectional CI. Our theoretical results provide sharp separatrices determining the competition outcome of the two Wolbachia strains. The results help choose appropriate Wolbachia strains and design optimal release strategies.
The theoretical results are offered in Section 3.1 and Section 3.2. Section 3.1 is focused on the case with complete CI. Theorem 1 shows that System (9) always generates four equilibria, among which the trivial equilibrium is a source, the two boundary equilibria and are sinks, and the unique interior equilibrium is a saddle. To characterize the competition outcome of the two Wolbachia strains, Theorem 2 offers a relatively sharp estimation of the stable curve of , above which w-B wins, and below which w-A wins. The stable curves of are located with two analytical functions and defined in Theorem 2. Thus, the global dynamics of System (9) can be totally determined by the stable curve.
To explore the result numerically, we take two Wolbachia strains, the benign wMel [13] and the virulent wMelPop [50] established in Aedes aegypti, as an example. Compared to wild Aedes aegypti, the wMel strain did not show significant reductions in fecundity and the egg hatch rate, together with a minor reduction in the mean longevity of adult mosquitoes. In contrast, the virulent wMelPop strain induced strong fitness cost and greatly reduced the fecundity and the egg hatch rate. Moreover, the wMelPop strain caused an approximately 40% reduction in mean longevity. In such a situation, we treat wMel and wMelPop as the best and the worst Wolbachia strains to estimate the range of and in System (9). Recalling that in [21], we estimated the birth rate constants and the death rate constants for wMel and wMelPop, and obtained
See Section 4.1 of [21] for a detailed conversion of these parameters based on the experimental data in [13,50]. This leads to
In the following numerical simulations, we take two parameter combinations with
such that and lie in the intervals in (44) and (45), respectively. We first let , and for case (i) and case (ii) we choose 11 points , and find , such that the solution initiated from of (10) tends to , while the solution initiated from tends to . If so, we claim that the point . The stable curves of are approximately plotted by seeking 11 points (red stars). In both cases, the stable curves of are sandwiched between and as shown in Figure 5, verifying the estimations of the stable curve shown in Theorem 2.
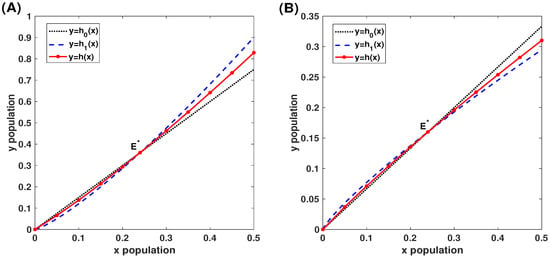
Figure 5.
For complete CI with , panel (A) is for the case with and , and panel (B) is for the case with and .
Regarding the situation with incomplete CI, the dynamics become much more complex. When , the dynamics of System (9) show the same pattern as that of System (8) with complete CI. However, when , the existence and stability of equilibria of System (9) depend on the other two thresholds on the CI intensity, denoted by and . See Table 3 for a summary of counting the equilibria of System (9), together with their stability analysis. The global dynamics of System (9) are proven in Theorems 3 and 4. To check these results numerically, we use pplane10 in Matlab to plot dynamics of System (9) when and . This yields . Fix . Panel A in Figure 6 shows that any solutions initiated from the interior of the first quadrant tends to ; that is, w-A outcompetes w-B and dominates the mosquito population. However, when taking , the saddle point appears. The stable curves of , shown in black curves in Panel B in Figure 6, determine the outcome of competition results between w-A and w-B strains. Similar figures can be plotted for the case , which we omit here.
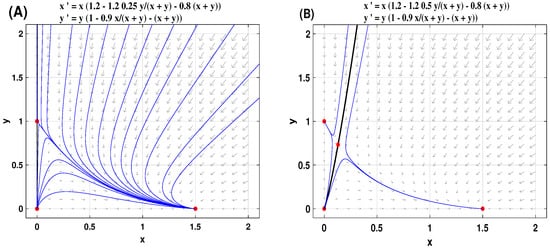
Figure 6.
Take and , we get . Fix . Panel (A) is for the case with , and panel (B) is for the case with .
It is well known that mosquitoes undergo four metamorphosis stages: egg, larva, pupa, and adult. Our current study only models the dynamics of adult mosquitoes, leaving the first three aquatic stages untouched. To characterize the developmental traits in aquatic stages, we have tried two modeling ideas in our previous works. The first one formulated stage-structured models by using difference equations that include the four stages of mosquitoes to predict the mosquitoes’ abundance in [51], together with an exploration of the optimal release strategies specific to Aedes albopictus in Guangzhou [52]. The second assumed that the average waiting time from mating to the emergence of reproductive progenies is kept as a constant and studied the interactive dynamics of Wolbachia-infected and wild mosquitoes [17,18,19,20,53]. To see the impact of aquatic stages on the competition between two Wolbachia strains, in our future work, we shall embed the aquatic stages into System (9), either by adding equations for aquatic stages or by introducing the maturation delays.
Author Contributions
Conceptualization, B.Z.; data curation, L.C.; formal analysis, L.C., Z.Z. and B.Z.; methodology, Q.H.; software, L.C.; supervision, B.Z.; visualization, Z.Z.; writing—original draft, Q.H., Z.Z. and B.Z.; writing—review and editing, Q.H., Z.Z. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 11971127, 12071095.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Vector-Borne Diseases. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 4 September 2022).
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef] [PubMed]
- Zoh, M.G.; Gaude, T.; Prudhomme, S.M.; Riaz, M.A.; David, J.P.; Reynaud, S. Molecular bases of P450-mediated resistance to the neonicotinoid insecticide imidacloprid in the mosquito Ae. aegypti. Aq. Toxicol. 2021, 236, 105860. [Google Scholar] [CrossRef]
- Fatima, A. Dengue vaccine fiasco leads to criminal charges for researcher in the Philippines. Science 2019, 364, 6438. [Google Scholar]
- Jon, C. Dengue may bring out the worst in Zika. Science 2017, 356, 175–180. [Google Scholar]
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia? A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Hertig, M.; Wolbach, S.B. Studies on rickettsia-like microorganisms in insects. J. Med. Res. 1924, 44, 329–374. [Google Scholar]
- Ghelelovitch, S. Genetic determinism of sterility in the cross-breeding of various strains of Culex autogenicus Roubaud. C. R. Hebd. Seances Acad. Sci. 1952, 234, 2386–2388. [Google Scholar]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. Biol. Sci. 2006, 273, 1317–1322. [Google Scholar]
- Zheng, B.; Yu, J.; Li, J. Modeling and analysis of the implementation of the Wolbachia incompatible and sterile insect technique for mosquito population suppression. SIAM J. Appl. Math. 2021, 81, 718–740. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, J.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti mosquitoes and reduction of local dengue transmission in Cairns and surrounding locations in northern Queensland. Gates Open Res. 2020, 3, 1547. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R. Modifying mosquitoes to suppress disease transmission: Is the long wait over? Genetics 2022, 221, 3. [Google Scholar] [CrossRef]
- Yu, J. Existence and stability of a unique and exact two periodic orbits for an interactive wild and sterile mosquito model. J. Differ. Equ. 2020, 269, 10395–10415. [Google Scholar] [CrossRef]
- Yu, J.; Li, J. A delay suppression model with sterile mosquitoes release period equal to wild larvae maturation period. J. Math. Biol. 2022, 84, 14. [Google Scholar] [CrossRef]
- Zheng, B. Impact of releasing period and magnitude on mosquito population in a sterile release model with delay. J. Math. Biol. 2022, 85, 18. [Google Scholar] [CrossRef]
- Zheng, B.; Li, J.; Yu, J. Existence and stability of periodic solutions in a mosquito population suppression model with time delay. J. Differ. Equ. 2022, 315, 159–178. [Google Scholar] [CrossRef]
- Zheng, B.; Tang, M.; Yu, J. Modeling Wolbachia spread in mosquitoes through delay differential equation. SIAM J. Appl. Math. 2014, 74, 743–770. [Google Scholar] [CrossRef]
- Hu, L.; Huang, M.; Tang, M.; Yu, J.; Zheng, B. Wolbachia spread dynamics in stochastic environments. Theor. Popul. Biol. 2015, 106, 32–44. [Google Scholar] [CrossRef]
- Huang, M.; Tang, M.; Yu, J. Wolbachia infection dynamics by reaction-diffusion equations. Sci. China Math. 2015, 58, 77–96. [Google Scholar] [CrossRef]
- Huang, M.; Hu, L.; Yu, J.; Zheng, B. Qualitative analysis for a Wolbachia infection model with diffusion. Sci. China Math. 2016, 59, 1249–1266. [Google Scholar] [CrossRef]
- Breeuwer, J.A.J.; Werren, J.H. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature 1990, 346, 558–560. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Karr, T.L. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature 1990, 348, 178–180. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 714–751. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Moretti, R.; Skidmore, A.R.; Dobson, S.L. Wolbachia strain wPip yields a pattern of cytoplasmic incompatibility enhancing a Wolbachia-based suppression strategy against the disease vector Aedes albopictus. Parasite Vectors 2012, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.; Louis, F.; Failloux, A.B.; Sinkins, S.P. Wolbachia transinfections in Culex quinquefasciatus generate cytoplasmic incompatibility. Insect Mol. Biol. 2020, 29, 1–8. [Google Scholar] [CrossRef]
- Sicard, M.; Bouchon, D.; Ceyrac, L.; Raimond, R.; Thierry, M.; Clec’h, L.W. Bidirectional cytoplasmic incompatibility caused by Wolbachia in the terrestrial isopod Porcellio dilatatus. J. Invertebr. Pathol. 2014, 121, 28–36. [Google Scholar] [CrossRef]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia strain wAu provides highly efficient virus transmission blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef]
- Ross, P.A.; Wiwatanaratanabutr, I.; Axford, J.K.; White1, V.L.; Endersby-Harshman, N.M.; Hoffmann, A.A. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog. 2016, 13, e1006006. [Google Scholar] [CrossRef] [PubMed]
- Bordenstein, S.R. Temperature affects the tripartite interactions between bacteriophage WO, Wolbachia, and cytoplasmic incompatibility. PLoS ONE 2011, 6, e29106. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Karr, T.L.; Yang, W.; Hoekstra, J.M.; James, A.C. Interaction of Drosophila and its endosymbiont Wolbachia: Natural heat shock and the overcoming of sexual incompatibility. Am. Zool. 1999, 39, 363–373. [Google Scholar] [CrossRef]
- Wiwatanaratanabutr, I.; Kittayapong, P. Effects of crowding and temperature on Wolbachia infection density among life cycle stages of Aedes albopictus. J. Invertebr. Pathol. 2009, 102, 220–224. [Google Scholar] [CrossRef]
- Turelli, M. Cytoplasmic incompatibility in populations with overlapping generations. Evolution 2010, 64, 232–241. [Google Scholar] [CrossRef]
- Turelli, M.; Hoffmann, A.A. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 1991, 353, 440–442. [Google Scholar] [CrossRef]
- Caspari, E.; Watson, G.S. On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 1959, 13, 568–570. [Google Scholar] [CrossRef]
- Aida, H.N.; Dieng, H.; Nurita, A.T.; Salmah, M.C.; Miake, F.; Norasmah, B. The biology and demographic parameters of Aedes albopictus in northern peninsular Malaysia. Asian Pac. J. Trop. Biomed. 2011, 1, 472–477. [Google Scholar] [CrossRef]
- Keeling, M.J.; Jiggins, F.M.; Read, J.M. The invasion and coexistence of competing Wolbachia strains. Heredity 2003, 90, 220–226. [Google Scholar] [CrossRef]
- Zheng, B.; Li, J.; Yu, J. One discrete dynamical model on Wolbachia infection frequency in mosquito populations. Sci. China Math. 2022, 65, 1749–1764. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, J. Existence and uniqueness of periodic orbits in a discrete model on Wolbachia infection frequency. Adv. Nonlinear Anal. 2022, 11, 212–224. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.C.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable Introduction of a Life-Shortening Wolbachia Infection into the Mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Calvitti, M.; Moretti, R.; Lampazzi, E.; Bellini, R.; Dobson, S.L. Characterization of a New Aedes albopictus (Diptera: Culicidae)-Wolbachia pipientis (Rickettsiales: Rickettsiaceae) Symbiotic Association Generated by Artificial Transfer of the wPip Strain from Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 2010, 47, 179–187. [Google Scholar] [CrossRef]
- Hirsch, M.W.; Smale, S.; Devaney, R.L. Differential Equations, Dynamical Systems, and an Introduction to Chaos, 2nd ed.; Elsevier: New York, NY, USA, 2004; pp. 166–174. [Google Scholar]
- Adams, K.L.; Abernathy, D.G.; Willett, B.C.; Selland, E.K.; Itoe, M.A.; Catteruccia, F. Wolbachia cifB induces cytoplasmic incompatibility in the malaria mosquito vector. Nat. Microbiol. 2021, 6, 11575–11582. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.B.; Stainton, K.; Harris, S.; Kambris, Z.; Sutton, E.R.; Bonsall, M.B.; Parkhill, J.; Sinkins, S.P. Transcriptional regulation of Culex pipiens mosquitoes by Wolbachia influences cytoplasmic incompatibility. PLoS Pathog. 2013, 9, e1003647. [Google Scholar] [CrossRef]
- Cai, L.; Ai, S.; Fan, G. Dynamics of delayed mosquitoes populations model with two different strategies of releasing sterile mosquitoes. Math. Biosci. Eng. 2008, 15, 1181–1202. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, Y.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q.; et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Yeap, H.L.; Mee, P.; Walker, T.; Weeks, A.R.; O’Neill, S.L.; Johnson, P.; Ritchie, S.A.; Richardson, K.M.; Doig, C.; Endersby, N.M.; et al. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 2011, 187, 583–595. [Google Scholar] [CrossRef]
- Zheng, B.; Yu, J.; Xi, Z.; Tang, M. The annual abundance of dengue and Zika vector Aedes albopictus and its stubbornness to suppression. Ecol. Model. 2018, 387, 38–48. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, X.; Tang, M.; Xi, Z.; Yu, J. Use of age-stage structural models to seek optimal Wolbachia-infected male mosquito releases for mosquito-borne disease control. J. Theor. Biol. 2019, 472, 95–109. [Google Scholar] [CrossRef]
- Yu, J.; Li, J. Global asymptotic stability in an interactive wild and sterile mosquito model. J. Differ. Equ. 2020, 269, 6193–6215. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).