Abstract
Infectious diseases include all diseases caused by the transmission of a pathogenic agent such as bacteria, viruses, parasites, prions, and fungi. They, therefore, cover a wide spectrum of benign pathologies such as colds or angina but also very serious ones such as AIDS, hepatitis, malaria, or tuberculosis. Many epidemic diseases exhibit seasonal peak periods. Studying the population behaviours due to seasonal environment becomes a necessity for predicting the risk of disease transmission and trying to control it. In this work, we considered a five-dimensional system for a fatal disease in a seasonal environment. We studied, in the first step, the autonomous system by investigating the global stability of the steady states. In a second step, we established the existence, uniqueness, positivity, and boundedness of a periodic orbit. We showed that the global dynamics are determined using the basic reproduction number denoted by and calculated using the spectral radius of an integral operator. The global stability of the disease-free periodic solution was satisfied if , and we show also the persistence of the disease once . Finally, we displayed some numerical investigations supporting the theoretical findings, where the trajectories converge to a limit cycle if .
Keywords:
SVEIR epidemic model; seasonal environment; periodic solution; Lyapunov stability; uniform persistence; extinction; basic reproduction number MSC:
92A15; 92A17; 92D30; 37N25; 34C13; 34C15; 34C35
1. Introduction
The flu is a seasonal disease. Every year, this viral and contagious disease reappears, and, at the same time, from the end of the year until the beginning of spring. It affects all categories of the population, young and old alike. This pathology has been monitored by the WHO (World Health Organization) since 1952 [1]. This monitoring makes it possible to identify viral strains but also to better understand and anticipate its consequences and its severity worldwide. Although the flu is most often a mild illness, it can lead to serious complications and can even be fatal. In Europe, the epidemic occurs every year, generally between November and April. There are three types of Influenza virus: viruses A and B are the cause of the “real” flu, while type C is responsible for infections similar to a common cold that go unnoticed. Influenza viruses are characterized by their significant genetic variability, with more or less frequent appearance depending on the virus of mutations [2]. Influenza viruses are easily transmitted through contact with respiratory secretions: by the projection of droplets, with direct contact and indirect contact (the virus can survive for up to 5 min on the skin, a few hours in dried secretions, and 48 h on objects). The incubation of the flu (period without symptoms during which the carrier subject is contagious) is about 3 days [2]. Every year, millions of people are affected by winter viruses. Influenza, gastroenteritis, and bronchiolitis are the main infections due to winter viruses, and they have a strong impact on healthcare structures during the winter. The most effective way to protect the fragile people around you is vaccination. Every year, a vaccination campaign is launched at the end of the year. The vaccine produced is different each year, in order to adapt to the genetic evolution of the virus. Thus, as the mutation of the virus is constant, it is necessary to be vaccinated every year to be protected and to protect those around you. If your child is less than 6 months old, it is recommended that you, the parents, and those around you get vaccinated to protect them. Indeed, before the age of 6 months, the vaccine is not suitable, but your child is vulnerable to the flu [2]. The seasonal flu vaccine is offered every year, especially to the elderly. Its formulation varies from year to year, as the strains in circulation change. The effectiveness of the vaccine varies according to the age of the patient but also to the formulation. In general, vaccination against seasonal flu is not 100% effective. A 2014 Cochrane review analysed 90 clinical trials including 70,000 people. The authors concluded that the efficacy of the vaccine was “modest”. In 2017, a Canadian study found the vaccine’s effectiveness to be 42%. In 2014–2015, it was not as good due to a mutation of the virus [3]. However, in people over 65, this efficiency decreases further, due to the ageing of their immune system. Finnish and Swedish data for 2016/2017 suggested protection in the order of 20–30% for people aged over 65. For the United States, the World Health Organization (WHO), or the European Union (EU), knowing the evolution of a human epidemic (H1N1 Flu, Ebola Virus, Coronavirus), animal (Bird Flu, Swine Fever, Rabies), or plant flu is essential.
The mathematical modelling in epidemiology is a way to study how a disease is spread, predict the future behaviour, and propose control strategies. Several works qualitatively proposed and studied some mathematical models describing the dynamical behaviour of infectious disease transmission (see, for example, [4,5,6,7,8,9,10]). In particular, the SVEIR epidemic models with constant coefficients have been analysed in several works (see, for example, [11,12,13,14,15,16,17]). However, seasonality is very repetitive in each of the ecological, biological, and human systems [18]. In particular, in the climate variation patterns repeated every year by the same way, bird migration is repeated according to the repeated season variation, schools open and close almost periodically each year, etc. Among other things, these seasonal factors affect the pathogens’ survival in the environment, host behaviour, and the abundance of vectors and non-human hosts. Therefore, several diseases show seasonal behaviours. Taking into account the seasonality in mathematical modelling becomes very important. Note that even the simplest mathematical models that take into account seasonality present many difficulties to study [6]. In [19], Bacaër and Gomes discussed the periodic S-I-R model, a simple generalization of the classical model of Kermack and McKendrick [20]. In [21], the authors studied a SEIRS epidemic model with periodic fluctuations. They calculated the basic reproduction number of the time-averaged system (autonomous). Then, they proved a sufficient but not necessary condition () such that the disease could not persist in the population in a seasonal environment. In [22], Guerrero-Flores et al. considered a class of SIQRS models with periodic variations in the contact rate. They proved the existence of periodic orbits by using Leray–Schauder degree theory. Zhang and Teng [23] studied an alternative SEIRS epidemic model in a seasonal environment and established some sufficient equivalent conditions for the persistence and the extinction of the disease. These results were improved by Nakata and Kuniya in [4] by giving a threshold value between the uniform persistence and the extinction of the disease. In [7], Bacaër and Guernaoui gave the definition of the basic reproduction number in seasonal environments. In 2008, Wang and Zhao [24] defined for several compartmental epidemic models in seasonal environments. All these definitions were different, in several cases, from the basic reproduction number defined for the time-averaged system. By considering general compartmental epidemic models in seasonal environments, Wang and Zhao [24] showed that was the threshold value for proving or not the local stability of the disease-free periodic trajectory.
As seasonality is very repetitive in the environment, which affects several diseases that show seasonal behaviours, taking seasonality into account in mathematical modelling becomes a necessity. In this paper, we proposed an extension of the SVEIR model proposed in [8] by taking into account the seasonal environment. We studied, in a first step, the autonomous system by investigating the global stability of the steady states. In a second step, we showed that the disease-free periodic solution is globally asymptotically stable if is less than 1, and, if is greater than 1, the disease persists. The rest of the paper is organized as follows. In Section 2, we introduced the mathematical model. In Section 3, we studied the case of an autonomous system, where all parameters are supposed to be constants. In Section 4, we considered the non-autonomous system, gave some basic results, and gave the definition of . We showed that the value of around one was a threshold value between the disease’s extinction and the disease’s uniform persistence. We gave numerical examples that supported the theoretical findings in Section 5. Section 6 provided a brief conclusions of our obtained results.
2. Mathematical Model and Properties
This study makes it possible to predict the evolution of the disease over time, and its main purpose is to guide leaders in decision-making in terms of public health. It should now be specified that mathematical models are simplified models, with limits that are necessary to be aware about. However, they remain useful in trying to predict the evolution of an epidemic. It would be tempting to mathematically model the spread of seasonal flu. realistically while increasing the complexity of the model. We will content ourselves below with an epidemic model with direct and non-vectorial transmission in a periodic seasonal environment, which can be applied to the case of seasonal flu. In particular, we consider a generalisation of the model given in [8], taking into account the epidemics’ seasonal features, and then we study the behaviour of solutions of the new model designed in Figure 1 and presented by the system of ordinary differential Equation (1).
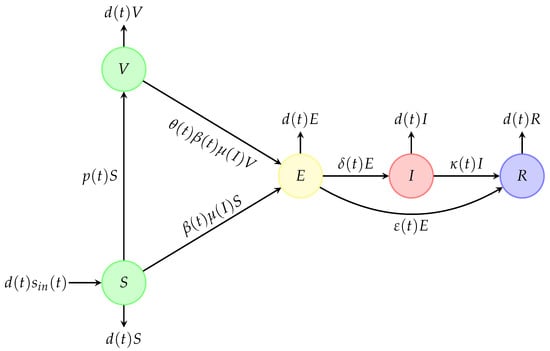
Figure 1.
Epidemic compartmental model with seasonal environment (inspired from El Hajji and Albargi [8], where the authors consider a similar model with fixed parameters).
, , , , and describe susceptible, vaccinated, exposed, infectious, and recovered compartments, respectively (see Table 1 for the epidemiological meaning of the model parameters).

Table 1.
Parameters and variables of System (1).
- All compartments have instantaneous common death rates, .
- Vaccination against seasonal flu is not 100% effective. Therefore, vaccinated individuals can be infected again.
- Recovered individuals will not re-infect.
The proposed “SVEIR” mathematical model for the spread of the seasonal flu is:
with the positive initial condition .
Assumption 1.
- The function μ is non-negative , increasing bounded and concave with .
- The functions , , , , , , , and are continuous, positive, and T-periodic.
satisfies the following results.
Lemma 1.
The function μ satisfies for all .
Proof.
See [25]. □
3. The Case of Constant Parameters
Consider System (1) for the case where all parameters are constants. The model becomes exactly the one considered in [8] but with a possible recovery of exposed individuals.
with the positive initial condition . It is necessary that the state variables and remain non-negative for all . We have the following result.
Proposition 1.
Proof.
See [8]. □
Proposition 2.
Hereafter, we discuss the global stability of the steady states, with respect to the value of . We started by giving the first main result for the autonomous System (2).
Theorem 1.
The equilibrium point is globally asymptotically stable once ; however, it is unstable once .
Proof.
Let the function be given by: . As , the time-derivative of is given by:
If then for all Let . Therefore, by applying the LaSalle’s invariance principle [26], we deduce that the steady state is globally asymptotically stable when . □
Give the second main result for the autonomous System (2), as, in the first phases of an epidemic, when the number of infections and exposed individuals remains small compared to their number more later, and as the number of susceptible and vaccinated individuals represents the most total size of the population. Thus, we consider the following set
Lemma 2.
The function μ satisfies for all .
Proof.
Let , and let us define the function then by Lemma 1 and then for all
. Note that for all . Therefore, for all . □
Theorem 2.
If , then the steady state is globally asymptotically stable in .
Proof.
Define the Lyapunov function in as follows
The function admits its minimum value once . Let us calculate the derivative of along trajectories of System (2).
As we have for all , and m is an integer, then , ,
, and . Therefore, by Lemma 2, in . Finally,
Thus, . Therefore, by applying LaSalle’s invariance principle [26], we deduce that the steady state is globally asymptotically stable in when it exists (). □
4. Seasonal Environment and Periodic Solution
Return now to the main Model (1), where all parameters are continuous and T-periodic positive functions. Let to be the ordered m-dimensional Euclidean space associated with the norm . For , we denote by if . We denote by if . We denote by if . Consider a T-periodic matrix function denoted by , which is continuous, irreducible, and cooperative. Let us denote by as the fundamental matrix, the solution of the following system
Let us denote the spectral radius of the matrix by . Therefore, all entries of are positive for each . Let us apply the theorem of Perron–Frobenius [27] to deduce that is the principal eigenvalue of (simple and admits an eigenvector ). For the rest of the paper, the following lemma will be useful.
Lemma 3
([28]). There exists a positive T-periodic function such that will be a solution of system (4) where .
Let us start by proving the existence (and uniqueness) of the disease-free periodic solution of System (1). Let us consider the following two-equations system
with the initial condition . System (5) admits a unique T-periodic trajectory , with , and it is globally attractive in . Therefore, System (1) admits a unique disease-free periodic trajectory . For any continuous, positive T-periodic function , let and .
Let be the population size at time t and . Then, we obtain the following lemma.
Lemma 4.
is a positively invariant attractor set for System (1). Furthermore, we have
Proof.
From System (1), we have
This means that is a forward invariant compact absorbing set of all solutions of System (1). Next, we set for . Then, we obtain , and this means that . □
Next, in Section 4.1, we will give the definition of the basic reproduction number , and we will prove that once is smaller than 1. Therefore, the disease-free periodic trajectory is globally asymptotically stable, which means that the disease dies out. Then, in Section 4.2, we will prove that once , then System (1) is uniformly persistent. Therefore, we deduce that is the threshold parameter between the uniform persistence and the extinction of the disease.
4.1. Disease-Free Periodic Solution
We start by giving the definition of the basic reproduction number of System (1), using the theory given in [24], where ,
and with .
Our aim is to satisfy Conditions (A1)–(A7) in [24] (Section 1). Note that System (1) can have the following form
The first five Conditions (A1)–(A5) are satisfied.
The System (8) admits a periodic solution (disease-free) . Let us define , and , where and are the i-th component of and , respectively. By a simple calculus, we obtain ; thus, . This means that is linearly asymptotically stable in the subspace
Therefore, Condition (A6) in [24] (Section 1) is satisfied.
Now, let us define and to be 2 by 2 matrices given by , and , where and are the j-th component of and , respectively. By an easy calculus, we obtain from System (8) that
Consider to be the two-by-two matrix solution of the system for any , with , the two by two identity matrix. Thus, condition (A7) was satisfied.
Let us define to be the ordered Banach space of T-periodic functions defined on , associated with the maximum norm and the positive cone . Consider the linear operator by
Let us now define the expression of the basic reproduction number, denoted by of the System (1), in terms of the spectral radius of the operator K as follows
Therefore, we can conclude on the local stability of the disease-free periodic trajectory for (1) as follows.
Theorem 3
([24] (Theorem 2.2)).
- .
- .
- .
Therefore, is unstable if , and it is asymptotically stable if .
Theorem 4.
The disease-free periodic solution is globally asymptotically stable once . It is unstable if .
Proof.
Using Theorem 3, is locally stable once , and it is unstable once . Therefore, it remains to prove the global attractivity of when . Consider the case where . Using the limit (6) in Lemma 4, for any , there exists a positive constant satisfying for . Then , and we deduce that
for . Let to be the following two by two matrix function
Again, using Theorem 3, we obtain . Let us choose so that
. Let the two-equations model be hereafter
Applying Lemma 3 and using the standard comparison principle, we deduce that there exists a positive T-periodic function , satisfying , where and . Thus, and . Therefore, we deduce that . Furthermore, we have . Therefore, we deduce that the disease-free periodic trajectory is globally attractive, which completes the proof. □
For the following subsection, we consider only the case where .
4.2. Endemic Periodic Solution
From Lemma 4, System (1) admits a positively invariant compact set . Now, since the recovery variable does not affect the other equations of System (1), then the Model (1) will be reduced as follows.
with the initial condition such that and .
Let us define function to be the Poincaré map associated with System (13) with , where is the unique solution of the reduced Model (13) with the initial value .
Let us define
Note that both and are positively invariant from Lemma 4. P is point dissipative. Define
In order to use the uniform persistence theory detailed by Zhao [29] (or in [28] (Theorem 2.3)), we prove that
Note that .
To show that , let us consider .
If and , then for any . Then, it holds that . If and , then and for any . Therefore, for any , we have
for all . This means that for . Therefore, is positively invariant, from which we deduce Equation (14). Using the previous discussion, we deduce that there exists one fixed point of P in . We deduce, therefore, the uniform persistence of the disease as follows.
Theorem 5.
Consider the case . Equation (13) admits at least one positive periodic trajectory and , satisfying ,
Proof.
Let us start by proving that P is uniformly persistent, respecting , which will prove that the trajectory of the reduced Equation (13) is uniformly persistent, respecting using [29] (Theorem 3.1.1). Recall that we obtain using Theorem 3. Therefore, small enough and satisfies . Let us consider the perturbed system
The function P associated with the perturbed System (15) has a unique positive fixed point that it is globally attractive in . Applying the implicit function theorem to deduce that is continuously respecting . Therefore, we can chose to be small enough, satisfying and . Let . As the trajectory is continuously respecting the initial condition, satisfies with , and it holds that
We prove by contradiction that
Suppose that for some . In particular, we can suppose that , . Therefore, we obtain For all , let , with and (greatest integer ). Then, we get Let . Therefore, for all and
The fixed point of the function P associated with the perturbed System (15) is globally attractive such that , then large enough and satisfies
Therefore, for
Note that we have . Applying Lemma 3 and the comparison principle, there exists a positive T-periodic trajectory , satisfying with , which implies that , which is impossible since the trajectories are bounded. Therefore, the inequality Equation (16) is satisfied, and P is weakly uniformly persistent respecting to . By applying Lemma 4, P has a global attractor. We deduce that is an isolated invariant set inside X and . All trajectory inside converges to , which is acyclic in . Applying [29] (Theorem 1.3.1 and Remark 1.3.1), we deduce that P is uniformly persistent, respecting . Furthermore, using [29] (Theorem 1.3.6), P admits a fixed point . Note that . We prove also by contradiction that . Assume that . Using the first equation of the reduced System (13), verifies
with . Applying Lemma 4, , there exists large enough and satisfying . Then, we have
There exists a large enough , satisfying for all . Applying the comparison principle, we deduce that
for any , which is impossible. Therefore, and is a positive T-periodic trajectory of the reduced System (13). □
5. More Examples, Numerical Results
We performed numerical simulations on the System (1) using the classical Monod function to express the transmission rate
where k and are constants. Note that satisfies Assumption A1. The parameters of the model are T-periodic functions having the following forms:
, , , , , , , and measure the amplitude of the seasonal variations in each of the parameters with , , , , , , , and . is the phase shift. Some fixed constants used for the numerical simulations are given in Table 2.

Table 2.
Some fixed parameters for numerical simulations.
We will consider three cases. The first case is dedicated for the case of constant parameters (autonomous system) to validate the obtained theoretical results, concerning the local and global stability of the equilibrium points and . The second case deals only with a seasonal contact ( is a periodic function), where the other parameters are constants (partially non-autonomous system). The third case considers all parameters as periodic functions (non-autonomous system). For the numerical simulation, we used the standard solver “ode45” of MATLAB for ordinary differential equations, which implements an explicit Runge–Kutta (4,5) formula with a variable time step for efficient computation.
5.1. The Case of the Autonomous System
In a first step, we consider that all parameters of the System (1) are constants (). Thus, the model is given by
with the positive initial condition .
We give some numerical results that confirm the stability of the equilibrium points of Equation (22). In Figure 2, we give the results for the case where . The approximated solution of the given Model (22) approaches the equilibrium , which confirms that is globally asymptotically stable once . In Figure 3, we give the results for the case where . The approximated solution of the given Model (22) approaches asymptotically to . In Figure 4, we give the results for different initial conditions, The components of the approximated solutions converge asymptotically to the components of , which confirms that is globally asymptotically stable once .
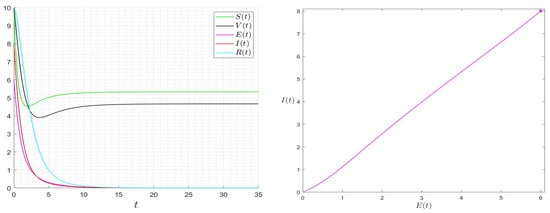
Figure 2.
Behaviour of the solution of System (1) for and then .
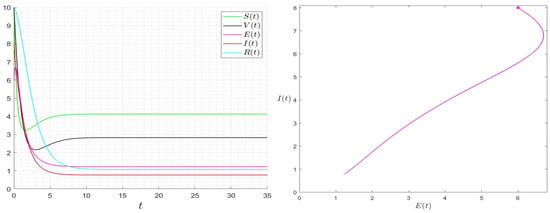
Figure 3.
Behaviour of the solution of System (1) for and then .
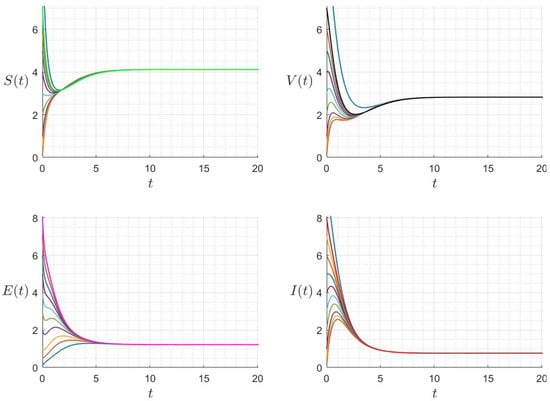
Figure 4.
Behaviour of the solution components for different initial conditions, where and (). The different graphs (colors) reflect different initial conditions.
5.2. The Case of the Partially Non-Autonomous System
In a second step, we perform numerical simulations on System (1), using a linear function to express the transmission rate, where only the seasonally forced T-periodic function depends on time, t. The other parameters are constant (). Thus, the model is given by
with positive initial condition , where . The basic reproduction number is approximated using the time-averaged system. We give some numerical results that confirm the asymptotic behaviour of the solution of Equation (23). In Figure 5, we give the results for the case where . The approximated solution of the given Model (23) asymptotically approaches the periodic solution with the persistence of the disease. In Figure 6, we give the results for different initial conditions. The components of the approximated solutions asymptotically converge to the components of the periodic solution with the persistence of the disease. In Figure 7, we give the results for the case where . The approximated solution of the given Model (23) approaches the disease-free trajectory once .
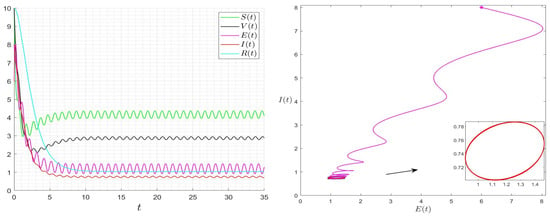
Figure 5.
Behaviour of the solution of System (1) for and then .
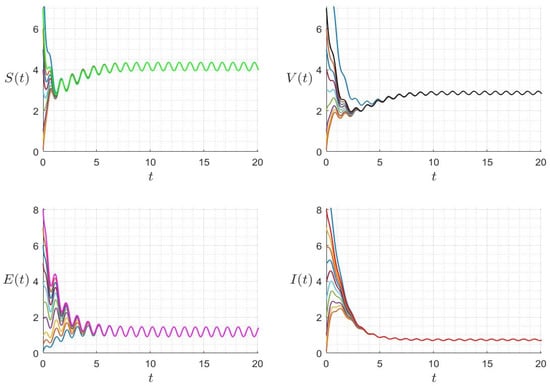
Figure 6.
Behaviour of the solution components for different initial conditions where and (). The different graphs (colors) reflect different initial conditions.
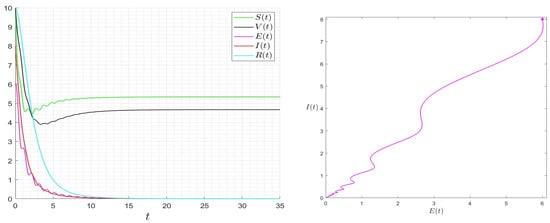
Figure 7.
Behaviour of the solution of System (1) for and then .
5.3. The Case of Totally Non-Autonomous System
In a third step, we performed numerical simulations on System (1) using the classical Monod function to express the transmission rate, where all parameters were T-periodic functions. Thus, the model was given by
with positive initial condition . Additional constants used for the numerical simulations in this step are given in Table 3.

Table 3.
Additional parameters for numerical simulations of the totally non-autonomous system.
We gave some numerical results that confirmed the asymptotic behaviour of the solution of Equation (24). The basic reproduction number was approximated using the time-averaged system.
In Figure 8, we give the results for the case where . The approximated solution of the given Model (24) approaches the disease-free periodic trajectory once . In Figure 9, we give the results for the case where . The approximated solution of the given Model (24) approaches asymptotically to a periodic solution with the persistence of the disease. In Figure 10, we give the results for different initial conditions. The components of the approximated solutions asymptotically converge to the components of the periodic solution with the persistence of the disease.
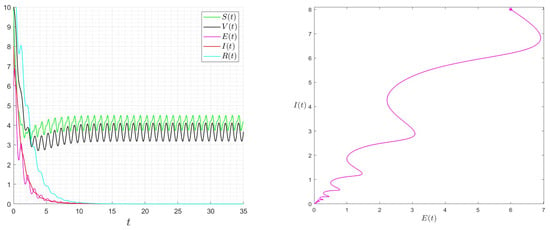
Figure 8.
Behaviour of the solution of System (1) for and then .
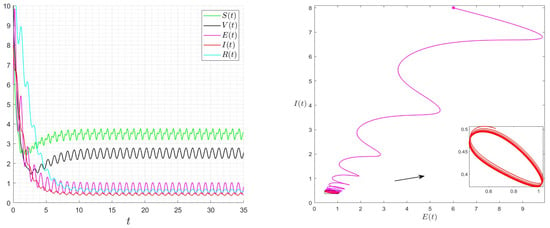
Figure 9.
Behaviour of the solution of System (1) for and then .
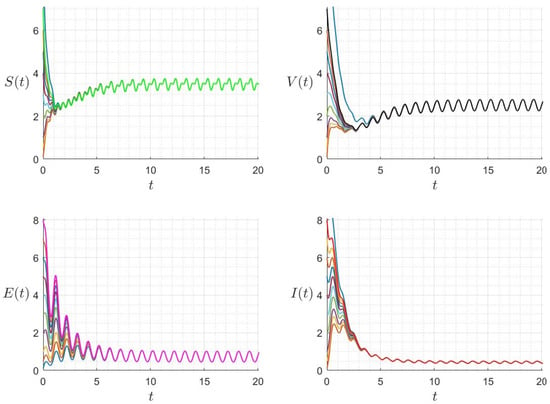
Figure 10.
Behaviour of the solution components for different initial conditions where and (). The different graphs (colors) reflect different initial conditions.
6. Conclusions
In this work, we proposed an extension of the SVEIR epidemic model already considered in [8] by taking into account the seasonal environment. This model could describe seasonal flu behaviour. In the first step, we studied the case of the autonomous system, where all parameters were supposed to be constants. In the second step, we considered the non-autonomous system, gave some basic results, and defined the basic reproduction number . We showed the global asymptotic stability of the disease-free periodic solution once ; however, the disease persisted once was greater than 1. Finally, we gave some numerical examples that supported the theoretical findings, including the autonomous system, the partially non-autonomous system, and the full non-autonomous system. It was deduced that the trajectories converged to one of the equilibriums of the System (2), according to Theorems 1 and 2, if the system was autonomous. However, if at least one of the model parameters was periodic, the trajectories converged to a limit cycle according to Theorems 4 and 5. This study remained theoretical, and we need real data to compare, improve, and validate the model.
Author Contributions
Conceptualization, M.E.H., D.M.A. and N.A.A.; methodology, M.E.H., D.M.A. and N.A.A.; writing—original draft, M.E.H., D.M.A. and N.A.A.; writing—review and editing, M.E.H., D.M.A. and N.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the unknown referees for the many constructive suggestions, which helped to improve the presentation of the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Influenza (Seasonal). Available online: https://www.emro.who.int/health-topics/influenza/influenza-seasonal.html (accessed on 30 March 2023).
- Centers for Disease Control and Prevention; National Center for Immunization and Respiratory Diseases (NCIRD). Types of Influenza Viruses. Available online: https://www.cdc.gov/flu/about/index.html (accessed on 30 March 2023).
- Demicheli, V.; Jefferson, T.; Ferroni, E.; Rivetti, A.; Pietrantonj, C.D. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst. Rev. 2018, 2018, CD001269. [Google Scholar] [CrossRef] [PubMed]
- Nakata, Y.; Kuniya, T. Global dynamics of a class of SEIRS epidemic models in a periodic environment. J. Math. Anal. Appl. 2010, 363, 230–237. [Google Scholar] [CrossRef]
- Bacaër, N.; Ouifki, R. Growth rate and basic reproduction number for population models with a simple periodic factor. Math. Biosci. 2007, 210, 647–658. [Google Scholar] [CrossRef]
- Bacaër, N. Approximation of the basic reproduction number R0 for vector-borne diseases with a periodic vector population. Bull. Math. Biol. 2007, 69, 1067–1091. [Google Scholar] [CrossRef] [PubMed]
- Bacaër, N.; Guernaoui, S. The epidemic threshold of vector-borne diseases with seasonality. J. Math. Biol. 2006, 53, 421–436. [Google Scholar] [CrossRef]
- El Hajji, M.; Albargi, A.H. A mathematical investigation of an “SVEIR” epidemic model for the measles transmission. Math. Biosci. Eng. 2022, 19, 2853–2875. [Google Scholar] [CrossRef]
- El Hajji, M. Modelling and optimal control for Chikungunya disease. Theory Biosci. 2021, 140, 27–44. [Google Scholar] [CrossRef]
- Alsolami, A.A.; El Hajji, M. Mathematical Analysis of a Bacterial Competition in a Continuous Reactor in the Presence of a Virus. Mathematics 2023, 11, 883. [Google Scholar] [CrossRef]
- Nkamba, L.; Ntaganda, J.; Abboubakar, H.; Kamgang, J.; Castelli, L. Global Stability of a SVEIR Epidemic Model: Application to Poliomyelitis Transmission Dynamics. Open J. Model. Simul. 2017, 5, 98–112. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, D.; Zhang, W.; Zhu, D. Dynamics of epidemic models with asymptomatic infection and seasonal succession. Math. Biosci. Eng. 2017, 14, 1407–1424. [Google Scholar] [CrossRef]
- Adda, P.; Nkague Nkamba, L.; Sallet, G.; Castelli, L. A SVEIR model with Imperfect Vaccine. In Proceedings of the CMPD 3 Conference on Computational and Mathematical Population Dynamics, Bordeaux, France, 31 May–4 June 2010. [Google Scholar]
- Wei, H.; Jiang, Y.; Song, X.; Su, G.; Qiu, S. Global attractivity and permanence of a SVEIR epidemic model with pulse vaccination and time delay. J. Comput. Appl. Math. 2009, 229, 302–312. [Google Scholar] [CrossRef]
- Gumel, A.B.; McCluskey, C.C.; Watmough, J. An SVEIR model for assessing potential impact of an imperfect anti-SARS vaccine. Math. Biosci. Eng. 2006, 3, 485–512. [Google Scholar] [PubMed]
- El Hajji, M.; Zaghdani, A.; Sayari, S. Mathematical analysis and optimal control for Chikungunya virus with two routes of infection with nonlinear incidence rate. Int. J. Biomath. 2022, 15, 2150088. [Google Scholar] [CrossRef]
- El Hajji, M.; Sayari, S.; Zaghdani, A. Mathematical analysis of an SIR epidemic model in a continuous reactor—Deterministic and probabilistic approaches. J. Korean Math. Soc. 2021, 58, 45–67. [Google Scholar]
- Xiao, D. Dynamics and bifurcations on a class of population model with seasonal constant-yield harvesting. Discret. Contin. Dyn. Syst. B 2016, 21, 699–719. [Google Scholar] [CrossRef]
- Bacaër, N.; Gomes, M. On the Final Size of Epidemics with Seasonality. Bull. Math. Biol. 2009, 71, 1954–1966. [Google Scholar] [CrossRef] [PubMed]
- Kermack, F.; McKendrick, D. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1927, 115, 700–721. [Google Scholar]
- Ma, J.; Ma, Z. Epidemic threshold conditions for seasonally forced SEIR models. Math. Biosci. Eng. 2006, 3, 161–172. [Google Scholar] [CrossRef]
- Guerrero-Flores, S.; Osuna, O.; de Leon, C.V. Periodic solutions for seasonal SIQRS models with nonlinear infection terms. Electron. J. Differ. Equations 2019, 2019, 1–13. [Google Scholar]
- Tailei Zhang, Z.T. On a nonautonomous SEIRS model in epidemiology. Bull. Math. Biol. 2007, 69, 2537–2559. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, X. Threshold dynamics for compartmental epidemic models in periodic environments. J. Dyn. Differ. Equ. 2008, 20, 699–717. [Google Scholar] [CrossRef]
- Alshehri, A.; El Hajji, M. Mathematical study for Zika virus transmission with general incidence rate. AIMS Math. 2022, 7, 7117–7142. [Google Scholar] [CrossRef]
- LaSalle, J. The Stability of Dynamical Systems; SIAM: Philadelphia, PA, USA, 1976. [Google Scholar]
- Frobenius, G. Uber Matrizen aus nicht negativen Elementen. Sitz. Preuss. Akad. Wiss. 1912, 26, 456–477. [Google Scholar]
- Zhang, F.; Zhao, X. A periodic epidemic model in a patchy environment. J. Math. Anal. Appl. 2007, 325, 496–516. [Google Scholar] [CrossRef]
- Zhao, X. Dynamical Systems in Population Biology; CMS Books in Mathematics; Springer: New York, NY, USA, 2003; Volume 16. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).