Electrochemical Detection of Furaltadone Antibiotic Drug by the Rare Earth Metal Tungstate Decorated Screen Printed Carbon Electrode
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Eu2(WO4)3 Nanoparticles by Hydrothermal Methods
2.2. Fabrication of SPCE/Eu2(WO4)3 Modified Electrode by Simple Drop-Casting Methods
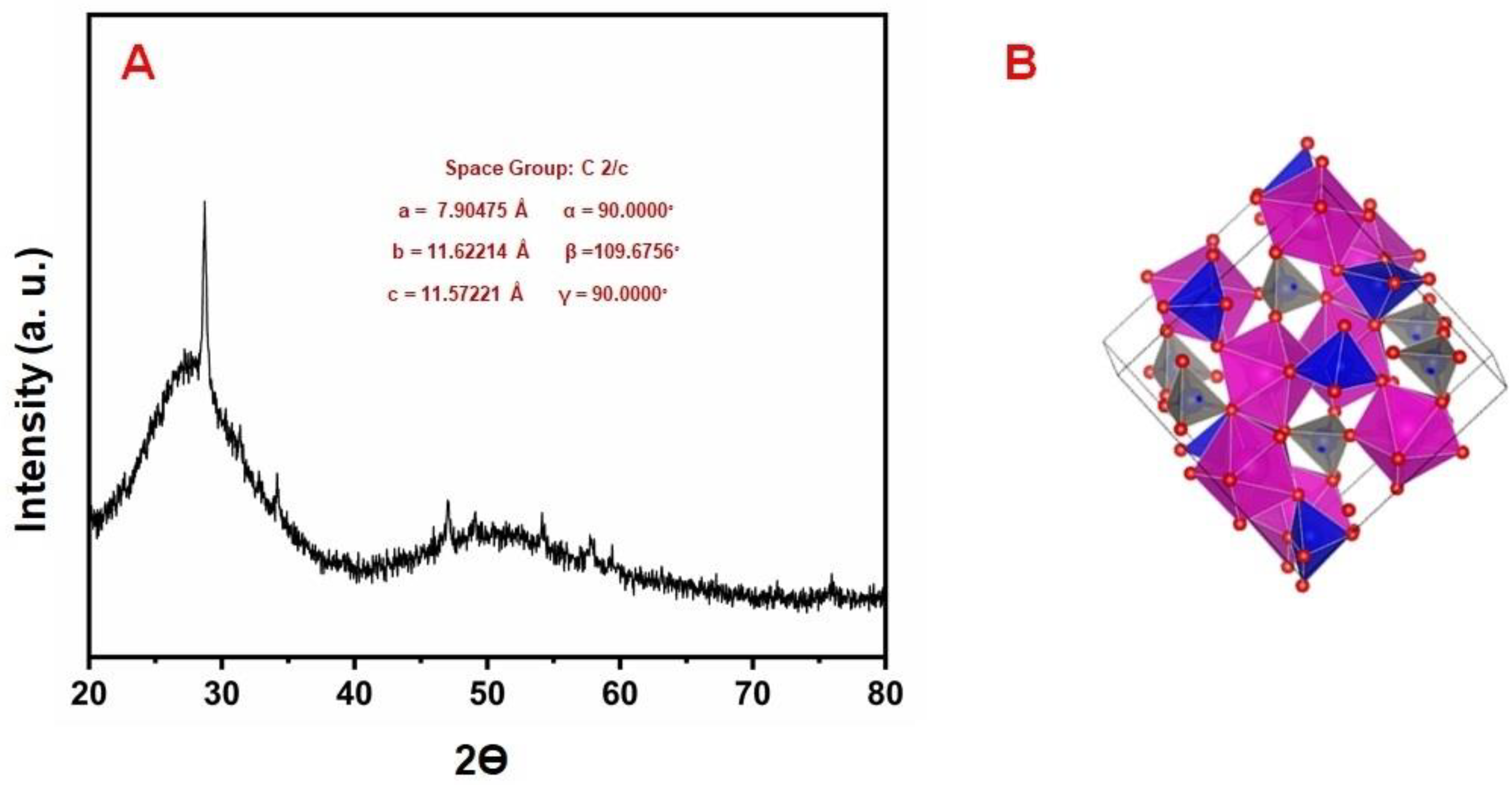
3. Characterization Studies of Materials
XRD, SEM, and EDX Studies
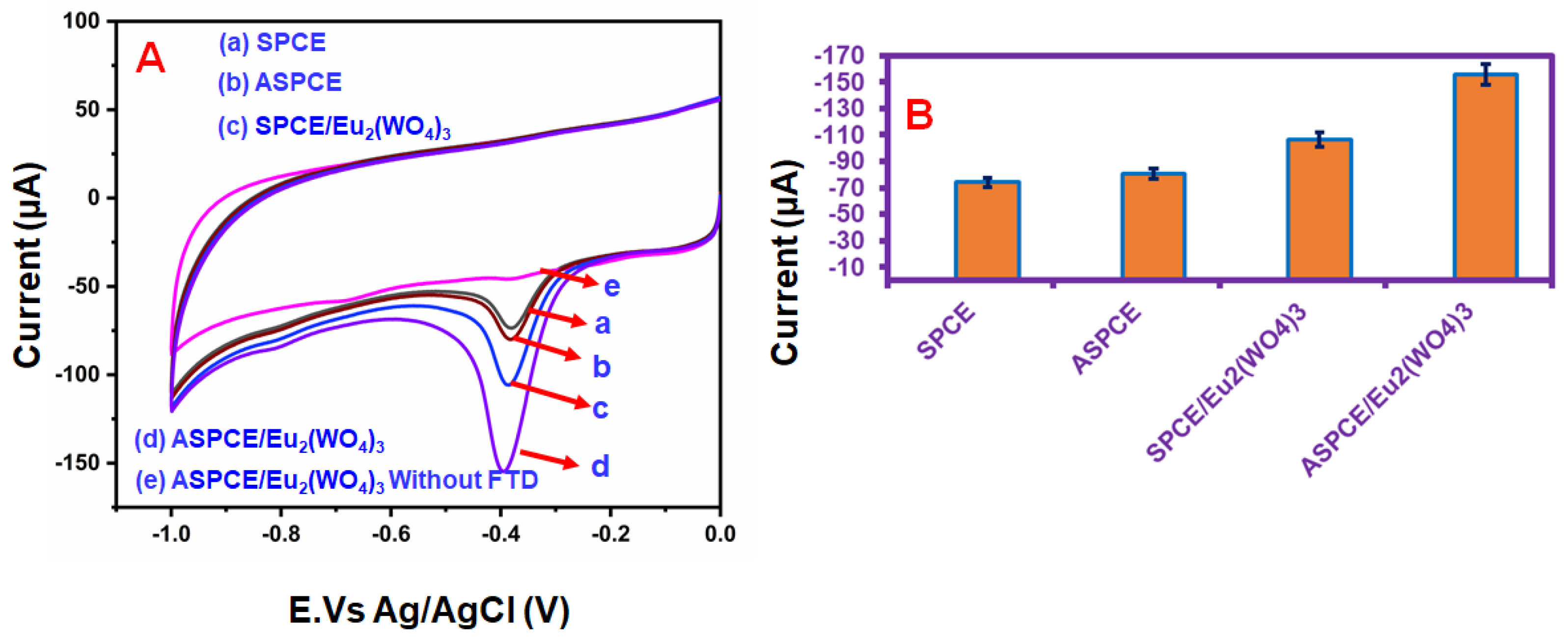
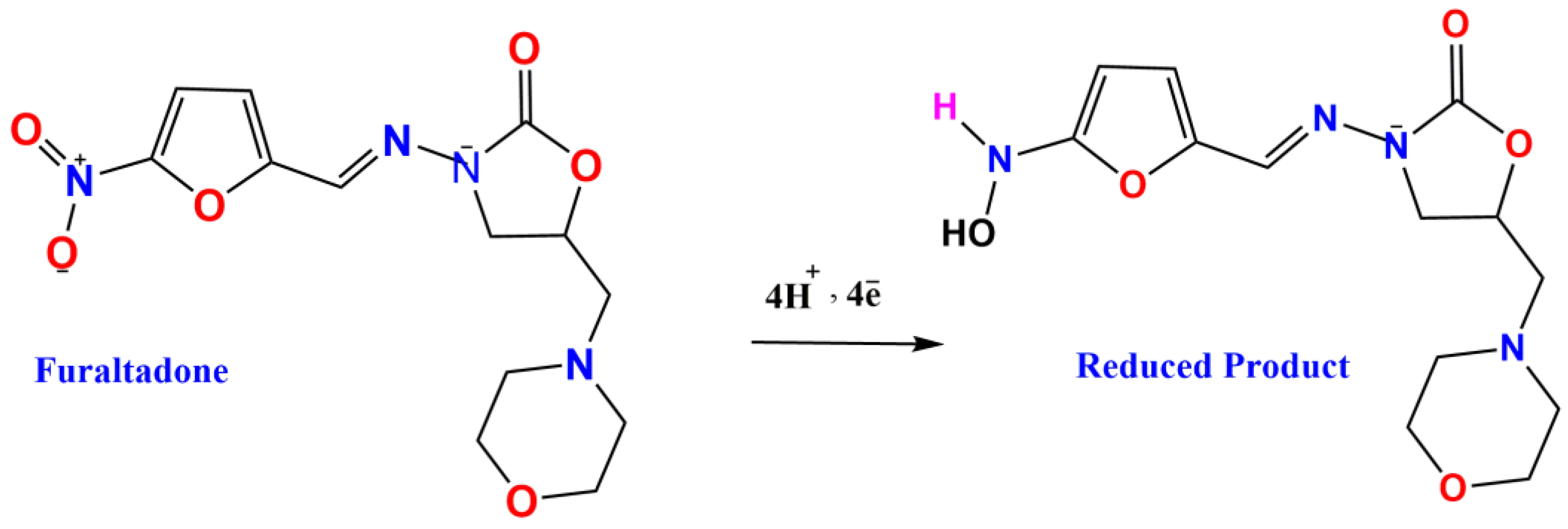
4. Electrochemical Studies of FTD on the ASPCE/Eu2(WO4)3 Electrode
4.1. Effects of Different Films and Effects of Different Concentrations
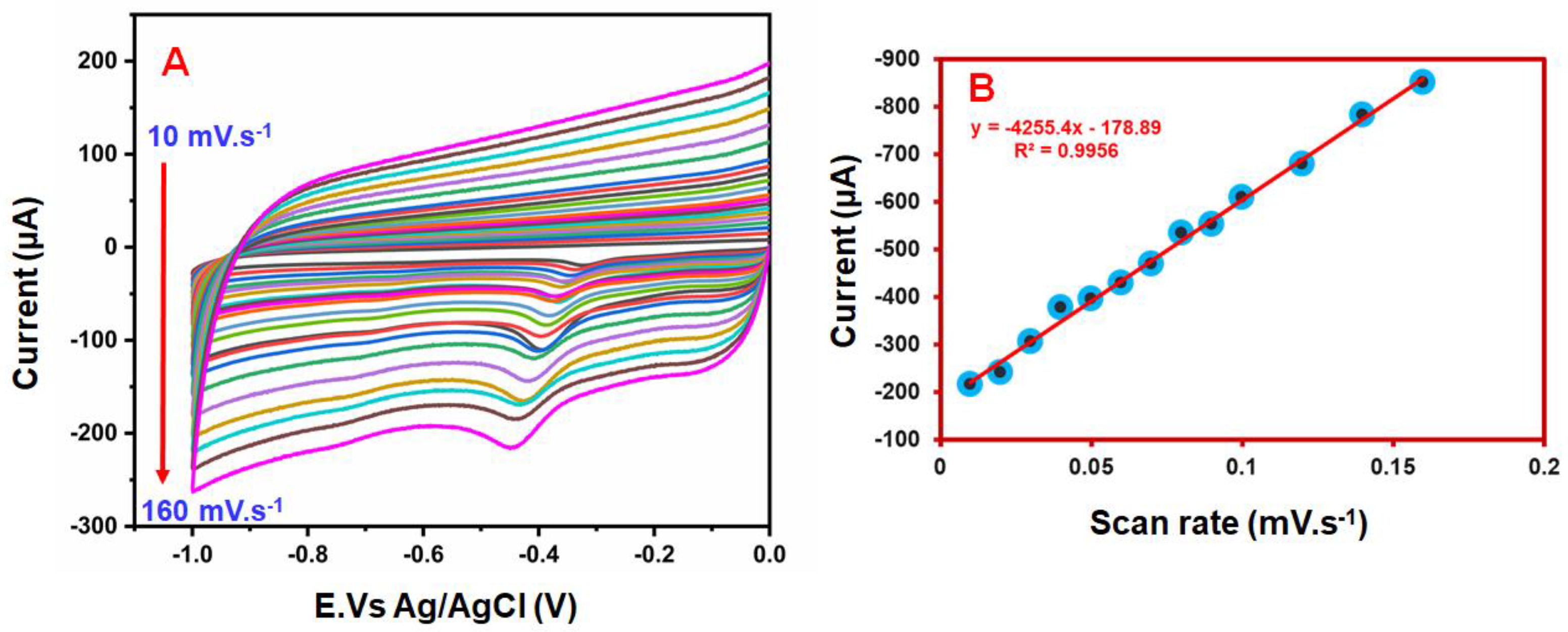
4.2. Effect of Different Scan Rates and Effect of Different pH Studies
4.3. DPV Studies for Detection of FLD
4.4. Repeatability, Cyclic Stability, Reproducibility, and Operational Stability Studies of Eu2(WO4)3 Coated Electrodes
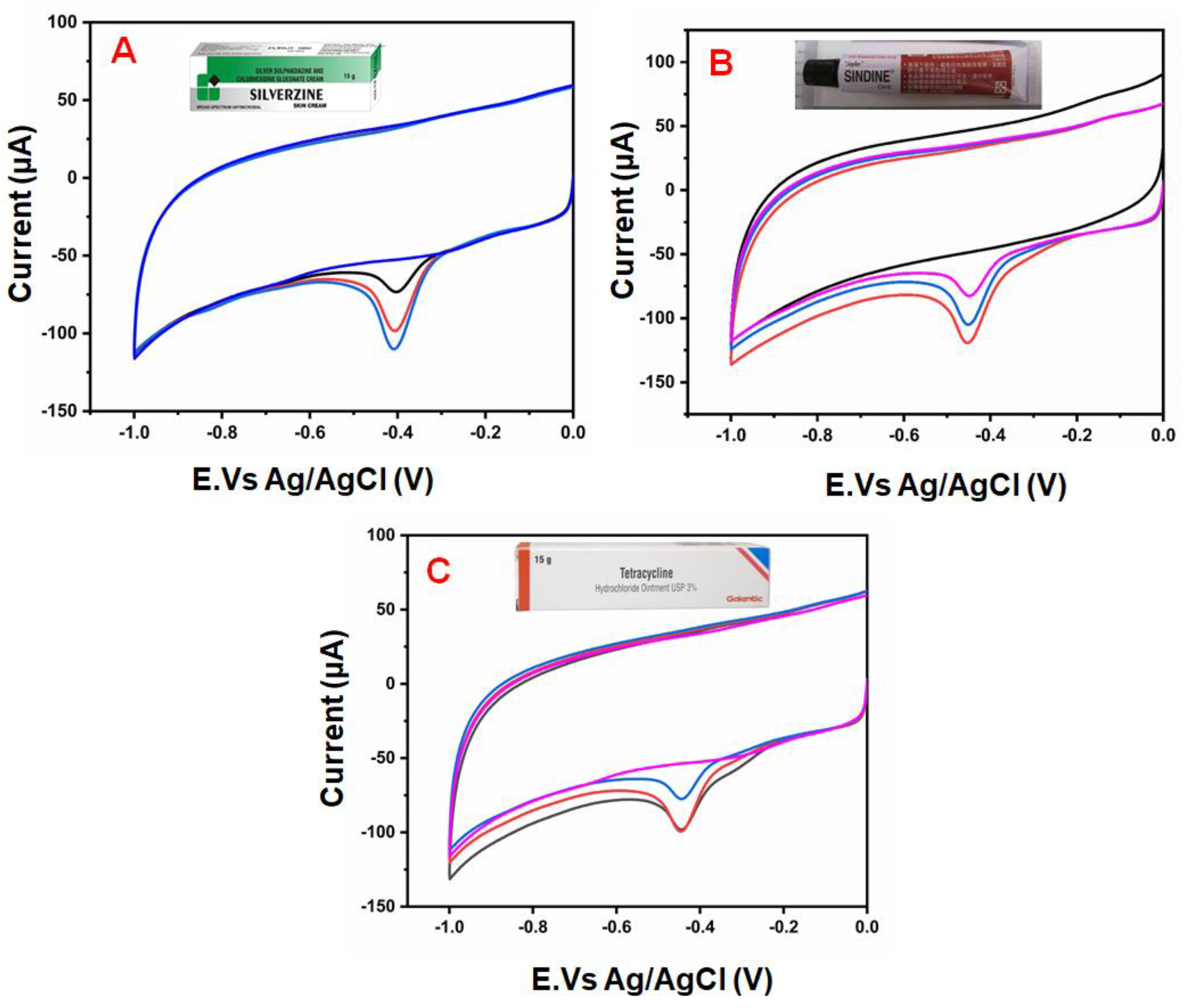
4.5. Real Sample Studies of FTD in Antibiotic Medicine
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diaz, T.G.; Cabanillas, A.G.; Valenzuela, M.I.A.; Correa, C.A.; Salinas, F. Determination of nitrofurantoin, furazolidone, and furaltadone in milk by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 1997, 764, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, K.; Chen, T.W.; Chen, S.M.; Tseng, T.W.; Bian, Y.; Sun, T.T.; Jiang, J.; Yu, J. Fabrication of Hexagonal CuCoO2 Modified Screen-Printed Carbon Electrode for the Selective Electrochemical Detection of Furaltadone. Int. J. Electrochem. Sci. 2022, 17, 220644. [Google Scholar] [CrossRef]
- Vasu, D.; Keyan, A.K.; Sakthinathan, S.; Chiu, T.W. Investigation of electrocatalytic and photocatalytic ability of Cu/Ni/TiO2/MWCNTs Nanocomposites for detection and degradation of antibiotic drug Furaltadone. Sci. Rep. 2022, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.J.J.; Murthy, U.N.; Fue, W.S. Ultrasensitive electrochemical detection of an antibiotic drug Furaltadone in fish tissue with a ZnO-ZnCo2O4 self-assembled nano-heterostructure as an electrode material. Microchem. J. 2021, 169, 106566. [Google Scholar] [CrossRef]
- Massa, A. Clinical trial of furaltadone, an antibacterial nitrofuran. Br. J. Clin. Pract. 1961, 15, 351–354. [Google Scholar] [CrossRef]
- Khodari, M.; Din, H.S.E.; Mersal, G.A.M. Electroreduction and quantification of furazolidone and furaltadone in different media. Mikrochim. Acta 2000, 135, 9–17. [Google Scholar] [CrossRef]
- Barbosa, J.; Freitas, A.; Moura, S.; Maria, J.L.M.; Silveira, I.N.; Ramos, F. Detection, accumulation, distribution, and depletion of furaltadone and nifursol residues in poultry muscle, liver, and gizzard. J. Agric. Food Chem. 2011, 59, 11927–11934. [Google Scholar] [CrossRef]
- Xu, Z.L.; Shen, Y.D.; Sun, Y.M.; Campbell, K.; Tian, Y.X.; Zhang, S.W.; Lei, H.T.; Jiang, Y.M. Novel hapten synthesis for antibody production and development of an enzyme-linked immunosorbent assay for determination of furaltadone metabolite 3-amino-5-morpholinomethyl-2-oxazolidinone (AMOZ). Talanta 2013, 103, 306–313. [Google Scholar] [CrossRef]
- Jester, E.L.E.; Abraham, A.; Wang, Y.; Steven, K.R.E.S.; Plakas, M. Performance evaluation of commercial ELISA kits for screening of furazolidone and furaltadone residues in fish. Food Chem. 2014, 145, 593–598. [Google Scholar] [CrossRef]
- Shi, S.; Cao, G.; Chen, Y.; Huang, J.; Tang, Y.; Jiang, J.; Gan, T.; Wan, C.; Wu, C. Facile synthesis of core-shell Co-MOF with hierarchical porosity for enhanced electrochemical detection of furaltadone in aquaculture water. Anal. Chim. Acta 2023, 1263, 341296. [Google Scholar] [CrossRef]
- Alawi, M.A. Analysis of furazolidone and furaltadone in chicken tissues and eggs using a modified HPLC/ELCD method. Fresenius Environ. Bull. 2000, 9, 508–514. [Google Scholar]
- Balamurugan, K.; Rajakumaran, R.; Chen, S.M.; Karthik, R.; Shim, J.J.; Shafi, P.M. Massive engineering of spinel cobalt tin oxide/tin oxide-based electrocatalyst for the selective voltametric determination of antibiotic drug furaltadone in water samples. J. Alloys Compd. 2021, 882, 160750. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Wang, T.J.; Ahmed, F.; Alshahrani, T. Synthesis of 3D flower-like zinc-chromium layered double hydroxides: A functional electrode material for furaltadone detection. Process Saf. Environ. Prot. 2023, 176, 889–897. [Google Scholar] [CrossRef]
- Keyan, A.K.; Sakthinathan, S.; Vasu, D.; Yu, C.L.; Vinothini, S.; Chiu, T.W. Gadolinium molybdate decorated graphitic carbon nitride composite: Highly visualized detection of nitrofurazone in water samples. RSC Adv. 2022, 12, 34066–34079. [Google Scholar] [CrossRef]
- Keyan, A.K.; Vasu, D.; Sakthinathan, S.; Chiu, T.W.; Lee, Y.H.; Lin, C.C. Facile synthesis of silver-doped copper selenide composite for enhanced electrochemical detection of ecological toxic nitrobenzene. Electrocatalysis 2023, 14, 448–462. [Google Scholar] [CrossRef]
- Sakthinathan, S.; Keyan, A.K.; Vasu, D.; Vinothini, S.; Nagaraj, K.; Mangesh, V.L.; Chiu, T.W. Graphitic carbon nitride incorporated europium molybdate composite as an enhanced sensing platform for electrochemical detection of carbendazim in agricultural products. J. Electrochem. Soc. 2022, 169, 127504. [Google Scholar] [CrossRef]
- Enayat, M.J. Controllable fabrication of europium tungstate nanoparticles in the presence of new capping agents and investigation of their photocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 16444–16449. [Google Scholar] [CrossRef]
- Nasrabadi, M.R.; Pourmohamadian, V.; Karimi, M.S.; Naderi, H.R.; Karimi, M.A.; Didehban, K.; Ganjali, M.R. Assessment of supercapacitive performance of europium tungstate nanoparticles prepared via hydrothermal method. J. Mater. Sci. Mater. Electron. 2017, 28, 12391–12398. [Google Scholar] [CrossRef]
- Templeton, D.H.; Zalkin, A. Crystal Structure of Europium Tungstate. Acta Cryst. 1963, 16, 762. [Google Scholar] [CrossRef]
- Naderi, H.R.; Nadri, P.; Sorouri, A.M.; Ganjali, M.R.; Ehrlich, H.; Nasrabadi, M.R.; Ahmadi, F. Evaluation of electrodes composed of europium tungstate/reduced graphene oxide nanocomposite for use as supercapacitors. Surf. Interfaces 2022, 31, 102002. [Google Scholar] [CrossRef]
- Kodairaa, C.A.; Britoa, H.F.; Maltab, O.L.; Serrac, O.A. Luminescence and energy transfer of the europium (III) tungstate obtained via the Pechini method. J. Lumin. 2003, 101, 11–21. [Google Scholar] [CrossRef]
- Karthikeyan, D.; Vijayakumar, K.; Suhasini, P.; Dhanusha, A.; Sabari, G.T.C. Third-order nonlinear optical responses of Cr3+ ions on La2(WO4)3 nanoparticles for optical limiting applications. J. Photochem. Photobiol. A Chem. 2023, 436, 114377. [Google Scholar] [CrossRef]
- Yun, F.; Cao, L.; Huang, J.; Wu, J. Effects of pH on the microstructures and optical property of FeWO4 nanocrystallites prepared via hydrothermal method. Ceram. Int. 2013, 39, 4133–4138. [Google Scholar]
- Kim, A.H.; Kang, J.H.; Hong, A.R.; Park, Y.J.; Jang, H.S.; Kang, Y.M.; Kim, D.H. Sputter-grown Eu-doped WO3-Eu2(WO4)3 composite red phosphor thin films. Opt. Mater. 2021, 122, 111721. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Sakthinathan, S.; Chen, S.M.; Lee, C.M.; Lou, B.S.; Sireesha, P.; Su, C. Electrochemically activated screen printed carbon electrode decorated with nickel nano particles for the detection of glucose in human serum and human urine sample. Int. J. Electrochem. Sci. 2016, 11, 7934–7946. [Google Scholar] [CrossRef]
- Rajakumaran, R.; Babulal, S.M.; Chen, S.M.; Sukanya, R.; Karthik, R.; Shafi, P.M.; Shim, J.J.; Shiuan, C.Y. Ingenious design of iron vanadate engulfed 3D porous reduced graphene oxide nanocomposites as a reliable electrocatalyst for the selective amperometric determination of furaltadone in aquatic environments. Appl. Surf. Sci. 2021, 569, 151046. [Google Scholar] [CrossRef]
- Horne, E.; Cadogan, A.; Keeffe, M.O.; Boom, L.A.P.H. Analysis of Protein-bound metabolites of Furazolidone and furaltadone in pig liver by high-performance liquid chromatography and liquid chromatography–mass spectrometry. Analyst 1996, 121, 1463–1468. [Google Scholar] [CrossRef]
- Yan, C.; Teng, J.; Liu, F.; Yao, B.; Xu, Z.; Yao, L.; Chen, W. Signal amplified enzyme-linked immunosorbent assay with gold nanoparticles for sensitive detection of trace furaltadone metabolite. Microchem. J. 2020, 159, 105414. [Google Scholar] [CrossRef]
- Barbosa, J.; Freitas, A.; Mourao, J.L.; Silveira, M.I.N.; Ramos, F. Determination of furaltadone and nifursol residues in poultry eggs by liquid chromatography-electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2012, 60, 4227–4234. [Google Scholar] [CrossRef]
- Bock, C.; Gowik, P.; Stachel, C. Matrix-comprehensive in-house validation and robustness check of a confirmatory method for the determination of four nitrofuran metabolites in poultry muscle and shrimp by LC-MS/MS. Biomed Life Sci. 2007, 856, 178–189. [Google Scholar] [CrossRef]





| Electrode and Technique | Linear Range | Limit of Detection | References |
|---|---|---|---|
| FeVO/p-rGO NCs | 0.584 | 138 nM | [26] |
| AOZ/AMOZ | 0.05–2.0 | 10.0 μg kg−1 | [27] |
| LC-MS/MSc | 1–800 | 0.5 μg kg−1 | [28] |
| ELISA | 0.9–105.3 | 0.3 μg kg−1 | [29] |
| LC-MS/MS | 0.98–0.99 | 0.2 μg/kg | [30] |
| ASPCE/Eu2(WO4)3 | 10 nM–300 µM | 97 µM | This work |
| Real Samples | Added | Found | Recovery (%) |
|---|---|---|---|
| Silverzine medicine | 10 | 9 | 90 |
| 20 | 18.5 | 92.5 | |
| 30 | 29.2 | 97.3 | |
| Sindine medicine | 10 | 8.5 | 85 |
| 20 | 19 | 95 | |
| 30 | 29.4 | 98 | |
| Tetracycline medicine | 10 | 9.3 | 93 |
| 20 | 19.3 | 96.5 | |
| 30 | 29.5 | 98.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinothini, S.; Chiu, T.-W.; Sakthinathan, S. Electrochemical Detection of Furaltadone Antibiotic Drug by the Rare Earth Metal Tungstate Decorated Screen Printed Carbon Electrode. Technologies 2023, 11, 105. https://doi.org/10.3390/technologies11040105
Vinothini S, Chiu T-W, Sakthinathan S. Electrochemical Detection of Furaltadone Antibiotic Drug by the Rare Earth Metal Tungstate Decorated Screen Printed Carbon Electrode. Technologies. 2023; 11(4):105. https://doi.org/10.3390/technologies11040105
Chicago/Turabian StyleVinothini, Sivaramakrishnan, Te-Wei Chiu, and Subramanian Sakthinathan. 2023. "Electrochemical Detection of Furaltadone Antibiotic Drug by the Rare Earth Metal Tungstate Decorated Screen Printed Carbon Electrode" Technologies 11, no. 4: 105. https://doi.org/10.3390/technologies11040105
APA StyleVinothini, S., Chiu, T.-W., & Sakthinathan, S. (2023). Electrochemical Detection of Furaltadone Antibiotic Drug by the Rare Earth Metal Tungstate Decorated Screen Printed Carbon Electrode. Technologies, 11(4), 105. https://doi.org/10.3390/technologies11040105









