Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation
Abstract
1. Introduction and Motivation
2. Science Goals and Objectives
2.1. Goal 1: Measure Habitability Indicators
2.1.1. Objective 1.1: Determine the Amount of Water Vapor in the Cloud Layers
2.1.2. Objective 1.2: Determine the Acidity of Single Cloud Particles
2.1.3. Objective 1.3: Detect and Identify Metals and Other Non-Volatile Elements in the Cloud Particles
2.1.4. Objective 1.4: Measure the Temperature, Pressure, and Windspeed as a Function of Altitude
2.2. Goal 2: Search for Evidence of Life in the Venusian Clouds
2.2.1. Objective 2.1: Search for Signs of Life via Gas Detection
2.2.2. Objectives 2.2 and 2.3: Detect and Identify or Constrain Organic Material within the Cloud Particles
2.3. Goal 3: Characterize Cloud Particles in Preparation for Sample Return
2.3.1. Objective 3.1: Determine If the Cloud Particles Are Liquid or Solid
2.3.2. Objective 3.2: Determine If the Venus Cloud Particles Are Homogeneous
3. Instrument Summary
3.1. Single Particle pH Meter
3.2. Autofluorescence Nephelometer
3.3. MEMS Aerosol Elemental Analyzer
3.4. Mini Tunable Laser Spectrometer
3.5. MEMS Gas Molecule Analyzer
3.6. Weather Instrument Suite
3.7. Conductivity Sensor
4. Mission Implementation Concepts
4.1. Fixed Altitude Balloon Concept
4.2. Variable Altitude Balloon Concept
4.3. VLF Habitability Parachute Probe
4.4. Alternative Science Payload
5. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.H.; Ehlmann, B.L.; Saikia, S.J.; Agrawal, R.; Buchanan, W.P.; Weber, M.U.; French, R.; et al. Venus Life Finder Missions Motivation and Summary. Aerospace 2022, 9, 385. [Google Scholar] [CrossRef]
- Dartnell, L.R.; Nordheim, T.A.; Patel, M.R.; Mason, J.P.; Coates, A.J.; Jones, G.H. Constraints on a potential aerial biosphere on Venus: I. Cosmic rays. Icarus 2015, 257, 396–405. [Google Scholar] [CrossRef]
- Patel, M.R.; Mason, J.P.; Nordheim, T.A.; Dartnell, L.R. Constraints on a potential aerial biosphere on Venus: II. Ultraviolet radiation. Icarus 2021, 114796. [Google Scholar] [CrossRef]
- Limaye, S.S.; Mogul, R.; Smith, D.J.; Ansari, A.H.; Słowik, G.P.; Vaishampayan, P. Venus’ Spectral Signatures and the Potential for Life in the Clouds. Astrobiology 2018, 18, 1181–1198. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Gao, P.; Bains, W.; Bryan, N.C.; Ranjan, S.; Greaves, J. The Venusian Lower Atmosphere Haze as a Depot for Desiccated Microbial Life: A Proposed Life Cycle for Persistence of the Venusian Aerial Biosphere. Astrobiology 2021, 21, 1206–1223. [Google Scholar] [CrossRef]
- Hallsworth, J.E.; Koop, T.; Dallas, T.D.; Zorzano, M.-P.; Burkhardt, J.; Golyshina, O.V.; Martín-Torres, J.; Dymond, M.K.; Ball, P.; McKay, C.P. Water activity in Venus’s uninhabitable clouds and other planetary atmospheres. Nat. Astron. 2021, 1–11. [Google Scholar] [CrossRef]
- Stevenson, A.; Hamill, P.G.; O’Kane, C.J.; Kminek, G.; Rummel, J.D.; Voytek, M.A.; Dijksterhuis, J.; Hallsworth, J.E. Aspergillus penicillioides differentiation and cell division at 0.585 water activity. Environ. Microbiol. 2017, 19, 687–697. [Google Scholar] [CrossRef]
- Bell, J.F., III; Crisp, D.; Lucey, P.G.; Ozoroski, T.A.; Sinton, W.M.; Willis, S.C.; Campbell, B.A. Spectroscopic observations of bright and dark emission features on the night side of Venus. Science 1991, 252, 1293–1296. [Google Scholar] [CrossRef]
- Petkowski, J.J.; Seager, S.; Grinspoon, D.H.; Bains, W.; Ranjan, S.; Rimmer, P.B.; Buchanan, W.P.; Agrawal, A.; Mogul, R.; Carr, C.E. Venus’ Atmosphere Anomalies as Motivation for Astrobiology Missions. Astrobiology 2022. in review. [Google Scholar]
- Titov, D.V.; Ignatiev, N.I.; McGouldrick, K.; Wilquet, V.; Wilson, C.F. Clouds and hazes of Venus. Space Sci. Rev. 2018, 214, 1–61. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Zhan, Z.; Seager, S. Evaluating Alternatives to Water as Solvents for Life: The Example of Sulfuric Acid. Life 2021, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Knollenberg, R.G.; Hunten, D.M. The microphysics of the clouds of Venus: Results of the Pioneer Venus particle size spectrometer experiment. J. Geophys. Res. Sp. Phys. 1980, 85, 8039–8058. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Jordan, S.; Constantinou, T.; Woitke, P.; Shorttle, O.; Paschodimas, A.; Hobbs, R. Hydroxide salts in the clouds of Venus: Their effect on the sulfur cycle and cloud droplet pH. Planet. Sci. J. 2021, 2, 133. [Google Scholar] [CrossRef]
- Bains, W.; Petkowski, J.J.; Rimmer, P.B.; Seager, S. Production of Ammonia Makes Venusian Clouds Habitable and Explains Observed Cloud-Level Chemical Anomalies. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Mogul, R.; Limaye, S.S.; Lee, Y.J.; Pasillas, M. Potential for Phototrophy in Venus’ Clouds. Astrobiology 2021, 21, 1237–1249. [Google Scholar] [CrossRef]
- Mendonça, J.M.; Buchhave, L.A. Modelling the 3D climate of Venus with OASIS. Mon. Not. R. Astron. Soc. 2020, 496, 3512–3530. [Google Scholar] [CrossRef]
- Ignatiev, N.I.; Moroz, V.I.; Moshkin, B.E.; Ekonomov, A.P.; Gnedykh, V.I.; Grigoriev, A.V.; Khatuntsev, I.V. Water vapour in the lower atmosphere of Venus: A new analysis of optical spectra measured by entry probes. Planet. Space Sci. 1997, 45, 427–438. [Google Scholar] [CrossRef]
- Cottini, V.; Ignatiev, N.I.; Piccioni, G.; Drossart, P.; Grassi, D.; Markiewicz, W.J. Water vapor near the cloud tops of Venus from Venus Express/VIRTIS dayside data. Icarus 2012, 217, 561–569. [Google Scholar] [CrossRef]
- Fedorova, A.; Marcq, E.; Luginin, M.; Korablev, O.; Bertaux, J.-L.; Montmessin, F. Variations of water vapor and cloud top altitude in the Venus’ mesosphere from SPICAV/VEx observations. Icarus 2016, 275, 143–162. [Google Scholar] [CrossRef]
- Marcq, E.; Mills, F.P.; Parkinson, C.D.; Vandaele, A.C. Composition and chemistry of the neutral atmosphere of Venus. Space Sci. Rev. 2018, 214, 10. [Google Scholar] [CrossRef]
- Encrenaz, T.; Moreno, R.; Moullet, A.; Lellouch, E.; Fouchet, T. Submillimeter mapping of mesospheric minor species on Venus with ALMA. Planet. Space Sci. 2015, 113, 275–291. [Google Scholar] [CrossRef]
- Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.; Ehlmann, B.; Saikia, S.J.; Agrawal, R.; Buchanan, W.; Weber, M.U.; French, R. Venus Life Finder Mission Study. arXiv 2021, arXiv:2112.05153. [Google Scholar]
- Schulze-Makuch, D.; Grinspoon, D.H.; Abbas, O.; Irwin, L.N.; Bullock, M.A. A sulfur-based survival strategy for putative phototrophic life in the Venusian atmosphere. Astrobiology 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Petrianov, I.V.; Andreichikov, B.M.; Korchuganov, B.N.; Ovsiankin, E.I.; Ogorodnikov, B.I.; Skitovich, V.I.; Khristianov, V.K. Iron in the Clouds of Venus; Akademiia Nauk SSSR Doklady: Moscow, USSR, 1981; Volume 260, pp. 834–836. [Google Scholar]
- Andreichikov, B.M. Chemical composition and structure of Venus clouds from results of X-ray radiometric experiments made with the Vega 1 and Vega 2 automatic interplanetary stations. Kosm. Issled. 1987, 25, 737–743. [Google Scholar]
- Krasnopolsky, V.A. On the iron chloride aerosol in the clouds of Venus. Icarus 2017, 286, 134–137. [Google Scholar] [CrossRef]
- Ando, H.; Imamura, T.; Tsuda, T.; Tellmann, S.; Pätzold, M.; Häusler, B. Vertical Wavenumber Spectra of Gravity Waves in the Venus Atmosphere Obtained from Venus Express Radio Occultation Data: Evidence for Saturation. J. Atmos. Sci. 2015, 72, 2318–2329. [Google Scholar] [CrossRef]
- Kaasik, L.; Rahu, I.; Roper, E.M.; Seeba, R.; Rohtsalu, A.; Pajusalu, M. Sensor for determining single droplet acidities in the Venusian atmosphere. Aerospace 2022, 9, 560. [Google Scholar] [CrossRef]
- Le Guern, F.; Mussard, V.; Gaucher, A.; Rottman, M.; Prim, D. Fluorescein Derivatives as Fluorescent Probes for pH Monitoring along Recent Biological Applications. Int. J. Mol. Sci. 2020, 21, 9217. [Google Scholar] [CrossRef]
- Lindqvist, L. The Triplet State Of Fluorescein In Sulfuric Acid. J. Phys. Chem. 1963, 67, 1701–1704. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, B.Y.; Yao, Q.; Zavabeti, A.; Huertas, C.S.; Brkljača, R.; Khan, M.W.; Nili, H.; Datta, R.S.; Khan, H. An ultrasensitive silicon photonic ion sensor enabled by 2D plasmonic molybdenum oxide. Small 2019, 15, 1805251. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Buchanan, W.P.; Arora, A.; Girija, A.P.; de Jong, M.; Seager, S.; Petkowski, J.J.; Saikia, S.J.; Carr, C.E.; Grinspoon, D.H.; et al. Mission Architecture to Characterize Habitability of Venus Cloud Layers via an Aerial Platform. Aerospace 2022, 9, 359. [Google Scholar] [CrossRef]
- French, R.; Mandy, C.; Hunter, R.; Mosleh, E.; Sinclair, D.; Beck, P.; Seager, S.; Petkowski, J.J.; Carr, C.E.; Grinspoon, D.H.; et al. Rocket Lab Mission to Venus. Aerospace 2022, 9, 445. [Google Scholar] [CrossRef]
- Beswick, K.; Baumgardner, D.; Gallagher, M.; Volz-Thomas, A.; Nedelec, P.; Wang, K.-Y.; Lance, S. The backscatter cloud probe–a compact low-profile autonomous optical spectrometer. Atmos. Meas. Tech. 2014, 7, 1443–1457. [Google Scholar] [CrossRef]
- Lloyd, G.; Gallagher, M.; Choularton, T.; Krämer, M.; Andreas, P.; Baumgardner, D. In Situ Measurements of Cirrus Clouds on a Global Scale. Atmosphere 2021, 12, 41. [Google Scholar] [CrossRef]
- Baumgardner, D.; Fisher, T.; Newton, R.; Roden, C.; Zmarzly, P.; Seager, S.; Petkowski, J.J.; Carr, C.E.; Špaček, J.; Benner, S.A.; et al. Deducing the Composition of Venus Cloud Particles with the Autofluorescence Nephelometer (AFN). Aerospace 2022, 9, 492. [Google Scholar] [CrossRef]
- Grzebyk, T.; Bigos, M.; Górecka-Drzazga, A.; Dziuban, J.A.; Hasan, D.; Lee, C. Mems Ion Sources For Spectroscopic Identification Of Gaseous And Liquid Samples. In Proceedings of the 2019 19th International Conference on Micro and Nanotechnology for Power Generation and Energy Conversion Applications (PowerMEMS), Kraków, Poland, 2–6 December 2019; pp. 1–3. [Google Scholar]
- Grzebyk, T.; Szyszka, P.; Dziuban, J. Identification of a gas composition based on an optical spectrum of plasma generated in MEMS ion spectrometer. In Proceedings of the 2021 IEEE 20th International Conference on Micro and Nanotechnology for Power Generation and Energy Conversion Applications (PowerMEMS), Exeter, UK, 6–8 December 2021; pp. 148–151. [Google Scholar]
- Lackner, M. Tunable diode laser absorption spectroscopy (TDLAS) in the process industries–a review. Rev. Chem. Eng 2007, 23, 65–147. [Google Scholar] [CrossRef]
- Du, Z.; Zhang, S.; Li, J.; Gao, N.; Tong, K. Mid-infrared tunable laser-based broadband fingerprint absorption spectroscopy for trace gas sensing: A review. Appl. Sci. 2019, 9, 338. [Google Scholar] [CrossRef]
- Mahaffy, P.R.; Webster, C.R.; Cabane, M.; Conrad, P.G.; Coll, P.; Atreya, S.K.; Arvey, R.; Barciniak, M.; Benna, M.; Bleacher, L. The sample analysis at Mars investigation and instrument suite. Space Sci. Rev. 2012, 170, 401–478. [Google Scholar] [CrossRef]
- Webster, C.R.; Mahaffy, P.R.; Flesch, G.J.; Niles, P.B.; Jones, J.H.; Leshin, L.A.; Atreya, S.K.; Stern, J.C.; Christensen, L.E.; Owen, T. Isotope ratios of H, C, and O in CO2 and H2O of the Martian atmosphere. Science 2013, 341, 260–263. [Google Scholar] [CrossRef]
- Pajusalu, M.; Borlina, C.S.; Seager, S.; Ono, S.; Bosak, T. Open-source sensor for measuring oxygen partial pressures below 100 microbars. PLoS ONE 2018, 13, e0206678. [Google Scholar] [CrossRef] [PubMed]
- Emerson Automation Solutions PaineTM 211-55-010 Series Pressure Transducer. 2017. Available online: https://www.emerson.com/documents/automation/product-data-sheet-211-55-010-series-pressure-transducer-paine-en-80014.pdf (accessed on 1 June 2022).
- Domínguez-Pumar, M.; Kowalski, L.; Jiménez, V.; Rodríguez, I.; Soria, M.; Bermejo, S.; Pons-Nin, J. Analyzing the Performance of a Miniature 3D Wind Sensor for Mars. Sensors 2020, 20, 5912. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.B.; Getty, S.A.; Arney, G.N.; Johnson, N.M.; Kohler, E.; Schwer, K.O.; Sekerak, M.; Bartels, A.; Saylor, R.S.; Elliott, V.E.; et al. Revealing the Mysteries of Venus: The DAVINCI Mission. Planet. Sci. J. 2022, 3, 117. [Google Scholar] [CrossRef]
- Buchanan, W.P.; de Jong, M.; Agrawal, R.; Petkowski, J.J.; Arora, A.; Saikia, S.J.; Seager, S.; Longuski, J. Aerial Platform Design Options for a Life-Finding Mission at Venus. Aerospace 2022, 9, 363. [Google Scholar] [CrossRef]
- Isaac, C.; Jones, N. Leading-Edge Vortex Lift (LEVL) Sample Probe for Venusian Atmosphere. Aerospace 2022, 9, 471. [Google Scholar] [CrossRef]
- Whitehead, S. LOVE-Bug Deployment Demonstrator. Aerospace 2022, 9, 573. [Google Scholar] [CrossRef]
- Marshall, S.M.; Mathis, C.; Carrick, E.; Keenan, G.; Cooper, G.J.T.; Graham, H.; Craven, M.; Gromski, P.S.; Moore, D.G.; Walker, S.I. Identifying molecules as biosignatures with assembly theory and mass spectrometry. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Willhite, L.; Ni, Z.; Arevalo, R.; Bardyn, A.; Gundersen, C.; Minasola, N.; Southard, A.; Briois, C.; Thirkell, L.; Colin, F. CORALS: A Laser Desorption/Ablation Orbitrap Mass Spectrometer for In Situ Exploration of Europa. In Proceedings of the 2021 IEEE Aerospace Conference (50100), Big Sky, MT, USA, 6–13 March 2021; pp. 1–13. [Google Scholar]
- Ligterink, N.F.W.; Grimaudo, V.; Moreno-García, P.; Lukmanov, R.; Tulej, M.; Leya, I.; Lindner, R.; Wurz, P.; Cockell, C.S.; Ehrenfreund, P. ORIGIN: A novel and compact Laser Desorption–Mass Spectrometry system for sensitive in situ detection of amino acids on extraterrestrial surfaces. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Ligterink, N.F.W.; Kipfer, K.A.; Gruchola, S.; Boeren, N.J.; Keresztes Schmidt, P.; de Koning, C.P.; Tulej, M.; Wurz, P.; Riedo, A. The ORIGIN Space Instrument for Detecting Biosignatures and Habitability Indicators on a Venus Life Finder Mission. Aerospace 2022, 9, 312. [Google Scholar] [CrossRef]
- Goesmann, F.; Brinckerhoff, W.B.; Raulin, F.; Goetz, W.; Danell, R.M.; Getty, S.A.; Siljeström, S.; Mißbach, H.; Steininger, H.; Arevalo Jr, R.D. The Mars Organic Molecule Analyzer (MOMA) instrument: Characterization of organic material in martian sediments. Astrobiology 2017, 17, 655–685. [Google Scholar] [CrossRef]
- Rohner, U.; Whitby, J.A.; Wurz, P. A miniature laser ablation time-of-flight mass spectrometer for in situ planetary exploration. Meas. Sci. Technol. 2003, 14, 2159. [Google Scholar] [CrossRef]
- Tulej, M.; Iakovleva, M.; Leya, I.; Wurz, P. A miniature mass analyser for in-situ elemental analysis of planetary material–performance studies. Anal. Bioanal. Chem. 2011, 399, 2185–2200. [Google Scholar] [CrossRef] [PubMed]
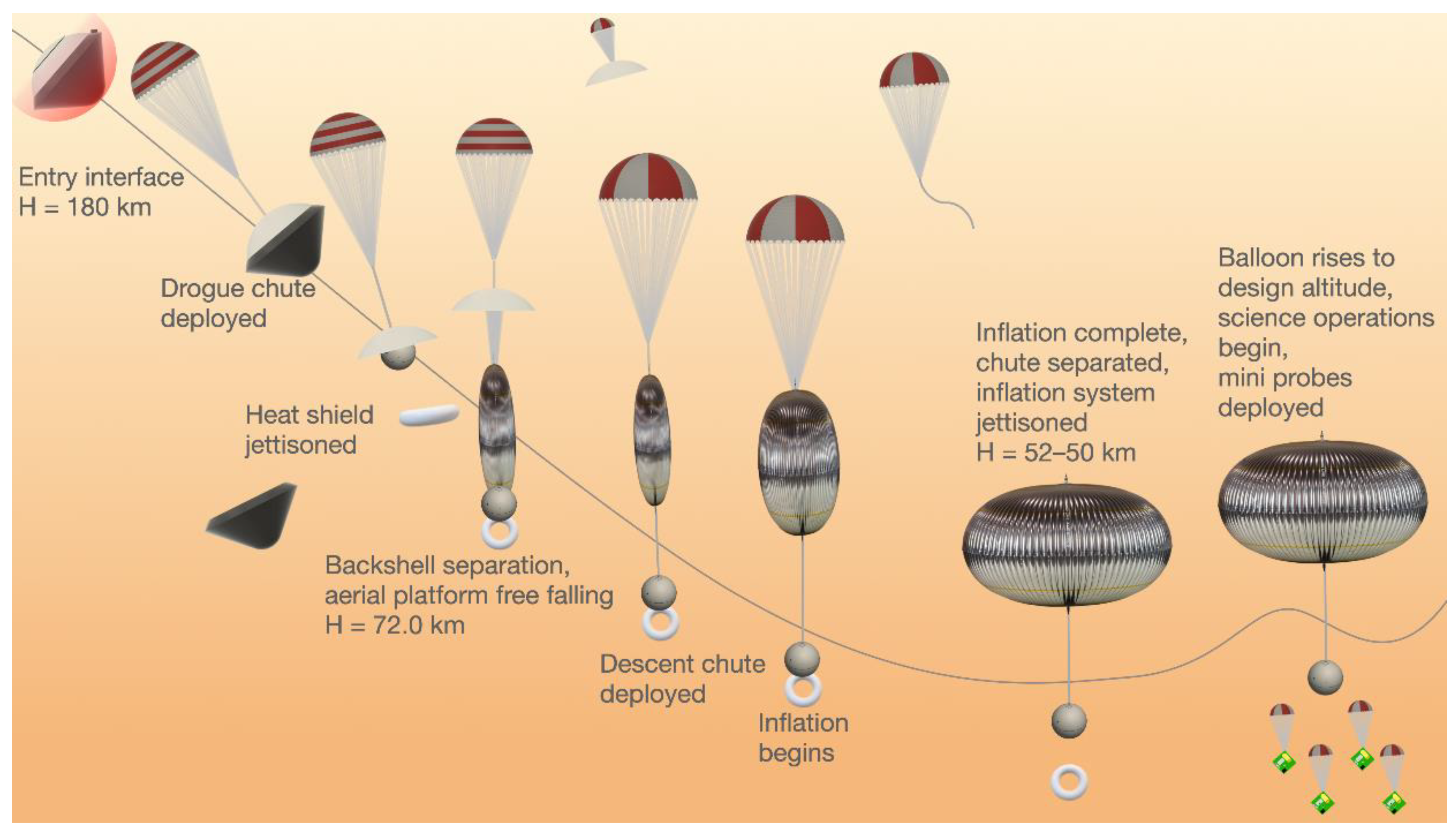
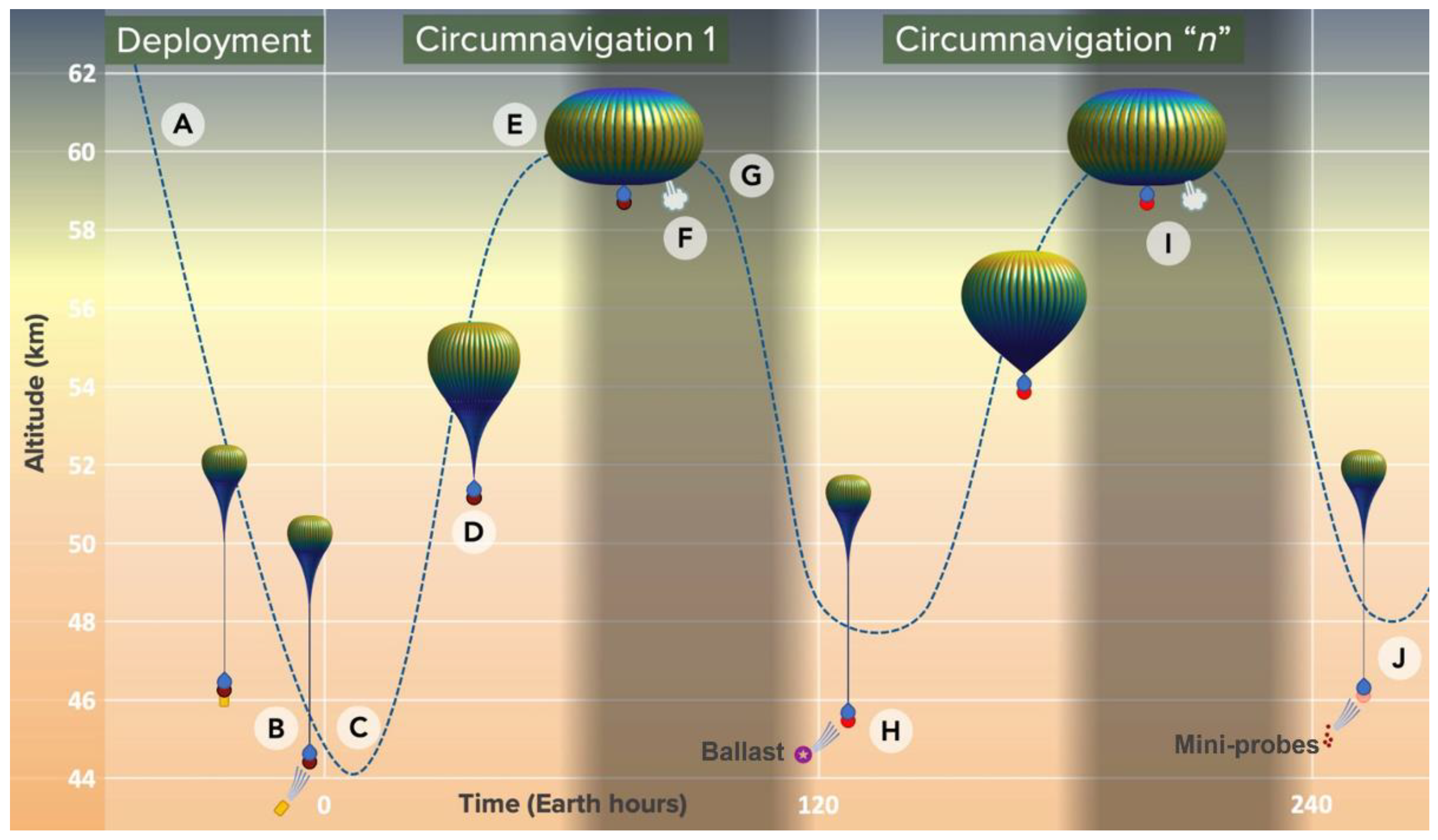
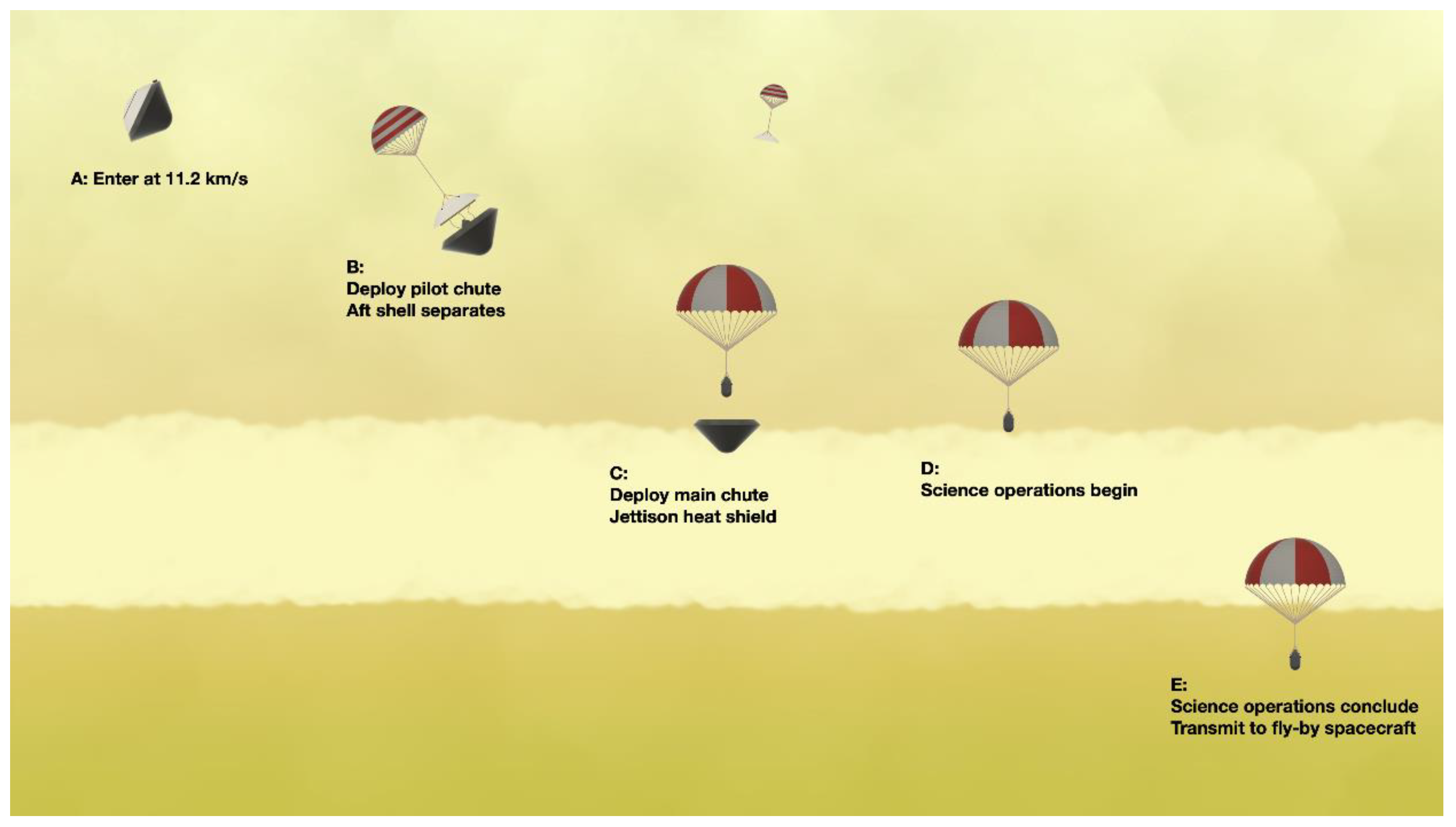

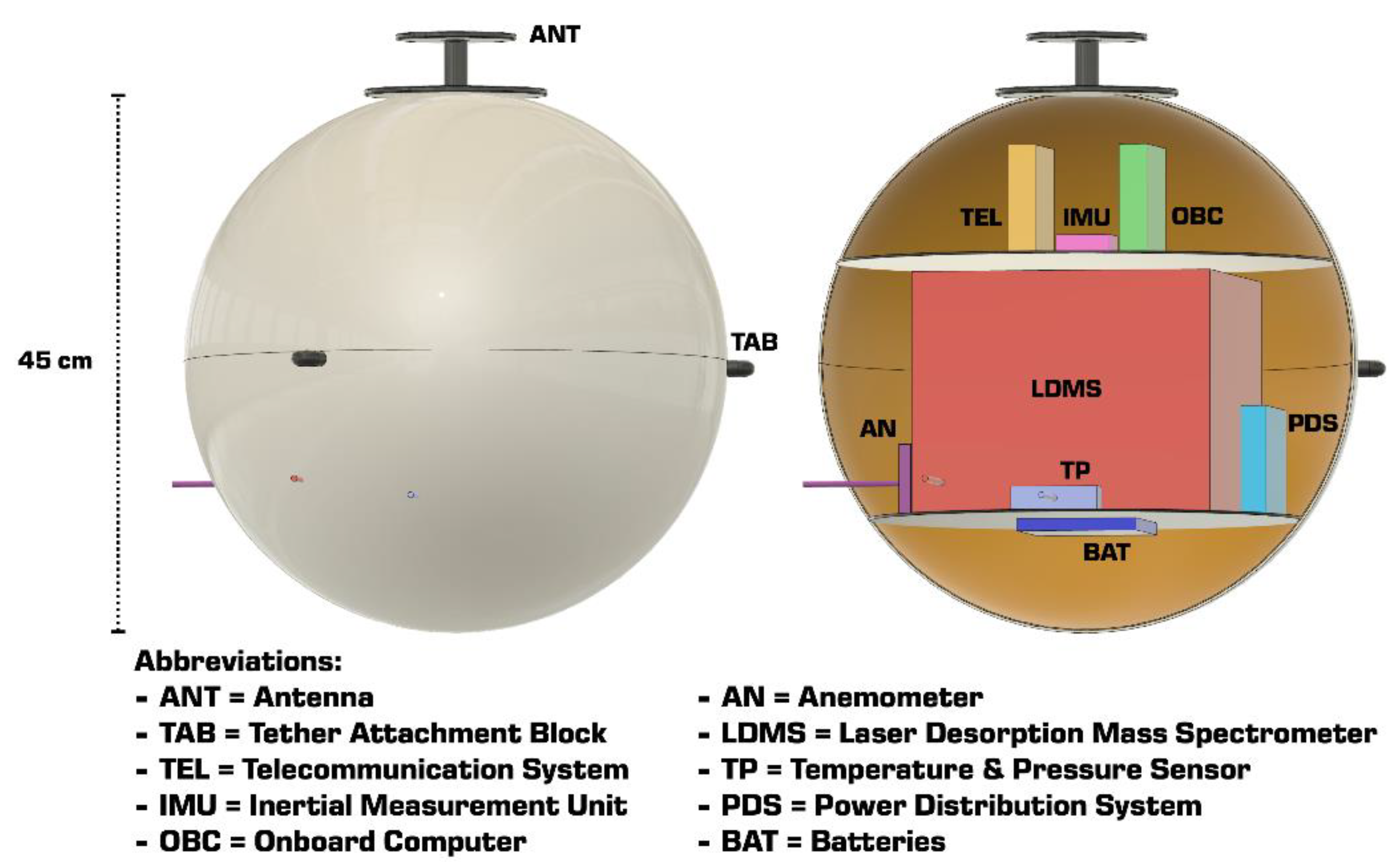
| Goals | Science Objectives | Instruments | |
|---|---|---|---|
| Habitability | 1. Measure Habitability Indicators | 1.1 Determine the amount of water in the cloud layers | TLS and Conductivity Sensor |
| 1.2 Determine the pH of single cloud particles | MoOSA and TOPS acidity sensors | ||
| 1.3 Determine and identify metals and other nonvolatile elements in the cloud particles | MEMS-A | ||
| 1.4 Measure the temperature, pressure, and windspeed as a function of altitude | Temperature and pressure sensor, anemometer | ||
| Biosignatures | 2. Search for Evidence of Life in the Venusian Clouds | 2.1 Search for signs of life via gas detection | TLS and MEMS-G |
| 2.2 Detect organic material within the cloud particles | AFN | ||
| 2.3 Identify organic material within the cloud particles | AFN, MEMS-A | ||
| Sample Return | 3. Characterize Cloud Particles in Preparation for Sample Return | 3.1 Determine if the cloud particles are liquid or solid | AFN |
| 3.2 Determine if the cloud particles are homogeneous | AFN, acidity sensors, MEMS-A |
| Goals | Science Objectives | Hypothesis | Science Outcomes | |
|---|---|---|---|---|
| Habitability | 1. Measure Habitability Indicators | 1.1 Determine the amount of water in the cloud layers | The amount of water in the clouds is not uniform and is locally variable. | Detection of anomalously high abundance values: The amount of water in the clouds is not uniform and is locally variable. No anomalously high values detected: Reconciles the previous measurements and upper limits. |
| 1.2 Determine the pH of single cloud particles | The acidity of cloud particles is variable and not all cloud particles are composed only of concentrated sulfuric acid. | Detection of variable acidity of cloud particles: The altitude profile of cloud acidity tests the validity of atmospheric and cloud models and model implications for the habitability of the clouds. Acidity of cloud particles is uniform and consistent with concentrated sulfuric acid: Puts clear constraints on the chemical processes in the atmosphere; confirms, for the first time by direct measurement, that the clouds are uniformly made of concentrated sulfuric acid particles. | ||
| 1.3 Determine and identify metals and other nonvolatile elements in the cloud particles | Cloud particles contain dissolved metal ions (e.g., Fe) and other ions of nonvolatile elements (e.g., P). | Metal ions detected: The composition of the cloud particles is chemically complex; Suggests efficient exchange of material between the surface (the presumed source of the non-volatile elements) and the clouds. No metal ions detected: The material exchange between the surface (the presumed source of the non-volatile elements) and the clouds is not efficient limiting the habitability of the clouds. | ||
| 1.4 Measure the temperature, pressure, and windspeed as a function of altitude | No specific hypothesis; more direct measurements are needed to understand the atmospheric dynamics. | Atmospheric dynamics can be compared to those predicted by current general circulation models of Venus (e.g., [16]) and are important to inform operations for future missions such as balloons or aerobots as well as aerosol sampling in support of in-situ analysis and/or sample return. | ||
| Biosignatures | 2. Search for Evidence of Life in the Venusian Clouds | 2.1 Search for signs of life via gas detection | Gases listed in Table 3 are signs of chemical disequilibria in the clouds that could be associated with life. | Disequilibrium gases (Table 3) detected: The abundance vs altitude profile constraints sources of gases and tests the validity of the atmospheric chemistry models and their implications. Non-detection ofdisequilibrium gases (Table 3): Reconciles the upper limits provided by the remote observations with the tentative in situ detections; Puts clear constraints on the chemical processes in the atmosphere. |
| 2.2 Detect organic material within the cloud particles | Clouds of Venus are not a chemically sterile environment and contain organic molecules. | Organics detected: The prospect for the habitability of Venus’ clouds increases as all life requires organic chemistry. No organics detected: The prospects of the clouds of Venus as a habitable environment diminish as we assume that all life, no matter its chemical makeup, requires organic chemistry. | ||
| 2.3 Identify organic material within the cloud particles | Cloud particles contain complex organic molecules that could be precursors to life or even be signs of life itself. | Complex and diverse organics identified: Potential for life in the cloud particles increases with the diversity and complexity of detected organics. Only simple and uniform organics identified: Abiotic processes are most likely responsible for organics formation. No organics identified: The prospects of the clouds of Venus as a habitable environment diminish as we assume that all life, no matter its chemical makeup, requires organic chemistry. | ||
| Sample Return | 3. Characterize Cloud Particles in Preparation for Sample Return | 3.1 Determine if the cloud particles are liquid or solid | Clouds are not homogenous and are composed of liquid concentrated sulfuric acid droplets and solid salt particles. | Solid Mode 3 particles composed of salts detected: Confirms the existence of the Mode 3 particles; the salt composition puts clear constraints on the chemical processes in the cloud droplets and the atmosphere; confirms that the clouds are not uniformly made of liquid concentrated sulfuric acid particles. No solid particles detected: Supports the model that the clouds of Venus are made of liquid droplets of concentrated sulfuric acid. |
| 3.2 Determine if the cloud particles are homogeneous | Cloud particles are not homogenous in terms of shape, size, acidity and chemical composition. | Cloud particles’ chemical composition and acidity vary: Cloud particles are non-homogenous. Informs the design of the future particle capture and storage technology for the atmospheric sample return mission. Cloud particles’ chemical composition and acidity are uniform: Supports the model that the clouds of Venus are made of liquid droplets of concentrated sulfuric acid. Informs the design of the future particle capture and storage technology for the atmospheric sample return mission. |
| Gas | Motivation | Scientific Outcomes | Instrument |
|---|---|---|---|
| O2 | Potential sign of life; prior in situ detection | Detection: The abundance vs altitude profile constraints the source of O2 and tests the validity of the models and their implications. Non-detection: Reconciles the upper limits provided by the remote observations with the tentative in situ detections; Puts clear constraints on the chemical processes in the atmosphere. | MEMS-G, TLS |
| SOx | Variable profile indicative of unknown cloud particle chemistry | Detection: The abundance vs altitude profile constraints the source of SO2 and other SOx gases and tests the validity of the models and their implications; Puts clear constraints on the chemical processes in the cloud droplets and the atmosphere. | MEMS-G |
| NOx | Important component of the nitrogen cycle; prior tentative detection | Detection: The abundance vs altitude profile constraints the source of NOx and tests the validity of the models and their implications. Non-detection: Puts clear constraints on the chemical processes in the cloud droplets and the atmosphere, including on the presence and intensity of lightning strikes. | MEMS-G |
| H2O | Variable profile indicative of unknown cloud particle chemistry, including some anomalously high values | Detection of anomalously high abundance values: Confirmation that the amount of water in the clouds is not uniform and is locally variable. No anomalously high values detected: Reconciles the values and upper limits provided by the remote and in situ spectroscopic observations with the tentative in situ detections. | TLS |
| NH3 | Potential sign of life; habitability indicator; potential neutralizing agent for cloud droplets; prior tentative detection | Detection: The abundance vs altitude profile constraints the source of NH3 and tests the validity of the models and their implications. Non-detection: The NOx species (if confirmed) could not be the result of oxidation of NH3; Reconciles the upper limits provided by the remote observations with the tentative in situ detections; Puts clear constraints on the chemistry of the cloud droplets and on the chemical processes in the atmosphere. | TLS or MEMS-G |
| PH3 | Potential sign of life; prior tentative detection | Detection: The abundance vs altitude profile constraints the source of PH3 and tests the validity of the models and their implications. Non-detection: Reconciles the remote observations, including the upper limits, with the tentative in situ detections; Puts clear constraints on the chemical processes in the atmosphere, including the availability of volatile P species. | TLS |
| CH4 | Potential sign of life; prior anomalous detection | Detection: The abundance vs altitude profile constraints the source of CH4 and tests the validity of the models and their implications; Provides a potential source for organic chemistry in the clouds. Non-detection: Reconciles the upper limits provided by the remote observations with the tentative in situ detections. | TLS or MEMS-G |
| Instrument | Mass ** (kg) | Volume (cm3) | Average Power (W) | * Data Vol. per Meas. (kB) | TRL |
|---|---|---|---|---|---|
| Mini tunable laser spectrometer (TLS) | 4.60 | 240 | 14.0 | 1000 | 4 |
| MEMS aerosol analyzer (MEMS-A) | 0.34 | 400 | 1.0 | 27 | 4 |
| Autofluorescing nephelometer (AFN) | 0.80 | 100 | 40.0 | 120 | 3 |
| Tartu Observatory pH Sensor (TOPS) | 0.35 | 844 | 2.0 | 1 | 2 |
| Imaging unit (IU) | 0.15 | 250 | 0.5 | 100 | 4 |
| Weather Instruments Suite (WIS) | 0.10 | 98 | 1.0 | 0.05 | 4 |
| Total Gondola Subsystem Mass | 6.34 | 1932 | 58.5 | 1248 |
| Instrument | Mass (kg) | Volume (cm3) | Average Power (W) | Data Vol. per Meas. (kB) | * TRL |
|---|---|---|---|---|---|
| MEMS gas analyzer (MEMS-G) ** | 0.34 | 400 | 0.8 | 27 | 4 |
| MEMS aerosol analyzer (MEMS-A) | 0.34 | 400 | 1.0 | 27 | 4 |
| Tartu Observatory pH Sensor (TOPS) | 0.35 | 844 | 2.0 | 1 | 2 |
| MoOSA pH sensor (MoOSA) ** | 0.20 | 10 | 2.0 | 1 | 2 |
| Weather Instruments Suite (WIS)—one in each mini probe | 0.10 | 98 | 1.0 | 0.05 | 4 |
| Total Mini Probe Instrument Mass | 1.63 | 2046 | 9.80 | 56.20 |
| Component | CBE Mass (kg) | Contingency | MEV Mass (kg) |
|---|---|---|---|
| Structure | 2.3 | 1.3 | 3.0 |
| Science Instruments | 6.4 | 1.3 | 8.3 |
| Battery + PDS | 0.4 | 1.3 | 0.5 |
| Communication | 3.7 | 1.3 | 4.8 |
| Thermal | 1.2 | 1.3 | 1.6 |
| C&DH | 3.1 | 1.3 | 4.0 |
| Total | 17.1 | 22.3 |
| Component | CBE Mass (kg) | Contingency | MEV Mass (kg) |
|---|---|---|---|
| Structure | 6.6 | 1.3 | 8.5 |
| Science instruments | 16.2 | 1.3 | 21.1 |
| Battery + PDS | 0.4 | 1.3 | 0.5 |
| Communication | 3.7 | 1.3 | 4.8 |
| Thermal | 4.2 | 1.3 | 5.5 |
| C&DH | 3.1 | 1.3 | 4.0 |
| Total | 34.2 | 44.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seager, S.; Petkowski, J.J.; Carr, C.E.; Saikia, S.J.; Agrawal, R.; Buchanan, W.P.; Grinspoon, D.H.; Weber, M.U.; Klupar, P.; Worden, S.P.; et al. Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation. Aerospace 2022, 9, 733. https://doi.org/10.3390/aerospace9110733
Seager S, Petkowski JJ, Carr CE, Saikia SJ, Agrawal R, Buchanan WP, Grinspoon DH, Weber MU, Klupar P, Worden SP, et al. Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation. Aerospace. 2022; 9(11):733. https://doi.org/10.3390/aerospace9110733
Chicago/Turabian StyleSeager, Sara, Janusz J. Petkowski, Christopher E. Carr, Sarag J. Saikia, Rachana Agrawal, Weston P. Buchanan, David H. Grinspoon, Monika U. Weber, Pete Klupar, Simon P. Worden, and et al. 2022. "Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation" Aerospace 9, no. 11: 733. https://doi.org/10.3390/aerospace9110733
APA StyleSeager, S., Petkowski, J. J., Carr, C. E., Saikia, S. J., Agrawal, R., Buchanan, W. P., Grinspoon, D. H., Weber, M. U., Klupar, P., Worden, S. P., Iakubivskyi, I., Pajusalu, M., Kaasik, L., & on behalf of the Venus Life Finder Mission Team. (2022). Venus Life Finder Habitability Mission: Motivation, Science Objectives, and Instrumentation. Aerospace, 9(11), 733. https://doi.org/10.3390/aerospace9110733










