Genome-Wide Association Analysis for Tuber Dry Matter and Oxidative Browning in Water Yam (Dioscorea alata L.)
Abstract
1. Introduction
2. Results
2.1. Genotypic Variability for Tuber DMC and OxB
2.2. Genotyping, Population Structure, and Linkage Disequilibrium
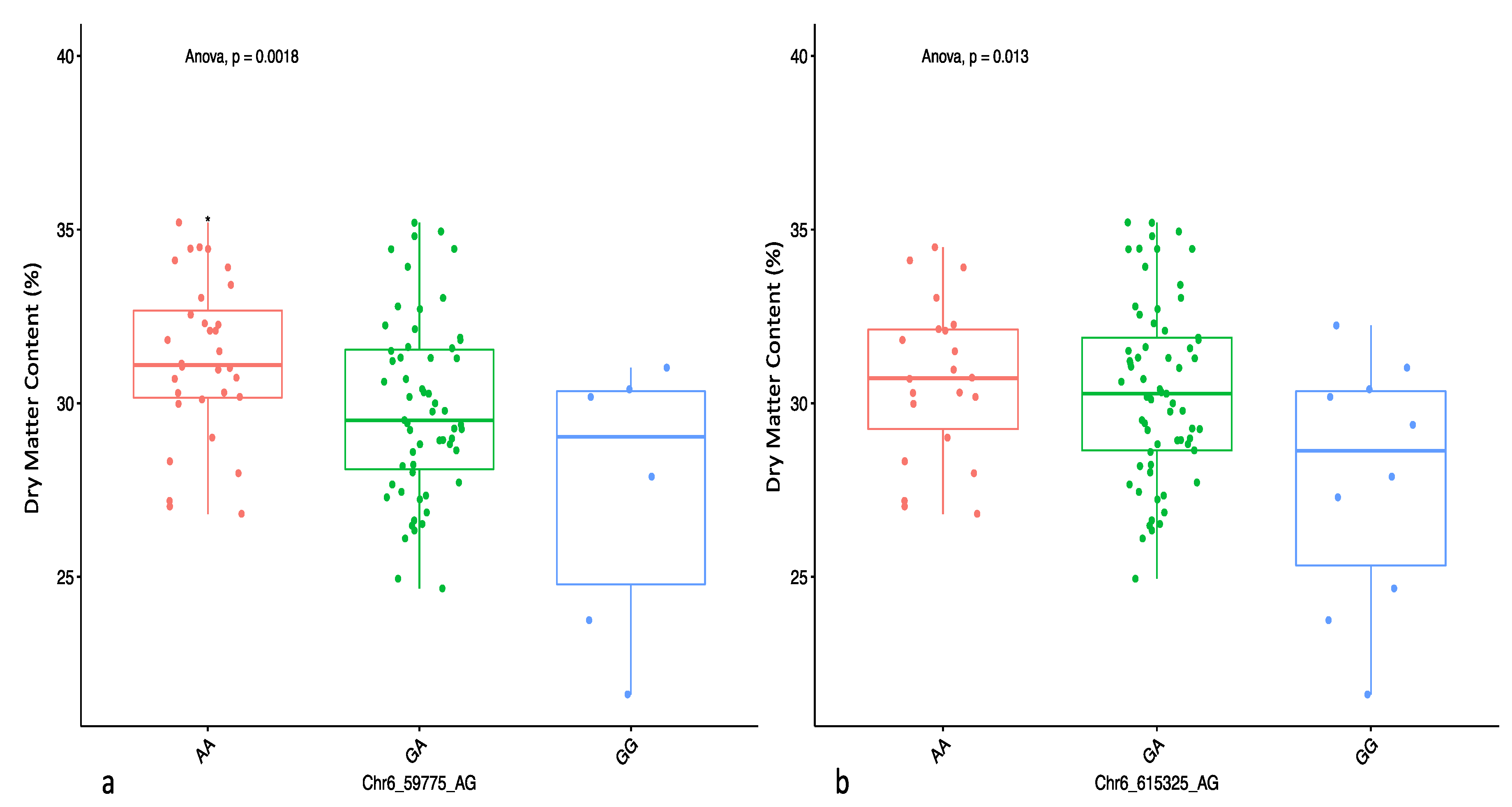
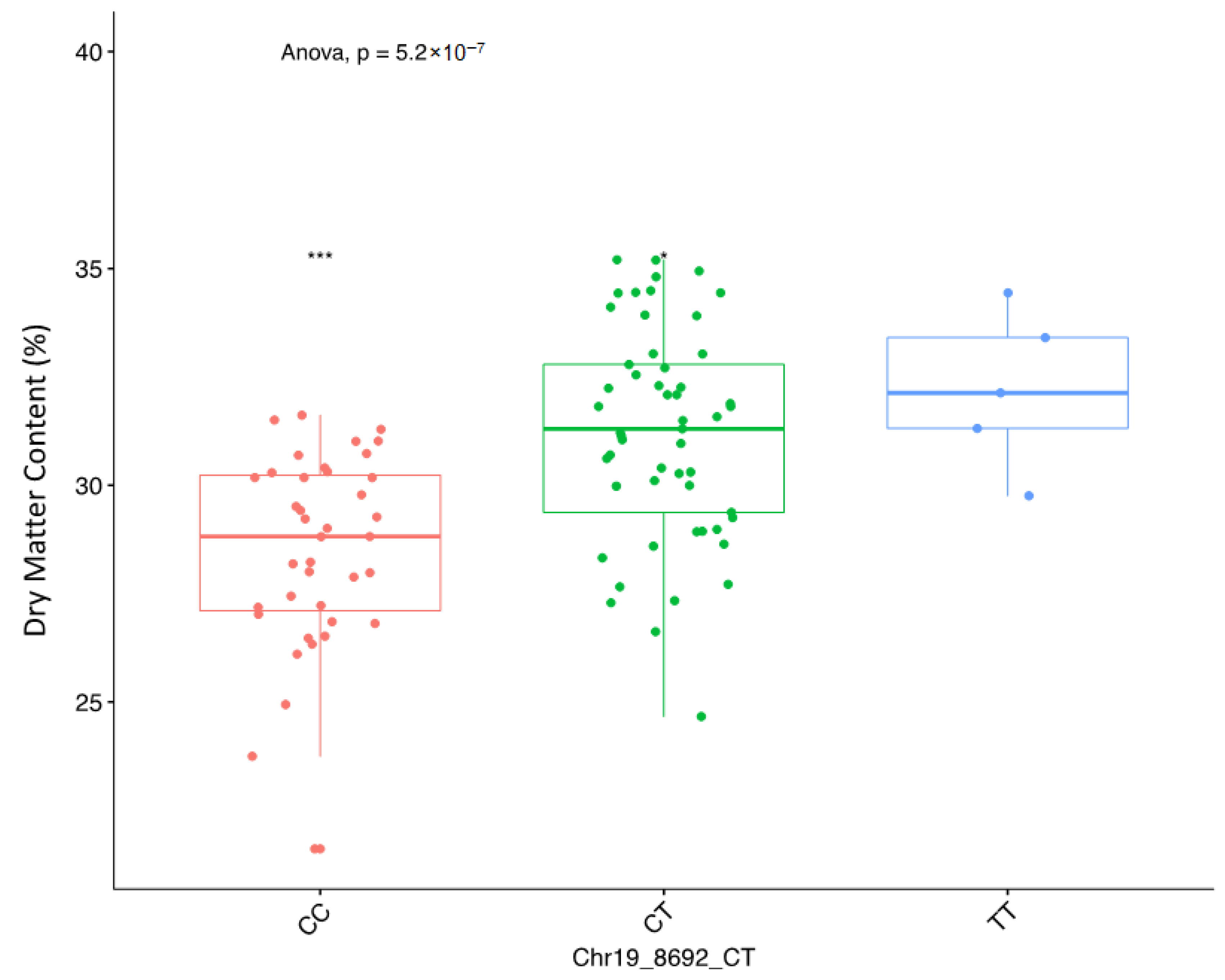
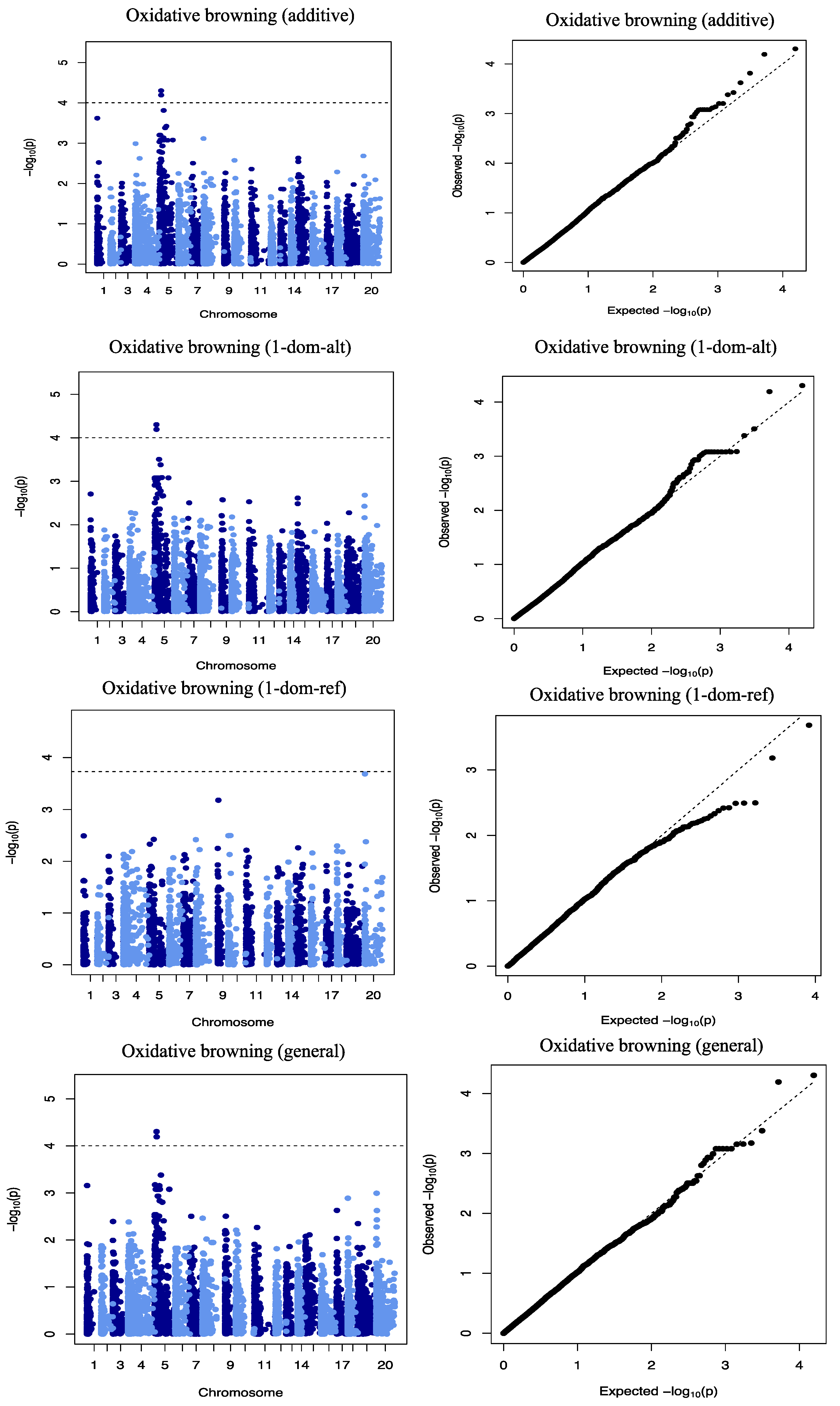
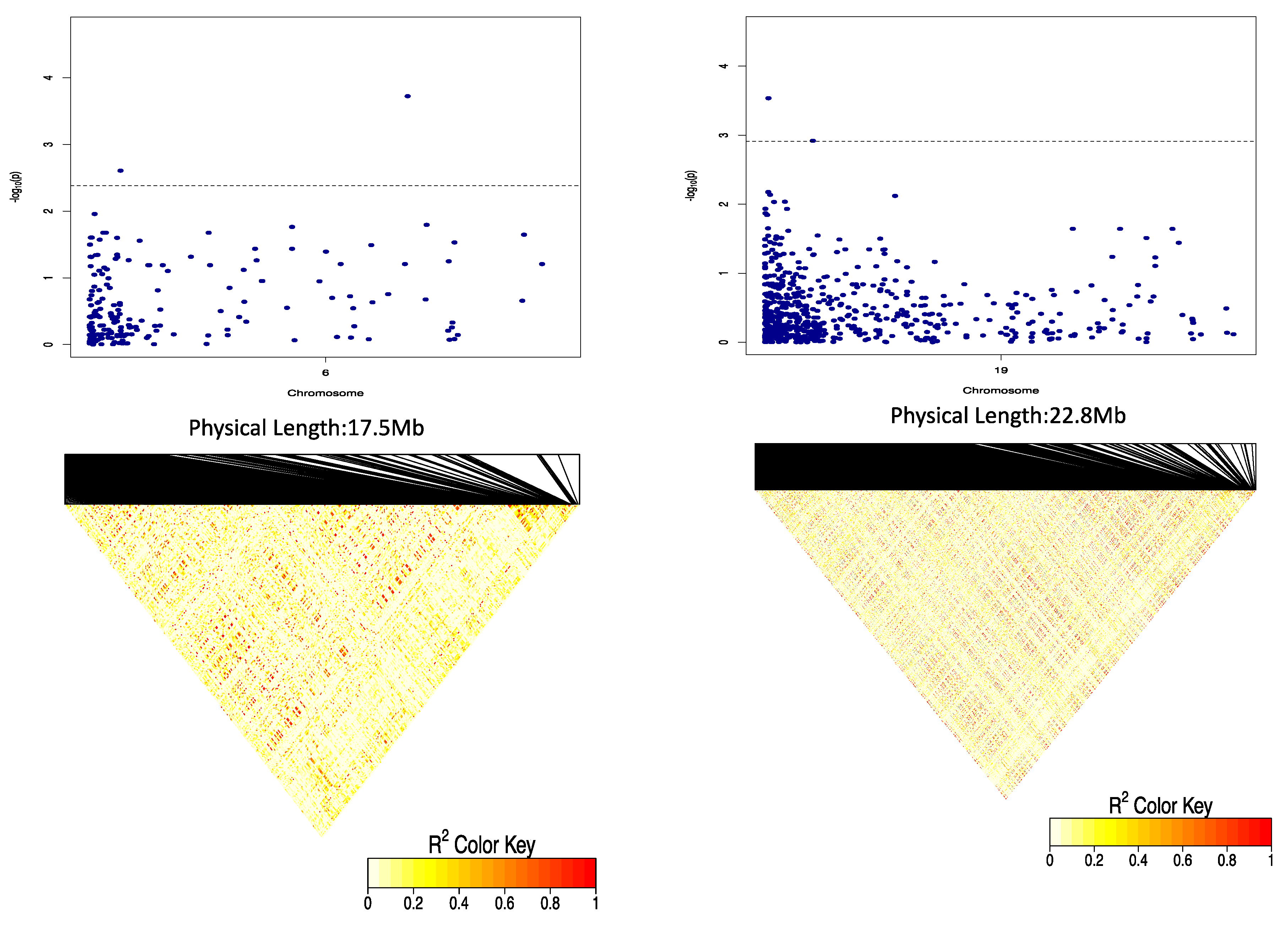
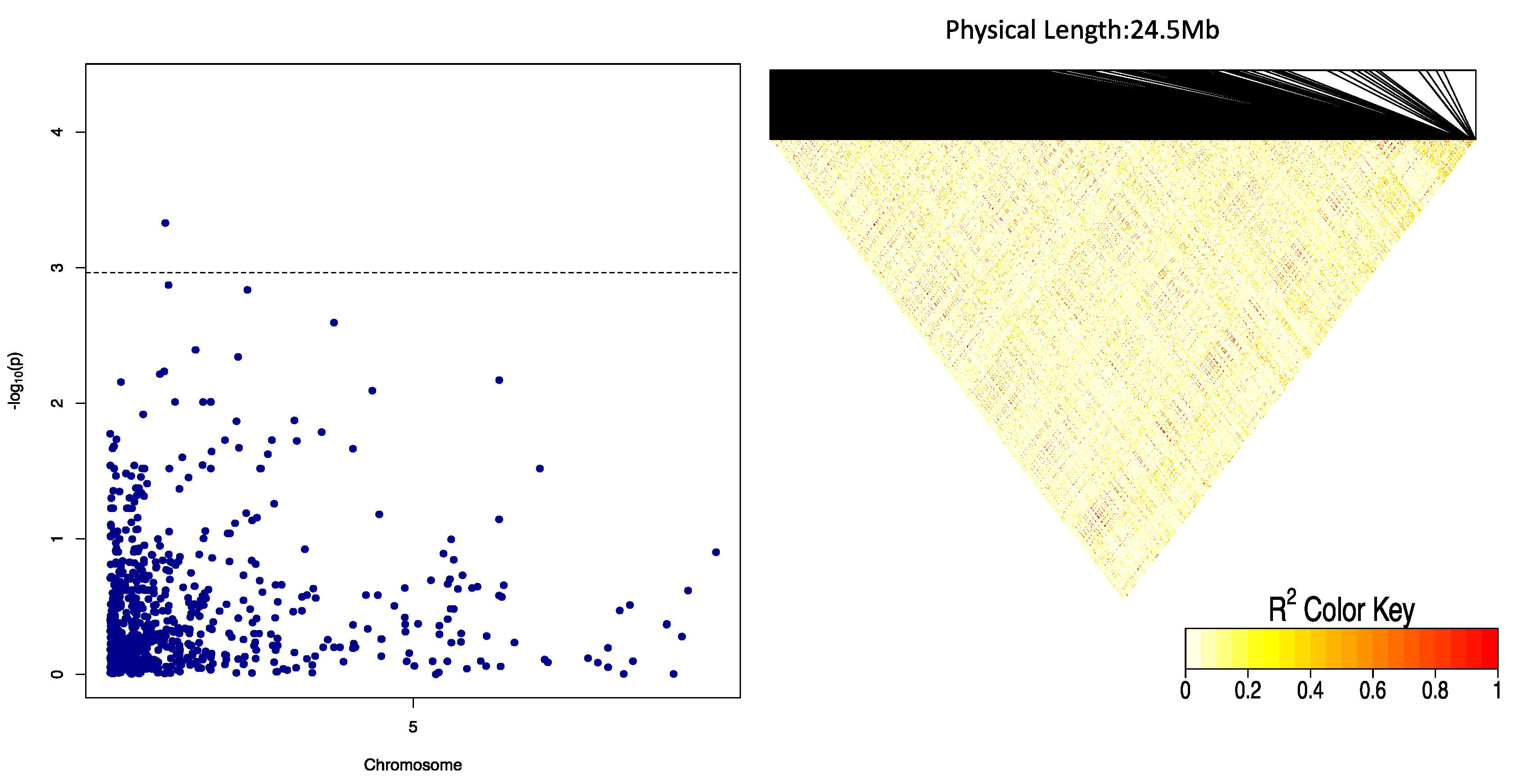
2.3. Genome-Wide Association Scan for Tuber Dry Matter Content
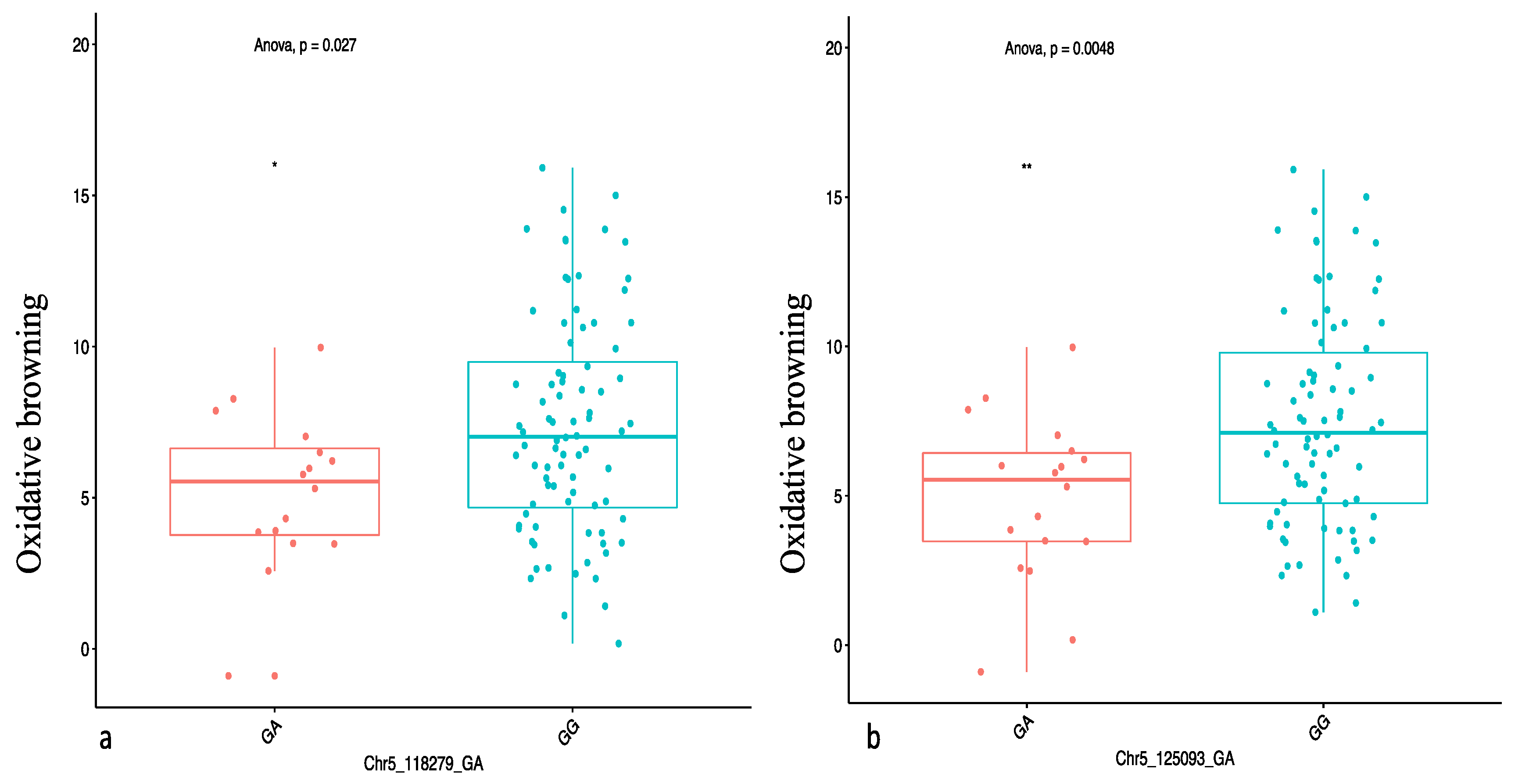
2.4. Genome-Wide Scan for Oxidative Browning
2.5. Gene Annotations
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Phenotypic Data Collection
4.2.1. Tuber Dry Matter Content
4.2.2. Oxidative Browning
4.3. DNA Extraction, Library Construction and SNP Calling
4.3.1. Phenotypic Data Analysis
4.3.2. Genotypic Data Analysis
4.3.3. Linkage Decay, GWAS Analysis and Gene Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CTAB | Cetyltrimethylammonium bromide |
| DAPC | Discriminant analysis of principal components |
| DArT | Diversity arrays technology |
| DMC | Dry matter content |
| DNA | Deoxyribonucleic acid |
| EH | Expected heterozygosity |
| EMBL-EBI | European Bioinformatics Institute |
| GWAS | Genome-wide association study |
| IITA | International institute of tropical agriculture |
| LD | Linkage disequilibrium |
| MAF | Minor allele frequency |
| MAS | Marker assisted selection |
| OH | Observed heterozygosity |
| OxB | Oxidative browning |
| PIC | Polymorphism information content |
| Quantile–quantile | |
| QTL | Quantitative trait locus |
| SNP | Single nucleotide polymorphism |
References
- Mahalakshmi, V.; Ng, Q.; Atalobhor, J.; Ogunsola, D.; Lawson, M.; Ortiz, R. Development of a West African yam Dioscorea spp. core collection. Genet. Resour. Crop Evol. 2007, 54, 1817–1825. [Google Scholar] [CrossRef]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Chopade, B.A. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483. [Google Scholar]
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Database, FAOSTAT. 2017. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 June 2020).
- Hahn, S.K.; Osiru, D.S.O.; Akoroda, M.O.; Otoo, J.A. Yam production and its future prospects. Outlook Agric. 1987, 16, 105–110. [Google Scholar] [CrossRef]
- Baah, F.D.; Maziya-Dixon, B.; Asiedu, R.; Oduro, I.; Ellis, W.O. Nutritional and biochemical composition of D. alata (Dioscorea spp.). Tubers 2009, 7, 373–378. [Google Scholar]
- Asiedu, R.; Wanyera, N.; Ng, S.Y.C.; Ng, N.Q. Yams; Cambridge University Press: Cambridge, UK, 1997; Volume 2, pp. 57–66. [Google Scholar]
- Egesi, C.N.; Asiedu, R.; Egunjobi, J.K.; Bokanga, M. Genetic diversity of organoleptic properties in water yam (Dioscorea alata L). J. Sci. Food Agric. 2003, 83, 858–865. [Google Scholar] [CrossRef]
- Lebot, V.; Malapa, R.; Molisalé, T.; Marchand, J.L. Physico-chemical characterisation of yam (Dioscorea alata L.) tubers from Vanuatu. Genet. Resour. Crop Evol. 2006, 53, 1199–1208. [Google Scholar] [CrossRef]
- Shittu, T.A.; Olaitan, O.F. Functional effects of dried okra powder on reconstituted dried yam flake and sensory properties of ojojo—A fried yam (Dioscorea alata L.) snack. J. Food Sci. Technol. 2014, 51, 359–364. [Google Scholar] [CrossRef][Green Version]
- Martin, F.W.; Ruberte, R. Polyphenol of Dioscorea alata (yam) tubers associated with oxidative browning. J. Agric. Food Chem. 1976, 24, 67–70. [Google Scholar] [CrossRef]
- Osagie, A.U. The Yam Tuber in Storage; Postharvest Research Unit, University of Benin: Benin City, Nigeria, 1992; pp. 174–191. [Google Scholar]
- Ukpabi, U.J.; Omodamiro, R.M.; Ikeorgu, J.G.; Asiedu, R. Sensory evaluation of amala from improved water yam (Dioscorea alata) genotypes in Nigeria. Afr. J. Biotechnol. 2008, 7, 1134–1138. [Google Scholar]
- Dufie, W.M.F.; Oduro, I.; Ellis, W.O.; Asiedu, R.; Maziya-Dixon, B. Potential health benefits of water yam (Dioscorea alata). Food Funct. 2013, 4, 1496–1501. [Google Scholar] [CrossRef]
- Sartie, A.; Asiedu, R. Segregation of vegetative and reproductive traits associated with tuber yield and quality in water yam (Dioscorea alata L.). Afr. J. Biotechnol. 2014, 13, 2807–2818. [Google Scholar]
- Asiedu, R.; Ng, S.Y.C.; Bai, K.V.; Ekanayake, I.J.; Wanyera, N.M.W. Genetic improvement. Food Yams 1998, 21, 63–104. [Google Scholar]
- Siqueira, M.V.; Bonatelli, M.L.; Günther, T.; Gawenda, I.; Schmid, K.J.; Pavinato, V.A.; Veasey, E.A. Water yam (Dioscorea alata L.) diversity pattern in Brazil: An analysis with SSR and morphological markers. Genet. Resour. Crop Evol. 2014, 61, 611–624. [Google Scholar] [CrossRef]
- Sartie, A.; Asiedu, R.; Franco, J. Genetic and phenotypic diversity in a germplasm working collection of cultivated tropical yams (Dioscorea spp.). Genet. Resour. Crop Evol. 2012, 59, 1753–1765. [Google Scholar] [CrossRef]
- Biscarini, F.; Cozzi, P.; Casella, L.; Riccardi, P.; Vattari, A.; Orasen, G.; Perrini, R.; Tacconi, G.; Tondelli, A.; Biselli, C.; et al. Genome-wide association study for traits related to plant and grain morphology, and root architecture in temperate rice accessions. PLoS ONE 2016, 11, e0155425. [Google Scholar] [CrossRef]
- Pétro, D.; Onyeka, T.J.; Etienne, S.; Rubens, S. An intraspecific genetic map of water yam (Dioscorea alata L.) based on AFLP markers and QTL analysis for anthracnose resistance. Euphytica 2011, 179, 405–416. [Google Scholar] [CrossRef]
- Bredeson, J.V.; Lyons, J.B.; Shu, S.; Ogunleye, I.; Bhattacharjee, R.; Obidiegwu, J.; Rokhsar, D.S. “The Genome Sequence of D. alata,” Manuscript in Preparation. Assembled Genome Sequence. Available online: https://phytozome-next.jgi.doe.gov/info/Dalata_v2_1 (accessed on 21 June 2020).
- Agre, P.; Asibe, F.; Darkwa, K.; Edemodu, A.; Bauchet, G.; Asiedu, R.; Asfaw, A. Phenotypic and molecular assessment of genetic structure and diversity in a panel of winged yam (Dioscorea alata) clones and cultivars. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Wanasundera, J.P.D.; Ravindran, G. Nutritional assessment of yam (Dioscorea alata) tubers. Plant Food Hum. Nutr. 1994, 46, 33–39. [Google Scholar] [CrossRef]
- Lebot, V.; Trilles, B.; Noyer, J.L.; Modesto, J. Genetic relationships between Dioscorea alata L. cultivars. Genet. Resour. Crop Evol. 1988, 45, 499–509. [Google Scholar] [CrossRef]
- Rabbi, I.Y.; Udoh, L.I.; Wolfe, M.; Parkes, E.Y.; Gedil, M.A.; Dixon, A.; Kulakow, P. Genome-wide association mapping of correlated traits in cassava: Dry matter and total carotenoid content. Plant Genome 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Wolfe, M.D.; Rabbi, I.Y.; Egesi, C.; Hamblin, M.; Kawuki, R.; Kulakow, P.; Jannink, J.L. Genome-wide association and prediction reveals genetic architecture of cassava mosaic disease resistance and prospects for rapid genetic improvement. Plant Genome 2016, 9, 1–13. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Lu, C.; Ye, J.; Zou, M.; Lu, K.; Ma, P.A. Genome-wide association studies of 11 agronomic traits in cassava (Manihot esculenta Crantz). Front. Plant Sci. 2018, 9, 503. [Google Scholar] [CrossRef]
- Uitdewilligen, J.G.; Wolters, A.M.A.; Bjorn, B.; Borm, T.J.; Visser, R.G.; van Eck, H.J. A next-generation sequencing method for genotyping-by-sequencing of highly heterozygous autotetraploid potato. PLoS ONE 2013, 8, e62355. [Google Scholar] [CrossRef]
- Björn, B.; Keizer, P.L.; Paulo, M.J.; Visser, R.G.; Van Eeuwijk, F.A.; Van Eck, H.J. Identification of agronomically important QTL in tetraploid potato cultivars using a marker–trait association analysis. Theor. Appl. Genet. 2014, 127, 731–748. [Google Scholar]
- Yu, D.; Lane, S.N. Urban fluvial flood modelling using a two-dimensional diffusion-wave treatment, part 2: Development of a sub-grid-scale treatment. Hydrol. Proccess. 2006, 20, 1567–1583. [Google Scholar] [CrossRef]
- Kayondo, S.I.; Del Carpio, D.P.; Lozano, R.; Ozimati, A.; Wolfe, M.; Baguma, Y.; Jannink, J.L. Genome-wide association mapping and genomic prediction for CBSD resistance in Manihot esculenta. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Jing, Y.; Zhao, X.; Wang, J.; Teng, W.; Qiu, L.; Han, Y.; Li, W. Identification of the genomic region underlying seed weight per plant in Soybean (Glycine max L. Merr.) via high-throughput single-nucleotide polymorphisms and a genome-wide association study. Front. Plant Sci. 2018, 9, 1392. [Google Scholar] [CrossRef]
- Knoll, J.; Ejeta, G. Marker-assisted selection for early-season cold tolerance in sorghum: QTL validation across populations and environments. Theor. Appl. Genet. 2008, 116, 541–553. [Google Scholar] [CrossRef]
- Li, L.; Tacke, E.; Hofferbert, H.R.; Lübeck, J.; Strahwald, J.; Draffehn, A.M.; Gebhardt, C. Validation of candidate gene markers for marker-assisted selection of potato cultivars with improved tuber quality. Theor. Appl. Genet. 2013, 126, 1039–1052. [Google Scholar] [CrossRef]
- Pryce, J.E.; Bolormaa, S.; Chamberlain, A.J.; Bowman, P.J.; Savin, K.; Goddard, M.E.; Hayes, B.J. A validated genome-wide association study in 2 dairy cattle breeds for milk production and fertility traits using variable length haplotypes. J. Dairy Sci. 2010, 93, 3331–3345. [Google Scholar] [CrossRef]
- Geigenberger, P. Regulation of sucrose to starch conversion in growing potato tubers. J. Exp. 2003, 54, 457–465. [Google Scholar] [CrossRef]
- Tiessen, A.; Prescha, K.; Branscheid, A.; Palacios, N.; McKibbin, R.; Halford, N.G.; Geigenberger, P. Evidence that SNF1-related kinase and hexokinase are involved in separate sugar-signalling pathways modulating post-translational redox activation of ADP-glucose pyrophosphorylase in potato tubers. Plant J. 2003, 35, 490–500. [Google Scholar] [CrossRef]
- McKibbin, R.S.; Muttucumaru, N.; Paul, M.J.; Powers, S.J.; Burrell, M.M.; Coates, S.; Halford, N.G. Production of high-starch, low-glucose potatoes through over-expression of the metabolic regulator SnRK1. Plant Biotechnol. J. 2006, 4, 409–418. [Google Scholar] [CrossRef]
- Purcell, P.C.; Smith, A.M.; Halford, N.G. Antisense expression of a sucrose non-fermenting-1-related protein kinase sequence in potato results in decreased expression of sucrose synthase in tubers and loss of sucrose-inducibility of sucrose synthase transcripts in leaves. Plant J. 1998, 14, 195–202. [Google Scholar] [CrossRef]
- Laurie, S.; McKibbin, R.S.; Halford, N.G. Antisense SNF1-related (SnRK1) protein kinase gene represses transient activity of an α-amylase (α-Amy2) gene promoter in cultured wheat embryos. J. Exp. 2003, 54, 739–747. [Google Scholar] [CrossRef]
- Lane, B.G. Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 1994, 8, 294–301. [Google Scholar] [CrossRef]
- Woo, E.J.; Dunwell, J.M.; Goodenough, P.W.; Marvier, A.C.; Pickersgill, R.W. Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities. Nat. Struct. Biol. 2000, 7, 1036–1040. [Google Scholar]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723. [Google Scholar] [CrossRef]
- Cantu, D.C.; Chen, Y.; Reilly, P.J. Thioesterases: A new perspective based on their primary and tertiary structures. Protein Sci. 2010, 19, 1281–1295. [Google Scholar] [CrossRef]
- Carrier, D.J.; Van Roermund, C.W.; Schaedler, T.A.; Rong, H.L.; IJlst, L.; Wanders, R.J.; Baker, A. Mutagenesis separates ATPase and thioesterase activities of the peroxisomal ABC transporter, Comatose. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- REQUENA, L.; BORNEMANN, S. Barley (Hordeum vulgare) oxalate oxidase is a manganese-containing enzyme. Biochem. J. 1999, 343, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Asfaw, A.; Ambachew, D.; Shah, T.; Blair, M.W. Trait associations in diversity panels of the two common bean (Phaseolus vulgaris L.) gene pools grown under well-watered and water-Stress conditions. Front. Plant Sci. 2017, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Akissoé, N.; Hounhouigan, J.; Mestres, C.; Nago, M. How blanching and drying affect the colour and functional characteristics of yam (Dioscorea cayenensis-rotundata) flour. Food Chem. 2003, 82, 257–264. [Google Scholar] [CrossRef]
- Mestres, C.; Dorthe, S.; Akissoé, N.; Hounhouigan, J.D. Prediction of sensorial properties (color and taste) of amala, a paste from yam chips flour of West Africa, through flour biochemical properties. Plant Food. Hum. Nutr. 2004, 59, 93–99. [Google Scholar] [CrossRef]
- Cavalcante, P.T.; Dondi, M.; Ercolani, G.; Guarini, G.; Melandri, C.; Raimondo, M.; Almendra, E.R. The influence of microstructure on the performance of white porcelain stoneware. Ceram. Int. 2004, 30, 953–963. [Google Scholar] [CrossRef]
- Hoffmann, G. Cielab Color Space. Wik., Encycl. Mht. 2003. Available online: http://docs-hoffmann.de/cielab03022003.pdf (accessed on 21 June 2020).
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team. 2018. nlme: Linear and nonlinear mixed effects models. R Package Version 2018, 3, 1–137. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Sham, P.C. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Yin, L. CMplot: Circle Manhattan Plot. 2018. Available online: https://github.com/YinLiLin/R-CMplot (accessed on 21 June 2020).
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; Buckler, E.S. A Unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef]
- Rosyara, U.R.; De Jong, W.S.; Douches, D.S.; Endelman, J.B. Software for genome-wide association studies in autopolyploids and its application to potato. Plant Genome 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Shin, J.H.; Blay, S.; McNeney, B.; Graham, J. LDheatmap: An R function for graphical display of pairwise linkage disequilibria between single nucleotide polymorphisms. J. Stat. Softw. 2006, 16, 1–10. [Google Scholar] [CrossRef]
- Hunter, S.; Jones, P.; Mitchell, A.; Apweiler, R.; Attwood, T.K.; Bateman, A.; Yong, S.Y. InterPro new developments in the family and domain prediction database. Nucleic Acids Res. 2011, 40, 306–312. [Google Scholar] [CrossRef] [PubMed]


| Source of Variance | Dry Matter Content | Oxidative Browning | ||
|---|---|---|---|---|
| Variance | Standard Deviation | Variance | Standard Deviation | |
| Genotype | 6.315 | 2.512901 | 47.8805 | 6.9196 |
| Location | 3.92 | 1.980232 | 7.8743 | 2.8061 |
| Replication | 7.294 × 10−2 | 0.270077 | 7.8743 | 2.8061 |
| Genotype × location | 1.806 | 1.343863 | 20.9957 | 4.5821 |
| Residual | 6.630 | 2.574859 | 75.4086 | 8.6838 |
| Chromosome | All SNPs | Filtered SNPs | Chr Size (Mb) | PIC of Filtered SNPs |
|---|---|---|---|---|
| 1 | 698 | 299 | 18.5 | 0.218 |
| 2 | 898 | 335 | 14.5 | 0.224 |
| 3 | 635 | 432 | 19.6 | 0.219 |
| 4 | 1225 | 893 | 33.4 | 0.241 |
| 5 | 1143 | 923 | 26.3 | 0.223 |
| 6 | 442 | 360 | 16.2 | 0.276 |
| 7 | 735 | 583 | 16.5 | 0.265 |
| 8 | 943 | 640 | 28.4 | 0.217 |
| 9 | 996 | 346 | 14 | 0.221 |
| 10 | 1096 | 326 | 23.6 | 0.277 |
| 11 | 1186 | 392 | 26.7 | 0.262 |
| 12 | 765 | 329 | 14 | 0.225 |
| 13 | 839 | 304 | 15 | 0.226 |
| 14 | 1080 | 528 | 11.2 | 0.237 |
| 15 | 583 | 424 | 20.1 | 0.255 |
| 16 | 944 | 386 | 19.3 | 0.243 |
| 17 | 1136 | 436 | 14.2 | 0.230 |
| 18 | 1113 | 477 | 12.2 | 0.208 |
| 19 | 1046 | 843 | 23.7 | 0.213 |
| 20 | 564 | 431 | 23.1 | 0.229 |
| Total | 18,067 | 9687 | 390.5 |
| Traits | SNP Markers | Chr | Alleles | Model | Physical Position (bp) | LOD | Effect | R2% |
|---|---|---|---|---|---|---|---|---|
| DMC | Chr19_8692 | 19 | CT | additive | 8692 | 4.01 | 1.79 | 30.37 |
| Chr6_59775 | 6 | GA | 1-dom-ref | 59,775 | 3.88 | −4.01 | 8.06 | |
| Chr6_615325 | 6 | GA | 1-dom-ref | 615,325 | 3.90 | −3.33 | 7.44 | |
| OxB | Chr5_118279 | 5 | GA | Additive, general, 1-dom-alt | 118,279 | 4.30 | −4.06 | 4.91 |
| Chr5_125093 | 5 | GA | Additive, general 1-dom-alt | 125,093 | 4.19 | −3.48 | 7.83 |
| Traits | Marker Interaction | MS | p-Value | Adjusted R |
|---|---|---|---|---|
| DMC | Chr6_59775: Location | 124.68 | 8.822 × 10−14 *** | 0.2139 |
| Chr6_615325: Location | 122.112 | 2.081 × 10−13 *** | 0.209 | |
| Chr19_8692: Location | 191.324 | 2.2 × 10−16 *** | 0.343 | |
| OxB | Chr5_118279: Location | 67.829 | 0.01475 * | 0.03051 |
| Chr5_125093: Location | 90.377 | 0.001929 ** | 0.0463 |
| Marker Location | p-Value | |
|---|---|---|
| DMC | Chr6_59775: Ikenne | 0.00532 ** |
| Chr6_59775: Ibadan | 0.00762 ** | |
| Chr6_59775: Ubiaja | 0.21641 | |
| Chr6_615325: Ikenne | 0.003599 ** | |
| Chr6_615325: Ibadan | 0.000982 *** | |
| Chr6_615325: Ubiaja | 0.467 | |
| Chr19_8692: Ikenne | 0.000207 *** | |
| Chr19_8692: Ibadan | 2.83 × 10−5 *** | |
| Chr19_8692: Ubiaja | 0.000617 *** | |
| OxB | Chr5_118279: Ikenne | 0.00699 ** |
| Chr5_118279: Ibadan | 0.02513 * | |
| Chr5_118279: Ubiaja | 0.19550 | |
| Chr5_125093: Ikenne | 0.002499 ** | |
| Chr5_125093: Ibadan | 0.012047 * | |
| Chr5_125093: Ubiaja | 0.146802 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatarira, C.; Agre, P.; Matsumoto, R.; Edemodu, A.; Adetimirin, V.; Bhattacharjee, R.; Asiedu, R.; Asfaw, A. Genome-Wide Association Analysis for Tuber Dry Matter and Oxidative Browning in Water Yam (Dioscorea alata L.). Plants 2020, 9, 969. https://doi.org/10.3390/plants9080969
Gatarira C, Agre P, Matsumoto R, Edemodu A, Adetimirin V, Bhattacharjee R, Asiedu R, Asfaw A. Genome-Wide Association Analysis for Tuber Dry Matter and Oxidative Browning in Water Yam (Dioscorea alata L.). Plants. 2020; 9(8):969. https://doi.org/10.3390/plants9080969
Chicago/Turabian StyleGatarira, Cobes, Paterne Agre, Ryo Matsumoto, Alex Edemodu, Victor Adetimirin, Ranjana Bhattacharjee, Robert Asiedu, and Asrat Asfaw. 2020. "Genome-Wide Association Analysis for Tuber Dry Matter and Oxidative Browning in Water Yam (Dioscorea alata L.)" Plants 9, no. 8: 969. https://doi.org/10.3390/plants9080969
APA StyleGatarira, C., Agre, P., Matsumoto, R., Edemodu, A., Adetimirin, V., Bhattacharjee, R., Asiedu, R., & Asfaw, A. (2020). Genome-Wide Association Analysis for Tuber Dry Matter and Oxidative Browning in Water Yam (Dioscorea alata L.). Plants, 9(8), 969. https://doi.org/10.3390/plants9080969







