Abstract
Low temperatures severely restrict plant growth and agricultural productivity, and exploring cold tolerance mechanisms is critical. This study investigated the combined effects of grape-derived transcription factor gene VyMYB24 and the synthetic cytokinin CPPU on cold tolerance in transgenic tobacco. Under low-temperature stress, tobacco plants overexpressing VyMYB24 and treated with CPPU exhibited significantly alleviated wilting, higher chlorophyll contents, and an improved net photosynthetic rate compared to controls. These plants also showed a lower relative conductivity and malondialdehyde (MDA) content, higher proline accumulation, and elevated activities of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), accompanied by reduced reactive oxygen species (ROS), hydrogen peroxide (H2O2), and superoxide anions (O2). The results confirm that VyMYB24 and CPPU synergistically improve cold tolerance via membrane stabilization, enhanced antioxidant defense, and maintained photosynthetic capacity, providing a theoretical foundation for the rational application of CPPU in the cultivation and management of grapes under low-temperature conditions.
1. Introduction
Grape (Vitis spp.) is a globally important fruit crop with significant economic value. Currently, the most widely cultivated cultivars with desirable fruit quality are predominantly European grapes (Vitis vinifera L.), yet their limited cold tolerance poses a major constraint to the sustainable development of the grape industry. As a primary center of grape origin, China harbors abundant wild grape germplasm resources rich in stress-resistant genes [1]. Among them, Vitis yeshanensis, commonly known as ‘Yanshan’, exhibits remarkable cold hardiness: its branches can tolerate temperatures as low as −35 °C, and its roots survive between −14 °C and −16 °C, ranking second only to Vitis amurensis Rupr. in cold resistance [2]. In addition to its strong cold tolerance, the ‘Yanshan’ grape produces significantly larger fruits than V. amurensis, rendering it highly suitable for cross-breeding and genetic studies aimed at enhancing cold resistance in grape hybrids [3].
Cold stress, typically induced by low temperatures above 0 °C, disrupts normal physiological metabolism, cellular homeostasis, and multiple physiological processes, ultimately leading to aberrant plant growth [4]. Short-term exposure results in wilting and leaf lesions, while prolonged cold stress induces metabolic dysfunction, suppresses growth and development, damages reproductive organs, and reduces seed set, thereby causing substantial yield losses in agricultural production [5]. Plants counteract cold stress primarily through the genetic regulation of metabolic pathways, with transcription factors playing a central role in cold signal transduction [6].
The MYB gene family represents one of the largest families of transcription factors in plants, and it plays critical roles in growth and development, cell morphogenesis, organ differentiation, secondary metabolism, and stress responses [7,8,9]. Agarwal et al. [10] demonstrated that AtMYB15 interacts with ICE1—a bHLH-type transcription factor and core regulator of cold acclimation—to bind specifically to the MYB cis-element in the promoter of CBF genes, thereby participating in the cold stress response. Jin et al. [11] recently reported a similar function in tomatoes: the overexpression of SpMYB1 (an R2R3-MYB from Solanum pennellii) enhanced cold tolerance by maintaining chlorophyll content, activating antioxidant enzyme genes (SlCAT, SlSOD), and upregulating stress-responsive genes (SlDREB2), which is consistent with the conserved role of R2R3-MYB transcription factors in plant cold stress responses [11]. Similarly, Laura et al. [12] reported that OsMYB4 enhances cold tolerance in rice by directly or indirectly regulating downstream target genes. Wang et al. [13] found that the apple MdMYB108L gene is induced by both light and low temperature, and its overexpression enhances cold tolerance in apple callus, underscoring the key role of R2R3-MYB transcription factors in plant responses to low-temperature stress.
Research on cytokinins (CTKs) spans more than a century. Since their isolation and identification by Skoog and Miller in 1955, cytokinins have been recognized as key signaling molecules widely applied in viticulture to promote root, stem, and leaf growth, fruit development, and fruit set [14]. Beyond their roles in growth and development, cytokinins are also involved in abiotic stress responses [15,16,17,18]. For instance, exogenous cytokinin application improved cold tolerance in tobacco cell cultures [19]. In rice, treatment with N6-benzyladenine (6-BA)—a synthetic cytokinin commonly used in stress studies to modulate growth and enhance abiotic stress tolerance via antioxidant activation and chloroplast stabilization—increased the activities of antioxidant enzymes (SOD, POD, CAT), reduced MDA content and lipid peroxidation, and protected chloroplast integrity under low-temperature stress, thereby promoting seedling growth [20]. In maize, 6-BA significantly boosted the activities of SOD, POD, and CAT and lowered MDA content, thereby improving drought resistance in seedlings [21]. Yan et al. [22] revealed that high salinity triggers plant adaptation by promoting the degradation of key cytokinin signaling components, offering new insights into hormone–gene crosstalk in stress resistance. Shen [23] compared a transgenic rice line overexpressing OsU496A (OX-43) with wild-type Oryza sativa ‘Nipponbare’ (WT) and found that exogenous 6-BA altered grain filling physiology and the expression of key enzymes, indicating that OsU496A mediates cytokinin-regulated grain filling. Bian et al. [24] cloned the cytokinin response regulator gene VlRR5 from the ‘Kyoho’ grape and showed that its expression was upregulated by exogenous CPPU [N-(2-chloro-4-pyridyl)-N′-phenylurea] but suppressed by the cytokinin biosynthesis inhibitor lovastatin, confirming that VlRR5 is responsive to cytokinin and regulates fruit set in grapes.
Recent studies have shown that cytokinins sustain active cell division in the Arabidopsis shoot apical meristem by fine-tuning the nucleocytoplasmic shuttling of the cell cycle transcription factor MYB3R4, highlighting the essential role of MYB proteins (e.g., MYB3R1, MYB3R4) in maintaining stem cell activity [25]. However, the synergistic interplay between grape MYB transcription factors and cytokinins in regulating growth, development, and stress adaptation remains poorly understood.
In this study, we applied exogenous CPPU—a cytokinin-like plant growth regulator known to promote root, stem, and leaf growth [26]—to both wild-type (WT) and VyMYB24-overexpressing transgenic tobacco plants. The VyMYB24 gene (VIT_14s0066g01090) was previously identified from the transcriptome of the ‘Yanshan’ grape as a drought-induced R2R3-MYB gene [27]. We investigated the roles of VyMYB24 and CPPU under low-temperature stress and analyzed their synergistic effects on cold tolerance. This work lays a theoretical foundation for understanding VyMYB24–cytokinin interactions in ‘Yanshan’ grape cold tolerance and for rational CPPU application in grape cultivation under low temperatures. Although this study primarily relies on physiological and biochemical data from a transgenic tobacco model system, this approach provides robust support for the synergistic mechanism: (1) the comprehensive dataset (covering photosynthesis, osmotic adjustment, and antioxidant defense) offers strong correlative evidence for the VyMYB24–CPPU interaction; (2) using tobacco as a model is rational for the initial mechanistic exploration, as it allows for efficient genetic manipulation and controlled stress imposition, providing a reliable platform to dissect gene–hormone interactions before validation in the more complex grape system.
2. Results
2.1. Synergistic Regulation of Transgenic Tobacco Phenotypes by VyMYB24 and Cytokinin
Cytokinins are known to regulate various aspects of plant growth and development. To evaluate their combined effect with VyMYB24 under cold stress, we compared the phenotypes of wild-type (WT) and VyMYB24-overexpressing (OE) tobacco plants, with or without CPPU treatment. Under normal conditions, both WT and OE plants displayed a similar growth and morphology (Figure 1A,D). After 28 h of low-temperature exposure, severe wilting was observed in WT plants sprayed with water (TWater/WT) (Figure 1B,C). In contrast, VyMYB24-overexpressing plants without CPPU (TWater/OE) exhibited only mild wilting. Notably, OE plants treated with CPPU (TCPPU/OE) showed the least phenotypic damage, with leaves maintaining nearly full turgidity (Figure 1E,F).

Figure 1.
Synergistic enhancement of cold tolerance by VyMYB24 and cytokinin in transgenic tobacco. (A) Top view of water-sprayed controls under normal conditions; (B) top and (C) front views of water-sprayed plants after 28 h at 0 °C; (D) top view of CPPU-sprayed plants under normal conditions, and (E) top and (F) front views of CPPU-sprayed plants after 28 h at 0 °C. Note: WT, wild-type; OE1/OE2/OE3, independent VyMYB24-overexpressing lines.
These results indicate that the combined application of VyMYB24 overexpression and exogenous CPPU synergistically enhances cold tolerance by maintaining cellular turgor, reducing dehydration, and minimizing growth inhibition—supporting the role of VyMYB24 and cytokinin in promoting cell division and plant growth under stress.
2.2. Synergistic Regulation of Photosynthetic Parameters in Transgenic Tobacco by VyMYB24 and Cytokinin
Low-temperature stress triggered a progressive decline in photosynthetic performance across all tobacco lines; however, this suppression was markedly attenuated in VyMYB24-overexpressing plants, especially under CPPU treatment. As shown in Table 1, the total chlorophyll content decreased gradually under prolonged cold exposure, yet the TCPPU/OE3 line consistently retained the highest chlorophyll (a + b) levels, reaching 1.601 mg g−1 FW by day 28—significantly greater than the 1.121 mg g−1 FW in cold-stressed wild-type plants (TWater/WT). Notably, even without CPPU, all VyMYB24-OE lines maintained a superior chlorophyll retention relative to WT, indicating an intrinsic protective role of VyMYB24. Exogenous CPPU further enhanced chlorophyll preservation in OE backgrounds, with TCPPU/OE2 and TCPPU/OE3 outperforming their water-sprayed OE counterparts across most time points (p < 0.05). By contrast, CPPU application in WT plants (TCPPU/WT) conferred only limited protection, underscoring that the full chlorophyll-stabilizing effect under cold stress depends on the synergistic action of VyMYB24 and cytokinin signaling.

Table 1.
Changes in total chlorophyll content (mg g−1 FW) of transgenic tobacco under low-temperature treatment.
A similar synergistic pattern was observed for the net photosynthetic rate (Pn, Table 2). The TCPPU/OE3 line exhibited the highest Pn values throughout the stress period, culminating in 8.29 mmol m−2 s−1 at day 28, significantly exceeding that of TWater/WT (6.23 mmol m−2 s−1). Both VyMYB24 overexpression and CPPU contributed to Pn maintenance, with their combination yielding the most robust photosynthetic performance under cold conditions.

Table 2.
Changes in net photosynthetic rate (Pn, mmol m−2 s−1) of transgenic tobacco under low-temperature treatment.
Conversely, stomatal conductance (Gs, Table 3) and transpiration rate (Tr, Table 4) were significantly lower in TCPPU/OE plants than in other groups during cold stress. This coordinated reduction in Gs and Tr likely contributed to improved water conservation, mitigating cold-induced dehydration without substantially compromising CO2 uptake—a physiological adjustment further reflecting the synergy between VyMYB24 and cytokinin in optimizing the water–carbon balance under stress.

Table 3.
Changes in stomatal conductance (Gs, mmol m−2 s−1) of transgenic tobacco under low-temperature treatment.

Table 4.
Changes in transpiration rate (Tr, g m−2 h−1) of transgenic tobacco under low-temperature treatment.
Collectively, these results demonstrate that VyMYB24 and CPPU act synergistically to maintain photosynthetic capacity under low-temperature stress, through mechanisms involving chlorophyll protection, enhanced carbon fixation, and optimized stomatal behavior.
2.3. Synergistic Regulation of Osmotic Regulators in Transgenic Tobacco by VyMYB24 and Cytokinin
Low-temperature stress significantly disrupted the cellular membrane integrity and osmotic balance; however, these adverse effects were effectively mitigated in the TCPPU/OE plants through the synergistic regulation of key osmotic substances.
Cold stress induced a marked increase in the relative electrolyte leakage across all plant groups (Figure 2A). However, the TCPPU/OE line exhibited the least membrane damage, with a significant reduction of approximately 30% in electrolyte leakage compared to the cold-stressed wild-type control TWater/WT by day 28 (p < 0.05).
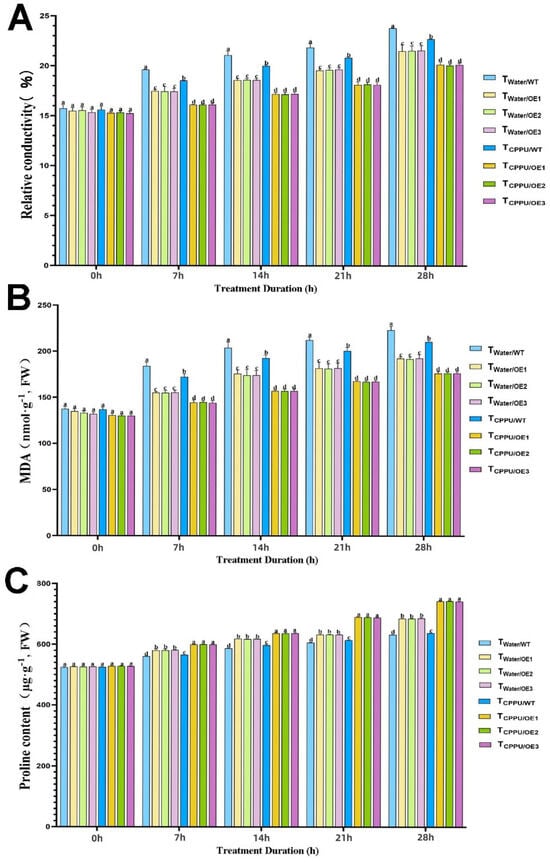
Figure 2.
Synergistic regulation of osmotic regulators in transgenic tobacco by VyMYB24 and cytokinin under low temperatures. (A) Relative conductivity; (B) MDA content; (C) proline content. Data—mean ± SD. Different lowercase letters indicate significant differences (p < 0.05). TWater/WT: cold-stressed WT, water-sprayed; TWater/OE: cold-stressed OE lines, water-sprayed; TCPPU/WT: cold-stressed WT, CPPU-sprayed; TCPPU/OE: cold-stressed OE lines, CPPU-sprayed.
Similarly, the malondialdehyde (MDA) content, an indicator of membrane lipid peroxidation, remained lowest in the TCPPU/OE plants (Figure 2B). At the end of the stress period, the MDA level in this group was about 25% lower than that in TWater/WT plants and was significantly reduced compared to all other treatments, indicating that the combination of VyMYB24 and CPPU significantly alleviated oxidative membrane damage.
In contrast, proline, an important osmoprotectant, accumulated most substantially in TCPPU/OE plants (Figure 2C). The proline content in this group was approximately 40% higher than in TWater/WT plants during the late stages of stress, and it remained significantly elevated at all time points compared to individual VyMYB24 overexpression or CPPU application alone. This suggests that the synergistic treatment strongly enhanced the osmotic adjustment capacity.
These results demonstrate that the synergistic action of VyMYB24 and CPPU effectively minimizes cold-induced membrane damage and optimizes osmotic balance through proline accumulation, which is critical for maintaining cellular homeostasis and improving cold tolerance.
2.4. Synergistic Regulation of Antioxidant Enzyme Activities in Transgenic Tobacco by VyMYB24 and Cytokinin
Under low-temperature stress, the activities of key antioxidant enzymes—superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT)—were significantly induced in all transgenic tobacco lines. However, the TCPPU/OE plants exhibited the most pronounced enhancement in this enzymatic antioxidant system throughout the treatment period (Figure 3A–C).
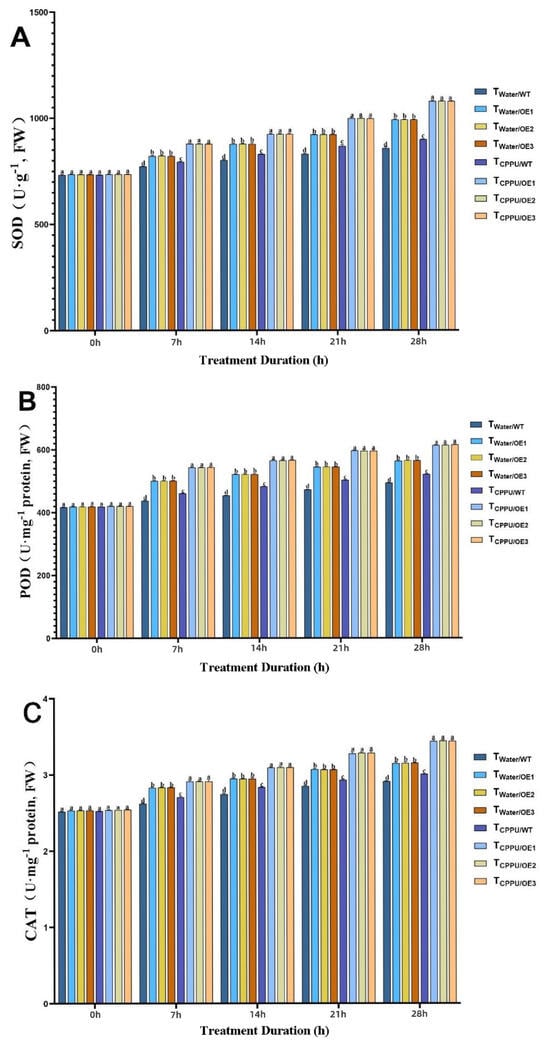
Figure 3.
Synergistic regulation of antioxidant enzyme activities in transgenic tobacco by VyMYB24 and cytokinin under low temperatures. (A) SOD; (B) POD; (C) CAT. Data—mean ± SD. Different lowercase letters indicate significant differences (p < 0.05). TWater/WT: cold-stressed WT, water-sprayed; TWater/OE: cold-stressed OE lines, water-sprayed; TCPPU/WT: cold-stressed WT, CPPU-sprayed; TCPPU/OE: cold-stressed OE lines, CPPU-sprayed.
SOD activity increased under cold conditions in all groups, but was most markedly elevated in TCPPU/OE plants, reaching a level approximately 35% higher than that in the cold-stressed wild-type control (TWater/WT) by the end of the experiment (Figure 3A). A similar trend was observed for POD activity (Figure 3B), which was about 28% greater in TCPPU/OE plants compared to TWater/WT, and also significantly surpassed the levels in plants with either VyMYB24 overexpression or CPPU treatment alone. CAT activity followed this pattern, being roughly 32% higher in the synergistic treatment group than in TWater/WT plants (Figure 3C).
The consistently highest activities of SOD, POD, and CAT in TCPPU/OE plants indicate a robustly potentiated antioxidant capacity, enabling the more efficient scavenging of reactive oxygen species (ROS) and reducing oxidative damage to cellular components under cold stress.
These findings confirm that VyMYB24 and CPPU act synergistically to activate a coordinated antioxidant enzyme response, which constitutes a critical mechanism in enhancing cold tolerance in transgenic tobacco.
2.5. Synergistic Regulation of Oxidative Substances in Transgenic Tobacco by VyMYB24 and Cytokinin
Under cold stress, a significant accumulation of reactive oxygen species (ROS), hydrogen peroxide (H2O2), and superoxide anions (O2) was observed in all tobacco plants; however, the TCPPU/OE line displayed the most effective suppression of these oxidative markers (Figure 4A–C).
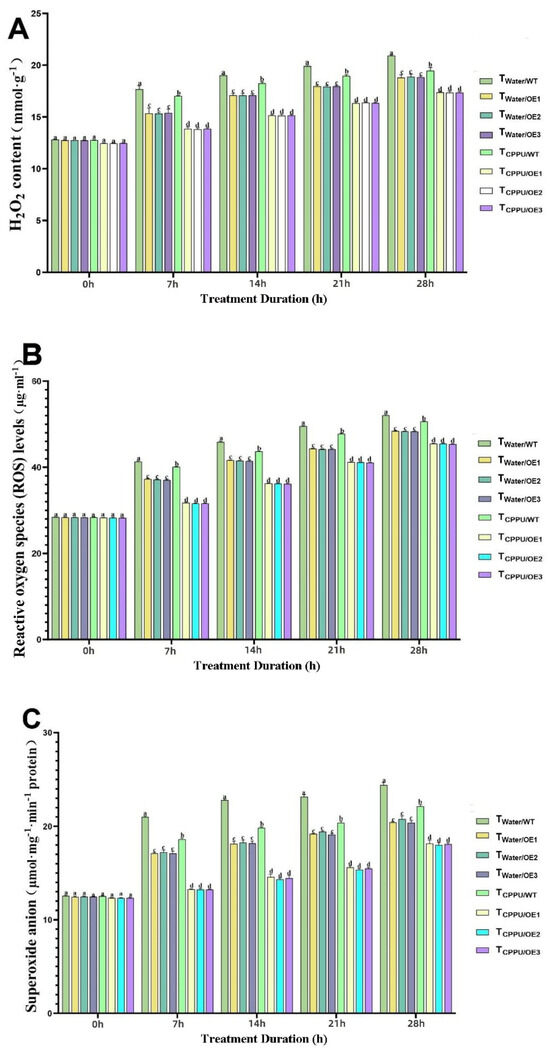
Figure 4.
Synergistic regulation of oxidative substances in transgenic tobacco by VyMYB24 and cytokinin under low temperatures. (A) H2O2 content; (B) reactive oxygen species (ROS) levels; (C) superoxide anion. Data—mean ± SD (n = 3). Different lowercase letters indicate significant differences (p < 0.05). TWater/WT: cold-stressed WT, water-sprayed; TWater/OE: cold-stressed OE lines, water-sprayed; TCPPU/WT: cold-stressed WT, CPPU-sprayed; TCPPU/OE: cold-stressed OE lines, CPPU-sprayed.
As shown in Figure 4A, the H2O2 content remained low under normal conditions but increased progressively during cold exposure. The TCPPU/OE plants consistently maintained the lowest H2O2 levels, showing a reduction of approximately 40% compared to the cold-stressed wild-type control (TWater/WT) at the end of the treatment period. A similar trend was observed for overall ROS levels (Figure 4B), which were about 35% lower in TCPPU/OE plants than in TWater/WT, and significantly lower than in all other treatment groups. Furthermore, the rate of O2 production (Figure 4C) was most effectively restrained in TCPPU/OE plants, being roughly 45% lower than that in TWater/WT plants under sustained low-temperature stress.
These results clearly demonstrate that the synergistic action of VyMYB24 and CPPU significantly alleviates cold-induced oxidative stress by suppressing the accumulation of H2O2, ROS, and O2, thereby contributing to the maintenance of redox homeostasis and cellular integrity under low-temperature conditions.
3. Discussion
Plants respond to low-temperature stress through integrated physiological adjustments involving the regulation of photosynthesis, maintenance of osmotic balance, and activation of antioxidant metabolism [28,29]. Notably, similar multi-pathway regulatory patterns mediated by exogenous substances have been reported in grapes: exogenous dopamine (0.4 mmol L−1) enhances cold tolerance in the Shine Muscat grape by protecting photosynthetic systems, optimizing ion homeostasis, activating antioxidant enzymes, and upregulating CBF-family cold-responsive genes, further confirming that exogenous regulators can coordinate multiple physiological processes to alleviate low-temperature damage [30]. This study systematically reveals, for the first time, the synergistic effect between the transcription factor gene VyMYB24 and the exogenous cytokinin CPPU in transgenic tobacco. Echoing the discovery by Agarwal et al. [10] regarding the involvement of AtMYB15 in the CBF regulatory pathway, our findings demonstrate that VyMYB24 interacts with cytokinin signaling to establish a multi-level regulatory network. This network synergistically enhances plant cold tolerance by stabilizing photosynthetic performance, improving osmotic adjustment, and strengthening the antioxidant defense system. This transcription factor–hormone synergy offers a novel perspective for understanding the regulatory mechanisms underlying plant responses to low-temperature stress.
Low-temperature stress usually reduces the chlorophyll content by accelerating pig-ment degradation and inhibiting synthesis [31,32]. In our study, TCPPU/OE plants main-tained the highest chlorophyll content and Pn under cold stress, which can be attributed to two synergistic effects: (1) VyMYB24-mediated chlorophyll protection: As an R2R3-MYB transcription factor, VyMYB24 may directly bind to the promoters of chlorophyll biosynthesis genes (e.g., CHLH, encoding Mg-chelatase) or repress chlorophyll degradation genes (e.g., SGR, a senescence-associated gene)—consistent with Wang et al. [20], who found MdMYB108L protects chlorophyll under cold stress; (2) CPPU-enhanced chloroplast stability: Cytokinins stabilize chloroplast morphology by upregulating Rubisco activity and protecting thylakoid membrane proteins from cold-induced denaturation [33]. CPPU likely enhances VyMYB24’s protective effect by preventing chloroplast swelling, maintaining a light-dependent reaction efficiency. Additionally, TCPPU/OE had lower Gs and Tr, optimizing water use efficiency: it avoids cellular dehydration caused by excessive transpiration while ensuring a sufficient CO2 supply for carbon fixation—this ‘water–carbon balance’ aligns with cross-stress tolerance mechanisms in tomatoes under nitrogen deficiency and drought [34].
The relative conductivity, MDA, and proline content reflect plants’ self-protection ability under stress [35,36]. Cold stress induces ROS accumulation (H2O2, O2), which damages biofilm structure and function [37,38]. Our results show that TCPPU/OE had the lowest relative conductivity/MDA and highest proline/SOD/POD/CAT activities, confirming a ‘dual protection mechanism’: (1) Membrane stabilization: The lower relative conductivity and MDA indicate reduced membrane leakage and lipid peroxidation—consistent with Gemrotová et al. [39], who found that cytokinins reduce membrane damage under stress. (2) ROS scavenging: The elevated antioxidant enzyme activities directly enhance ROS clearance, while proline acts as a ‘dual-function protectant’ [40]. This mechanism forms a multi-layered defense against cold-induced damage, and is supported by Zong et al. [41], who found that low-concentration 6-BA reduces ROS and MDA content in cold-stressed rice.
Compared to the adaptation mechanism involving cytokinin signaling degradation under salt stress revealed by Yan et al. [22], the VyMYB24-CPPU synergy identified in our study presents a distinctly different regulatory mode, suggesting that plants may employ diverse hormonal regulatory strategies to cope with different environmental stresses. Furthermore, referencing the mechanism of the cytokinin-regulated nucleocytoplasmic shuttling of MYB3R4 reported by Yang et al. [25], we speculate that VyMYB24 might participate in the low-temperature stress response through similar mechanisms of transcription factor localization control.
Our study focuses on physiological mechanisms of VyMYB24-CPPU synergy in tobacco. Although we used tobacco as a model, our results provide a theoretical basis for grape cold resistance research. CPPU is a practical tool for grape cultivation under low temperatures: exogenous CPPU application can alleviate cold damage in grapes—this is particularly valuable in regions prone to spring frosts (e.g., Xinxiang, Henan), where low temperatures cause severe grape yield losses. Future work will (1) verify the mechanism in the ‘Yanshan’ grape (e.g., analyze VlRR5 and antioxidant enzyme gene expression under VyMYB24 overexpression and CPPU treatment); (2) identify the direct target genes of VyMYB24 via ChIP-seq, and explore protein–protein interactions between VyMYB24 and cytokinin signaling components via co-IP to fully elucidate this regulatory module [10,24,27].
4. Materials and Methods
4.1. Plant Materials
Wild-type tobacco (Nicotiana benthamiana L., WT) and VyMYB24-overexpressing transgenic tobacco lines (OE1, OE2, OE3) were obtained from our previous study [27]. All plants were grown in a growth chamber under a 16 h light/8 h dark photoperiod, 25–28 °C, and 70–80% relative humidity (RH).
4.2. Low-Temperature Treatment and Exogenous Cytokinin Application
Overexpressed VyMYB24 transgenic tobacco (OE1, OE2, OE3) plants and wild-type tobacco (WT) plants, with uniform growth 4 weeks after germination, were selected and sprayed with exogenous cytokinin, which comprised 100 mol L−1 N-(2-chloro-4-pyridyl)-N′-phenylurea (CPPU) plus Silwet-77 surfactant (final concentration 0.03%, volume ratio). Spraying was performed once daily for 7 consecutive days, with a volume of 10 mL per plant until leaves were uniformly wet without dripping. Controls were sprayed with clean water using the same frequency and volume. And control overexpressed VyMYB24 transgenic tobacco (OE1, OE2, OE3) plants and WT were sprayed with clean water for 7 days. Low-temperature treatment was administered after 7 days of treatment.
Four treatments were designed. TCK represents the clear water control group, that is, no cytokinin was sprayed; TCPPU represents that cytokinin was sprayed. (1) TWater/WT—WT without spraying exogenous cytokinin at low temperatures; (2) TWater/OE (TWater/OE1, TWater/OE2, TWater/OE3)—overexpressed VyMYB24 transgenic tobacco plants (OE1, OE2, OE3) without exogenous cytokinin sprayed at low temperatures; (3) TCPPU/WT—WT sprayed with exogenous cytokinin at low temperatures; (4) TCPPU/OE (TCPPU/OE1, TCPPU/OE2, TCPPU/OE3)—overexpressed VyMYB24 transgenic tobacco plants (OE1, OE2, OE3) with exogenous cytokinin sprayed at low temperatures. Tobacco leaves were collected at 0 h, 7 h, 14 h, 21 h, and 28 h after 0 °C treatment. Low-temperature treatment was conducted with a BPHJS-120B High and Low Temperature Alternating Humidity Test Kit (Shanghai Yiheng, Shanghai, China) at 0 °C, with a 16/8 h light/dark cycle, light intensity of 2000 lx, and relative humidity of 70–80%. Temperature was monitored every 30 min using built-in sensors to ensure stability (fluctuation ≤ ±0.5 °C). We randomly selected three leaves from the upper-middle part of the plant that were healthy, spot-free, pest-free, and fully mature.
The 0 h sampling time point (before initiating 0 °C treatment) for each group served as its own non-low-temperature control, providing baseline physiological data to quantify the impact of low-temperature stress and the regulatory effects of VyMYB24 and CPPU. All plants were grown under identical normal conditions (25–28 °C, 16/8 h photoperiod, 70–80% RH) before 0 h sampling, ensuring consistency in initial growth status.
4.3. Phenotypic Analysis
Phenotypes of wild-type (WT) and VyMYB24-overexpressing transgenic lines (OE1, OE2, OE3) were visually assessed and recorded photographically at 0, 7, 14, 21, and 28 h of low-temperature exposure. The evaluation focused on symptoms of chilling injury, including wilting severity, turgor pressure loss, and the development of chlorosis or necrosis.
4.4. Measurement of Photosynthetic Parameters
An LI-6400 portable photosynthetic instrument (Beijing Ligotai Technology Co., Ltd. (Beijing, China)) was used to determine photosynthetic indexes, including the net photosynthetic rate (Pn, mmol m−2 s−1), stomatal conductance (Gs, mmol m−2 s−1), and transpiration rate (Tr, g m−2 h−1). The measuring time was 14:00 on a sunny day; all the parameters for each leaf were measured three times, and the average value was recorded.
4.5. Analysis of Physiological and Biochemical Indices
The relative conductivity (RC) was measured using a conductivity meter (DDS-308A, Shanghai Precision Scientific Instrument Co., Ltd. (Shanghai, China)). Relative conductivity = conductivity before boiling water bath R1/conductivity after boiling water bath R2 × 100%. The chlorophyll content, malondialdehyde (MDA) content, proline (Pro) content, superoxide dismutase (SOD) activity, peroxidase (POD) activity, catalase (CAT) activity, hydrogen peroxide (H2O2) content, and superoxide anion (O2) production rate (test kit number BC0990, BC0020, BC0290, BC5160, BC0090, BC0200, BC3590, and BC1290, respectively; Beijing Solarbio Technology Co., Ltd. (Beijing, China)) were all determined through spectrophotometry with an ultraviolet–visible spectrophotometer (TU-1810, Beijing Puxi General Instrument Co., Ltd. (Beijing, China)).
4.6. Statistical Analysis
All experimental data were derived from three independent biological replicates and are expressed as the mean ± standard deviation (SD). Statistical analyses were performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Significant differences among treatment groups were determined through one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with a significance level set at p < 0.05. Figures were generated using GraphPad Prism 10.1.2 (GraphPad Software, San Diego, CA, USA).
5. Conclusions
Under low-temperature stress, the application of exogenous CPPU reduced the stomatal conductance and transpiration rate in tobacco, thereby minimizing water loss and alleviating wilting symptoms. Concurrently, the combination of VyMYB24 overexpression and CPPU significantly enhanced the activities of key antioxidant enzymes (SOD, POD, CAT) and promoted proline accumulation, while markedly reducing relative electrolyte leakage, MDA content, and the levels of H2O2, ROS, and O2. These coordinated physiological adjustments collectively contributed to an improved cellular membrane stability, mitigated oxidative damage, and supported photosynthetic performance. Notably, the observed improvements were more pronounced in VyMYB24-overexpressing transgenic tobacco than in wild-type plants, supporting a synergistic interaction between VyMYB24 and cytokinin signaling in enhancing low-temperature tolerance. This study provides a theoretical foundation for cold resistance breeding in grapes and supports the rational application of CPPU in grape cultivation under low-temperature conditions.
Author Contributions
All authors contributed to the study conception and design. G.L. conceived and designed the experiments. K.L. carried out the experiments. K.L., Y.L. (Yihai Lu), J.L., Y.L. (Yongmu Li), L.W. and Z.Z. analyzed the data and prepared figures and tables. K.L. wrote the manuscript. G.L. and X.F. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Henan Provincial Key Research and Development Special Project (241111113200).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
6-BA: 6-benzylaminopurine; SOD: superoxide dismutase; POD: peroxidase; CAT: catalase; MDA: malondialdehyde; CPPU: N-(2-chloro-4-Pyridyl)-N′-phenylurea; WT: wild-type tobacco plants; TWater: the clear water control group; TCPPU: cytokinin was sprayed; TCK/WT: WT without spraying exogenous cytokinin at low temperatures; TWater/OE: overexpressed VyMYB24 transgenic tobacco plants; TCPPU/WT: WT sprayed with exogenous cytokinin at low temperatures; TCPPU/OE: overexpressed VyMYB24 transgenic tobacco plants (OE1, OE2, OE3) with exogenous cytokinin sprayed at low temperatures.
References
- He, P. Grape Science; Agriculture Press: Beijing, China, 1999. [Google Scholar]
- He, P.; Niu, L. Study on cold bhresistance of wild Vitis species in China. Acta Hortic. Sin. 1989, 6, 81–88. [Google Scholar]
- Wu, C. Study on Cold Resistance of Grape Germplasm Resources. Master’s Thesis, Northwest A&F University, Xianyang, China, 2011. [Google Scholar]
- Guy, C. Molecular responses of plants to cold shock and cold acclimation. J. Mol. Microbiol. Biotechnol. 1999, 1, 231–242. [Google Scholar] [PubMed]
- Huner, N.; Oquist, G.; Hurry, V.; Krol, M.; Falk, S.; Griffith, M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth. Res. 1993, 37, 19–39. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, F.; Xiao, L.; Gao, W.; Lu, L. Transcription factors and regulatory mechanisms of plant responses to abiotic stresses. Acta Bot. Boreali-Occident. Sin. 2006, 26, 1295–1300. [Google Scholar] [CrossRef]
- Li, M.; Qiao, Y.; Li, Y.; Shi, Z.; Zhang, N.; Bi, C.; Gao, J. A R2R3-MYB transcription factor gene in common wheat (namely TaMYBsm1) involved in enhancement of drought tolerance in transgenic Arabidopsis. J. Plant Res. 2016, 129, 1097–1107. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, F.; Sun, F.; Luo, Q.; Wang, R.; Hu, R.; He, G. A wheat MYB tran-scriptional repressor TaMyb1D regulates phenylpropanoid metabolism and enhances tolerance to drought and oxidative stresses in transgenic tobacco plants. Plant Sci. 2017, 265, 112–123. [Google Scholar] [CrossRef]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Guan, Q. MdMYB88 and MdMYB124 enhance drought tolerance by modulating root vessels and cell wall in apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.; Fujii, H.; Zheng, X.; Zhu, J. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Jin, R.; Muhammad, T.; Jia, C.; Yang, T.; Yang, H.; Wang, J.; Wang, B.; Yu, Q. Overexpression of R2R3-MYB Type Transcription Factor SpMYB1 Enhances Cold and Drought Tolerance in Tomato. Plant Physiol. Biochem. 2025, 229, 110326. [Google Scholar] [CrossRef]
- Laura, M.; Consonni, R.; Locatelli, F.; Fumagalli, E.; Allavena, A.; Coraggio, I.; Mattana, M. Metabolic response to cold and freezing of Osteospermum ecklonis overexpressing Osmyb4. Plant Physiol. Biochem. 2010, 48, 764–771. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, Z.; Jiang, H.; Zhang, Z.; Chen, X. A feedback loop involving MdMYB108L and MdHY5 controls apple cold tolerance. Biochem. Biophys. Res. Commun. 2019, 512, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; An, J.; You, C.; Wang, X.; Hao, Y. Molecular cloning and tolerance identification of apple cytokinin oxidase gene MdCKX7.2. Acta Hortic. Sin. 2019, 46, 409–420. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, M.; Tian, Y.; He, W.; Han, L.; Xia, G. Over-expression of TaMYB33 encoding a novel wheat MYB tran-scription factor increases salt and drought tolerance in Arabidopsis. Mol. Biol. Rep. 2012, 39, 7183–7192. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Guo, X.; Wang, Q.; Wang, Y.; Zhao, D.; Yao, L.; Li, T. Overexpression of MsDREB6.2 results in cytokinin-deficient developmental phenotypes and enhances drought tolerance in transgenic apple plants. Plant J. 2016, 89, 510–526. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.; Braga Rocha, G.; Polverari, L.; Pinheiro Brito, D.; Batista, D.; Notini, M.; Nogueira, F. Mir156-targeted SPL10 controls Arabidopsis root meristem activity and root-derived de novo shoot regeneration via cytokinin responses. J. Exp. Bot. 2020, 71, 934–950. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, H.; Wang, Q.; Zhang, G. Roles of cytokinins in root growth and abiotic stress response of Arabidopsis thaliana. Plant Growth Regul. 2021, 94, 151–160. [Google Scholar] [CrossRef]
- Chen, J.N. Research on effect of external cytokinin on strengthening cold resistance of cultured cell line in tobacco. Hereditas 1993, 15, 24–27. [Google Scholar] [CrossRef]
- Wang, S.; Liang, Y. Protective effect of 6-BA on cell membrane system of rice seedlings under low temperature. Chin. J. Paddy Sci. 1995, 9, 223–229. [Google Scholar] [CrossRef]
- Dong, Y.; Shi, J.; Li, G.; Han, J.; Shang, Z. Effect of applying 6-BA and ABA to improve drought resistance of maize seedlings. Acta Bot. Boreali-Occident. Sin. 1998, 18, 202–206. [Google Scholar]
- Yan, Z.; Wang, J.; Wang, F.; Xie, C.; Lv, B.; Yu, Z.; Ding, Z. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis. EMBO Rep. 2021, 22, e52457. [Google Scholar] [CrossRef]
- Shen, W. Role of OsU496A in the Regulation of Grain Filling by Auxin and Cytokinin in Rice. Master’s Thesis, Yangzhou University, Yangzhou, China, 2018. [Google Scholar]
- Bian, L.; Guo, D.; Yu, K.; Wei, T.; Pei, M.; Liu, H.; Yu, Y. Cloning and expression analysis of the cytokinin response regulator VlRR5 in ‘JuFeng’ grape. Acta Hortic. Sin. 2021, 48, 1437–1445. [Google Scholar] [CrossRef]
- Yang, W.; Cortijo, S.; Korsbo, N.; Roszak, P.; Schiessl, K.; Gurzadyan, A.; Meyerowitz, E. Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 2021, 371, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhao, J. Research progress of cytokinin and its application in crop production. J. Zhejiang Agric. Sci. 2017, 58, 1411–1414. [Google Scholar] [CrossRef]
- Zhu, Z.; Quan, R.; Chen, G.; Yu, G.; Li, X.; Han, Z.; Li, B. An R2R3-MYB transcription factor VyMYB24, isolated from wild grape Vitis yanshanesis J.X. Chen., regulates the plant development and confers the tolerance to drought. Front. Plant Sci. 2022, 13, 966641. [Google Scholar] [CrossRef]
- Aslam, M.; Fakher, B.; Ashraf, M.; Yan, C.; Wang, B.; Qin, Y. Plant low-temperature stress: Signaling and response. Agronomy 2022, 12, 702. [Google Scholar] [CrossRef]
- Meng, A.; Wen, D.; Zhang, C. Maize seed germination under low-temperature stress impacts seedling growth under normal temperature by modulating photosynthesis and antioxidant metabolism. Front. Plant Sci. 2022, 13, 843033. [Google Scholar] [CrossRef]
- Li, J.; Wu, Q.; Cheng, J.; Zhu, J.; Su, P.; Wu, J.; Fan, X.; Li, G. Mechanism of Exogenous Dopamine Regulating Shine Muscat Grape in Response to Low-Temperature Stress. Plants 2025, 14, 3225. [Google Scholar] [CrossRef]
- ElSayed, A.; Rafudeen, M.; Gomaa, A.; Hasanuzzaman, M. Exogenous melatonin enhances the ROS metabolism, antioxidant defense-related gene expression and photosynthetic capacity of Phaseolus vulgaris L. to confer salt stress tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Korkmaz, A.; Değer, Ö.; Szafrańska, K.; Köklü, Ş.; Karaca, A.; Yakupoğlu, G. Melatonin effects in enhancing chilling stress tolerance of pepper. Sci. Hortic. 2021, 289, 110434. [Google Scholar] [CrossRef]
- Chang, C.; Lu, J.; Zhang, H.; Ma, C.; Sun, G. Copy number variation of cytokinin oxidase gene Tackx4 associated with grain weight and chlorophyll content of flag leaf in common wheat. PLoS ONE 2015, 10, e0145970. [Google Scholar] [CrossRef]
- Safavi-Rizi, V.; Uellendahl, K.; Öhrlein, B.; SafaviRizi, H.; Stöhr, C. Cross-stress tolerance: Mild nitrogen (N) deficiency effects on drought stress response of tomato (Solanum lycopersicum L.). Plant Environ. Interact. 2021, 2, 217–228. [Google Scholar] [CrossRef]
- Zhang, T.; Mo, J.; Zhou, K.; Chang, Y.; Liu, Z. Overexpression of Brassica campestris BcICE1 gene increases abiotic stress tolerance in tobacco. Plant Physiol. Biochem. 2018, 132, 515–523. [Google Scholar] [CrossRef]
- Lian, X.; Zhao, X.; Zhao, Q.; Wang, G.; Li, Y.; Hao, Y. MdDREB2A in apple is involved in the regulation of multiple abiotic stress responses. Hortic. Plant J. 2021, 7, 197–208. [Google Scholar] [CrossRef]
- Yu, Y.; Bian, L.; Yu, K.; Yang, S.; Zhang, H.; Wang, L. Vitis vinifera bZIP14 functions as a transcriptional activator and enhances drought stress resistance via suppression of reactive oxygen species. J. Berry Res. 2020, 10, 547–558. [Google Scholar] [CrossRef]
- Zhai, J.; Qi, Q.; Wang, M.Q.; Yan, J.; Li, K.; Xu, H. Overexpression of tomato thioredoxin h (SlTrxh) enhances excess nitrate stress tolerance in transgenic tobacco interacting with SlPrx protein. Plant Sci. 2022, 315, 111137. [Google Scholar] [CrossRef]
- Gemrotová, M.; Kulkarni, M.; Stirk, W.; Strnad, M. Seedlings of medicinal plants treated with either a cytokinin antagonist (PI-55) or an inhibitor of cytokinin degradation (INCYDE) are protected against the negative effects of cadmium. Plant Growth Regul. 2013, 71, 137–145. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.; Zhao, W.; Popp, M.; Porat, R. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Zong, X.; Liu, D.; Wang, S.; Wu, W.; Hu, X. Effects of cytokinins on heat stable protein and energy metabolism of membrane protective enzymes in chilling damaged paddy seedlings. J. Southwest Agric. Univ. 1998, 20, 3–6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).