Biochemical Responses of Atacama and Blesbok Sweet Potato (Ipomoea batatas L.) Cultivars to Early Drought Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sweet Potato Planting
2.2. Metabolite Extraction
2.3. UHPLC-ESI-MS Analysis
3. Data Analysis
3.1. Raw Data Pre-Processing
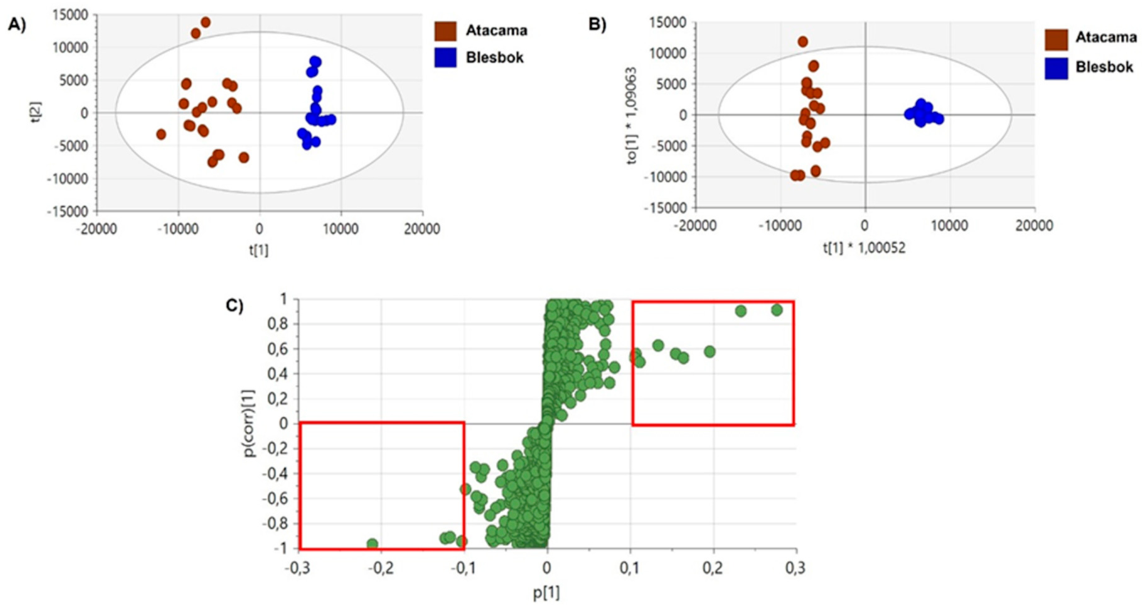
3.2. Multivariate Data Analysis
3.3. Metabolite Annotation and Pathway Analysis
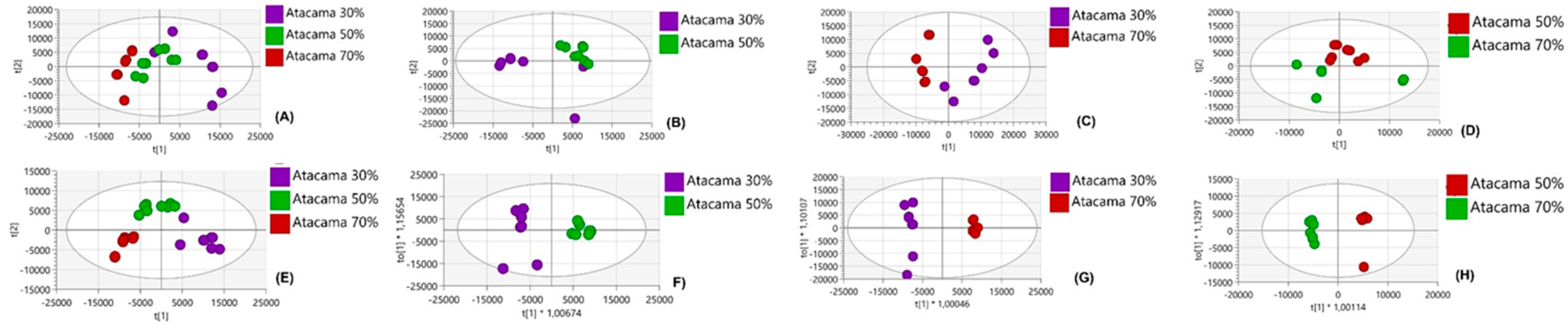
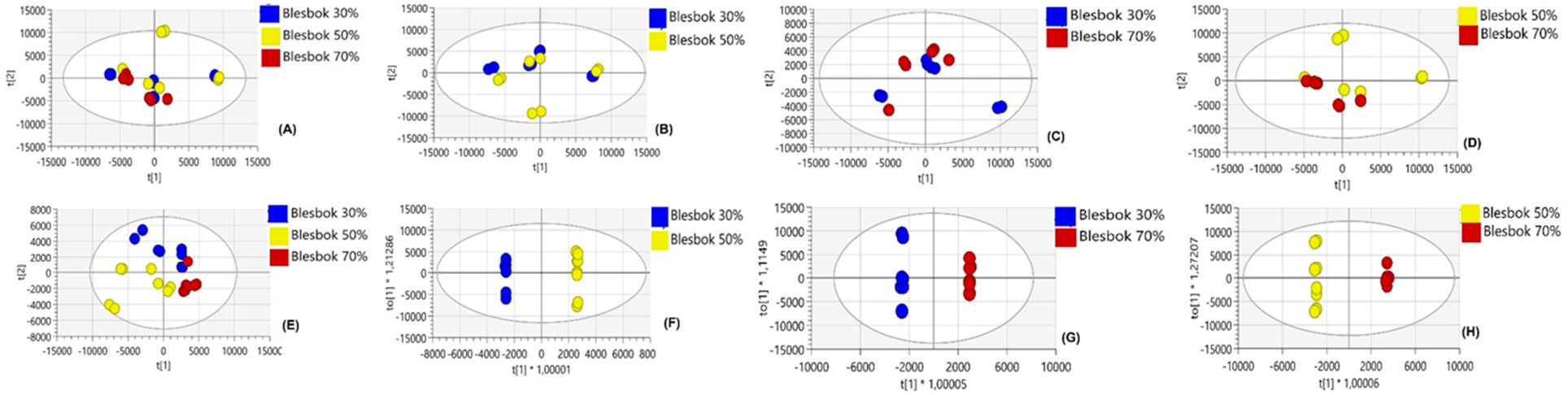
4. Results
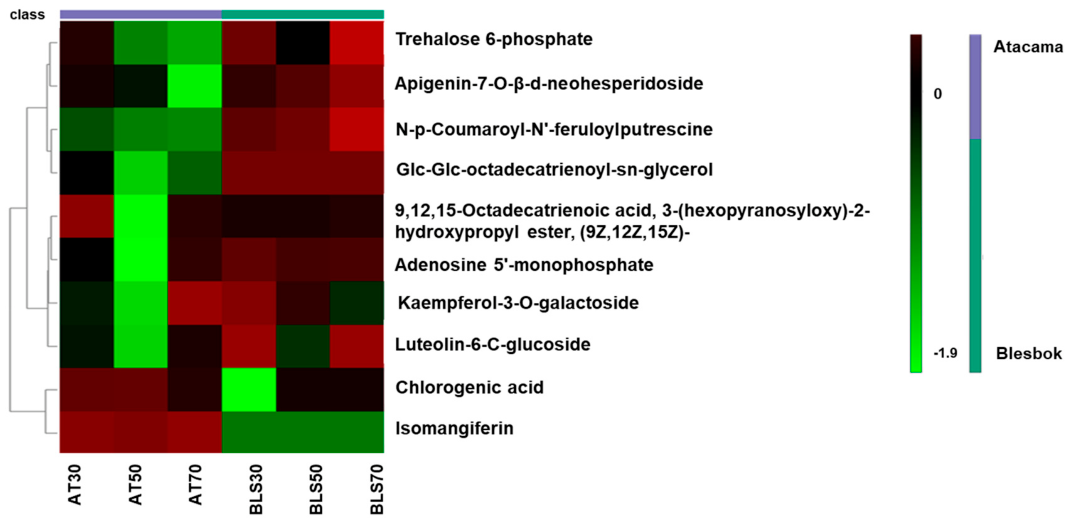
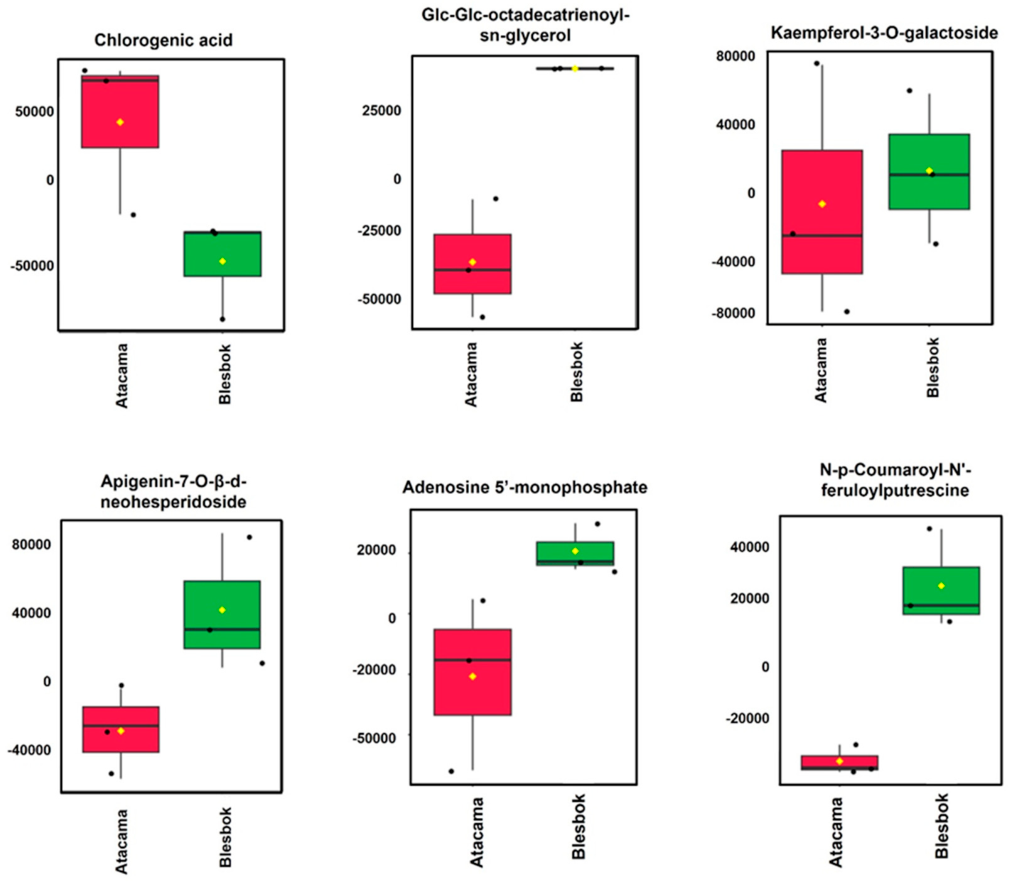
4.1. Comparative Analysis of Metabolites Under Non-Drought Stress Conditions
4.2. Metabolic Differences Between Sweet Potato Cultivars Under Drought Stress
| Compound Name | Experimental Mass (m/z) | Rt (min) | Molecular Formula | Log2Fold Change | VIP Value | p-Value | Class |
|---|---|---|---|---|---|---|---|
| Isolariciresinol 9′-O-alpha-L-arabinofuranoside | 492.031 | 4.66 | C25H32O10 | −10.64 | 2.23 | 4.10 × 10−4 | Lignan glycosides |
| alpha-Tocotrienol | 423.040 | 4.65 | C29H44O2 | −17.95 | 2.23 | 4.10 × 10−4 | Vitamin E derivatives |
| Octadecyl ferulic acid | 445.022 | 4.67 | C28H46O4 | −11.84 | 2.22 | 4.10 × 10−4 | Coumaric acids and derivatives |
| Lupeol | 425.548 | 4.65 | C30H50O | −7.66 | 2.21 | 4.10 × 10−4 | Triterpenoid |
| 10-Octacosene-1,12-diol | 424.731 | 4.65 | C28H56O2 | −7.96 | 2.21 | 4.10 × 10−4 | Fatty alcohol |
| Tricin 7-neohesperidoside | 638.366 | 7.80 | C29H34O16 | 8.71 | 2.01 | 5.54 × 10−4 | Flavonoid-7-o-glycosides |
| Tryptophan | 203.092 | 3.73 | C11H12N2O2 | −0.82 | 2.20 | 1.55 × 10−4 | Indolyl carboxylic acids and derivatives |
| epsilon-Tocopherol | 410.330 | 4.42 | C28H42O2 | −7.77 | 2.20 | 4.09 × 10−4 | Vitamin E derivatives |
| Gibberellin A23 | 378.146 | 7.79 | C20H26O7 | 11.63 | 2.22 | 4.09 × 10−4 | c20-gibberellin 6-carboxylic acids |
| Peonidin 3-sambubioside 5-glucoside | 758.35 | 6.91 | C33H41O20 | −11.28 | 2.20 | 4.09 × 10−4 | Anthocyanidin-5-o-glycosides |
| Glc-Glc-octadecatrienoyl-sn-glycerol (isomer 2) | 722.273 | 6.89 | C33H56O14 | −9.07 | 2.19 | 8.26 × 10−4 | Glycolipids |
| PC(20:1(13Z)/22:0) | 871.071 | 4.65 | C50H98NO8P | −11.50 | 2.19 | 4.09 × 10−4 | Glycerophospholipid |
| Metabolites | Experimental Mass (m/z) | Rt (min) | Molecular Formula | Log2Fold Change | p-Value |
|---|---|---|---|---|---|
| Glc-Glc-octadecatrienoyl-sn-glycerol | 721.444 | 6.34 | C33H56O14 | 3.14 | 3.55 × 10−15 |
| Chlorogenic acid | 353.142 | 3.17 | C16H18O9 | 3.34 | 4.09 × 10−14 |
| Luteolin-6-C-glucoside | 447.253 | 8.61 | C21H20O11 | 0.47 | 7.93 × 10−5 |
| 9,12,15-Octadecatrienoic acid, 3-(hexopyranosyloxy)-2-hydroxypropyl ester, (9Z,12Z,15Z)- | 559.314 | 6.59 | C27H46O9 | 1.07 | 1.42 × 10−4 |
| Apigenin-7-O-β-d-neohesperidoside | 577.339 | 6.98 | C27H30O14 | 2.71 | 1.70 × 10−4 |
| Trehalose 6-phosphate | 421.237 | 8.81 | C12H23O14P | 0.48 | 1.77 × 10−4 |
| Kaempferol-3-O-galactoside | 447.314 | 8.58 | C21H20O11 | 3.48 | 2.27 × 10−10 |
| Isomangiferin | 423.252 | 9.22 | C19H18O11 | −15.80 | 0 |
| N,-p-Coumaroyl-N’-feruloylputrescine | 409.024 | 4.38 | C23H26N2O5 | −5.40 | 4.44 × 10−15 |
| Adenosine 5′-monophosphate | 347.081 | 4.08 | C10H14N5O7P | −9.18 | 1.19 × 10−13 |
4.3. Metabolic Variations Within Atacama and Blesbok in Response to Drought Stress
4.4. Pathway Analysis of Metabolites Under Drought Stress
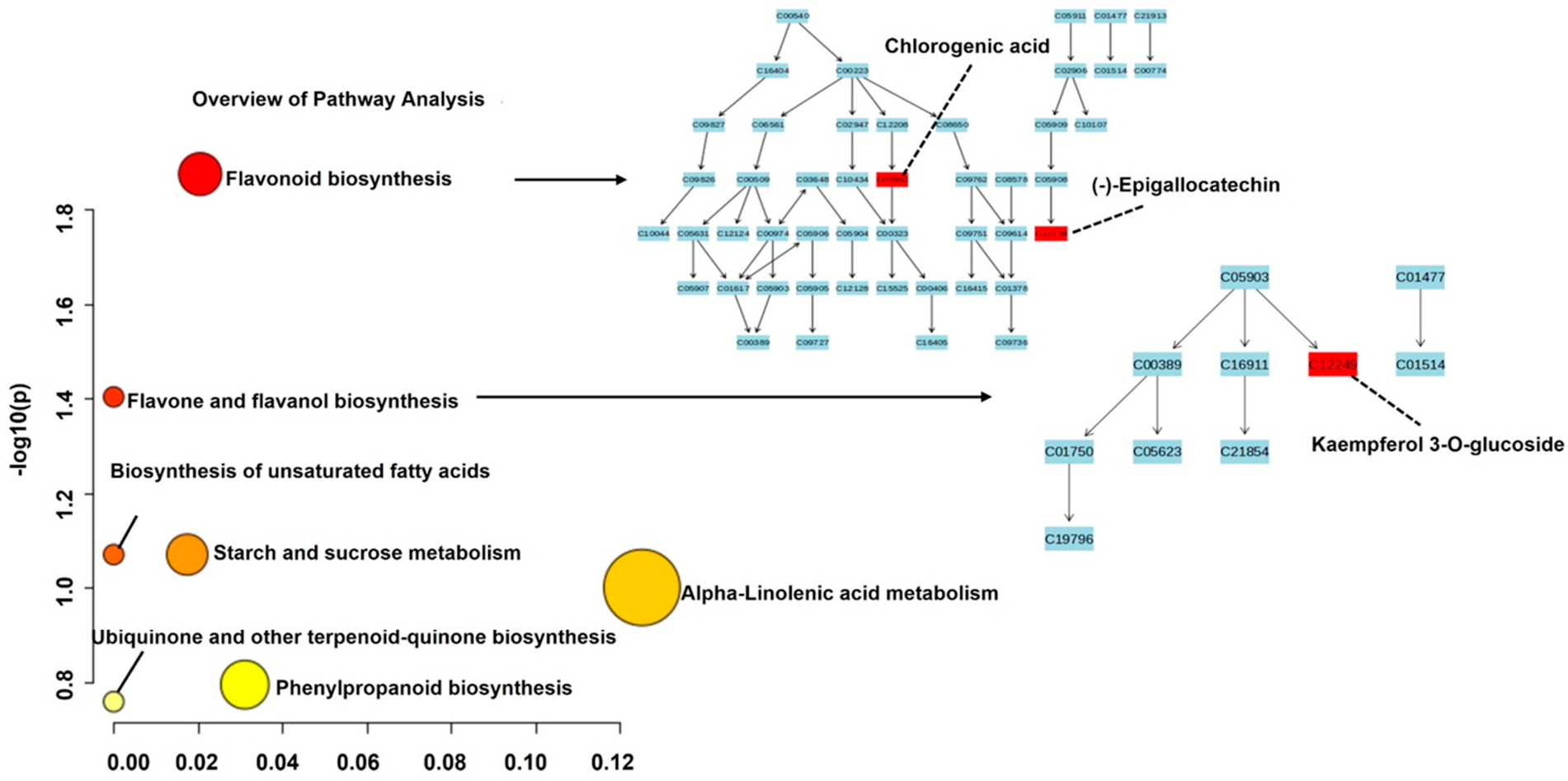
5. Discussion
5.1. Polyphenolic Compounds as Key Regulators of Drought Stress
5.2. The Role of Other Metabolites in Response to Drought Stress
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohammadi, R.; Amri, A. Genotype × Environment Interaction and Genetic Improvement for Yield and Yield Stability of Rainfed Durum Wheat in Iran. Euphytica 2013, 192, 227–249. [Google Scholar] [CrossRef]
- Berger, J.; Palta, J.; Vadez, V. An Integrated Framework for Crop Adaptation to Dry Environments: Responses to Transient And Terminal Drought. Plant Sci. 2016, 253, 58–67. [Google Scholar] [CrossRef]
- Yin, Y.; Qiao, S.; Kang, Z.; Luo, F.; Bian, Q.; Cao, G.; Zhao, G.; Wu, Z.; Yang, G.; Wang, Y.; et al. Transcriptome and Metabolome Analyses Reflect the Molecular Mechanism of Drought Tolerance in Sweet Potato. Plants 2024, 13, 351. [Google Scholar] [CrossRef]
- Patel, M.K.; Kumar, M.; Li, W.; Luo, Y.; Burritt, D.J.; Alkan, N.; Tran, L.-S.P. Enhancing Salt Tolerance of Plants: From Metabolic Reprogramming to Exogenous Chemical Treatments and Molecular Approaches. Cells 2020, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Elansary, H.O.; Mattar, M.A.; Elhindi, K.A.M.; Alotaibi, M.; Mishra, A. Differential accumulation of metabolites in Suaeda species provides new insights into abiotic stress tolerance in C4-halophytic species in elevated CO2 conditions. Agronomy 2021, 11, 131. [Google Scholar] [CrossRef]
- Llanes, A.; Andrade, A.; Alemano, S.; Luna, V. Metabolomic Approach to Understand Plant Adaptations to Water and Salt Stress. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 133–144. [Google Scholar] [CrossRef]
- Faostat. Food and Agriculture Organisation. 2021. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 12 July 2024).
- Laveriano-Santos, E.P.; López-Yerena, A.; Jaime-Rodríguez, C.; González-Coria, J.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A.; Romanyà, J.; Pérez, M. Sweet Potato Is Not Simply an Abundant Food Crop: A Comprehensive Review of Its Phytochemical Constituents, Biological Activities, and the Effects of Processing. Antioxidants 2022, 11, 1648. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Toyama, J.; Islam, M.S.; Yoshimoto, M.; Kumagai, T.; Kai, Y.; Nakazawa, Y.; Yamakawa, O. Suioh, a New Sweetpotato Cultivar for Utilization in Vegetable Greens. Acta Hortic. 2004, 339–346. [Google Scholar] [CrossRef]
- Flores, G.; Wu, S.-B.; Negrin, A.; Kennelly, E.J. Chemical Composition and Antioxidant Activity of Seven Cultivars of Guava (Psidium guajava) Fruits. Food Chem. 2015, 170, 327–335. [Google Scholar] [CrossRef]
- Shekhar, S.; Mishra, D.; Buragohain, A.K.; Chakraborty, S.; Chakraborty, N. Comparative Analysis of Phytochemicals and Nutrient Availability in Two Contrasting Cultivars of Sweet Potato (Ipomoea batatas L.). Food Chem. 2015, 173, 957–965. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A Comparative Metabolomics Study of Flavonoids in Sweet Potato with Different Flesh Colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. A Review of Therapeutic Potentials of Sweet Potato: Pharmacological Activities and Influence of the Cultivar. Trop. J. Pharm. Res. 2017, 15, 2751. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The Link between Genotypes and Phenotypes. In Functional Genomics; Springer: Dordrecht, The Netherlands, 2002; pp. 155–171. [Google Scholar] [CrossRef]
- Lebot, V.; Michalet, S.; Legendre, L. Identification and Quantification of Phenolic Compounds Responsible for the Antioxidant Activity of Sweet Potatoes with Different Flesh Colours Using High Performance Thin Layer Chromatography (HPTLC). J. Food Compos. Anal. 2016, 49, 94–101. [Google Scholar] [CrossRef]
- Drapal, M.; Rossel, G.; Heider, B.; Fraser, P.D. Metabolic Diversity in Sweet Potato (Ipomoea batatas, Lam.) Leaves and Storage Roots. Hortic. Res. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.A.; Mahood, E.H.; Fan, K.; Moghe, G.D. Untargeted Metabolomics of Purple and Orange-Fleshed Sweet Potatoes Reveals a Large Structural Diversity of Anthocyanins and Flavonoids. Sci. Rep. 2021, 11, 16408. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, T.; Wu, H.; Ge, Y.; Zhao, X.; Shen, X.; Zhou, W.; Wang, T.; Zhang, Y.; Ma, D.; et al. Exploring the Metabolic Changes in Sweet Potato during Postharvest Storage Using a Widely Targeted Metabolomics Approach. J. Food Process. Preserv. 2021, 45, e15118. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Liu, Y.; Zhang, A.; Xiao, S.; Dai, X.; Yuan, R.; Zhou, Z.; Cao, Q. Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway for the Accumulation of Anthocyanins and Other Flavonoids in Sweetpotato Root Skin and Leaf Vein Base. J. Agric. Food Chem. 2022, 70, 2574–2588. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, J.; Cao, Q.; Li, Z.; Ma, D. Differential response of physiology and metabolic response to drought stress in different sweetpotato cultivars. PLoS ONE 2022, 17, e0264847. [Google Scholar] [CrossRef]
- Oloka, B.M.; da Silva, C.C.; Azevedo, C.F.; Unzimai, I.V.; Unzimai, I.V.; Yada, B.; Grüneberg, W.; Andrade, M.; Pecota, K.V.; da Silva Pereira, G.; et al. The Future of Crop Improvement in Sweetpotato: Merging Traditional and Genomic-Assisted Breeding Methods. In The Sweetpotato Genome; Yencho, G.C., Olukolu, B.A., Isobe, S., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 147–164. [Google Scholar] [CrossRef]
- Omotobora, B.O.; Adebola, P.O.; Modise, D.M.; Laurie, S.M.; Gerrano, A.S. Greenhouse and Field Evaluation of Selected Sweetpotato (Ipomoea batatas (L.) LAM) Accessions for Drought Tolerance in South Africa. Am. J. Plant Sci. 2014, 5, 3328–3339. [Google Scholar] [CrossRef]
- Naidoo, S.I.; Laurie, S.M.; Shimelis, H.; Laing, M.D. Morpho-Agronomical Characterisation of Local and International Sweetpotato Germplasm from the South African Collection. S. Afr. J. Plant Soil 2020, 37, 308–320. [Google Scholar] [CrossRef]
- Laurie, S.M.; Mulabisana, J.; Sutherland, R.; Sivakumar, D.; Pofu, K.; Mphela, W.M.; Truter, M.; du Plooy, I.; Araya, N.; Araya, H.; et al. Seventy Years of Sweet Potato [ Ipomoea batatas L. (LAM)] Research in South Africa. Crop Sci. 2023, 64, 1112–1128. [Google Scholar] [CrossRef]
- Ma, J.; Li, R.; Wang, H.; Li, D.; Wang, X.; Zhang, Y.; Zhen, W.; Duan, H.; Yan, G.; Li, Y. Transcriptomics Analyses Reveal Wheat Responses to Drought Stress during Reproductive Stages under Field Conditions. Front. Plant Sci. 2017, 8, 592. [Google Scholar] [CrossRef] [PubMed]
- Maserumule, M.; Rauwane, M.; Madala, N.E.; Ncube, E.; Figlan, S. Defence-Related Metabolic Changes in Wheat (Triticum aestivum L.) Seedlings in Response to Infection by Puccinia graminis f. Sp. tritici. Front. Plant Sci. 2023, 14, 1166813. [Google Scholar] [CrossRef] [PubMed]
- Makhubu, F.N.; Mutanda, M.; Madala, N.E.; Figlan, S. Metabolite profiling in ten bread wheat (Triticum aestivum L.) genotypes in response to drought stress. Plant Stress 2024, 14, 100680. [Google Scholar] [CrossRef]
- Ramabulana, A.-T.; Petras, D.; Madala, N.E.; Tugizimana, F. Metabolomics and Molecular Networking to Characterize the Chemical Space of Four Momordica Plant Species. Metabolites 2021, 11, 763. [Google Scholar] [CrossRef]
- Tugizimana, F.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. A Conversation on Data Mining Strategies in LC-MS Untargeted Metabolomics: Pre-Processing and Pre-Treatment Steps. Metabolites 2016, 6, 40. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Wu, R.; Liu, Y.; Hu, X.; Yan, Y.; Ling, X. A Novel Strategy for Rapidly and Accurately Screening Biomarkers Based on Ultraperformance Liquid Chromatography-Mass Spectrometry Metabolomics Data. Anal. Chim. Acta 2019, 1063, 47–56. [Google Scholar] [CrossRef]
- Liu, E.; Xu, L.; Luo, Z.; Li, Z.; Zhou, G.; Gao, H.; Fang, F.; Tang, J.; Zhao, Y.; Zhou, Z.; et al. Transcriptomic Analysis Reveals Mechanisms for the Different Drought Tolerance of Sweet Potatoes. Front. Plant Sci. 2023, 14, 1136709. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Carvalho, L.M.; Duque, P. Effect of Photoperiod on Flavonoid Pathway Activity in Sweet Potato (Ipomoea batatas (L.) Lam.) Leaves. Food Chem. 2010, 118, 384–390. [Google Scholar] [CrossRef]
- Althwab, S.A.; Mousa, H.M.; El-Zaha, K.M.; Zaher, A.A. Protective Effect of Sweet Potato Peel against Oxidative Stress in Hyperlipidemic Albino Rats. Food Nutr. Sci. 2019, 10, 503–516. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as Potent Scavengers of Hydroxyl Radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of Oxidative and Drought Tolerance in Arabidopsis by Overaccumulation of Antioxidant Flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Groenbaek, M.; Tybirk, E.; Neugart, S.; Sundekilde, U.K.; Schreiner, M.; Kristensen, H.L. Flavonoid Glycosides and Hydroxycinnamic Acid Derivatives in Baby Leaf Rapeseed from White and Yellow Flowering Cultivars with Repeated Harvest in a 2-Years Field Study. Front. Plant Sci. 2019, 10, 355. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar] [CrossRef]
- Kourouma, V.; Mu, T.; Zhang, M.; Sun, H. Comparative Study on Chemical Composition, Polyphenols, Flavonoids, Carotenoids and Antioxidant Activities of Various Cultivars of Sweet Potato. Int. J. Food Sci. Technol. 2020, 55, 369–378. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Shen, X.; Xie, Y.; Yang, Y.; Bian, R.; Gao, Y.; Li, P.; Sun, L.; Feng, H.; Ma, F.; et al. Regulation of Phenylpropanoid Biosynthesis by MdMYB88 and MdMYB124 Contributes to Pathogen and Drought Resistance in Apple. Hortic. Res. 2020, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chen, J.; Lv, Z.; Gao, X.; Guo, S.; Xu, R.; Deng, Z.; Yao, S.; Chen, Z.; Kang, Y.; et al. Staged and Repeated Drought-Induced Regulation of Phenylpropanoid Synthesis Confers Tolerance to a Water Deficit Environment in Camellia sinensis. Ind. Crops Prod. 2023, 201, 116843. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The Effect of Drought Stress on Polyphenolic Compounds and Expression of Flavonoid Biosynthesis Related Genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- de Albuquerque, T.M.R.; Sampaio, K.B.; de Souza, E.L. Sweet Potato Roots: Unrevealing an Old Food as a Source of Health Promoting Bioactive Compounds—A Review. Trends Int. J. Food Sci. Technol. 2019, 85, 277–286. [Google Scholar] [CrossRef]
- Zheng, W.; Clifford, M.N. Profiling the Chlorogenic Acids of Sweet Potato (Ipomoea batatas) from China. Food Chem. 2008, 106, 147–152. [Google Scholar] [CrossRef]
- Zhao, J.-G.; Yan, Q.-Q.; Xue, R.-Y.; Zhang, J.; Zhang, Y.-Q. Isolation and Identification of Colourless Caffeoyl Compounds in Purple Sweet Potato by HPLC-DAD–ESI/MS and Their Antioxidant Activities. Food Chem. 2014, 161, 22–26. [Google Scholar] [CrossRef]
- Tošović, J.; Marković, S.; Dimitrić Marković, J.M.; Mojović, M.; Milenković, D. Antioxidative Mechanisms in Chlorogenic Acid. Food Chem. 2017, 237, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Seo, J.; Le, J.; Lee, E.J. Chilling stress induces decreased abscisic acid and elevated salicylic acid levels in harvested sweet potato (Ipomoea batatas L.) roots. Postharvest Biol. Technol. 2025, 226, 113557. [Google Scholar] [CrossRef]
- Yan, K.; Cui, M.; Zhao, S.; Chen, X.; Tang, X. Salinity stress is beneficial to the accumulation of chlorogenic acids in honeysuckle (Lonicera japonica Thunb.). Front. Plant Sci. 2016, 7, 1563. [Google Scholar] [CrossRef] [PubMed]
- Talukder, P.; Chanda, S.; Sinha, B. Boosting Biotic Stress Resistance in Solanum melongena L.: The Role of Exogenous Chlorogenic Acid in Enhancing Secondary Metabolite Production. Appl. Biochem. Biotechnol. 2025, 197, 3407–3430. [Google Scholar] [CrossRef]
- Lebot, V.; Leo, P.; Legendre, L. Phenotyping chlorogenic acids and coumarins in sweet potato [Ipomoea batatas (L.) Lam.] Breed. Lines Enhanc. Toler. Periderm pathogens. Euphytica 2021, 217, 59. [Google Scholar] [CrossRef]
- Liao, Y.; Zeng, L.; Rao, S.; Gu, D.; Liu, X.; Wang, Y.; Zhu, H.; Hou, X.; Yang, Z. Induced biosynthesis of chlorogenic acid in sweetpotato leaves confers the resistance against sweetpotato weevil attack. J. Adv. Res. 2020, 24, 513–522. [Google Scholar] [CrossRef]
- Dos Santos, I.C.; de Almeida, A.A.F.; Pirovani, C.P.; Costa, M.G.C.; Bellete, B.S.; Freschi, L.; Soares Filho, W.; Coelho Filho, M.A.; da Silva Gesteira, A. Differential Accumulation of Flavonoids and Phytohormones Resulting from the Canopy/Rootstock Interaction of Citrus Plants Subjected to Dehydration/Rehydration. Plant Physiol. Biochem. 2017, 119, 147–158. [Google Scholar] [CrossRef]
- Hernández, I.; Alegre, L.; Van Breusegem, F.; Munné-Bosch, S. How Relevant Are Flavonoids as Antioxidants in Plants? Trends Plant Sci. 2009, 14, 125–132. [Google Scholar] [CrossRef]
- Marzouk, M.; Khalifa, S.M.; Ahmed, A.H.; Metwaly, A.M.; Mohammed, H.S.; Taie, H.A.A. LC/HRESI-MS/MS Screening, Phytochemical Characterization, and In Vitro Antioxidant and Cytotoxic Potential of Jatropha integerrima Jacq. Extracts. Bioorganic Chem. 2023, 140, 106825. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, Ultrasound-Assisted, and Accelerated-Solvent Extractions of Anthocyanins from Purple Sweet Potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rodríguez-Werner, M.; Schlösser, A.; Winterhalter, P.; Rimbach, G. Fractionation, Enzyme Inhibitory and Cellular Antioxidant Activity of Bioactives from Purple Sweet Potato (Ipomoea batatas). Food Chem. 2017, 221, 447–456. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative Role of Sugar Signaling and Transport during Drought Stress Responses in Plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of Drought Stress on Sugar Metabolism in Leaves and Roots of Soybean Seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Beena, R. Sucrose Metabolism in Plants under Drought Stress Condition: A Review. Indian J. Agric. Res. 2021, 58, 943. [Google Scholar] [CrossRef]
- Lunn, J.E.; Feil, R.; Hendriks, J.H.; Gibon, Y.; Morcuende, R.; Osuna, D.; Scheible, W.R.; Carillo, P.; Hajirezaei, M.R.; Stitt, M. Sugar-Induced Increases in Trehalose 6–Phosphate Are Correlated with Redoxactivation of ADP-Glucose Pyrophosphorylase and Higher Rates of Starchsynthesis in Arabidopsis thaliana. Biochem. J. 2006, 397, 139–148. [Google Scholar] [CrossRef]
- Eastmond, P.J.; Graham, I.A. Trehalose Metabolism: A Regulatory Role for Trehalose-6-Phosphate? Curr. Opin. Plant Biol. 2003, 6, 231–235. [Google Scholar] [CrossRef]
- Fichtner, F.; Lunn, J.E. The Role of Trehalose 6-Phosphate (Tre6P) in Plant Metabolism and Development. Annu. Rev. Plant Biol. 2021, 72, 737–760. [Google Scholar] [CrossRef]
- Henry, C.; Bledsoe, S.W.; Griffiths, C.A.; Kollman, A.; Paul, M.J.; Sakr, S.; Lagrimini, L.M. Differential Role for Trehalose Metabolism in Salt-Stressed Maize. Plant Physiol. 2015, 169, 1072–1089. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, W.; Gao, J.; Yang, F.; Zhuang, C. Overexpression of the Trehalose-6-Phosphate Phosphatase OsTPP3 Increases Drought Tolerance in Rice. Plant Biotechnol. Rep. 2019, 13, 285–292. [Google Scholar] [CrossRef]
- Cao, Y.; Tanaka, K.; Nguyen, C.T.; Stacey, G. Extracellular ATP Is a Central Signaling Molecule in Plant Stress Responses. Curr. Opin. Plant Biol. 2014, 20, 82–87. [Google Scholar] [CrossRef]
- Shu, J.; Zhang, L.; Liu, G.; Wang, X.; Liu, F.; Zhang, Y.; Chen, Y. Transcriptome Analysis and Metabolic Profiling Reveal the Key Regulatory Pathways in Drought Stress Responses and Recovery in Tomatoes. Int. J. Mol. Sci. 2024, 25, 2187. [Google Scholar] [CrossRef]
- Yang, F.; Lv, G. Metabolomic Analysis of the Response of Haloxylon ammodendron and Haloxylon persicum to Drought. Int. J. Mol. Sci. 2023, 24, 9099. [Google Scholar] [CrossRef]
| Metabolite | EM (m/z) | Rt (min) | Molecular Formula | Adduct | Class | Cultivars Log2Fold Changes | |||
|---|---|---|---|---|---|---|---|---|---|
| Atacama 50% | Atacama 70% | Blesbok 50% | Blesbok 70% | ||||||
| Apigenin-7-O-β-d-neohesperidoside | 577.339 | 6.80 | C27H30O14 | [M − H]− | Flavonoid glycoside | 5.27 | 3.31 | 0.29 | 0.75 |
| Quercetin 3-O-malonylglucoside | 549.285 | 7.47 | C24H22O15 | [M − H]− | Flavonoid glycoside | 2.39 | −5.51 | ND | ND |
| 9,12,15-Octadecatrienoic acid, 3-(hexopyranosyloxy)-2-hydroxypropyl ester, (9Z,12Z,15Z)- | 559.314 | 7.45 | C27H46O9 | M + FA − H | Fatty acid | 1.17 | 1.97 | −0.14 | ND |
| Glc-Glc-octadecatrienoyl-sn-glycerol | 721.366 | 6.91 | C33H56O14 | M + FA − H | Glycolipids | 1.20 | 1.66 | ND | ND |
| Dicaffeoylquinic acid | 561.258 | 7.48 | C25H24O12 | M + FA − H | Phenolic acids | −2.35 | ND | ND | ND |
| Isovitexin 7-O-glucoside | 593.274 | 6.81 | C27H30O15 | [M − H]− | Flavonoid glycoside | −1.75 | ND | ND | ND |
| Chlorogenic acid | 353.088 | 3.16 | C16H18O9 | [M − H]− | Polyphenols | −0.36 | 1.95 | 0.23 | −0.39 |
| Isomangiferin | 423.252 | 9.22 | C19H18O11 | [M + H]− | Xanthones | ND | 2.32 | −0.38 | 0.47 |
| (-)-Epigallocatechin | 305.143 | 3.51 | C15H14O7 | [M − H]− | Flavonoids | ND | ND | −0.38 | ND |
| Glutamyltyrosine | 309.207 | 5.44 | C14H18N2O6 | [M − H]− | Dipeptides | ND | ND | 2.76 | ND |
| Kaempferol-3-O-glucoside | 447.253 | 7.88 | C21H20O11 | [M − H]− | Flavonoid glycoside | ND | ND | 0.15 | 0.15 |
| Trehalose 6-phosphate | 421.237 | 8.00 | C12H23O14P | [M − H]− | Disaccharide phosphate | ND | ND | −0.26 | ND |
| Menatetrenone | 445.237 | 7.43 | C31H40O2 | [M + H]− | Menaquinones | ND | ND | −0.16 | ND |
| LysoPC (15:0) | 481.258 | 7.62 | C23H48NO7P | [M − H]− | Glycerophosphocholines | ND | ND | ND | −0.76 |
| 1,3-Dicaffeoylquinic acid | 515.121 | 3.81 | C25H24O12 | [M − H]− | Quinic acids and derivatives | ND | ND | ND | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makhubu, F.N.; Siviya, L.E.; Rauwane, M.E.; Laurie, S.M.; Madala, N.E.; Figlan, S. Biochemical Responses of Atacama and Blesbok Sweet Potato (Ipomoea batatas L.) Cultivars to Early Drought Stress. Plants 2025, 14, 3532. https://doi.org/10.3390/plants14223532
Makhubu FN, Siviya LE, Rauwane ME, Laurie SM, Madala NE, Figlan S. Biochemical Responses of Atacama and Blesbok Sweet Potato (Ipomoea batatas L.) Cultivars to Early Drought Stress. Plants. 2025; 14(22):3532. https://doi.org/10.3390/plants14223532
Chicago/Turabian StyleMakhubu, Fikile N., Lebogang E. Siviya, Molemi E. Rauwane, Sunette M. Laurie, Ntakadzeni E. Madala, and Sandiswa Figlan. 2025. "Biochemical Responses of Atacama and Blesbok Sweet Potato (Ipomoea batatas L.) Cultivars to Early Drought Stress" Plants 14, no. 22: 3532. https://doi.org/10.3390/plants14223532
APA StyleMakhubu, F. N., Siviya, L. E., Rauwane, M. E., Laurie, S. M., Madala, N. E., & Figlan, S. (2025). Biochemical Responses of Atacama and Blesbok Sweet Potato (Ipomoea batatas L.) Cultivars to Early Drought Stress. Plants, 14(22), 3532. https://doi.org/10.3390/plants14223532








