Genome-Wide Identification of ABSCISIC ACID-INSENSITIVE (ABI) Genes and Their Response to MeJA During Early Somatic Embryogenesis in Longan (Dimocarpus longan L.)
Abstract
1. Introduction
2. Results
2.1. Identification and Physicochemical Characteristics of DlABI Genes
2.2. Phylogenetic Analysis of DlABI Family Members
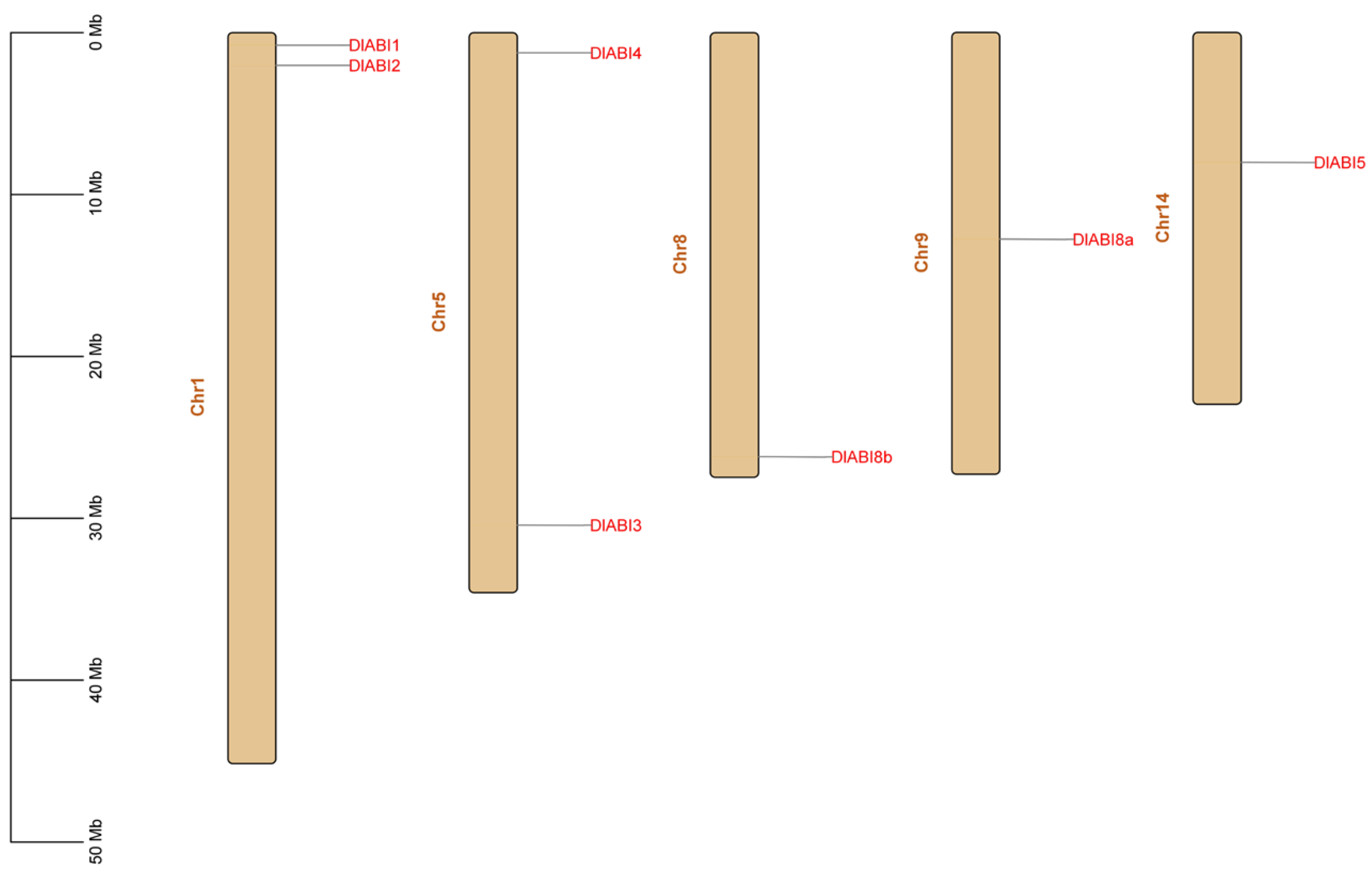
2.3. Chromosomal Location, Conserved Motifs, and Gene Structure of DlABI Family Members
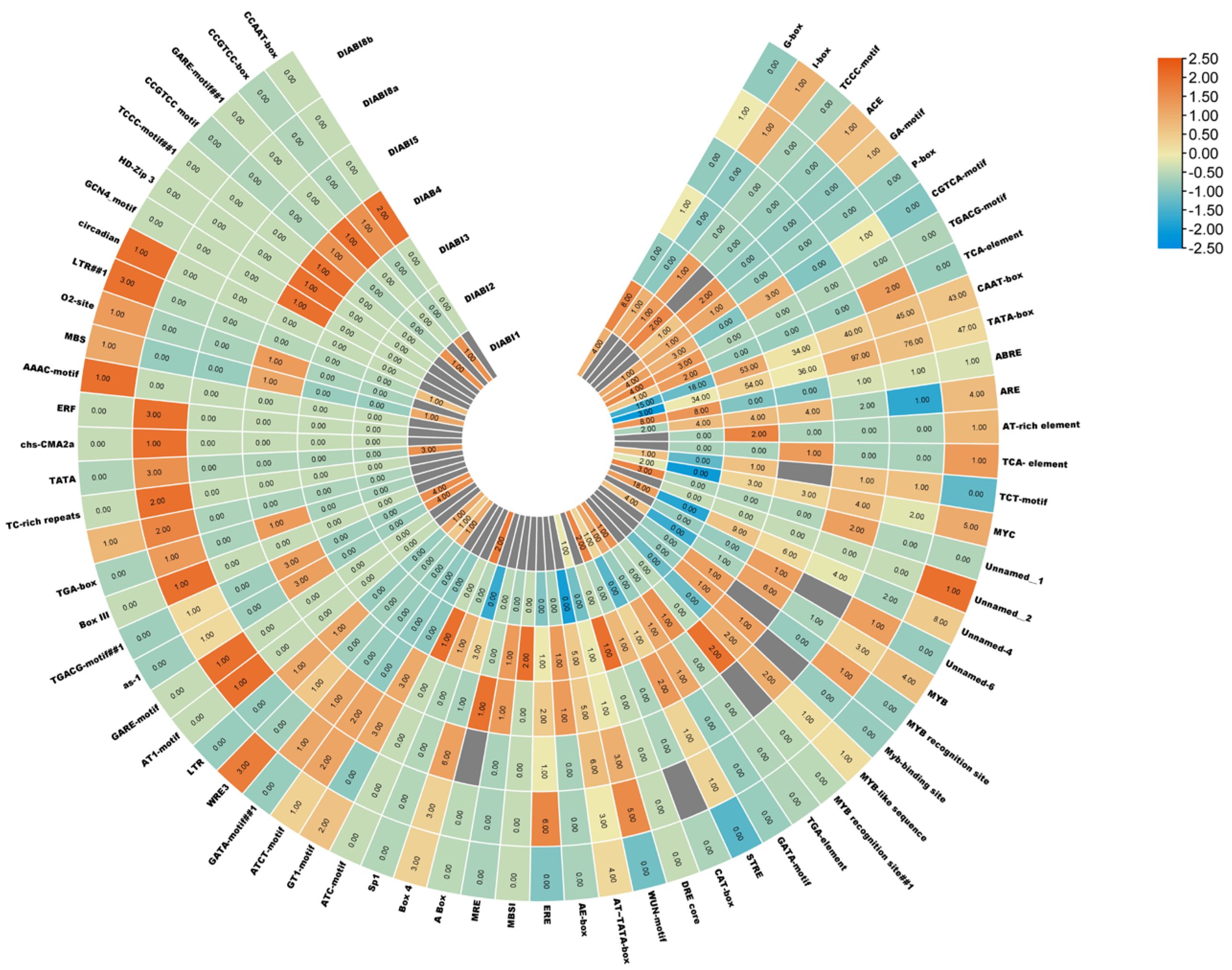
2.4. Cis Element Analysis of DlABI Genes
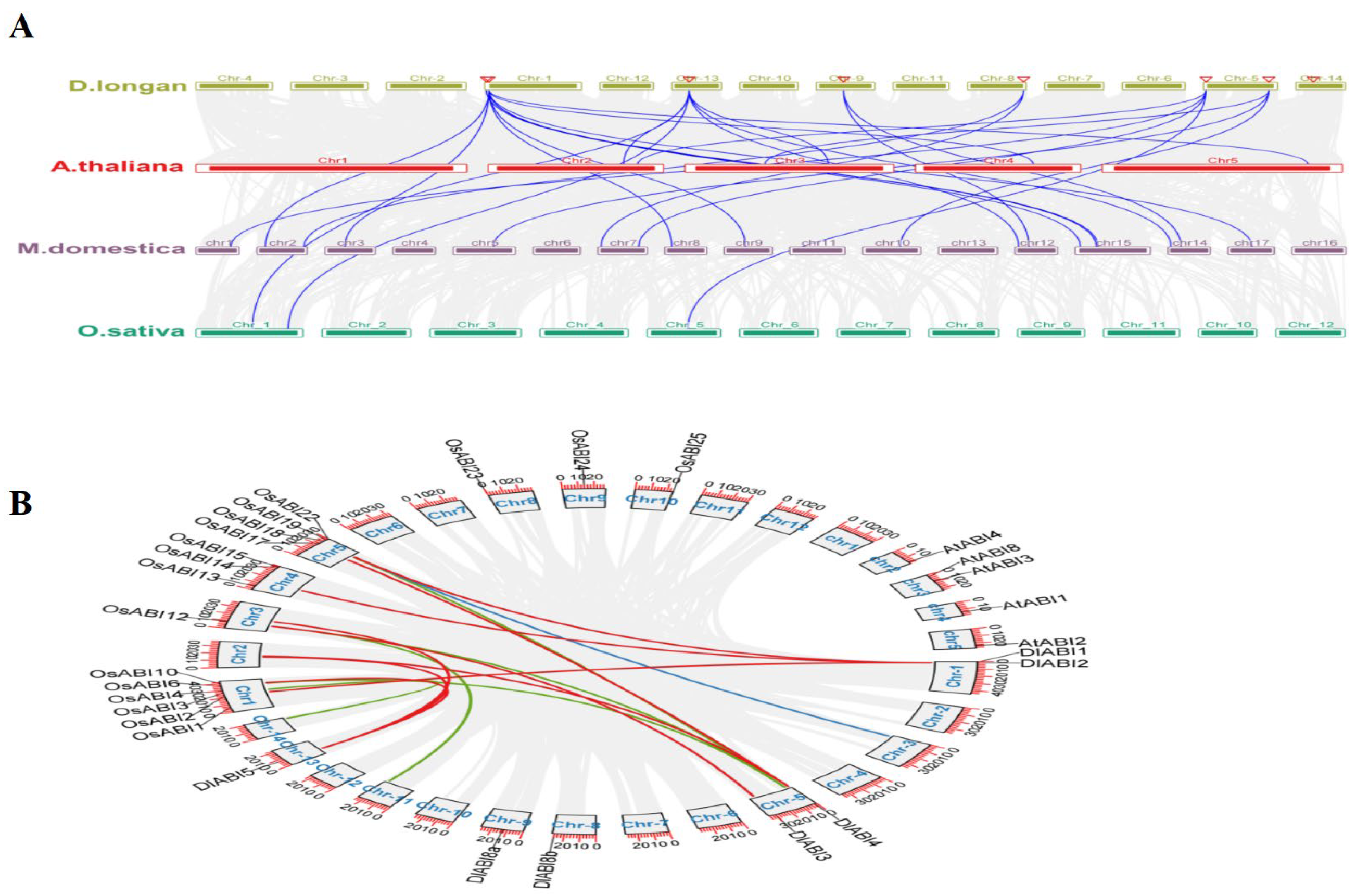
2.5. Synteny Analysis and Chromosomal Duplication of DlABI Genes
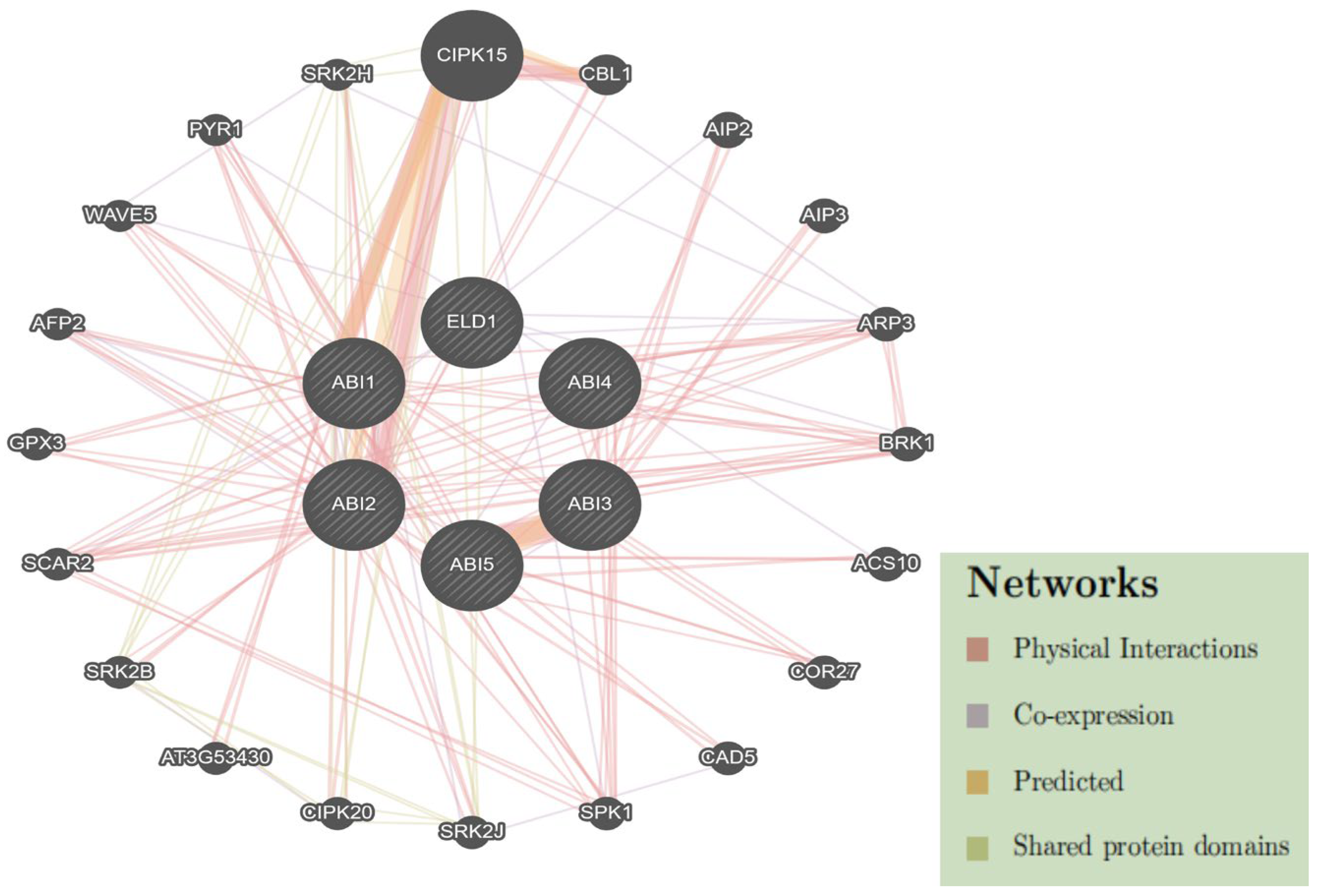
2.6. Protein–Protein Interaction Network of DlABI Genes
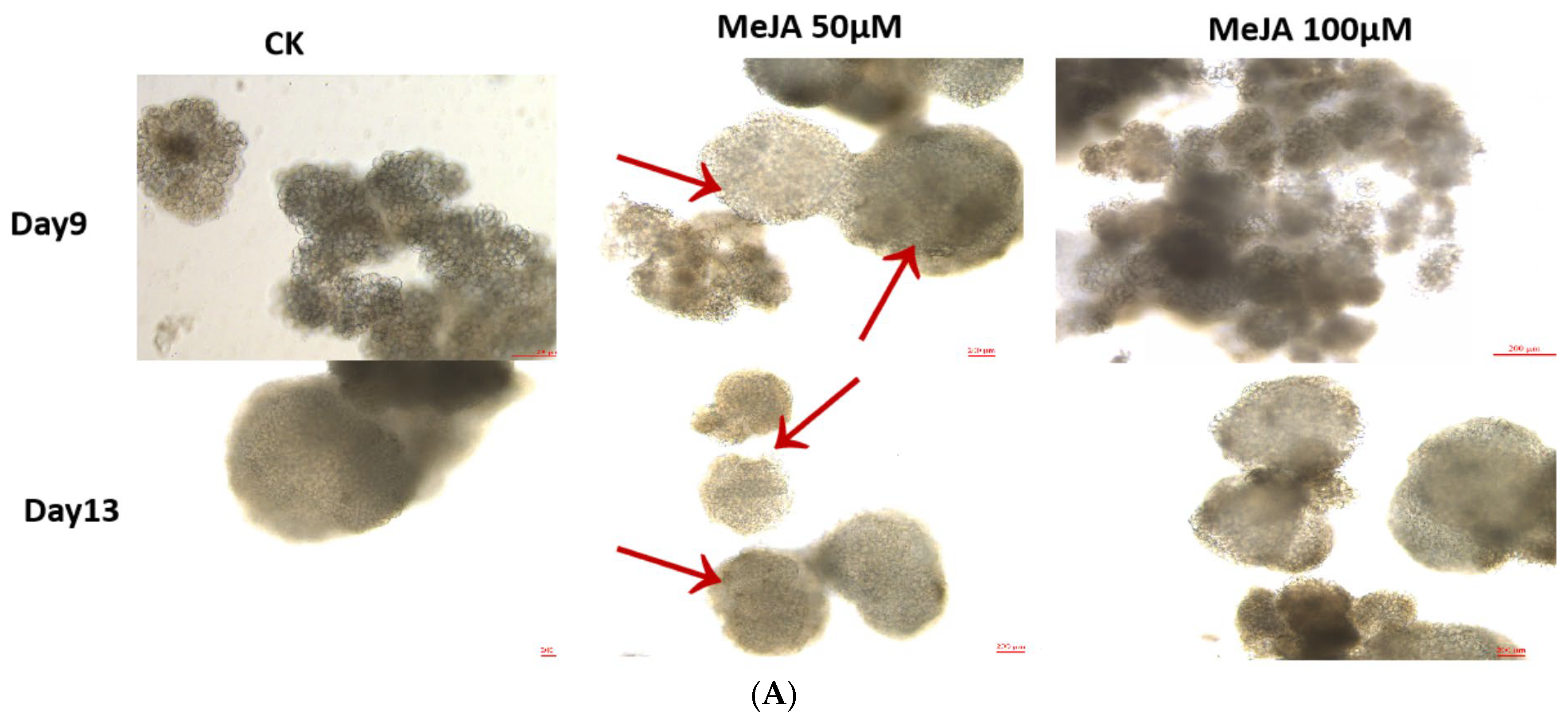
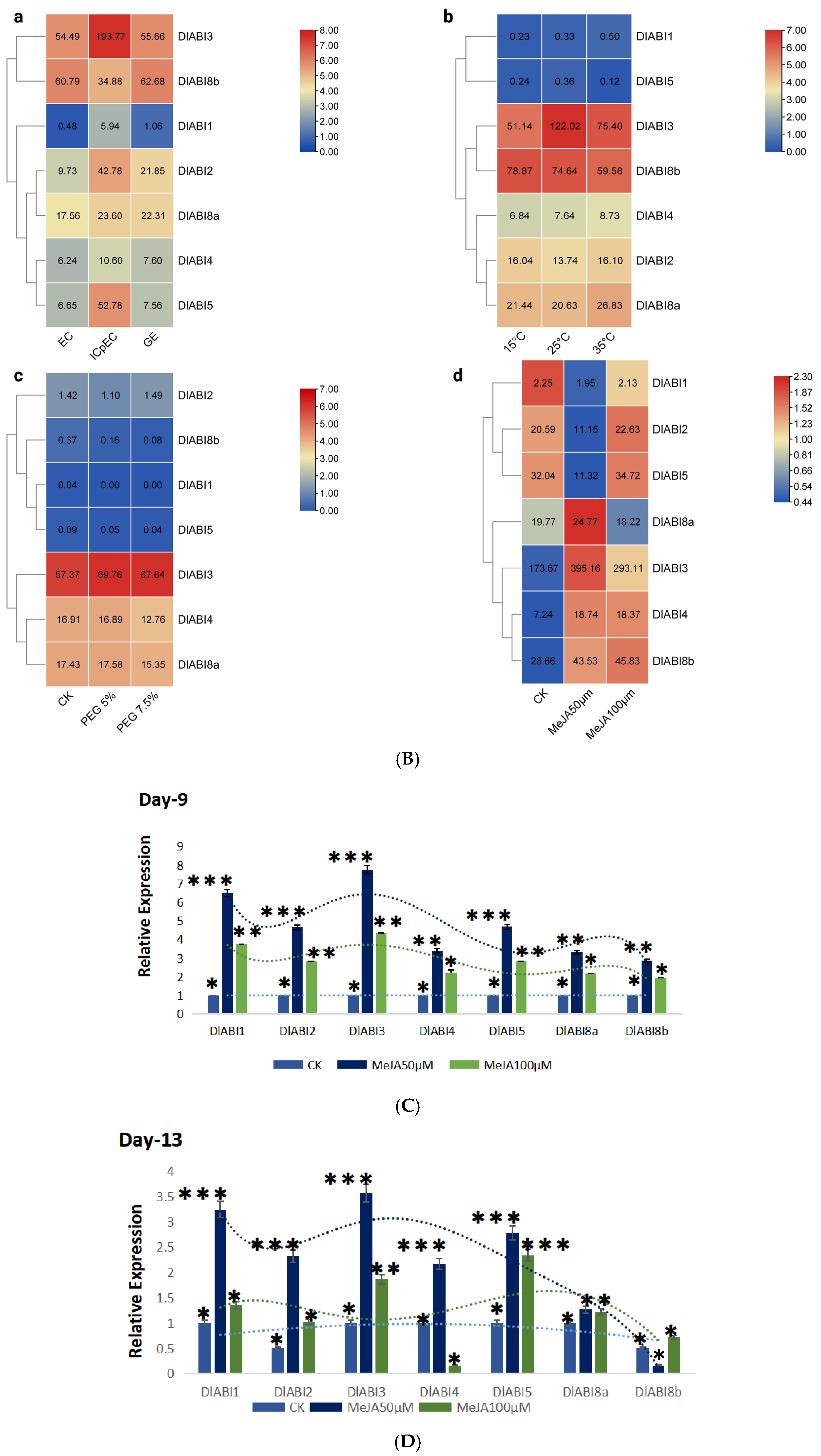
2.7. Morphological Changes Assessment, Heat Map Expression Profiling, and the qRT-PCR Analysis of DlABI Genes in Response to MeJA Treatment
2.8. Subcellular Localization Investigation of DlABI3
2.9. Determination of Reactive Oxygen Species (ROS) Enzymes Activity and Malondialdehyde (MDA)
3. Discussion
3.1. Evolutionary Conservation and Functional Diversity of the DlABI Genes
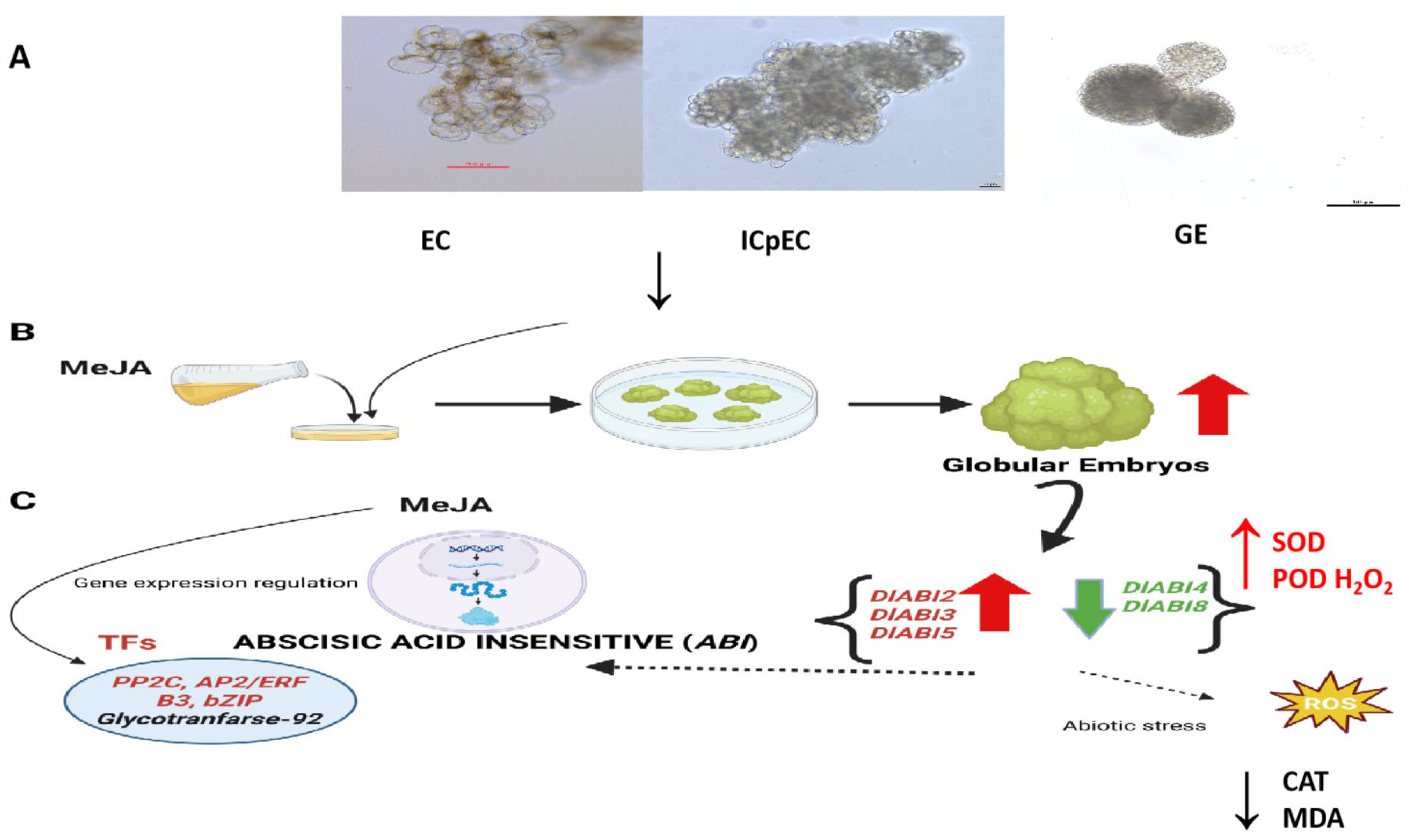
3.2. DlABI May Regulate the Early Somatic Embryogenesis of Longan in Response to MeJA by Maintaining ROS Activity
4. Materials and Methods
4.1. Plant Materials
4.2. Physicochemical Properties and Phylogenetic Analysis of DlABI Genes
4.3. Domain Analysis and Gene Structure View of DlABI Genes
4.4. Analysis of Cis-Regulatory Elements and Synteny Visualization of Longan ABI Genes
4.5. Analysis of the Specific Expression and the qRT-PCR Results of DlABI
4.6. The Subcellular Localization Analysis of DlABI3
4.7. Measurement of Reactive Oxygen Species (ROS) Activity and Malondialdehyde (MDA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Dl | Dimocarpus longan Lour |
| NEC | non-embryogenic callus |
| EC | embryogenic callus |
| ICpEC | incomplete compact pro-embryogenic culture |
| GE | globular embryos |
| SE | somatic embryogenesis |
| ABA | abscisic acid |
| ABI | abscisic acid-insensitive |
| TFs | transcription factors |
| CDS | coding sequence |
| UTR | untranslated region |
| ORF | open reading frame |
| PI | isoelectric point |
| MW | molecular weight |
| GA | gibberellin |
| SA | salicylic acid |
| qRT-PCR | real-time reverse transcription PCR |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| POD | peroxidase |
| CAT | catalase |
| MDA | malondialdehyde |
References
- Wang, J.; Song, L.; Gong, X.; Xu, J.; Li, M. Functions of Jasmonic Acid in Plant Regulation and Response to Abiotic Stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.A.; Uddin, M.; Khan, M.M.A.; Hakeem, K.R.; Jaleel, H. Jasmonates Counter Plant Stress: A Review. Environ. Exp. Bot. 2015, 115, 49–57. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Chen, J.; Yao, S.; Li, L.; Huang, R.; Tan, Y.; Ming, R.; Huang, Y. Genome-Wide Identification and Characterization of the JAZ Gene Family in Gynostemma Pentaphyllum Reveals the COI1/JAZ/MYC2 Complex Potential Involved in the Regulation of the MeJA-Induced Gypenoside Biosynthesis. Plant Physiol. Biochem. 2024, 214, 108952. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-Regulated Root Growth Inhibition and Root Hair Elongation. J. Exp. Bot. 2023, 74, 1176–1185. [Google Scholar] [CrossRef]
- Howe, G.A.; Major, I.T.; Koo, A.J. Modularity in Jasmonate Signaling for Multistress Resilience. Annu. Rev. Plant Biol. 2018, 69, 387–415. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Li, S.; Gao, X.; Zhang, K.; Shen, X. Transcriptome Analysis of Artemisia Argyi Following Methyl Jasmonate (MeJA) Treatment and the Mining of Genes Related to the Stress Resistance Pathway. Front. Genet. 2023, 14, 1279850. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The Roles of Methyl Jasmonate to Stress in Plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef]
- Jo, L.; Pelletier, J.M.; Harada, J.J. Central Role of the LEAFY COTYLEDON1 Transcription Factor in Seed Development. J. Integr. Plant Biol. 2019, 61, 564–580. [Google Scholar] [CrossRef]
- Iluz, D. Zoochory: The Dispersal of Plants by Animals. In All Flesh Is Grass: Plant-Animal Interrelationships; Springer: Dordrecht, The Netherlands, 2010; pp. 199–214. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Champagne, C.E.M.; Townsley, B.T.; Park, S.; Malhó, R.; Pedroso, M.C.; Harada, J.J.; Sinha, N.R. Evolution of Asexual Reproduction in Leaves of the Genus Kalanchoe. Proc. Natl. Acad. Sci. USA 2007, 104, 15578–15583. [Google Scholar] [CrossRef]
- Garcês, H.M.P.; Koenig, D.; Townsley, B.T.; Kim, M.; Sinha, N.R. Truncation of LEAFY COTYLEDON1 Protein Is Required for Asexual Reproduction in Kalanchoë daigremontiana. Plant Physiol. 2014, 165, 196–206. [Google Scholar] [CrossRef][Green Version]
- Corredoira, E.; Merkle, S.A.; Martínez, M.T.; Toribio, M.; Canhoto, J.M.; Correia, S.I.; Ballester, A.; Vieitez, A.M. Non-Zygotic Embryogenesis in Hardwood Species. Crit. Rev. Plant Sci. 2019, 38, 29–97. [Google Scholar] [CrossRef]
- Corredoira, E.; Toribio, M.; Vieitez, A.M. Clonal Propagation via Somatic Embryogenesis in Quercus spp. In Tree Biotechnology; CRC Press: Boca Raton, FL, USA, 2014; pp. 262–302. [Google Scholar] [CrossRef]
- Martínez, M.T.; San-José, M.d.C.; Arrillaga, I.; Cano, V.; Morcillo, M.; Cernadas, M.J.; Corredoira, E. Holm Oak Somatic Embryogenesis: Current Status and Future Perspectives. Front. Plant Sci. 2019, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Pipatchananan, P.; Lithanatudom, P.; Jaitan, N.; Patawang, I.; Lithanatudom, S.K. Karyological Analysis in Eight Cultivars of Dimocarpus longan and a Rare Species, Dimocarpus obtusus (Sapindaceae). Cytologia 2022, 87, 231–238. [Google Scholar] [CrossRef]
- Afrin, M.; Ahmed, S.; Ahamed, T.; Rahman, M.M.M.; Khatun, S.M.; Mohiuddin, A.K.M.; Shohael, A.M. Optimization of Plant Growth Regulators for Efficient Embryogenic Callus Induction and Subsequent Plant Regeneration in Two Indigenous Aromatic Rice Varieties of Bangladesh. Plant Tissue Cult. Biotechnol. 2024, 34, 153–164. [Google Scholar] [CrossRef]
- Lai, Z.; Lin, Y. Analysis of the Global Transcriptome of Longan (Dimocarpus longan Lour.) Embryogenic Callus Using Illumina Paired-End Sequencing. BMC Genom. 2013, 14, 561. [Google Scholar] [CrossRef]
- Lai, Z.X.; Pan, L.Z.; Chen, Z.G. Establishment and maintenance of longan embryogenic cell lines. J. Fujian Agric. Univ. 1997, 2, 33–40. [Google Scholar]
- Lai, Z.; Chen, Z. Somatic embryogenesis of high frequency from longan embryogenic calli. J. Fujian Agric. Univ. 1997, 26, 271–276. [Google Scholar]
- Koornneef, M.; Reuling, G.; Karssen, C.M. The Isolation and Characterization of Abscisic Acid-insensitive Mutants of Arabidopsis Thaliana. Physiol. Plant 1984, 61, 377–383. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Vashisth, A.; Singh, V.K.; Sirohi, P.; Tomar, R.S. Genome-Wide Study of the ABI3 Gene Family and Identification of Putative miRNA Targeting ABI3 Gene in Oryza sativa ssp. indica. Plants 2021, 10, 2036. [Google Scholar] [CrossRef]
- Bedi, S.; Sengupta, S.; Ray, A.; Chaudhuri, R.N. ABI3 Mediates Dehydration Stress Recovery Response in Arabidopsis thaliana by Regulating Expression of Downstream Genes. Plant Sci. 2016, 250, 125–140. [Google Scholar] [CrossRef]
- Suzuki, M.; Kao, C.Y.; McCarty, D.R. The Conserved B3 Domain of VIVIPAROUS1 Has a Cooperative DNA Binding Activity. Plant Cell 1997, 9, 799–807. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Signora, L.; Beeckman, T.; Inzé, D.; Foyer, C.H.; Zhang, H. An Abscisic Acid-Sensitive Checkpoint in Lateral Root Development of Arabidopsis. Plant J. 2003, 33, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Siklnik-Inbar, D.; Bar-Zvi, D. ABI4 Mediates Abscisic Acid and Cytokinin Inhibition of Lateral Root Formation by Reducing Polar Auxin Transport in Arabidopsis. Plant Cell 2010, 22, 3560–3573. [Google Scholar] [CrossRef]
- Skubacz, A.; Daszkowska-Golec, A.; Szarejko, I. The Role and Regulation of ABI5 (ABA-Insensitive 5) in Plant Development, Abiotic Stress Responses and Phytohormone Crosstalk. Front. Plant Sci. 2016, 7, 1884. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Lynch, T.J. The Arabidopsis Abscisic Acid Response Gene ABI5 Encodes a Basic Leucine Zipper Transcription Factor. Plant Cell 2000, 12, 599–609. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Seeley, K.A.; Sung, Z.R. The Role of the Arabidopsis ELD1 Gene in Cell Development and Photomorphogenesis in Darkness. Plant Physiol. 2000, 123, 509–520. [Google Scholar] [CrossRef]
- Brocard-Gifford, I.; Lynch, T.J.; Garcia, M.E.; Malhotra, B.; Finkelstein, R.R. The Arabidopsis thaliana ABSCISIC ACID-INSENSITIVE8 Locus Encodes a Novel Protein Mediating Abscisic Acid and Sugar Responses Essential for Growth. Plant Cell 2004, 16, 406–421. [Google Scholar] [CrossRef]
- Perruc, E.; Kinoshita, N.; Lopez-Molina, L. The Role of Chromatin-Remodeling Factor PKL in Balancing Osmotic Stress Responses during Arabidopsis Seed Germination. Plant J. 2007, 52, 927–936. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Wang, W.; Xu, D. Computational Methods for Protein Localization Prediction. Comput. Struct. Biotechnol. J. 2021, 19, 5834–5844. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and Validation of Promoters and Cis-Acting Regulatory Elements. Plant Sci. 2014, 217, 109–119. [Google Scholar] [CrossRef]
- Walther, D.; Brunnemann, R.; Selbig, J. The Regulatory Code for Transcriptional Response Diversity and Its Relation to Genome Structural Properties in A. thaliana. PLoS Genet. 2007, 3, e11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; Wang, X.; Sun, Y.; Joseph, P.V.; Paterson, A.H. Detection of Colinear Blocks and Synteny and Evolutionary Analyses Based on Utilization of MCScanX. Nat. Protoc. 2024, 19, 2206–2229. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, C.; Shan, H.; Kong, H. Divergence of Duplicate Genes in Exon–Intron Structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Lyons, E.; Pedersen, B.; Kane, J.; Alam, M.; Ming, R.; Tang, H.; Buelow, M.; Freeling, M.; Rounsley, S.D.; Deynze, A.V. Finding and Comparing Syntenic Regions among Arabidopsis and the Outgroups Papaya, Poplar, and Grape: CoGe with Rosids. Plant Physiol. 2008, 148, 1772–1781. [Google Scholar] [CrossRef]
- Waltner, J.K.; Peterson, F.C.; Lytle, B.L.; Volkman, B.F. Structure of the B3 Domain from Arabidopsis thaliana Protein At1g16640. Protein Sci. 2005, 14, 2478–2483. [Google Scholar] [CrossRef]
- Hofmann, F.; Schon, M.A.; Nodine, M.D. The Embryonic Transcriptome of Arabidopsis thaliana. Plant Reprod. 2019, 32, 77–91. [Google Scholar] [CrossRef]
- Indoliya, Y.; Tiwari, P.; Chauhan, A.S.; Goel, R.; Shri, M.; Bag, S.K.; Chakrabarty, D. Decoding Regulatory Landscape of Somatic Embryogenesis Reveals Differential Regulatory Networks between Japonica and Indica Rice Subspecies. Sci. Rep. 2016, 6, 23050. [Google Scholar] [CrossRef]
- Schweighofer, A.; Hirt, H.; Meskiene, I. Plant PP2C Phosphatases: Emerging Functions in Stress Signaling. Trends Plant Sci. 2004, 9, 236–243. [Google Scholar] [CrossRef]
- Axtell, M.J.; Snyder, J.A.; Bartel, D.P. Common Functions for Diverse Small RNAs of Land Plants. Plant Cell 2007, 19, 1750–1769. [Google Scholar] [CrossRef]
- Mazzella, M.A.; Arana, M.V.; Staneloni, R.J.; Perelman, S.; Rodriguez Batiller, M.J.; Muschietti, J.; Cerdan, P.D.; Chen, K.; Sanchez, R.A.; Zhu, T.; et al. Phytochrome Control of the Arabidopsis Transcriptome Anticipates Seedling Exposure to Light. Plant Cell 2005, 17, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.M.; Lynch, T.J.; Mobin, R.; Finkelstein, R.R. Direct Targets of the Transcription Factors ABA-Insensitive (ABI)4 and ABI5 Reveal Synergistic Action by ABI4 and Several bZIP ABA Response Factors. Plant Mol. Biol. 2011, 75, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhu, C.; Lyu, Y.; Chen, Y.; Zhang, Z.; Lai, Z.; Lin, Y. Genome-Wide Identification, Molecular Evolution, and Expression Analysis Provide New Insights into the APETALA2/Ethylene Responsive Factor (AP2/ERF) Superfamily in Dimocarpus longan Lour. BMC Genom. 2020, 21, 62. [Google Scholar] [CrossRef]
- Nakamura, S.; Lynch, T.J.; Finkelstein, R.R. Physical Interactions between ABA Response Loci of Arabidopsis. Plant J. 2001, 26, 627–635. [Google Scholar] [CrossRef]
- Chiu, R.S.; Nahal, H.; Provart, N.J.; Gazzarrini, S. The Role of the Arabidopsis FUSCA3 Transcription Factor during Inhibition of Seed Germination at High Temperature. BMC Plant Biol. 2012, 12, 15. [Google Scholar] [CrossRef]
- McSteen, P.; Zhao, Y. Plant Hormones and Signaling: Common Themes and New Developments. Dev. Cell 2008, 14, 467–473. [Google Scholar] [CrossRef]
- Kim, O.-T.; Bang, K.-H.; Shin, Y.-S.; Lee, M.-J.; Jung, S.-J.; Hyun, D.-Y.; Kim, Y.-C.; Seong, N.-S.; Cha, S.-W.; Hwang, B. Enhanced Production of Asiaticoside from Hairy Root Cultures of Centella asiatica (L.) Urban Elicited by Methyl Jasmonate. Plant Cell Rep. 2007, 26, 1941–1949. [Google Scholar] [CrossRef]
- Kamińska, M. Role and Activity of Jasmonates in Plants under in Vitro Conditions. Plant Cell Tissue Organ Cult. (PCTOC) 2021, 146, 425–447. [Google Scholar] [CrossRef]
- Schlögl, P.S.; Nogueira, F.T.S.; Drummond, R.; Felix, J.M.; De Rosa, V.E., Jr.; Vicentini, R.; Menossi, M.; Ulian, E.C. Identification of New ABA- and MEJA-Activated Sugarcane bZIP Genes by Data Mining in the SUCEST Database. Plant Cell Rep. 2008, 27, 335–345. [Google Scholar] [CrossRef]
- Tao, L.; Yang, Y.; You, X.; Wang, Q. Stress-Related Genes and Proteins of Somatic Embryogenesis. Afr. J. Biotechnol. 2012, 11, 11728–11735. [Google Scholar] [CrossRef]
- Horstman, A.; Bemer, M.; Boutilier, K. A Transcriptional View on Somatic Embryogenesis. Regeneration 2017, 4, 201–216. [Google Scholar] [CrossRef]
- Karami, O.; Saidi, A. The Molecular Basis for Stress-Induced Acquisition of Somatic Embryogenesis. Mol. Biol. Rep. 2010, 37, 2493–2507. [Google Scholar] [CrossRef]
- Yao, D.; Chen, Y.; Xu, X.; Lin, Y.; Lai, Z. Exploring the Effect of Methyl Jasmonate on the Expression of microRNAs Involved in Biosynthesis of Active Compounds of Rosemary Cell Suspension Cultures through RNA-Sequencing. Int. J. Mol. Sci. 2022, 23, 3704. [Google Scholar] [CrossRef]
- Kępczyńska, E.; Ruduś, I.; Kępczyński, J. Abscisic Acid and Methyl Jasmonate as Regulators of Ethylene Biosynthesis during Somatic Embryogenesis of Medicago sativa L. Acta Physiol. Plant. 2009, 31, 1263–1270. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, G.; Pan, D.; Wang, X.; Han, Q.; Qin, Y.; Li, K.; Huang, G. Analysis of the Plant Hormone Expression Profile during Somatic Embryogenesis Induction in Teak (Tectona grandis). Front. Plant Sci. 2024, 15, 1429575. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shariatpanahi, M.E.; Teixeira da Silva, J.A. Efficient Induction of Microspore Embryogenesis Using Abscisic Acid, Jasmonic Acid and Salicylic Acid in Brassica napus L. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 116, 343–351. [Google Scholar] [CrossRef]
- Blázquez, S.; Piqueras, A.; Serna, M.D.; Casas, J.L.; Fernández, J.A. Somatic Embryogenesis in Saffron: Optimisation through Temporary Immersion and Polyamine Metabolism. Acta Hortic. 2004, 650, 269–276. [Google Scholar] [CrossRef]
- Białecka, B.; Kępczyński, J. Regulation of α-Amylase Activity in Amaranthus caudatus Seeds by Methyl Jasmonate, Gibberellin A3, Benzyladenine and Ethylene. Plant Growth Regul. 2003, 39, 51–56. [Google Scholar] [CrossRef]
- Ali, M.B.; Yu, K.-W.; Hahn, E.-J.; Paek, K.-Y. Methyl Jasmonate and Salicylic Acid Elicitation Induces Ginsenosides Accumulation, Enzymatic and Non-Enzymatic Antioxidant in Suspension Culture Panax ginseng Roots in Bioreactors. Plant Cell Rep. 2006, 25, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; Polverino de Laureto, P.; Caruso, C. Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. Int. J. Mol. Sci. 2019, 20, 2525. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Guo, Y.; Yang, M.; Zhang, X.; Lin, Y.; Cai, D.; Lan, S.; Tang, M.; Ma, W.; Wang, S.; et al. The bZIP20 Transcription Factor Enhances Thermotolerance in Dimocarpus longan by Maintaining ROS Homeostasis and Involving the MeJA Pathway. Plant Physiol. Biochem. 2025, 223, 109945. [Google Scholar] [CrossRef]
- Selwal, N.; Supriadi, K.; Rahayu, F.; Sukmadjaja, D.; Khamidah, A.; Budiaarto, K.; Purnobasuki, H. Elicitation Strategies for Enhanced Secondary Metabolite Synthesis in Plant Cell Cultures and Its Role in Plant Defense Mechanism. Plant Gene 2025, 41, 100485. [Google Scholar] [CrossRef]
- Mangas, S.; Bonfill, M.; Osuna, L.; Moyano, E.; Tortoriello, J.; Cusido, R.M.; Piñol, M.T.; Palazón, J. The Effect of Methyl Jasmonate on Triterpene and Sterol Metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca Cultured Plants. Phytochemistry 2006, 67, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Demirci, T. Determination of Secondary Metabolite Production Efficiency in Echinacea purpurea Callus, Shoot, and Root in Vitro Cultures with Methyl Jasmonate Applications. Acta Physiol. Plant 2022, 44, 128. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Mahran, E.; Chowdhury, K.; Boroujerdi, A.; El-Bakry, A. Effect of Exogenous Methyl Jasmonate on in Vitro Propagation, Metabolic Profiling and Proximadiol Production from Cymbopogon schoenanthus subsp. proximus. Plant Physiol. Rep. 2021, 26, 548–560. [Google Scholar] [CrossRef]
- Gao, E.; Zhao, Y.; Wu, M.; Qu, X.; Wu, X.; Guo, W.; Zhang, J.; Zhang, S.; Wu, X.; Wang, H.; et al. Enhanced Storage Lipid Biosynthesis Promotes Somatic Embryogenesis in Citrus. Plant Physiol. 2025, 197, kiaf049. [Google Scholar] [CrossRef]
- Kersey, P.J.; Allen, J.E.; Allot, A.; Barba, M.; Boddu, S.; Bolt, B.J.; Carvalho-Silva, D.; Christensen, M.; Davis, P.; Grabmueller, C.; et al. Ensembl Genomes 2018: An Integrated Omics Infrastructure for Non-Vertebrate Species. Nucleic Acids Res. 2018, 46, D802–D808. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; He, Y.; Xia, R. TBtools, a Toolkit for Biologists Integrating Various Biological Data Handling Tools with a User-Friendly Interface. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein Identification and Analysis Tools on the ExPASy Server. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent Updates to the Phylogenetic Tree Display and Annotation Tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Li, H.; Lyu, Y.; Chen, X.; Wang, C.; Yao, D.; Ni, S.; Huang, X.; Lai, Z.; Lin, Y. Exploration of the Effect of Blue Light on Functional Metabolite Accumulation in Longan Embryonic Calli via RNA Sequencing. Int. J. Mol. Sci. 2019, 20, 441. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, C.; Lai, C.; Zhang, Z.; Wu, J.; Su, Q.; Lai, Z.; Lin, Y. Genome-Wide Identification and Expression Analysis of Bx Involved in Benzoxazinoids Biosynthesis Revealed the Roles of DIMBOA during Early Somatic Embryogenesis in Dimocarpus longan Lour. Plants 2024, 13, 1373. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Lai, Z.X. Reference Gene Selection for qPCR Analysis during Somatic Embryogenesis in Longan Tree. Plant Sci. 2010, 178, 359–365. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, T.; Deng, L.; Lv, D.; Zhang, X.; Pan, X.; Zhou, Y.; Liu, P.; Zhang, J.; Li, M.; et al. Visualizing the Essential Role of Complete Virion Assembly Machinery in Efficient Hepatitis C Virus Cell-to-Cell Transmission by a Viral Infection-Activated Split-Intein-Mediated Reporter System. J. Virol. 2017, 91, e01720-16. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awais, M.; Xu, X.; Zhang, C.; Chen, Y.; Liu, S.; Lin, Y.; Lai, Z. Genome-Wide Identification of ABSCISIC ACID-INSENSITIVE (ABI) Genes and Their Response to MeJA During Early Somatic Embryogenesis in Longan (Dimocarpus longan L.). Plants 2025, 14, 3508. https://doi.org/10.3390/plants14223508
Awais M, Xu X, Zhang C, Chen Y, Liu S, Lin Y, Lai Z. Genome-Wide Identification of ABSCISIC ACID-INSENSITIVE (ABI) Genes and Their Response to MeJA During Early Somatic Embryogenesis in Longan (Dimocarpus longan L.). Plants. 2025; 14(22):3508. https://doi.org/10.3390/plants14223508
Chicago/Turabian StyleAwais, Muhammad, Xiaoqiong Xu, Chunyu Zhang, Yukun Chen, Shengcai Liu, Yuling Lin, and Zhongxiong Lai. 2025. "Genome-Wide Identification of ABSCISIC ACID-INSENSITIVE (ABI) Genes and Their Response to MeJA During Early Somatic Embryogenesis in Longan (Dimocarpus longan L.)" Plants 14, no. 22: 3508. https://doi.org/10.3390/plants14223508
APA StyleAwais, M., Xu, X., Zhang, C., Chen, Y., Liu, S., Lin, Y., & Lai, Z. (2025). Genome-Wide Identification of ABSCISIC ACID-INSENSITIVE (ABI) Genes and Their Response to MeJA During Early Somatic Embryogenesis in Longan (Dimocarpus longan L.). Plants, 14(22), 3508. https://doi.org/10.3390/plants14223508









