Effect of Brassinolide on the Growth and Physiological Indicators of Foxtail Millet Under Cyhalofop-Butyl Damage
Abstract
1. Introduction
2. Results
2.1. Effect of BR Spraying on Agronomic Traits of Jingu 21 Under Cyhalofop-Butyl Damage
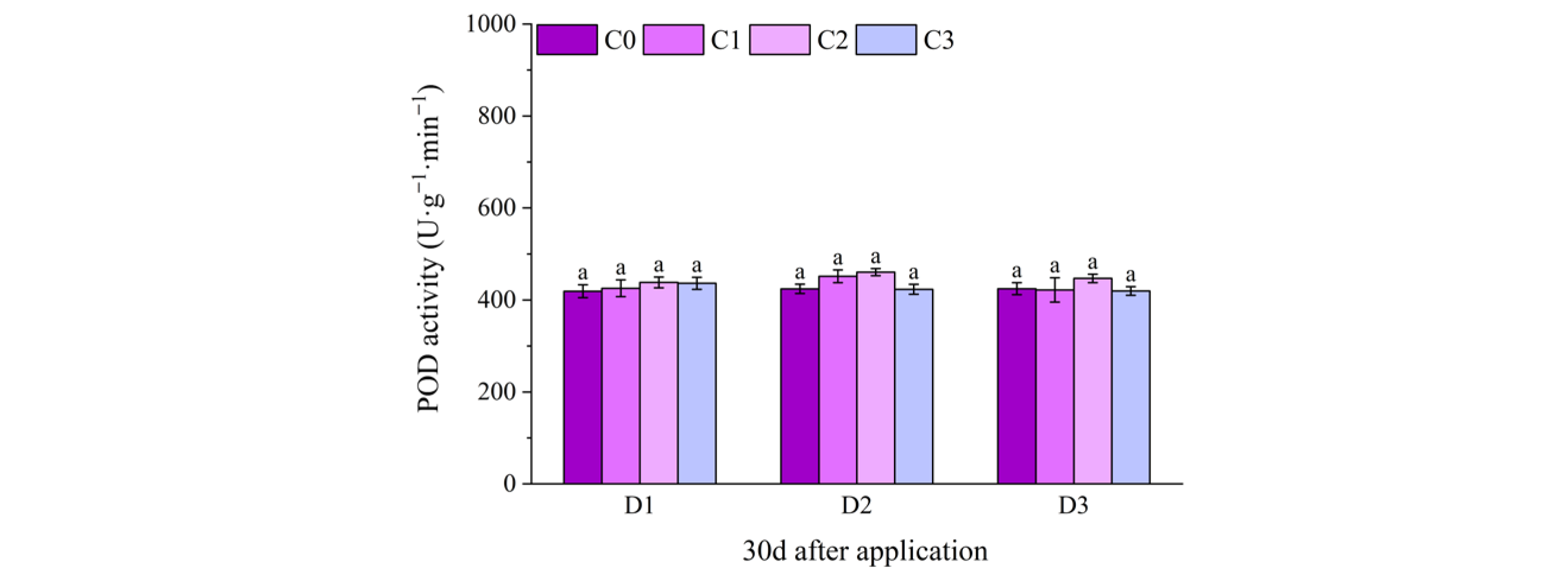
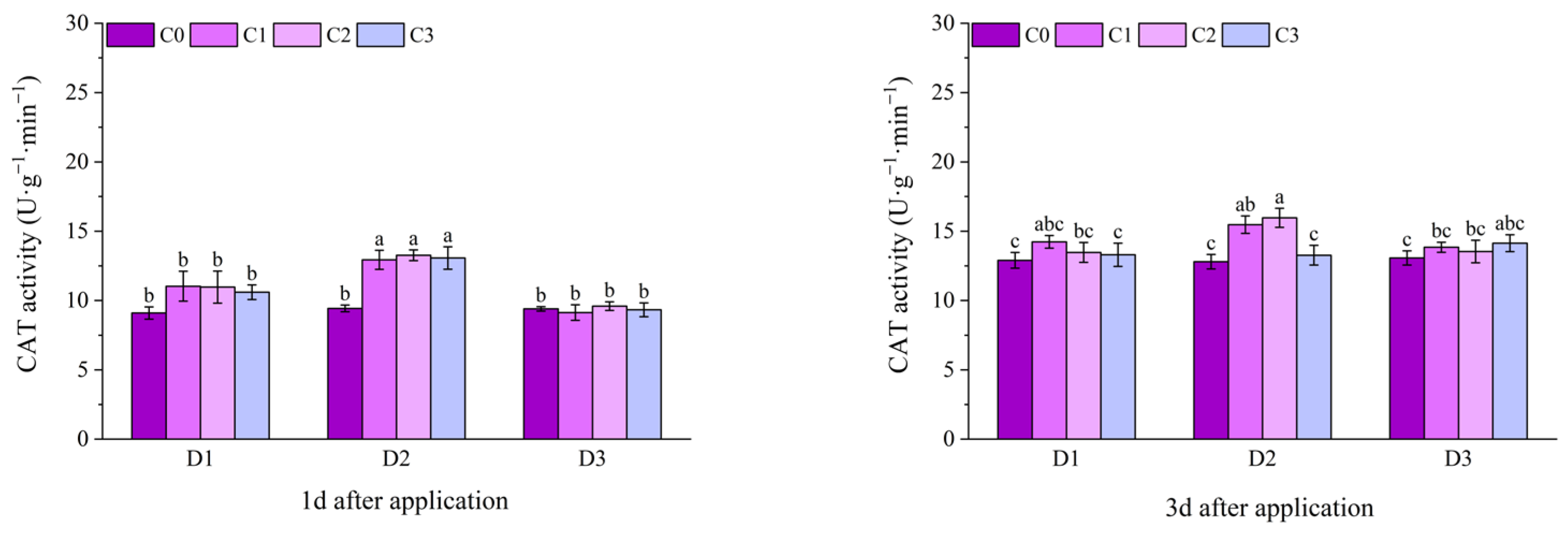
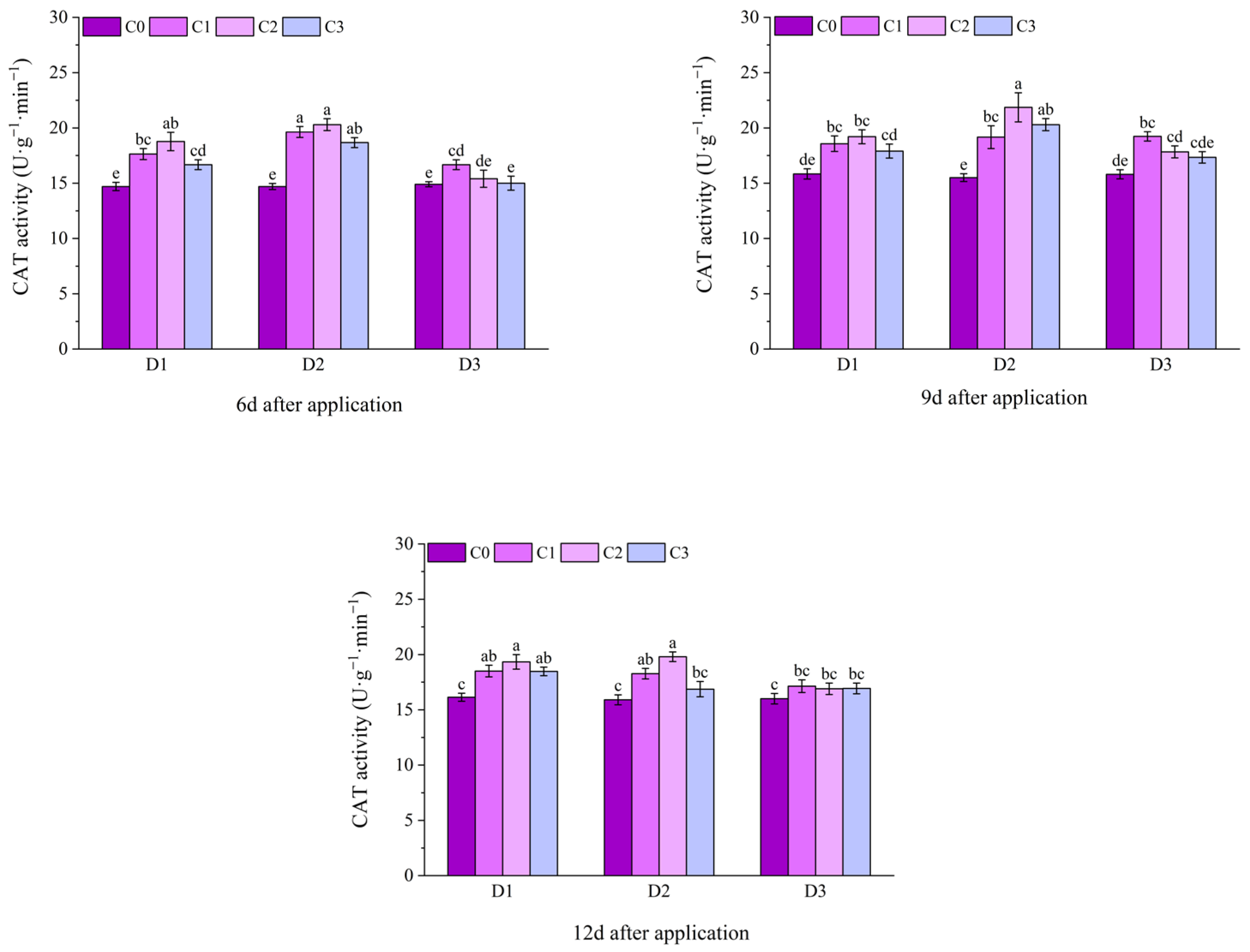
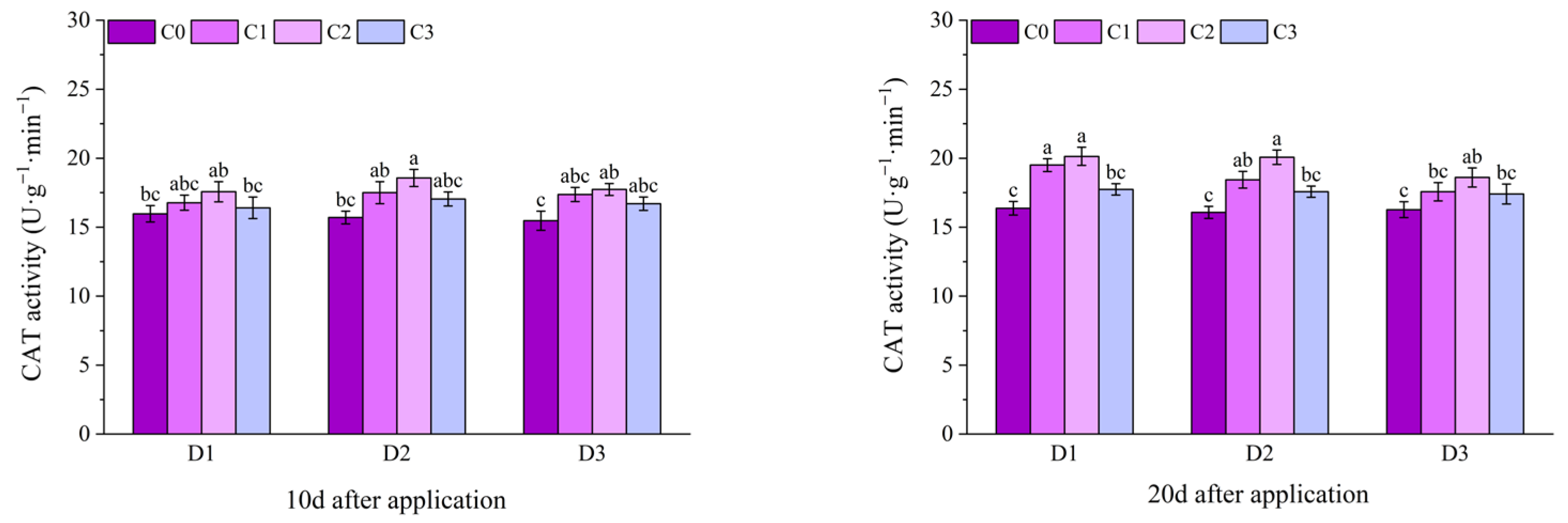
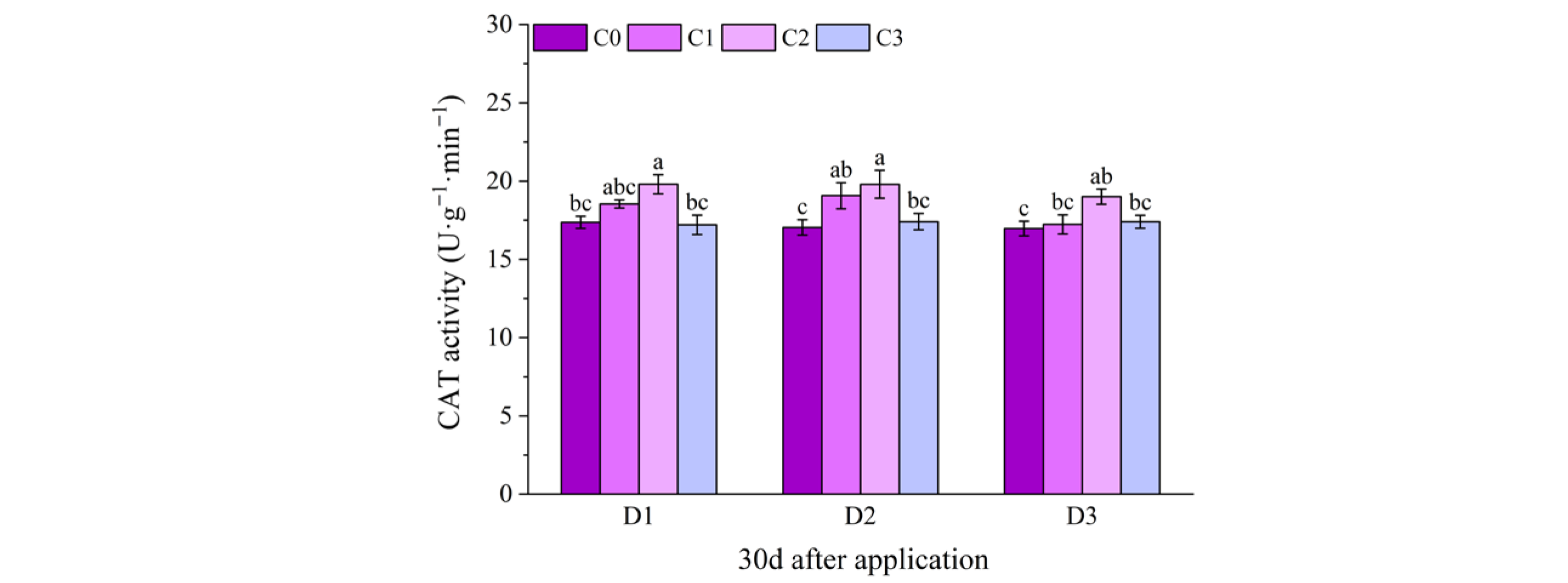
2.2. Effect of BR Spraying on Antioxidant Enzymes of Jingu 21 Under Cyhalofop-Butyl Damage
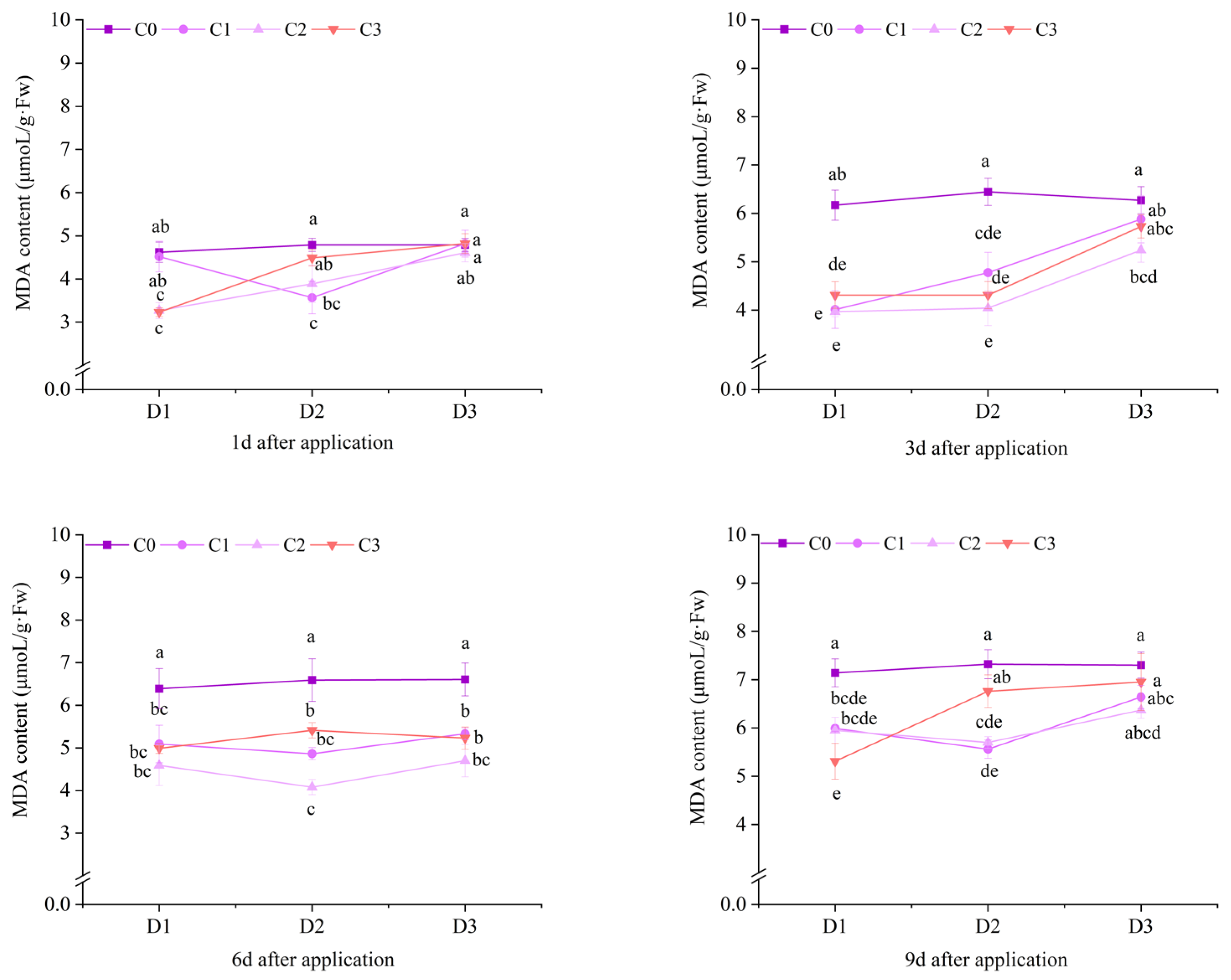
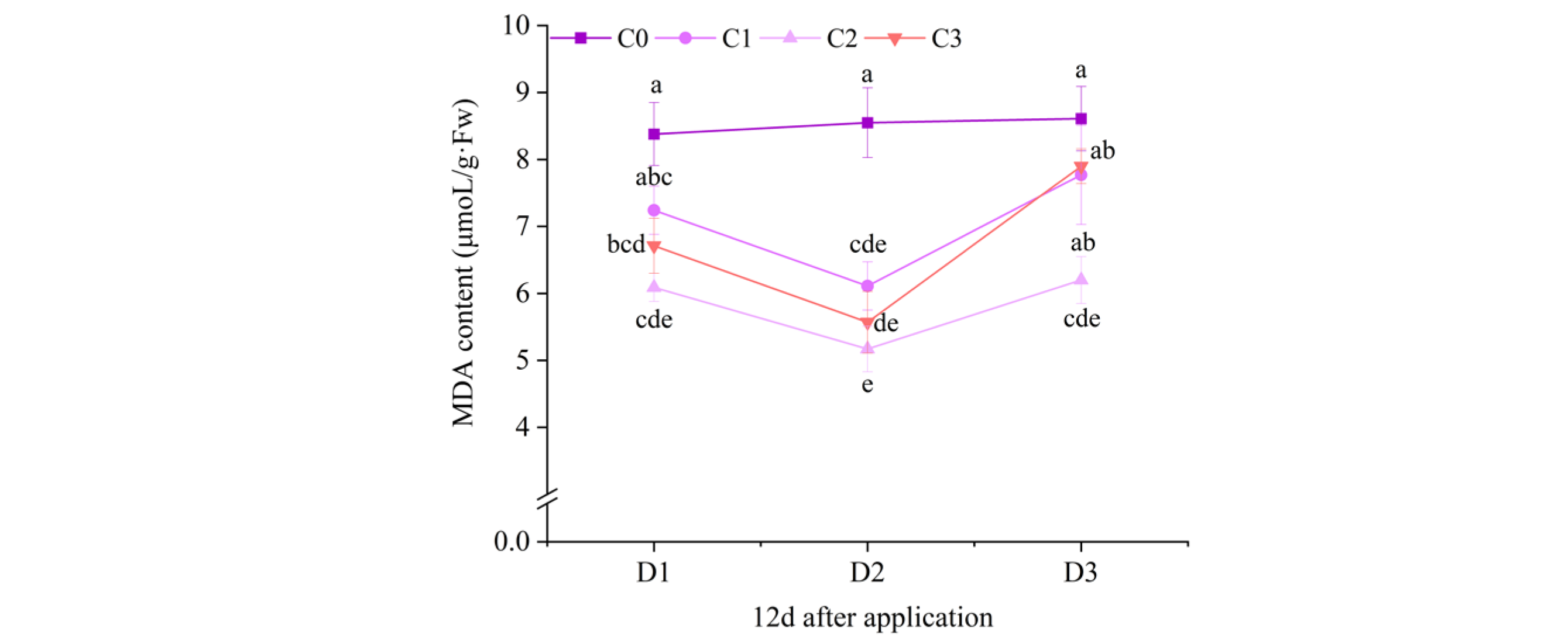
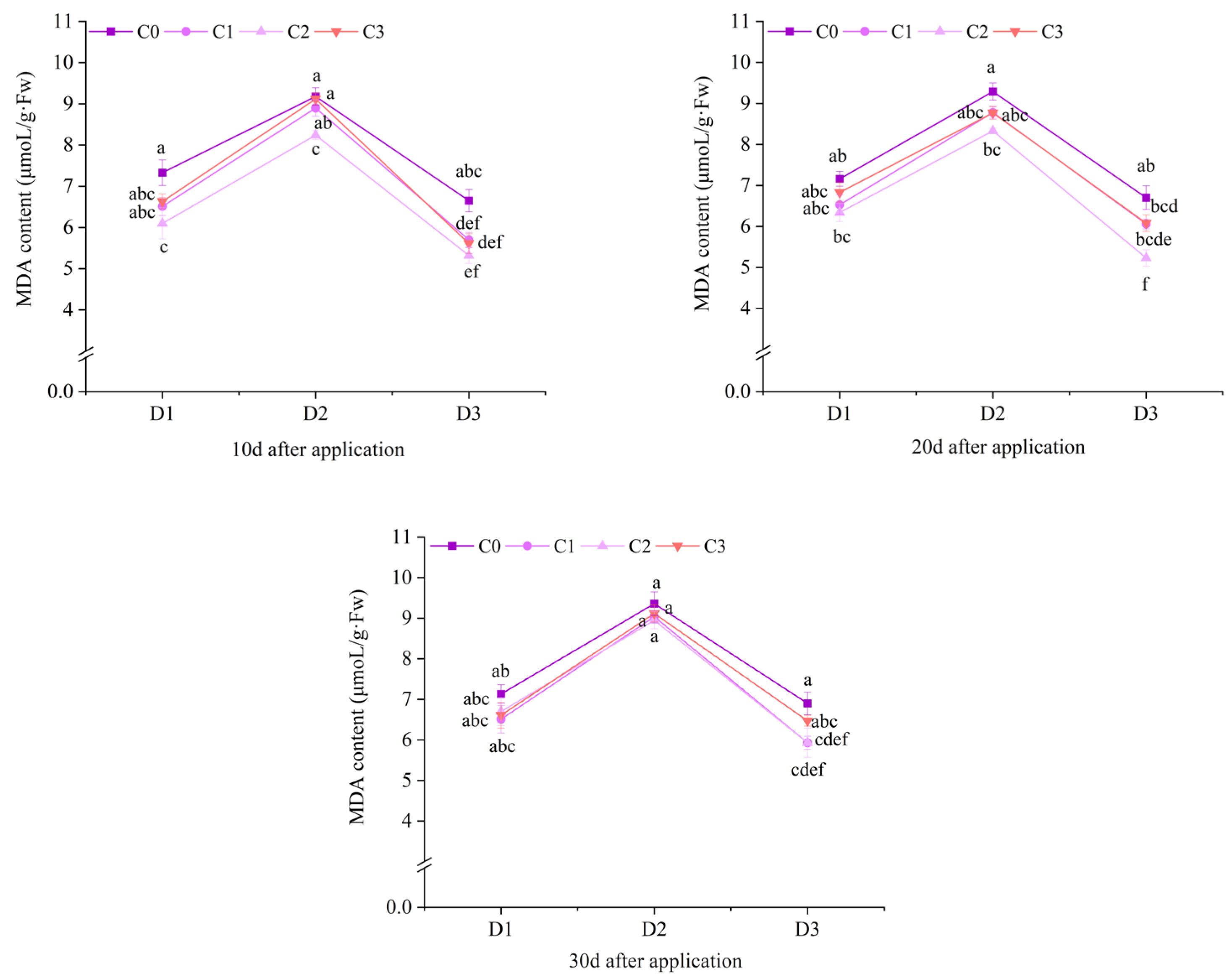
2.3. Effect of BR Spraying on MDA Content of Jingu 21 Under Cyhalofop-Butyl Damage
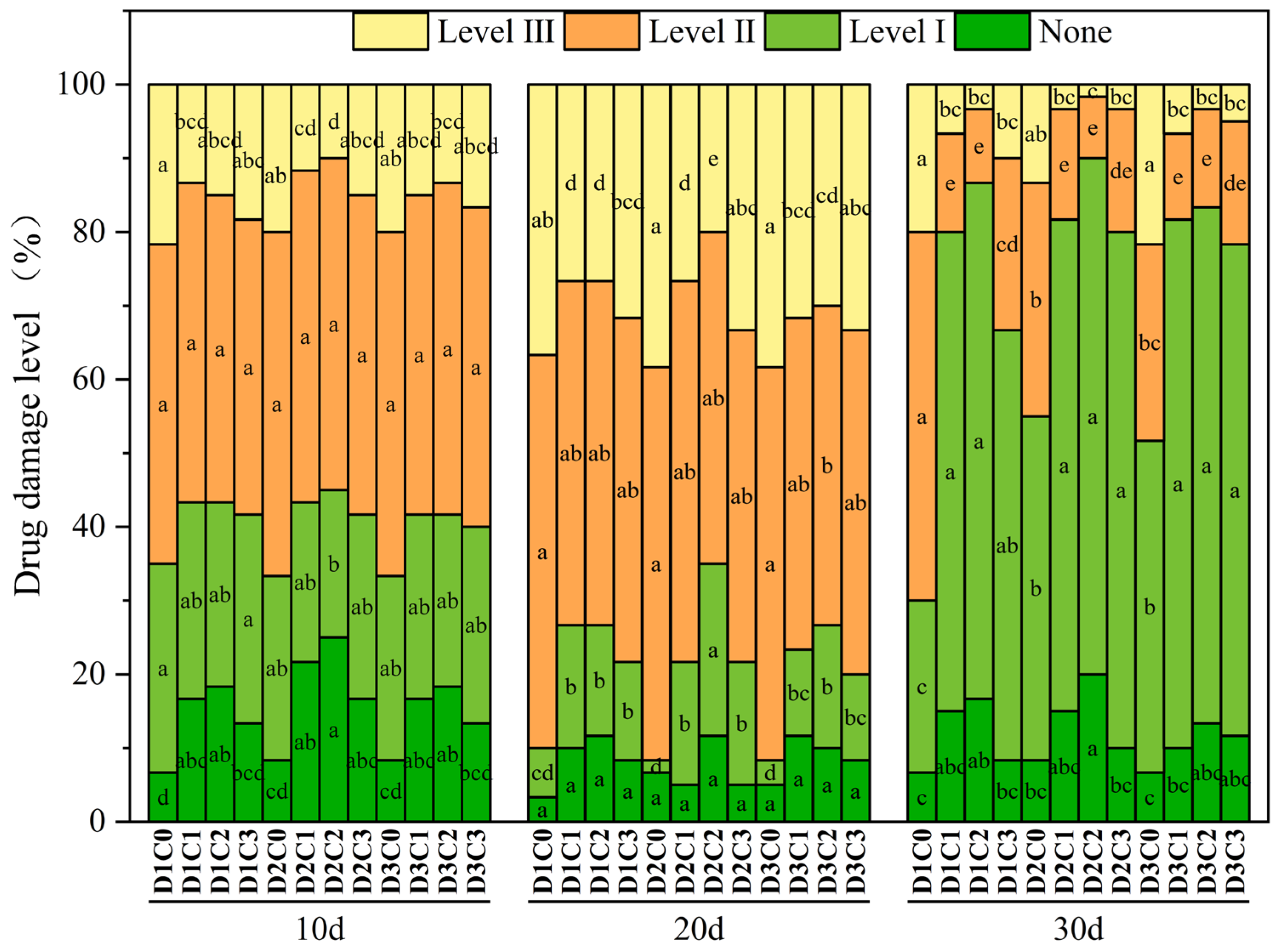
2.4. Effect of BR Spraying on Drug Damage Level of Jingu 21 Under Cyhalofop-Butyl Damage
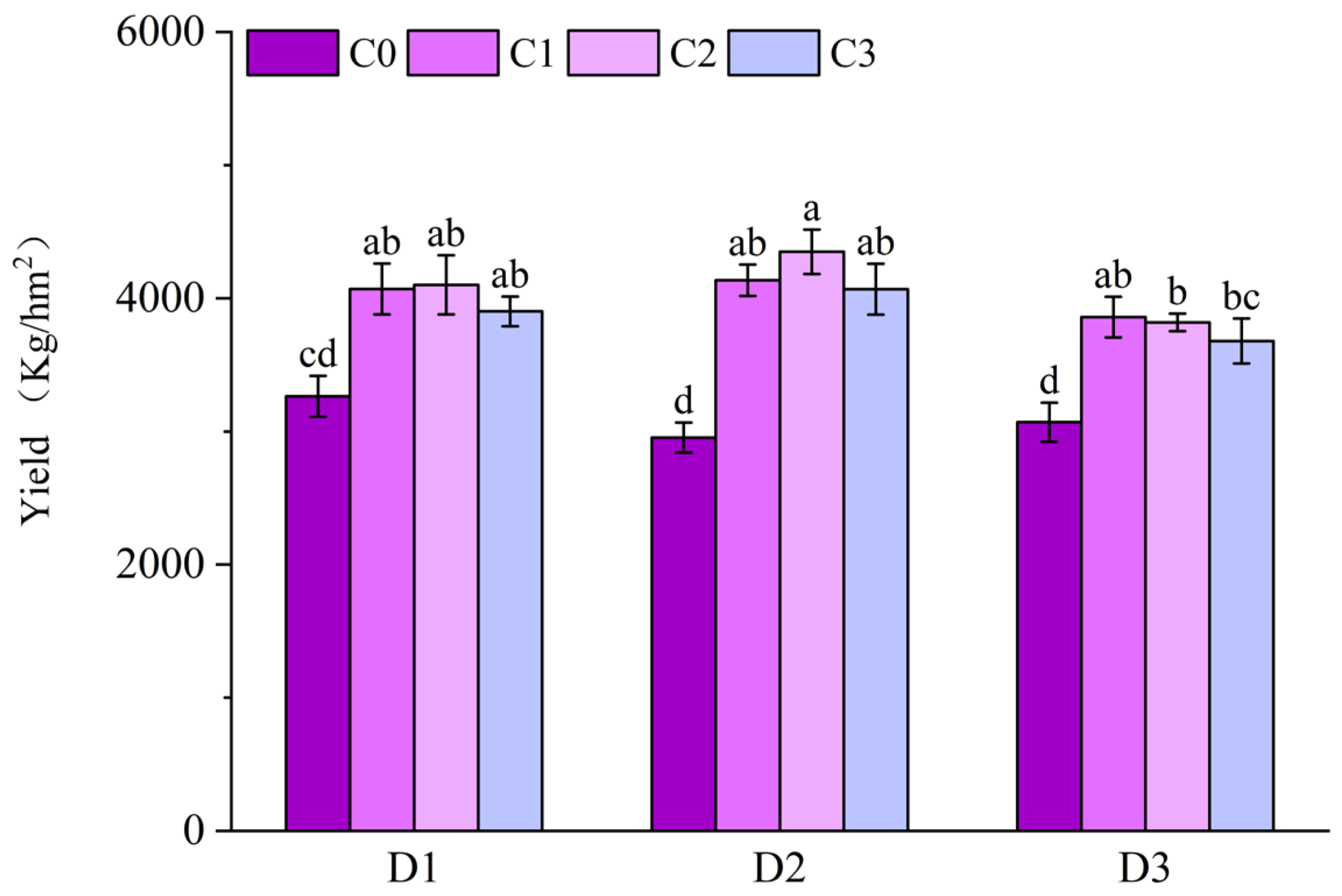
2.5. Effect of BR Spraying on Yield of Jingu 21 Under Cyhalofop-Butyl Damage
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Design
4.3. Test Indicators and Analysis Methods
4.3.1. Agronomic Traits
4.3.2. Antioxidant Enzyme Activities and MDA Content
4.3.3. Drug Damage Level
- None: The plants grow normally, the leaves are healthy, and there is no obvious damage.
- Level I: Less than 20% of the leaves of foxtail millet are withered and yellow or have spots.
- Level II: 20–50% of the leaves of foxtail millet are withered, yellow, and curled.
- Level III: 50–70% of the leaves of foxtail millet are withered, yellow, and deformed.
- Level IV: More than 70% of the leaves of foxtail millet are withered or the plants die.
4.3.4. Yield
4.4. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atzberger, C.; Guérif, M.; Baret, F.; Werner, W. Comparative analysis of three chemometric techniques for the spectroradiometric assessment of canopy chlorophyll content in winter wheat. Comput. Electron. Agric. 2010, 73, 165–173. [Google Scholar] [CrossRef]
- Cutti, L.; Rigon, C.A.G.; Girelli, N.; Angonese, P.S.; Ulguim, A.D.R.; Merotto, A. The safener isoxadifen-ethyl confers fenoxaprop-p-ethyl resistance on a biotype of Echinochloa crus-galli. Pest. Manag. Sci. 2022, 78, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Bera, A.; Panda, D. Response of Different Sources of Phosphate Fertilizers and Homo-brassinolide on Total Chlorophyll Content, Yield Attributes and Yield of Hybrid Rice under Lateritic Zone of West Bengal, India. Int. J. Bio-Resour. Stress. Manag. 2013, 4, 14–18. [Google Scholar]
- Nakagawa, Y.; Nishikawa, B.; Miyagawa, H. Effects of brassinolide on the growing of rice plants. J. Pestic. Sci. 2021, 46, 274–277. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.; Wang, J.; Liang, D.; Xia, H.; Lv, X.; Tang, Y.; Wang, X.; Deng, Q.; Liao, M. 24-epibrassinolide promotes selenium uptake in grapevine under selenium stress. Sci. Hortic. 2023, 308, 111564. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, Y.; Hu, Z.; Gao, Y.; Zhang, Y.; Chen, M.; Khaldun, A.B.M.; Yan, X.; Fan, J. Transcriptomic profiling revealed the role of 24-epibrassinolide in alleviating salt stress damage in tall fescue (Festuca arundinacea). Front. Plant Sci. 2022, 13, 976341. [Google Scholar] [CrossRef]
- Lu, R.; Zhang, J.; Liu, D.; Wei, Y.; Wang, Y.; Li, X. Characterization of bHLH/HLH genes that are involved in Brassinolide (BR) signaling in fiber development of cotton (Gossypium hirsutum). BMC Plant Biol. 2018, 18, 304. [Google Scholar] [CrossRef]
- Zhong, X.; Lan, R.; Su, G.; Hao, L.; Xu, H.; Zhou, H.; Zhou, X. Enhancing the salt stress resistance of seeds and seedlings via a brassinolide sustained release agent system. Chem. Biol. Technol. Agric. 2023, 10, 140. [Google Scholar] [CrossRef]
- Chakma, S.P.; Chileshe, S.M.; Thomas, R.; Krishna, P. Cotton Seed Priming with Brassinolide Promotes Germination and Seedling Growth. Agronomy 2021, 11, 566. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, D.; Li, K.; Liu, Z.; Liu, X.; Song, C.; Li, G.; Zhao, C.; Ma, J.; Han, M. Effect of exogenous Brassinolide (BR) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Plant Growth Regul. 2017, 82, 391–401. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Wang, M.; Du, Y.; Wu, D.; Chu, J.; Yao, X. Exogenous brassinolide improves the antioxidant capacity of Pinellia ternata by enhancing the enzymatic and nonenzymatic defense systems under non-stress conditions. Front. Plant Sci. 2022, 13, 917301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, L.; Yuan, C.; Zhai, K.; Xia, W.; Duan, Y.; Zhao, B.; Chu, J.; Yao, X. Brassinolide as potential rescue agent for Pinellia ternata grown under microplastic condition: Insights into their modulatory role on photosynthesis, redox homeostasis, and AsA-GSH cycling. J. Hazard. Mater. 2024, 470, 134116. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.J.D.; Almeida, A.R.; Layane, S.D.S.; Alves, A.S.D.; Carlos, A.C.D.; Fátima, D.J.S. Physiological performance of brassinolide-conditioned green soybean seeds. S. Afr. J. Bot. 2024, 165, 237–245. [Google Scholar]
- Guo, C.; Zhang, Y.; Wu, D.; Wang, M.; Du, Y.; Chu, J.; Yao, X. Principal Component Analysis to Assess the Changes of Yield and Quality in Pinellia ternata at Different Stages after Brassinolide Treatments. Int. J. Mol. Sci. 2022, 23, 15375. [Google Scholar] [CrossRef]
- Nasser, S.E.; Sarhan, I.A. Effect of Foliar Nutrition with Brassinolide on Vegetative Growth Characteristics of Several Sesame Cultivars. IOP Conf. Ser. Earth and Environ. Sci. 2023, 1158, 062034. [Google Scholar] [CrossRef]
- Yadav, P.N.; Bahadur, V.; Singh, G.; Singh, V.N. Influence of Foliar Spray of Brassinolides (BR), Salicylic Acid (SA) and Gibberellic Acid (GA3) on Vegetative Growth and Flowering Parameters of Cucumber (Cucumis sativus L) cv. Arpit. Int. J. Environ. Clim. Change. 2022, 12, 607–615. [Google Scholar] [CrossRef]
- Narayan, K.M.; Jaswal, A.; Singh, A. Effect of Soil and Foliar Application of Humic Acid and Brassinolide on Morpho-physiological and Yield Parameters of Black Gram (Vigna mungo). Nat. Env. Poll. Tech. 2023, 22, 2201–2208. [Google Scholar] [CrossRef]
- Galal, A. 24-epibrassinolide application enhances growth and biochemical aspects of squash under salt stress conditions. Acta Biol. Hung. 2018, 69, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Ren, L.; Peng, T.; Xin, L.; Qian, H.; Fu, Z. Carbon dioxide enrichment and Brassinolide pretreatment alleviate chlorpyrifos damage under suboptimal light and temperature conditions in tomato. Sci. Hortic. 2015, 192, 256–263. [Google Scholar] [CrossRef]
- Tofighi, C.; Khavari-nejad, R.A.; Najafi, F.; Razavi, K.; Rejali, F. Brassinolide (BR) and arbuscular mycorrhizal (AM) fungi alleviate salinity in wheat. J. Plant Nutr. 2017, 40, 1091–1098. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Chu, W.; Chang, S.; Lin, J.; Zhang, C.; Li, J.; Liu, X.; Liu, Z.; Liu, D.; Yang, Q.; Zhao, D.; et al. Methyltransferase TaSAMT1 mediates wheat freezing tolerance by integrating Brassinolide and salicylic acid signaling. Plant Cell. 2024, 36, 2607–2628. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Xiang, J.; Zhang, Y.; Wang, Z.; Zhu, D.; Wang, J.; Zhang, Y.; Wang, Y. Rice spikelet formation inhibition caused by decreased sugar utilization under high temperature is associated with Brassinolide decomposition. Environ. Exp. Bot. 2021, 190, 104585. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of Brassinolides on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Sun, J.; Guo, R.; Jiang, Q.; Chen, C.; Gao, Y.; Jiang, M.; Shen, R.; Zhu, X.; Huang, J. Brassinolide decreases cadmium accumulation via regulating gibberellic acid accumulation and Cd fixation capacity of root cell wall in rice (Oryza sativa). J. Hazard. Mater. 2024, 469, 133862. [Google Scholar] [CrossRef]
- Zhou, Y.; You, X.; Wang, X.; Cui, L.; Jiang, Z.; Zhang, K. Exogenous 24-Epibrassinolide Enhanced Drought Tolerance and Promoted Brassinolide-INSENSITIVE2 Expression of Quinoa. Plants 2024, 13, 873. [Google Scholar] [CrossRef]
- Garrido-Auñón, F.; Puente-Moreno, J.; García-Pastor, M.E.; Zapata, P.J.; Martínez-Romero, D.; Serrano, M.; Valero, D. Brassinolide preharvest treatments as a useful tool to increase crop yield and red colour in blood orange fruits. Front. Plant Sci. 2025, 16, 1654517. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yu, Z.; Zhang, G.; Yin, W.; Li, L.; Niu, M.; Meng, W.; Zhang, X.; Dong, N.; Liu, J.; et al. Diversification of plant agronomic traits by genome editing of Brassinolide signaling family genes in rice. Plant physiol. 2021, 187, 2563–2576. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Qi, S.; Zhang, X.; Mu, H. Effects of Exogenous Brassinolide on Prolonging Flowering Period and Physiological Characteristics of Freesia hybrida Klatt. (Iridaceae). Russ. J. Plant Physiol. 2025, 72, 144. [Google Scholar] [CrossRef]
- Nie, S.; Nie, S.; Huang, S.; Wang, S.; Cheng, D.; Liu, J.; Lv, S.; Li, Q.; Wang, X. Enhancing Brassinosteroid Signaling via Overexpression of Tomato (Solanum lycopersicum) SlBRI1 Improves Major Agronomic Traits. Front. Plant Sci. 2017, 8, 1386. [Google Scholar] [CrossRef]
- Tian, Q.; Luan, J.; Guo, C.; Shi, X.; Deng, P.; Zhou, Z.; Zhang, W.; Shen, L. A bHLH protein, OsBIM1, positively regulates rice leaf angle by promoting Brassinolide signaling. Biochem. Biophys. Res. Commun. 2021, 578, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yang, Y.; Li, X.; Hu, L.; Sun, C.; Liu, X.; Wei, L.; Zhu, J. Brassinolide Promotes the Growth of Zanthixylum schinifolium by Improving Photosynthetic Efficiency and Antioxidant Capacity. Agronomy 2024, 14, 2892. [Google Scholar] [CrossRef]
- Hamed, M.A.; Abdullah, B.H. Effect of Brassinolide Levels on Some Growth of Sunflower Hellanthus annuus L. IOP Conf. Ser. Earth Environ. Sci. 2021, 761, 012084. [Google Scholar]
- Cui, J.; Zhang, L.; Li, Q.; Qi, Y.; Ma, J.; Guo, D.; Zhang, P.; Xu, Y.; Gu, Y.; Wang, H. Seed Dressing Containing Gibberellic Acid, Indole-3-Acetic Acid, and Brassinolide Improves Maize Seed Germination and Seedling Growth Under Cold Stress. Agronomy 2024, 14, 2933. [Google Scholar] [CrossRef]
- Yao, L.; Liu, S.; Shi, W.; Gan, Y.; Fan, M.; Rolland, F.; Bai, M.; Han, C. Dampened Nuclear Localization of SnRK1 by Brassinolide Signaling Inhibits Stomatal Development in Arabidopsis. Mol. Plant. 2025, 18, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ma, X.; Tang, D.; Chen, Y.; Chen, G.; Zou, J.; Tan, L.; Tang, Q.; Chen, W. Effects of Brassinolide on the Physiological Changes on Two Varieties of Tea Plants Under Salt Stress. Int. J. Mol. Sci. 2024, 25, 13445. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Wang, C.; Cai, Q.; Ma, Y.; Ma, K.; Xie, J. Effect of 2, 4-epizoobrassinolide on fruit growth and capsaicin metabolism in peppers (Capsicum annuum L.). Plant Physiol. Biochem. 2025, 229, 110466. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Ma, Y.; Wu, X.; Wang, W.; Liu, H.; Li, X. Brassinolide Coordinates with ROS, Auxin and Gibberellin to Promote Mesocotyl Elongation and Deep-Sowing Tolerance in Maize. Curr. Issues Mol. Biol. 2025, 47, 668. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Ren, Y.; Jiang, Z.; Chen, H.; Hu, W.; Tang, M. The alleviation mechanisms of cadmium toxicity in Broussonetia papyrifera by arbuscular mycorrhizal symbiosis varied with different levels of cadmium stress. J. Hazard. Mater. 2023, 459, 132076. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, W.; Zhang, L.; He, X.; Fan, Y.; Alam, S.; Yuan, X. Effect of pyrazosulfuron-methyl on the photosynthetic characteristics and antioxidant systems of foxtail millet. Front. Plant Sci. 2021, 12, 696169. [Google Scholar]
- Zhou, Y.; Xia, X.; Yu, G.; Wang, J.; Wu, J.; Wang, M.; Yang, Y.; Shi, K.; Yu, Y.; Chen, Z.; et al. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015, 5, 9018. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Liu, X.; Li, C.; Zhang, Y. 24-epibrassinolide as a multidimensional regulator of rice (Oryza sativa) physiological and molecular responses under isoproturon stress. Ecotox. Environ. Safe. 2024, 281, 116575. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, X.; Mu, H.; Liu, S.; Qi, S.; Wang, J.; Wei, S.; Wang, Y. Exogenous brassinolide alleviates the autotoxicity induced by vanillin in Lagenaria siceraria. Plant Soil. 2025, 326, 112615. [Google Scholar] [CrossRef]
- Xiang, L.; Yu, Z.; Wang, H.; Cai, L.; Li, W.; Guo, M.; Li, T.; Sun, M. Effect of azoxystrobin on the physiology, morphology, and sclerotial formation of Rhizoctonia solani from tobacco. Eur. J. Plant Pathol. 2024, 168, 109–119. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
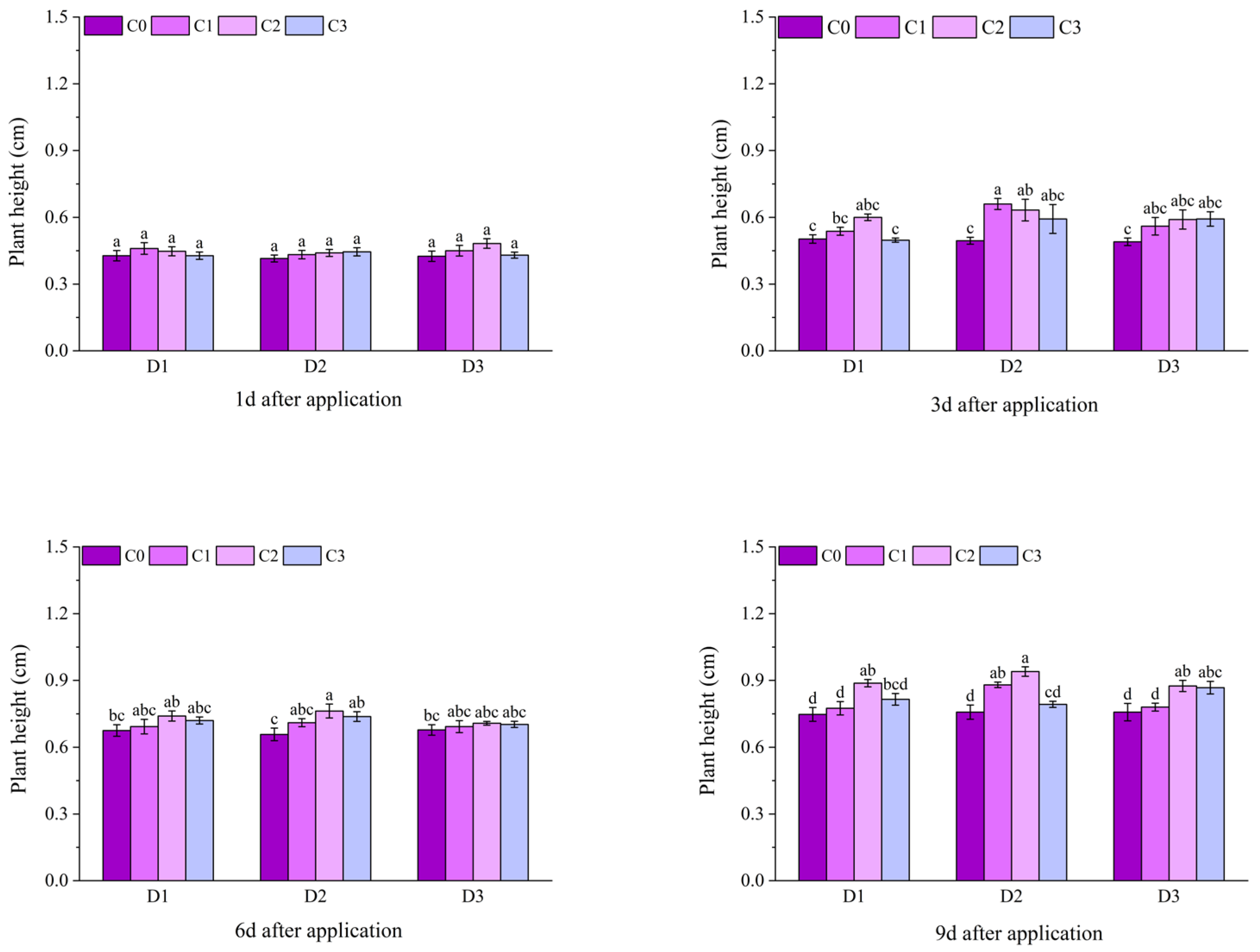
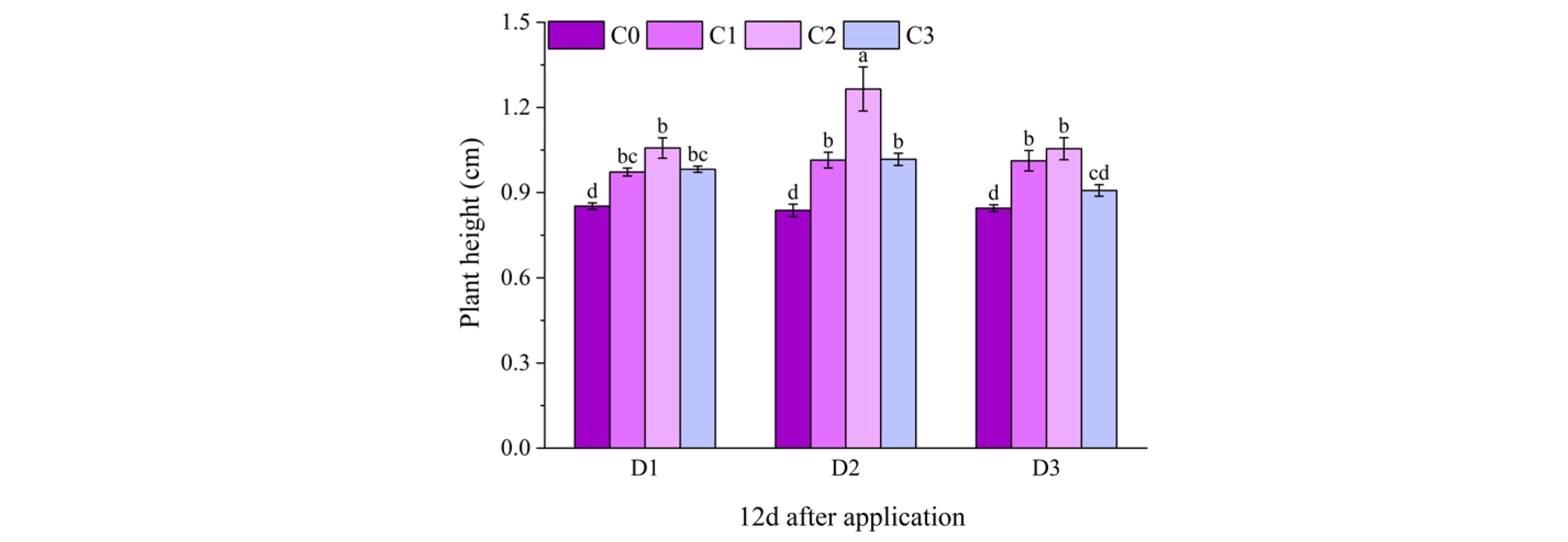
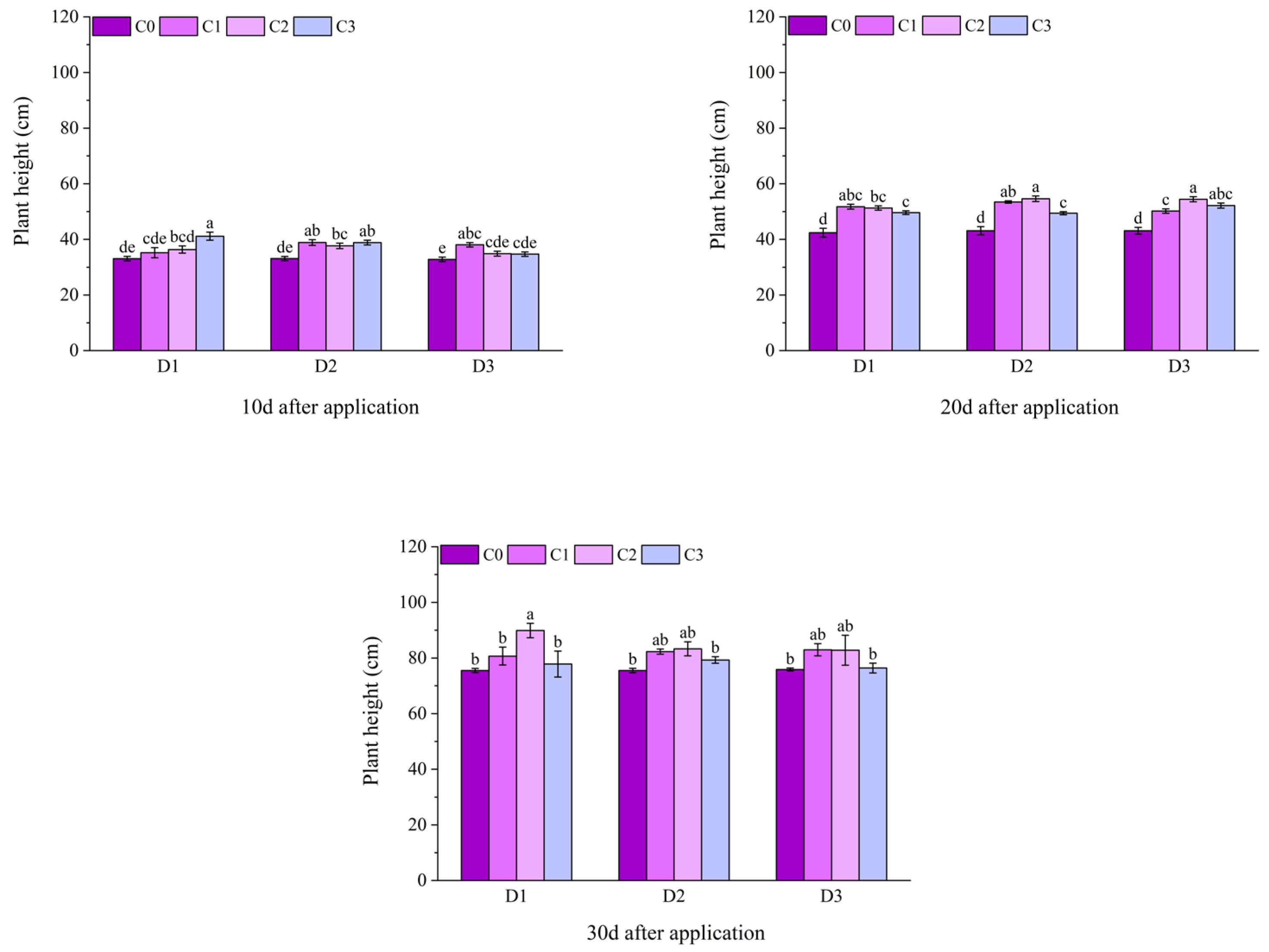
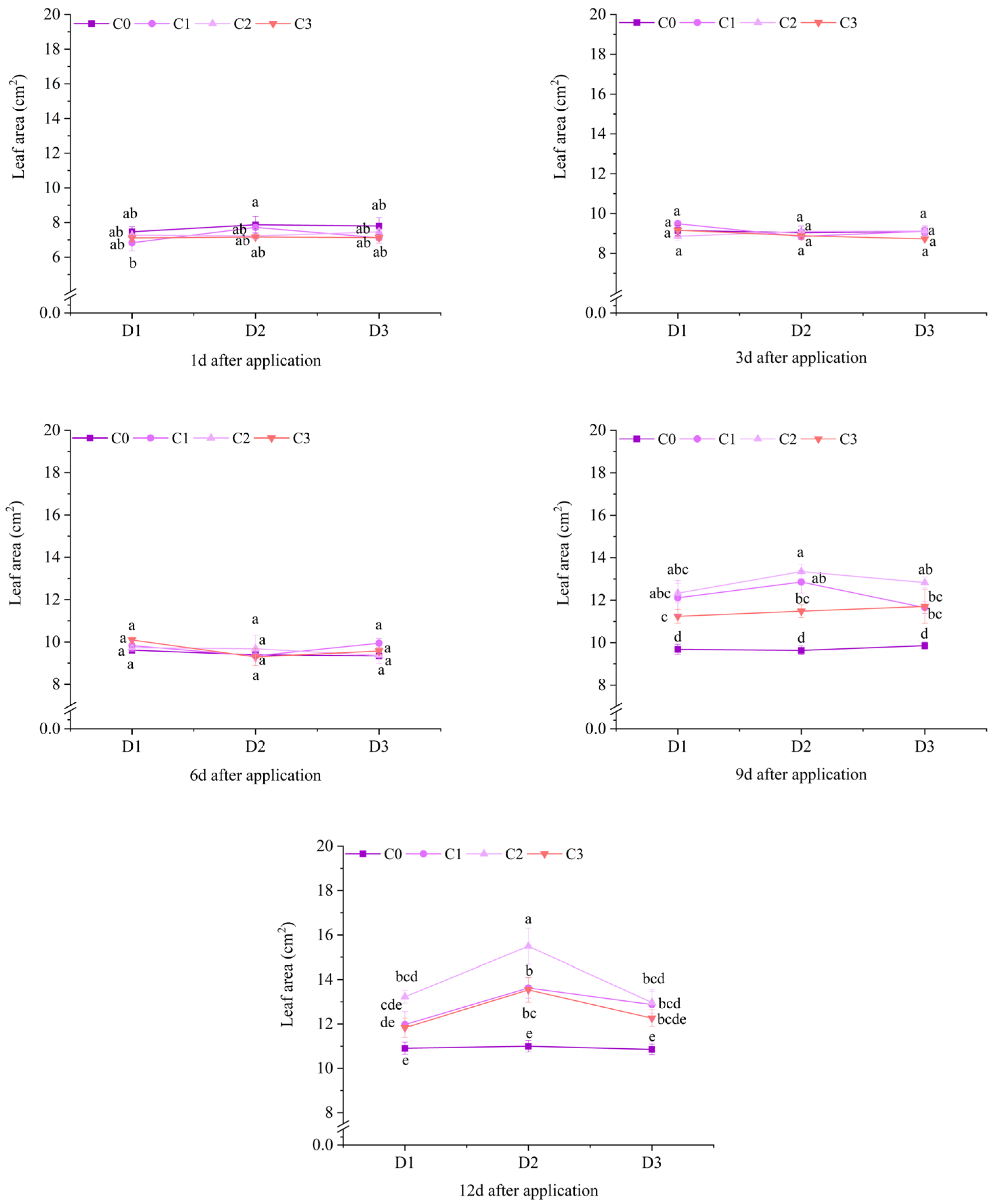
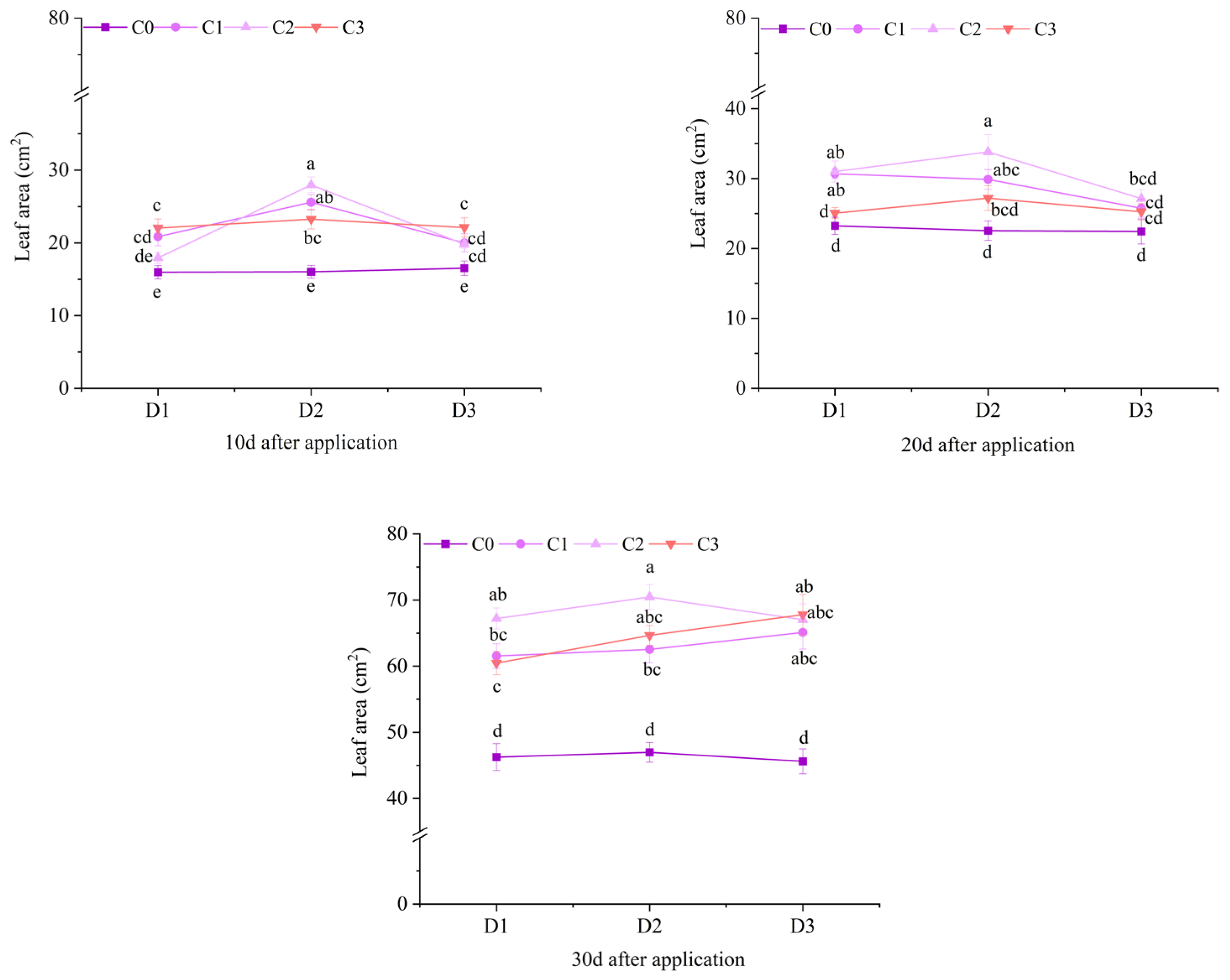
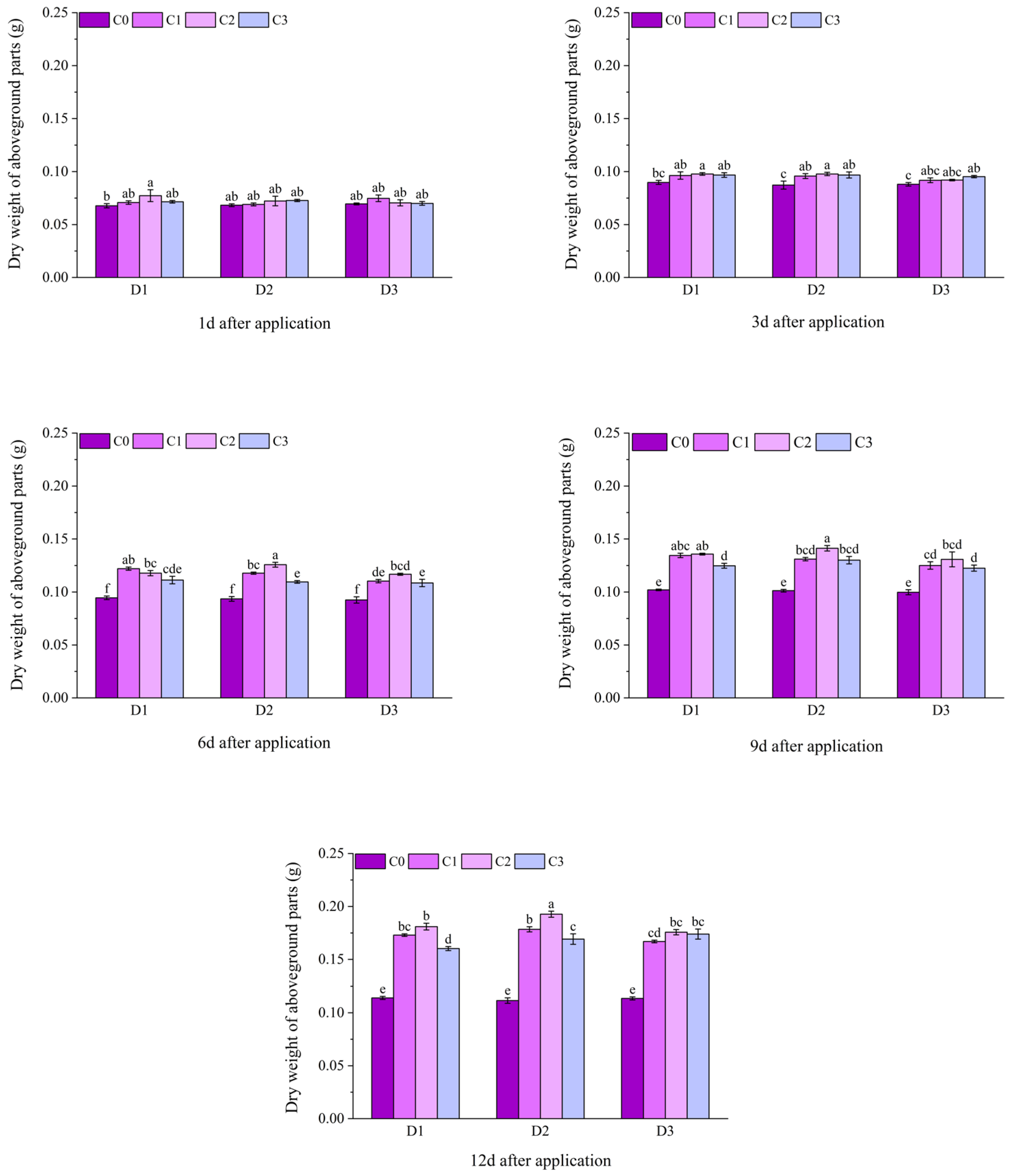
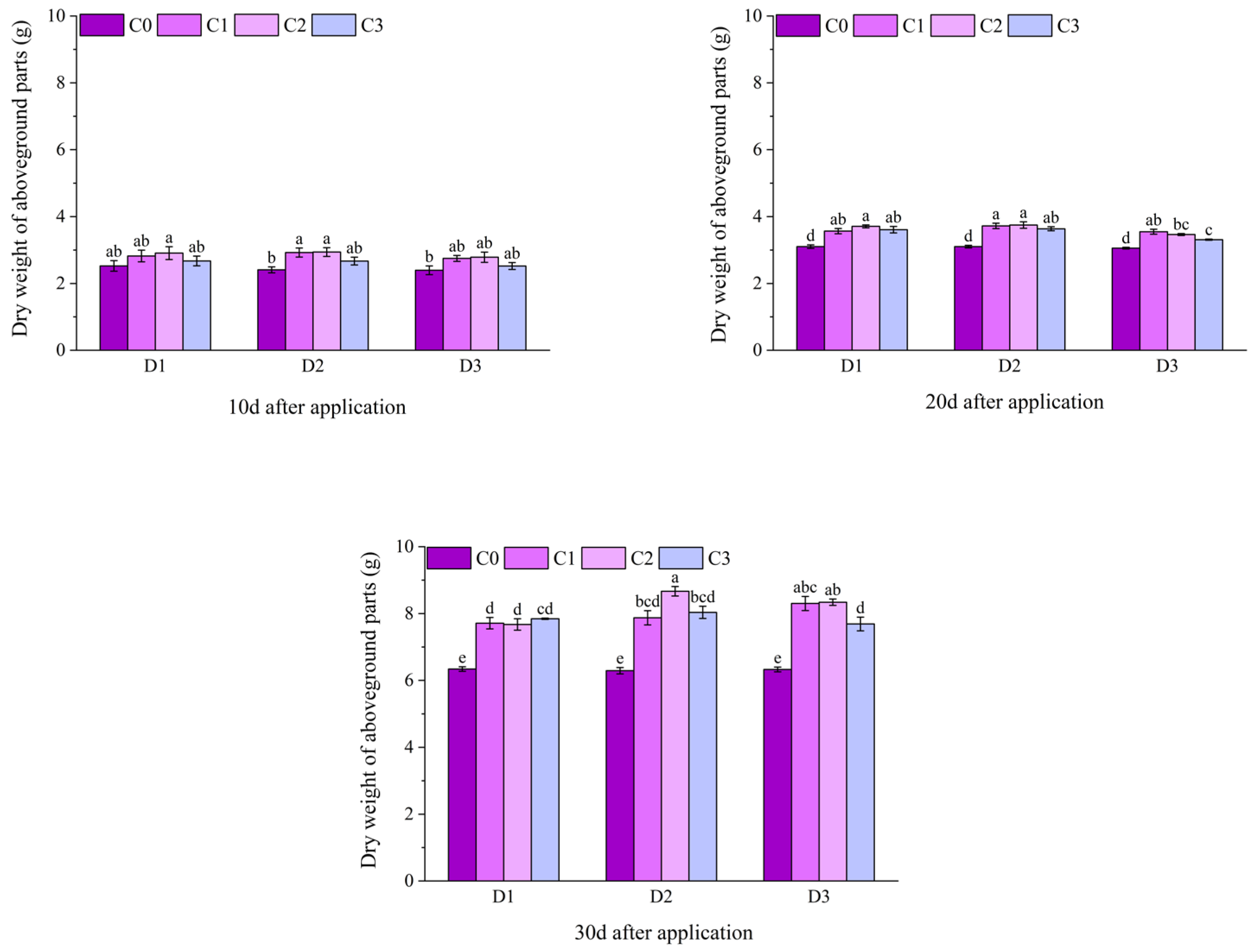
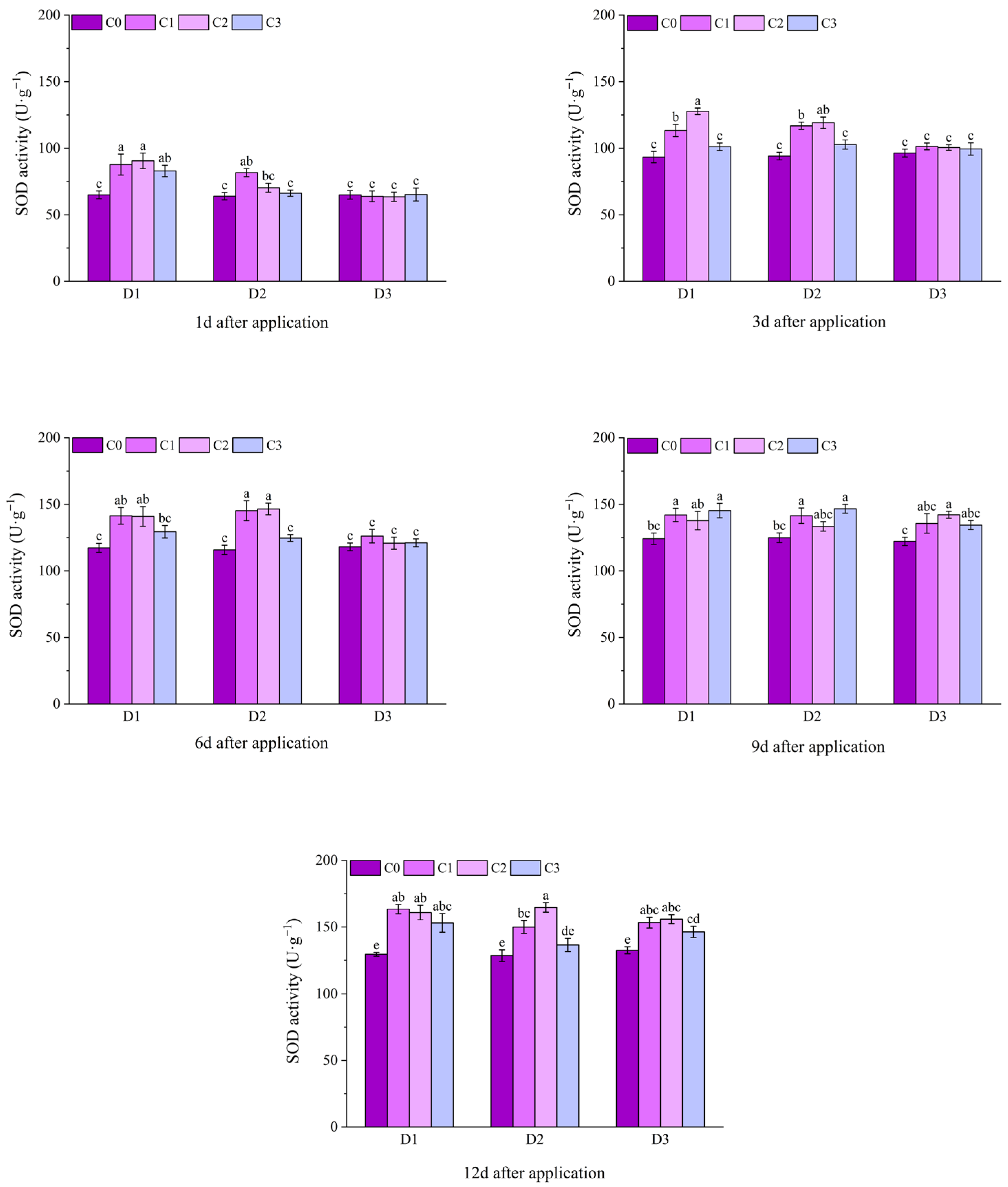
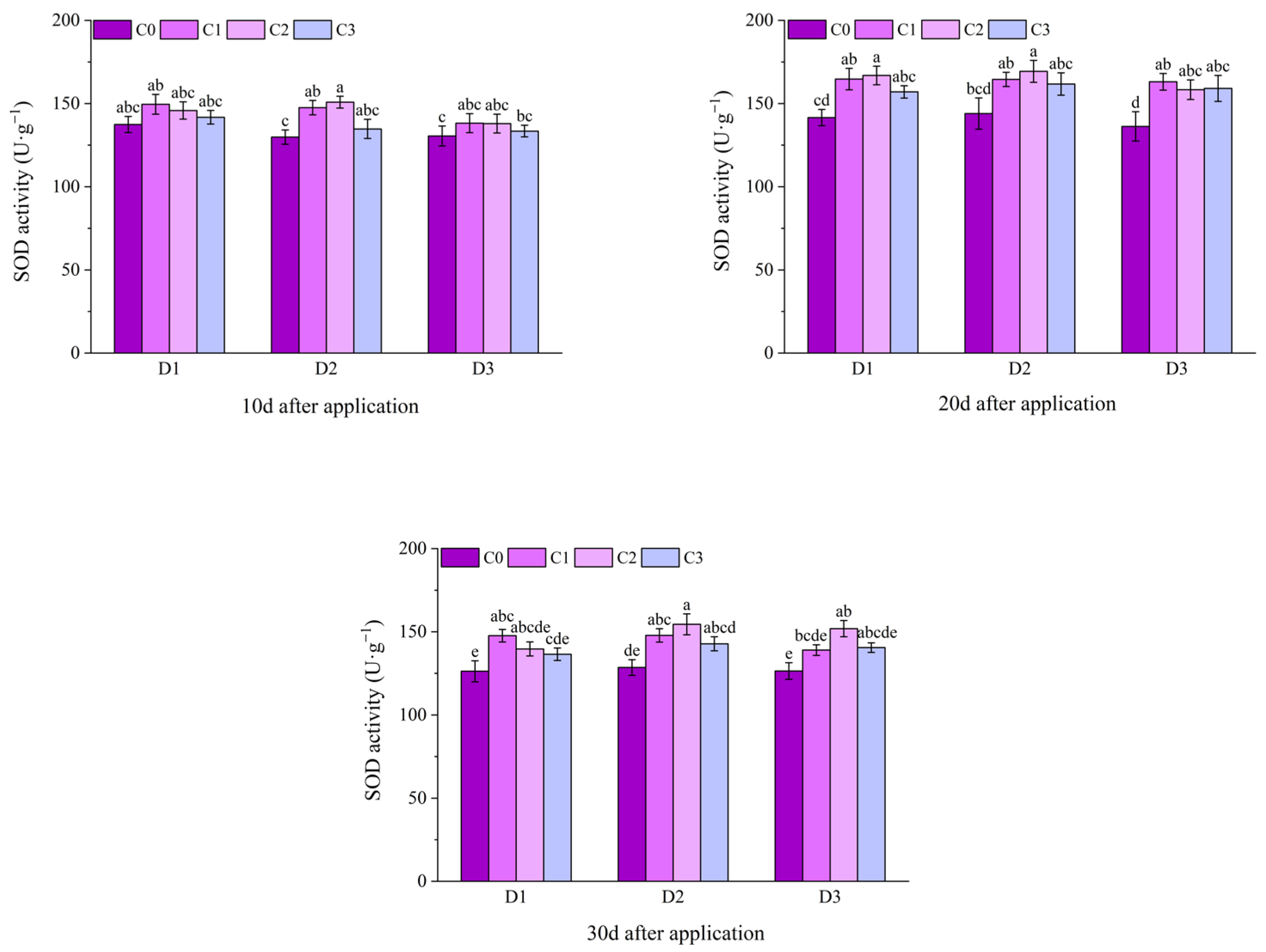
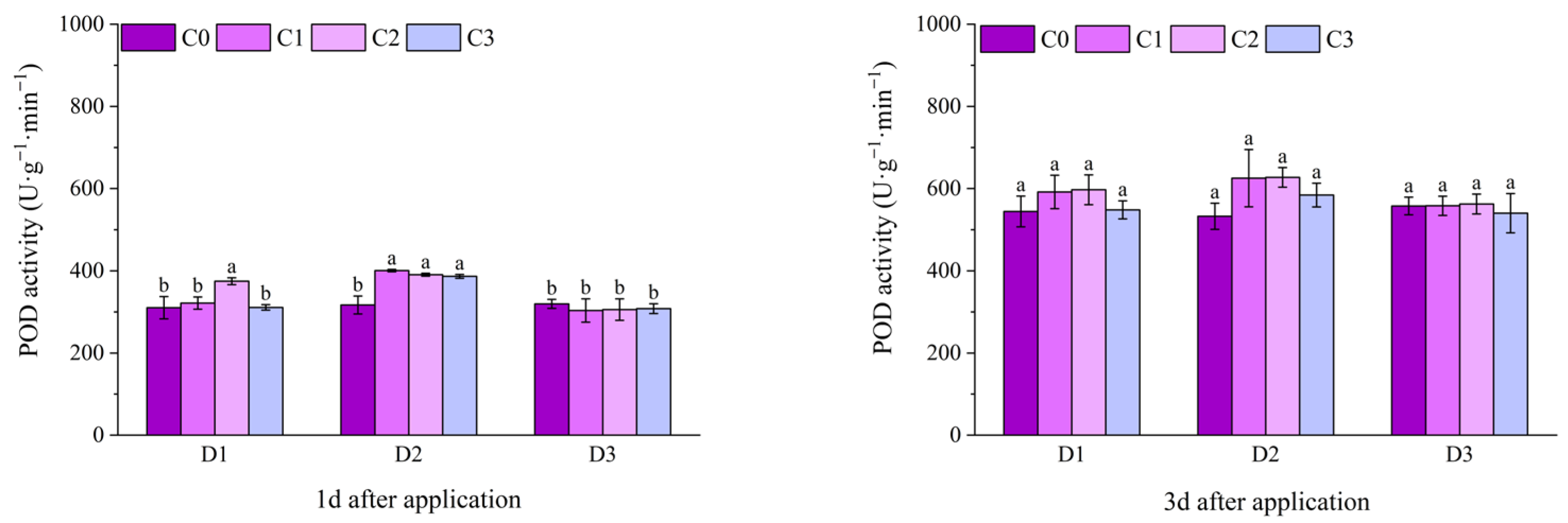
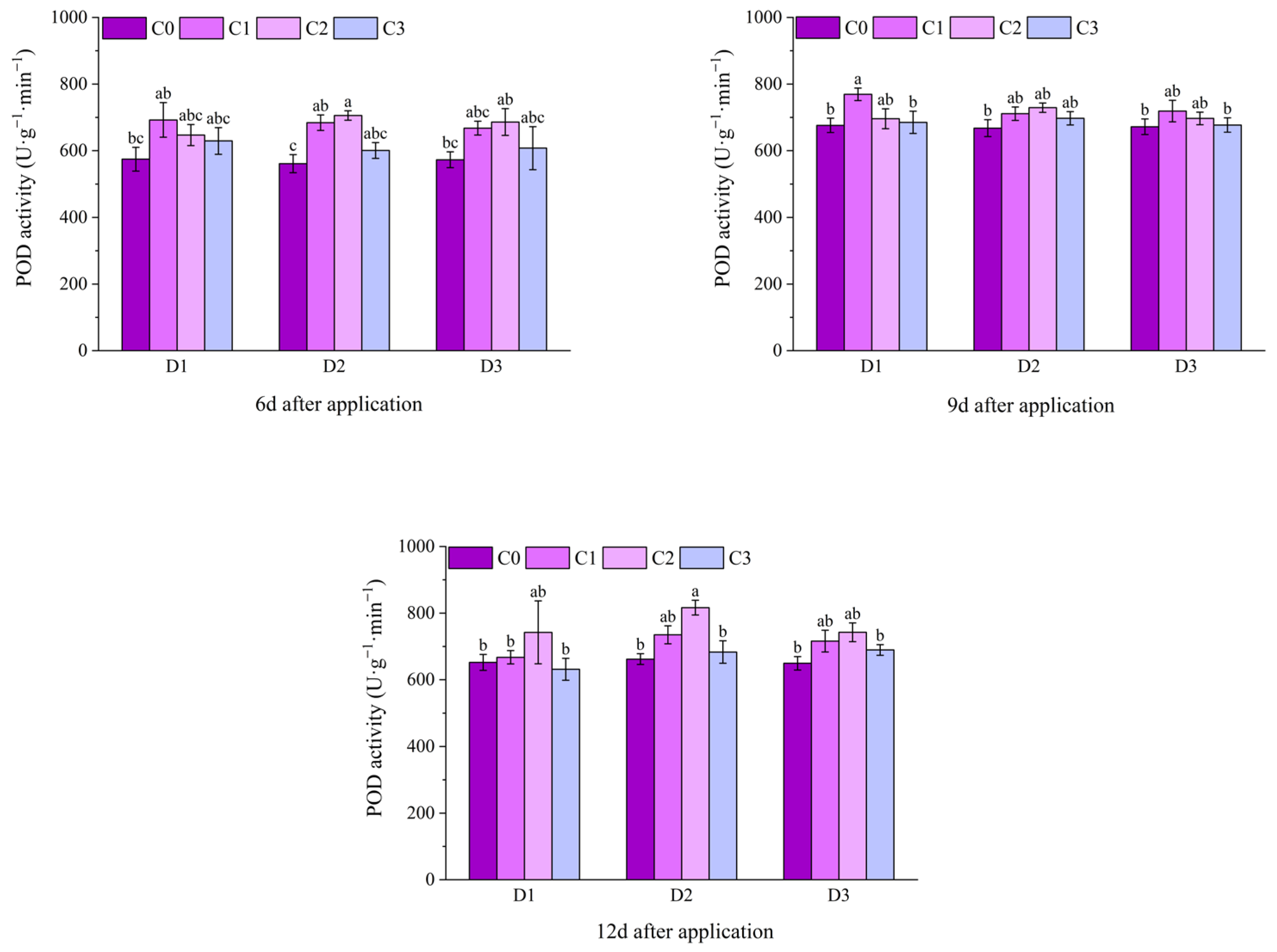
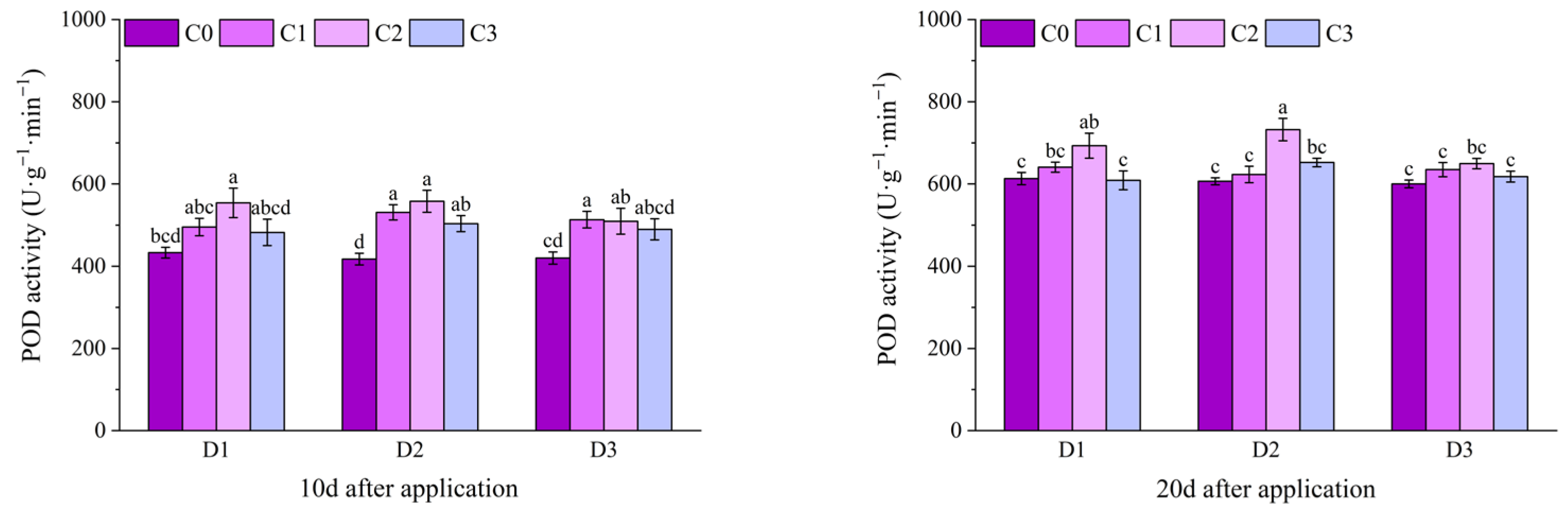
| Analysis of Variance | Plant Height | Leaf Area | Dry Weight of Aboveground Parts | |
|---|---|---|---|---|
| spraying time (D) | 1 d | NS | NS | NS |
| 3 d | ** | NS | NS | |
| 6 d | ** | NS | * | |
| 9 d | ** | NS | * | |
| 12 d | * | ** | ** | |
| spraying concentration (C) | 1 d | ** | NS | NS |
| 3 d | ** | NS | ** | |
| 6 d | ** | NS | ** | |
| 9 d | ** | ** | ** | |
| 12 d | ** | ** | ** | |
| Time × concentration (D × C) | 1 d | NS | NS | NS |
| 3 d | * | NS | NS | |
| 6 d | NS | NS | NS | |
| 9 d | NS | NS | NS | |
| 12 d | NS | NS | ** | |
| Analysis of Variance | Plant Height | Leaf Area | Dry Weight of Aboveground Parts | |
|---|---|---|---|---|
| spraying time (D) | 10 d | * | ** | NS |
| 20 d | NS | * | ** | |
| 30 d | NS | NS | ** | |
| spraying concentration (C) | 10 d | ** | ** | ** |
| 20 d | ** | ** | ** | |
| 30 d | ** | ** | ** | |
| Time × concentration (D × C) | 10 d | ** | ** | NS |
| 20 d | NS | NS | NS | |
| 30 d | NS | NS | ** | |
| Analysis of Variance | SOD Activity | POD Activity | CAT Activity | |
|---|---|---|---|---|
| spraying time (D) | 1 d | ** | ** | ** |
| 3 d | ** | NS | NS | |
| 6 d | ** | NS | ** | |
| 9 d | NS | NS | ** | |
| 12 d | NS | NS | ** | |
| spraying concentration (C) | 1 d | ** | * | ** |
| 3 d | ** | NS | * | |
| 6 d | ** | ** | ** | |
| 9 d | ** | * | ** | |
| 12 d | ** | ** | ** | |
| Time × concentration (D × C) | 1 d | * | * | NS |
| 3 d | ** | NS | NS | |
| 6 d | NS | NS | ** | |
| 9 d | NS | NS | * | |
| 12 d | NS | NS | NS | |
| Analysis of Variance | SOD Activity | POD Activity | CAT Activity | |
|---|---|---|---|---|
| spraying time (D) | 10 d | NS | NS | NS |
| 20 d | NS | NS | NS | |
| 30 d | NS | NS | NS | |
| spraying concentration (C) | 10 d | ** | ** | ** |
| 20 d | ** | ** | ** | |
| 30 d | ** | NS | ** | |
| Time × concentration (D × C) | 10 d | NS | NS | NS |
| 20 d | NS | NS | NS | |
| 30 d | NS | NS | NS | |
| Analysis of Variance | Spraying Time (D) | Spraying Concentration (C) | Time × Concentration (D × C) | |
|---|---|---|---|---|
| pot experiments | 1 d | ** | ** | ** |
| 3 d | ** | ** | NS | |
| 6 d | NS | ** | NS | |
| 9 d | ** | ** | * | |
| 12 d | ** | ** | NS | |
| field experiments | 10 d | NS | ** | NS |
| 20 d | * | ** | NS | |
| 30 d | * | ** | NS | |
| Years | pH | Available P mg/kg | Available K mg/kg | Total N g/kg | Total P g/kg | Total K g/kg | Organic Matter g/kg | Hydrolyzable Nitrogen mg/kg |
|---|---|---|---|---|---|---|---|---|
| 2024 | 8.29 | 13.4 | 189.98 | 1.01 | 0.81 | 20.44 | 17.26 | 69.74 |
| Spraying Amount of Cyhalofop-Butyl | Time of Spraying BR | Concentration of Spraying BR |
|---|---|---|
| 67.5 g a.i./ha | The same day as cyhalofop-butyl spraying (D1) | 0 mg/L (C0) |
| 0.05 mg/L (C1) | ||
| 0.1 mg/L (C2) | ||
| 0.2 mg/L (C3) | ||
| One day after cyhalofop-butyl spraying (D2) | 0 mg/L (C0) | |
| 0.05 mg/L (C1) | ||
| 0.1 mg/L (C2) | ||
| 0.2 mg/L (C3) | ||
| Three days after cyhalofop-butyl spraying (D3) | 0 mg/L (C0) | |
| 0.05 mg/L (C1) | ||
| 0.1 mg/L (C2) | ||
| 0.2 mg/L (C3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Dong, J.; Yuan, J.; Shang, S.; Zhai, X.; Wen, Y.; Song, X.; Zhao, J.; Cao, H.; Dong, S. Effect of Brassinolide on the Growth and Physiological Indicators of Foxtail Millet Under Cyhalofop-Butyl Damage. Plants 2025, 14, 3421. https://doi.org/10.3390/plants14223421
Hu C, Dong J, Yuan J, Shang S, Zhai X, Wen Y, Song X, Zhao J, Cao H, Dong S. Effect of Brassinolide on the Growth and Physiological Indicators of Foxtail Millet Under Cyhalofop-Butyl Damage. Plants. 2025; 14(22):3421. https://doi.org/10.3390/plants14223421
Chicago/Turabian StyleHu, Chunyan, Jiaxin Dong, Jingtao Yuan, Suqi Shang, Xutao Zhai, Yinyuan Wen, Xi’e Song, Juan Zhao, Hui Cao, and Shuqi Dong. 2025. "Effect of Brassinolide on the Growth and Physiological Indicators of Foxtail Millet Under Cyhalofop-Butyl Damage" Plants 14, no. 22: 3421. https://doi.org/10.3390/plants14223421
APA StyleHu, C., Dong, J., Yuan, J., Shang, S., Zhai, X., Wen, Y., Song, X., Zhao, J., Cao, H., & Dong, S. (2025). Effect of Brassinolide on the Growth and Physiological Indicators of Foxtail Millet Under Cyhalofop-Butyl Damage. Plants, 14(22), 3421. https://doi.org/10.3390/plants14223421






