“Radix Saniculae”: Phytochemical Characterization and Potential Adulteration of an Austrian Traditional Wound-Healing Agent
Abstract
1. Introduction
2. Results
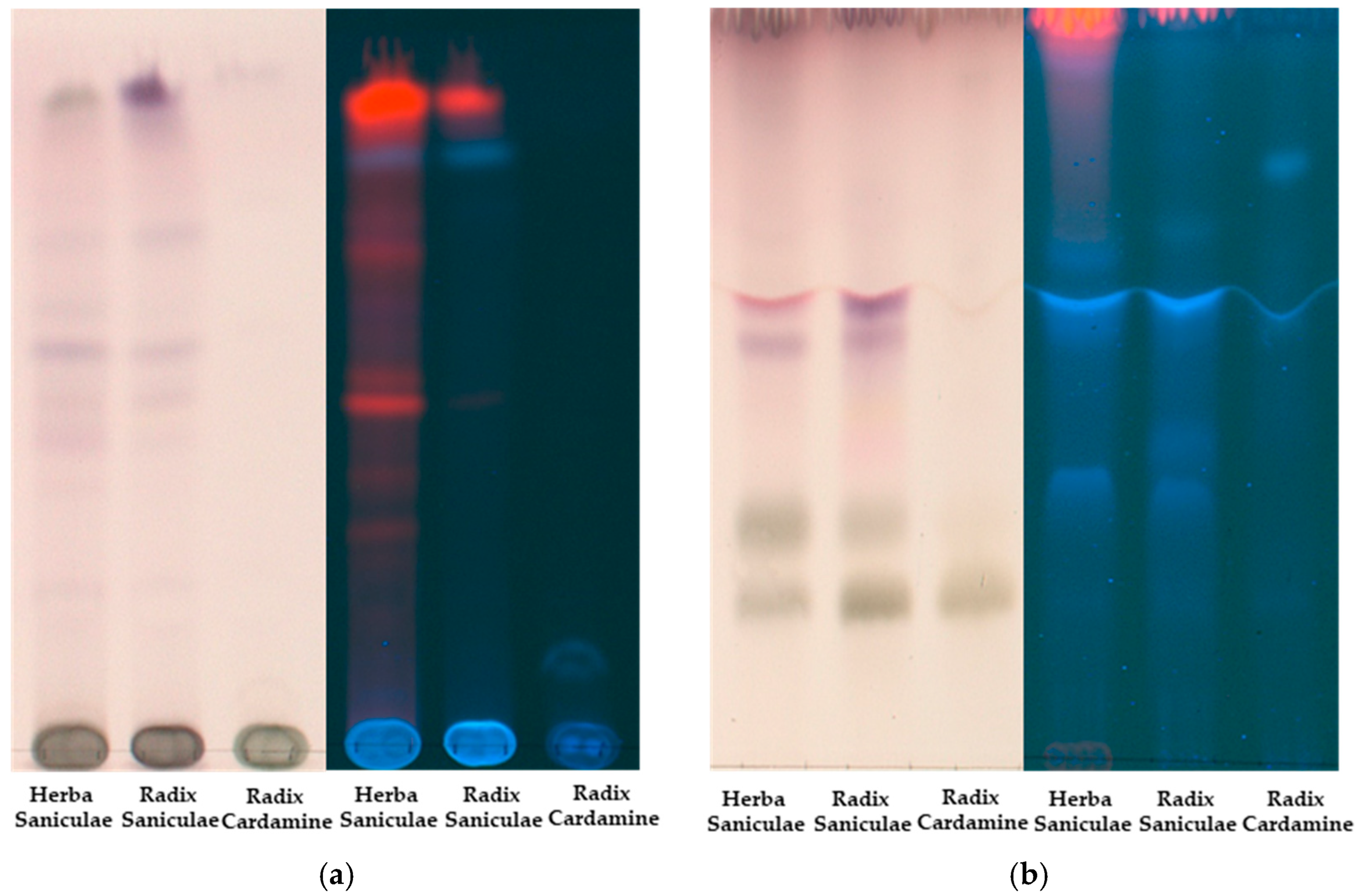
2.1. Thin-Layer Chromatography (TLC)
2.2. High-Performance Liquid Chromatography–Diode Array Detection/Evaporative Light Scattering Detector (HPLC-DAD/ELSD) and Ultra High-Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry (UHPLC-ESIMS)
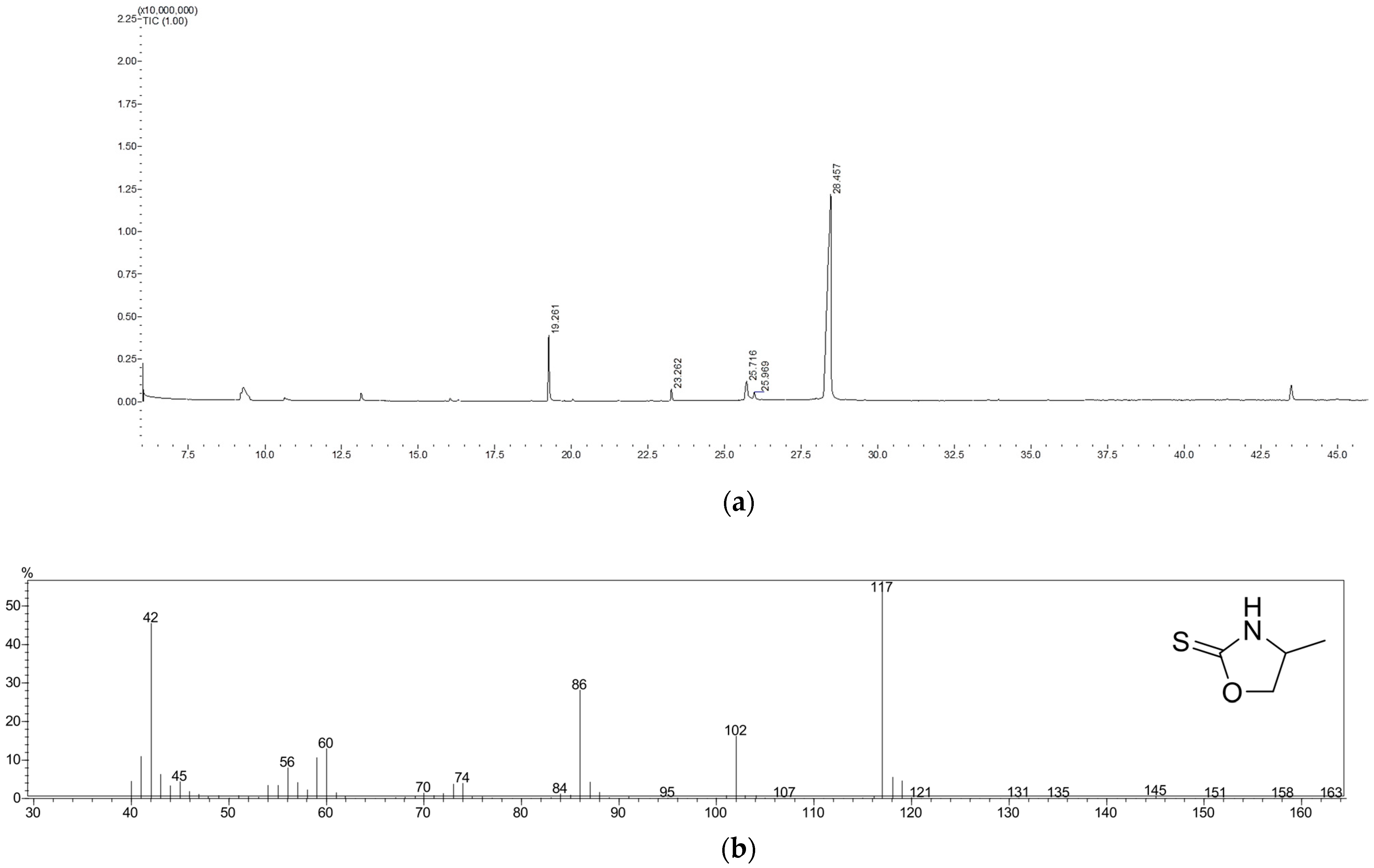
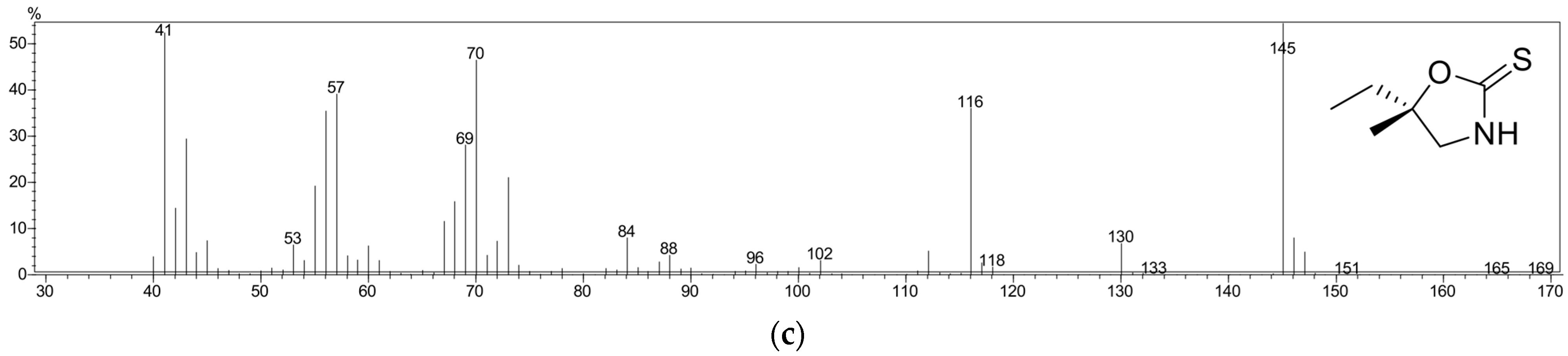
2.3. Gas Chromatography–Mass Spectrometry (GC-MS)
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Thin-Layer Chromatography (TLC)
4.3. High-Performance Liquid Chromatography—Diode Array Detection/Evaporative Light Scattering Detector (HPLC-DAD/ELSD)
4.4. Gas Chromatography–Mass Spectrometry (GC-MS)
4.5. Ultra High-Performance Liquid Chromatography–Electrospray Ionization Mass Spectrometry (UHPLC-ESIMS)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbeni, S.A.; Negm, W.A. The Wound Healing Effect of Botanicals and Pure Natural Substances Used in in Vivo Models. Inflammopharmacology 2023, 31, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Eichenauer, E.; Saukel, J.; Glasl, S. VOLKSMED Database: A Source for Forgotten Wound Healing Plants in Austrian Folk Medicine. Planta Med. 2024, 90, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Wikispecies. Available online: https://species.wikimedia.org/ (accessed on 5 August 2024).
- Kew Royal Botanic Gardens. Plants of the World Online (Kew Science). Available online: https://powo.science.kew.org/ (accessed on 5 August 2024).
- Hiller, K. Sanicula europaea L.—Sanikel—Porträt einer Arzneipflanze. Z. Phytother. 2005, 26, 251–254. [Google Scholar] [CrossRef]
- Blaschek, W. Wichtl–Teedrogen Und Phytopharmaka; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany, 2016; pp. 592–593. [Google Scholar]
- Arda, N.; Gören, N.; Kuru, A.; Pengsuparp, T.; Pezzuto, J.M.; Qiu, S.-X.; Cordell, G.A. Saniculoside N from Sanicula europaea L. J. Nat. Prod. 1997, 60, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Turan, K.; Nagata, K.; Kuru, A. Antiviral Effect of Sanicula europaea L. Leaves Extract on Influenza Virus-Infected Cells. Biochem. Biophys. Res. Commun. 1996, 225, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Grytsyk, L.; Legin, N.; Svirska, S.; Grytsyk, A. The Study of Phenolic Compounds of Sanicula europaea L. Sci. Eur. 2020, 60, 31–35. [Google Scholar]
- Pavlović, M.; Kovačević, N.; Tzakou, O.; Couladis, M. Essential Oil Composition of Sanicula europaea L. Flavour Fragr. J. 2006, 21, 687–689. [Google Scholar] [CrossRef]
- Beggs, C.B.; Denyer, M.C.T.; Lemmerz, A.; Sefat, F.; Wright, C.; Youseffi, M. The Effect of Transforming Growth Factor Beta (TGF-Β3) and Sanicle on Wound Healing. In Proceedings of the World Congress on Engineering, London, UK, 30 June–2 July 2010; p. 6. [Google Scholar]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in Vitro Studies on Austria’s Folk Medicine—An Unexplored Lore in Vitro Anti-Inflammatory Activities of 71 Austrian Traditional Herbal Drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef]
- Encyclopedia of Life. Available online: https://eol.org/ (accessed on 5 August 2024).
- Fischer, M.A.; Adler, W.; Oswald, K. Exkursionsflora Für Österreich, Liechtenstein Und Südtirol, 2nd ed.; Land Oberösterreich, OÖ Landesmuseen: Linz, Austria, 2005. [Google Scholar]
- Ricek, E.W. Mundartliche Pflanzennamen Aus Dem Attergau. In Jahrbuch des OÖ Musealvereines; Land Oberösterreich, OÖ Landesmuseen: Linz, Austria, 1981; pp. 189–228. [Google Scholar]
- Fischer, M.A. Zur Typologie Und Geschichte Deutscher Botanischer Gattungsnamen Mit Einem Anhang Über Deutsche Infraspezifische Namen; Stapfia: Linz, Austria, 2002; Volume 80, pp. 125–200. [Google Scholar]
- Jurenitsch, J.; Deichstetter, W.M.; Robien, W.; Kubelka, W. Über Die “Alkaloide” von Dentaria enneaphyllos L. Sci. Pharm. 1985, 53, 163–168. [Google Scholar]
- Kroeber, L. Pharmakochemische Ergebnisse Der Untersuchung Heimischer Pflanzen. Pharm. Ztg. 1931, 76, 539–541. [Google Scholar]
- Smola, G. Volkstümliche Pflanzennamen Der Steiermark. In Mitteilungen der Abteilung für Zoologie und Botanik am Landesmuseum Joanneum Graz; H07-08; Steiermärkisches Landesmuseum Joanneum: Graz, Austria, 1958; pp. 21–80. [Google Scholar]
- Kroeber, L. Sanicula Europaea—Dentaria Enneaphyllos (Ein Beitrag Zur Verwechslung von Sanikel Mit Zahnwurz). Dtsch. Apoth.-Ztg. 1929, 44, 106. [Google Scholar]
- Vogl, A. Ueber Radix Saniculi Der Apotheken. Z. Des Allgem. Österr. Apoth. 1904, 42, 501–503. [Google Scholar]
- Lémery, N. Dizionario Overo Trattato Universale Delle Droghe Semplici; Stamperia dell’Hertz: Venezia, Italy, 1737; pp. 119–120. [Google Scholar]
- Schultz, O.-E.; Wagner, W. Trennung der Senfölglucoside durch absteigende Papierchromatographie: XI. Mitt. über Senfölglucoside. Z. Naturforsch. B 1956, 11, 73–78. [Google Scholar] [CrossRef][Green Version]
- Merck, D.E. Anfärbereagenzien für Dünnschicht- und Papier-Chromatographie; Merck: Darmstadt, Germany, 1980. [Google Scholar]
- Vo, N.N.Q.; Fukushima, E.O.; Muranaka, T. Structure and Hemolytic Activity Relationships of Triterpenoid Saponins and Sapogenins. J. Nat. Med. 2017, 71, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, A.; Miyase, T.; Noguchi, H.; Vander Velde, D. Oleanane Saponins from Sanicula Elata Var. Chinensis. J. Nat. Prod. 2004, 67, 377–383. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The Cellular and Subcellular Organization of the Glucosinolate–Myrosinase System against Herbivores and Pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in Wound Healing: An Updated Review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-Y.; Liu, H.-Y.; Xie, B.-B.; Liu, Z.-H.; Chen, C.-X. Two New Glycosides from Sanicula Lamelligera. Z. Naturforsch. B 2006, 61, 607–610. [Google Scholar] [CrossRef]
- Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum Ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef] [PubMed]
- Budzianowski, J.; Romaniuk-Drapała, A.; Kikowska, M.; Budzianowska, A.; Thiem, B.; Lisiak, N.; Rubiś, B.; Jacczak, B.; Kosmalska, I.; Totoń, E. Rosmarinic Acid 4′-O-β-Glucoside—A Compound with Prospective Medicinal and Cosmetological Applications—Its Isolation from Callus and Root Cultures of Eryngium Species and Biological Activity. Ind. Crops Prod. 2023, 193, 116138. [Google Scholar] [CrossRef]
- Iqbal, H.; Wright, C.L.; Jones, S.; Da Silva, G.R.; McKillen, J.; Gilmore, B.F.; Kavanagh, O.; Green, B.D. Extracts of Sida cordifolia Contain Polysaccharides Possessing Immunomodulatory Activity and Rosmarinic Acid Compounds with Antibacterial Activity. BMC Complement. Med. Ther. 2022, 22, 27. [Google Scholar] [CrossRef]
- Al-Ghanayem, A.A.; Alhussaini, M.S.; Ibrahim Alyahya, A.A.; Asad, M.; Joseph, B. Wound Healing Activity of Chlorogenic Acid in Diabetic Rats Is Mediated Through Antibacterial, Antioxidant, and Proliferative Effects. Online J. Biol. Sci 2024, 24, 255–262. [Google Scholar] [CrossRef]
- Küba, M.C.; Türkoğlu, A.; Oğuz, A.; Tuncer, M.C.; Kaya, Ş.; Başol, Ö.; Bilge, H.; Tatlı, F. Comparison of Local Rosmarinic Acid and Topical Dexpanthenol Applications on Wound Healing in a Rat Experimental Wound Model. Folia Morphol. 2021, 80, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Liou, S.-S.; Tzeng, T.-F.; Lee, S.-L.; Liu, I.-M. Effect of Topical Application of Chlorogenic Acid on Excision Wound Healing in Rats. Planta Med. 2013, 79, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yuan, W.; Deng, G.; Su, Z.; Li, S. Triterpenoid Saponins from Eryngium yuccifolium ‘Kershaw Blue’. Phytochem. Lett. 2013, 6, 306–309. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Masullo, M.; Thiem, B.; Piacente, S.; Stochmal, A.; Oleszek, W. Three New Triterpene Saponins from Roots of Eryngium planum. Nat. Prod. Res. 2014, 28, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Scott Beasley, R. Phenolic Compounds and Rare Polyhydroxylated Triterpenoid Saponins from Eryngium Yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Kartal, M.; Mitaine-Offer, A.-C.; Paululat, T.; Abu-Asaker, M.; Wagner, H.; Mirjolet, J.-F.; Guilbaud, N.; Lacaille-Dubois, M.-A. Triterpene Saponins from Eryngium campestre. J. Nat. Prod. 2006, 69, 1105–1108. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Yang, Y.; Wang, D.; Gao, H. Natural Barrigenol–like Triterpenoids: A Comprehensive Review of Their Contributions to Medicinal Chemistry. Phytochemistry 2019, 161, 41–74. [Google Scholar] [CrossRef] [PubMed]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.M.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Argentieri, M.P. Brassicaceae: A Rich Source of Health Improving Phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Arora, R. Glucosinolate Hydrolytic Products—A Multi-Arm Warrior. J. AOAC Int. 2024, 107, qsae054. [Google Scholar] [CrossRef] [PubMed]
- Opretzka, L.C.F.; Viana, M.D.M.; De Lima, A.A.; De Souza, T.A.; Scotti, M.T.; Tavares, J.F.; Da Silva, M.S.; Soares, M.B.P.; Villarreal, C.F. Cleomin Exerts Acute Antinociceptive Effects in Mice via GABAB and Muscarinic Receptors. Pharmaceuticals 2023, 16, 1547. [Google Scholar] [CrossRef]

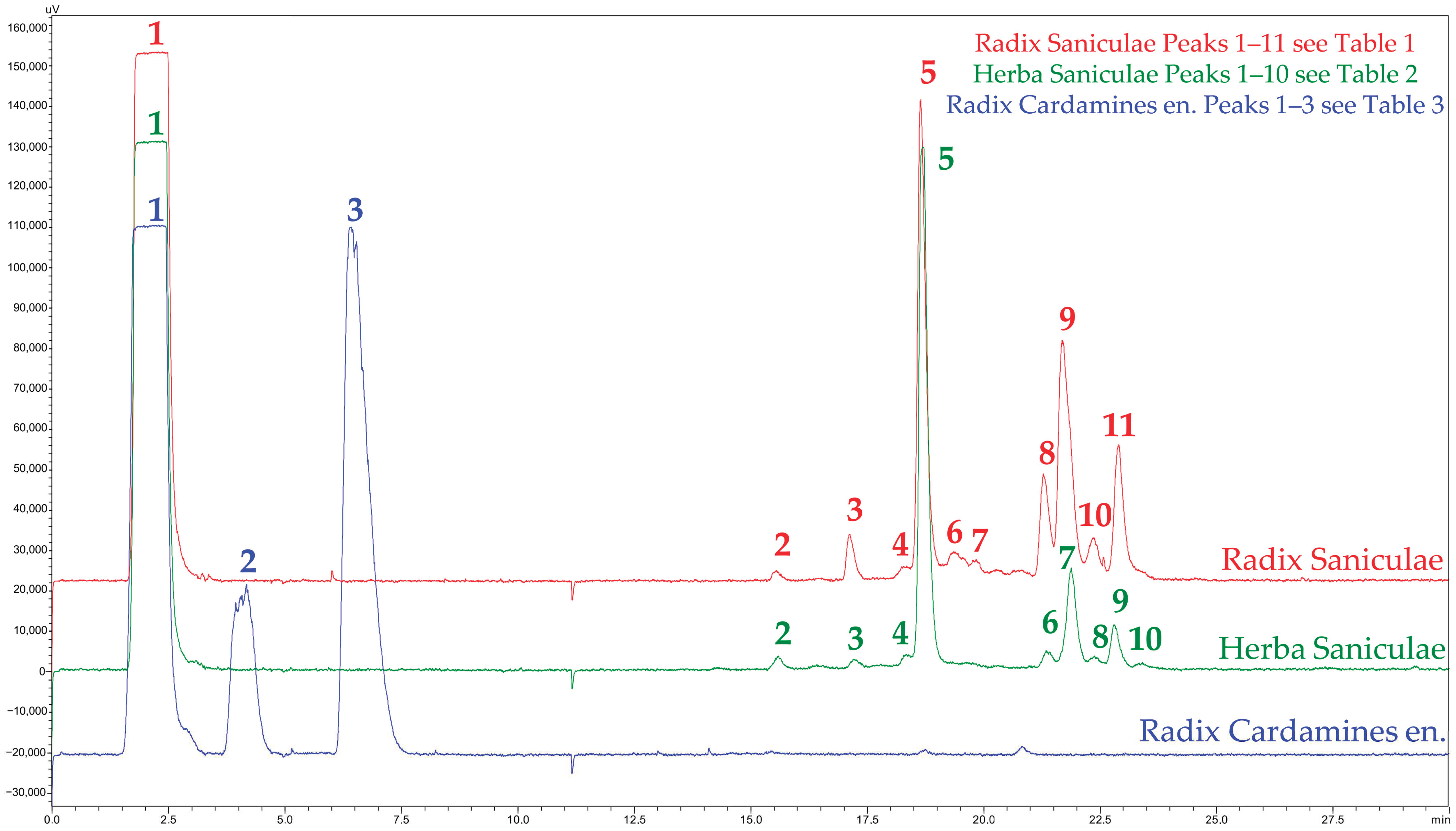
| Peak ID 1 | Rt. [min] 1 | UV max [nm] 1 | m/z [neg.] | m/z [pos.] | Putatively Assigned Compound/Compound Class |
|---|---|---|---|---|---|
| 1 | 2.29 | nd | 341.31 [M-H]−, 387.27 [M+FA-H]−, 683.42 [2M-H]−, 729.20 [2M+FA-H]−, 1025.27 [3M-H]−, 1071.15 [3M+FA-H]− | 365.37 [M+Na]+, 381.30 [M+K]+, 707.37 [2M+Na]+, 723.15 [2M+K]+ | Sucrose 2 (sugars) |
| 2 | 15.55 | 203, 243, 326 | 191.15 [M-H]− Quinic acid, 353.27 [M-H]−, 707.34 [2M-H]−, 1061.12 [3M-H]− | 355.24 [M+H]+, 377.32 [M+Na]+ | Chlorogenic acid 2 |
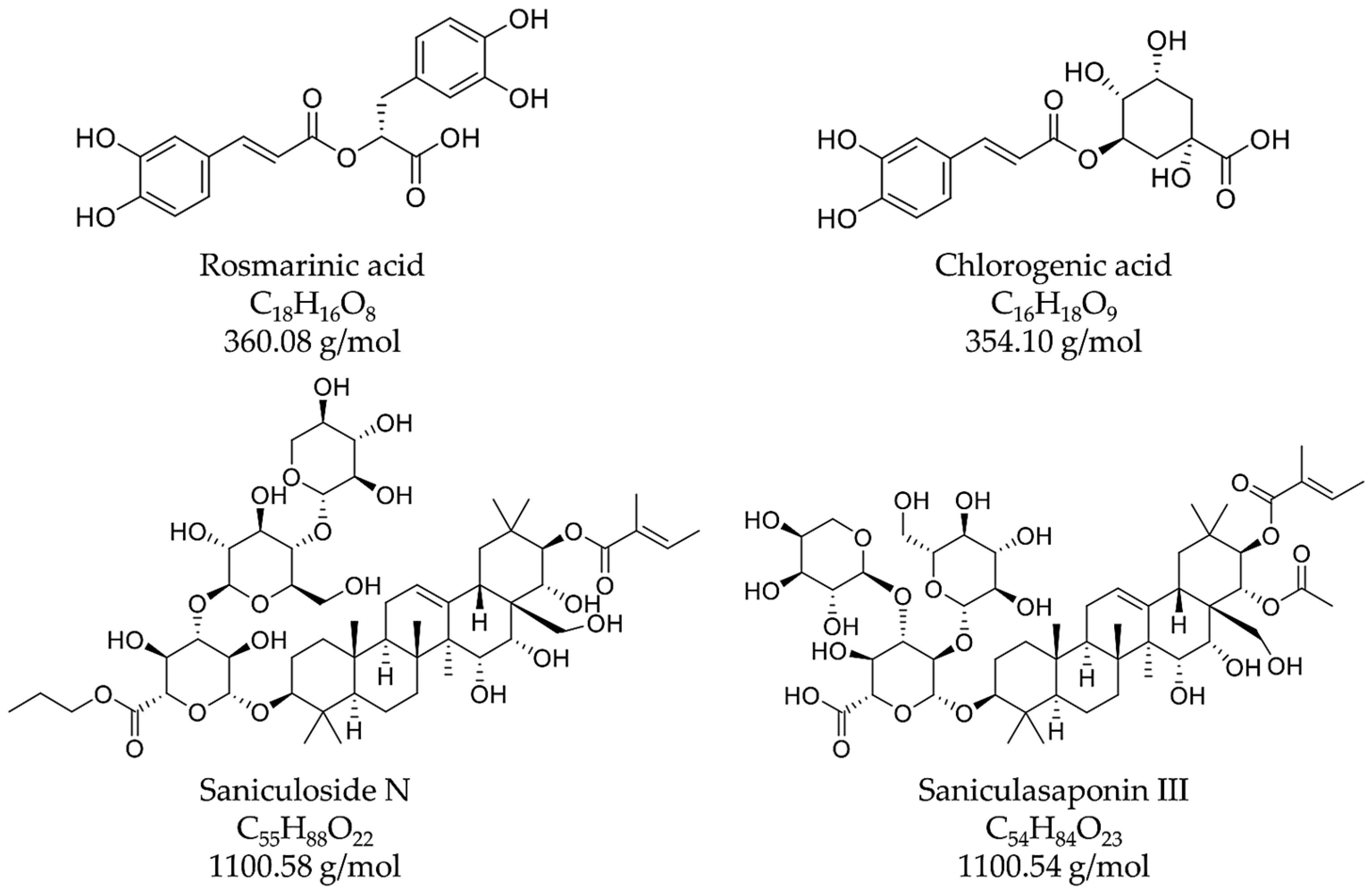
| 3 | 17.12 | 200, 329 | 161.09 [M-H]− Glucopyranose, 359.36 [M-H]− Rosmarinic acid, 521.40 [M-H]−, 1043.39 [2M-H]− | 163.05 [M+H]+ Glucopyranose, 361.11 [M+H]+ Rosmarinic acid, 523.16 [M+H]+, 721.12 [2M+H]+ Rosmarinic acid, 1045.06 [2M+H]+ | 4-O-ß-D-glucopyranosyl rosmarinic acid (or another rosmarinic acid derivative) |
| 4 | 18.36 | 203, 241, 326 | 353.33 [M-Caffeoyl-H]−, 515.35 [M-H]− | 499.38 [M-H2O+H]+, 517.20 [M+H]+ | 3,4-Dicaffeoylquinic acid 2 |
| 5 | 18.65 | 196, 212, 329 | 359.29 [M-H]−, 719.32 [2M-H]−, 1079.05 [3M-H]− | 361.14 [M+H]+, 721.13 [2M+H]+, 1081.07 [3M+H]+ | Rosmarinic acid 2 |
| 6 | 19.37 | 202, 328 | 1099.87 [M-H]− | 1101.49 [M+H]+ | Saponins |
| 7 | 19.84 | 203, 323 | 925.83 [M-H]− | 927.48 [M+H]+ | Saponins |
| 8 | 21.29 | 203, 329 | 1099.91 [M-H]− | 1101.37 [M+H]+ | Saponins |
| 9 | 21.69 | 205 | 967.93 [M-H]− | 969.42 [M+H]+ | Saponins |
| 10 | 22.35 | 203, 322 | 969.86 [M-H]− | 971.17 [M+H]+ | Saponins |
| 11 | 22.91 | 205 | 909.88 [M-H]− | 911.42 [M+H]+ | Saponins |
| Peak ID 1 | Rt. [min] 1 | UV max [nm] 1 | m/z [neg.] | m/z [pos.] | Putatively Assigned Compound/Compound Class |
|---|---|---|---|---|---|
| 1 | 2.29 | nd | 341.31 [M-H]−, 387.25 [M+FA-H]−, 683.41 [2M-H]−, 729.20 [2M+FA-H]−, 1025.23 [3M-H]−, 1071.20 [3M+FA-H]− | 365.36 [M+Na]+, 381.28 [M+K]+, 707.21 [2M+Na]+, 723.13 [2M+K]+ | Sucrose 2 (sugars) |
| 2 | 15.60 | 202, 242, 325 | 191.11 [M-H]− Quinic acid, 353.29 [M-H]−, 707.39 [2M-H]−, 1061.03 [3M-H]− | 355.24 [M+H]+, 377.30 [M+Na]+ | Chlorogenic acid 2 |
| 3 | 17.24 | 200, 283, 324 | 161.08 [M-H]− Glucopyranose, 359.31 [M-H]− Rosmarinic acid, 521.39 [M-H2O-H]−, 539.34 [M-H]− | 163.06 [M+H]+ Glucopyranose, 361.22 [M+H]+ Rosmarinic acid, 541.30 [M+H]+ | Rosmarinic acid derivative |
| 4 | 18.35 | 201, 244, 328 | 353.29 [M-Caffeoyl-H]−, 515.36 [M-H]− | 499.36 [M-H2O+H]+, 517.15 [M+H]+ | 3,4-Dicaffeoylquinic acid 2 |
| 5 | 18.70 | 196, 211, 329 | 359.28 [M-H]−, 719.27 [2M-H]−, 1079.04 [3M-H]− | 361.12 [M+H]+, 721.09 [2M+H]+, 1081.09 [3M+H]+ | Rosmarinic acid 2 |
| 6 | 21.35 | 203, 323 | 1099.89 [M-H]− | 1101.26 [M+H]+ | Saponins |
| 7 | 21.89 | 201 | 1101.91 [M-H]− | 1085.38 [M-H2O+H]+, 1103.34 [M-+H]+ | Saponins |
| 8 | 22.37 | 203, 315 | 969.83 [M-H]− | 953.16 [M-H2O+H]+, 971.15 [M+H]+ | Saponins |
| 9 | 22.90 | 196 | 1001.89 [M-H]− | 1003.35 [M+H]+ | Saponins |
| 10 | 23.41 | 202, 322 | 969.85 [M-H]− | 971.42 [M+H]+ | Saponins |
| Peak ID 1 | Rt. [min] 1 | UV max [nm] 1 | m/z [neg.] | m/z [pos.] | Putatively Assigned Compound/Compound Class |
|---|---|---|---|---|---|
| 1 | 2.29 | nd | 341.33 [M-H]−, 387.27 [M+FA-H]−, 683.45 [2M-H]−, 729.23 [2M+FA-H]−, 1025.26 [3M-H]−, 1071.23 [3M+FA-H]− | 365.35 [M+Na]+, 381.32 [M+K]+, 707.37 [2M+Na]+, 723.19 [2M+K]+ | Sucrose 2 (sugars) |
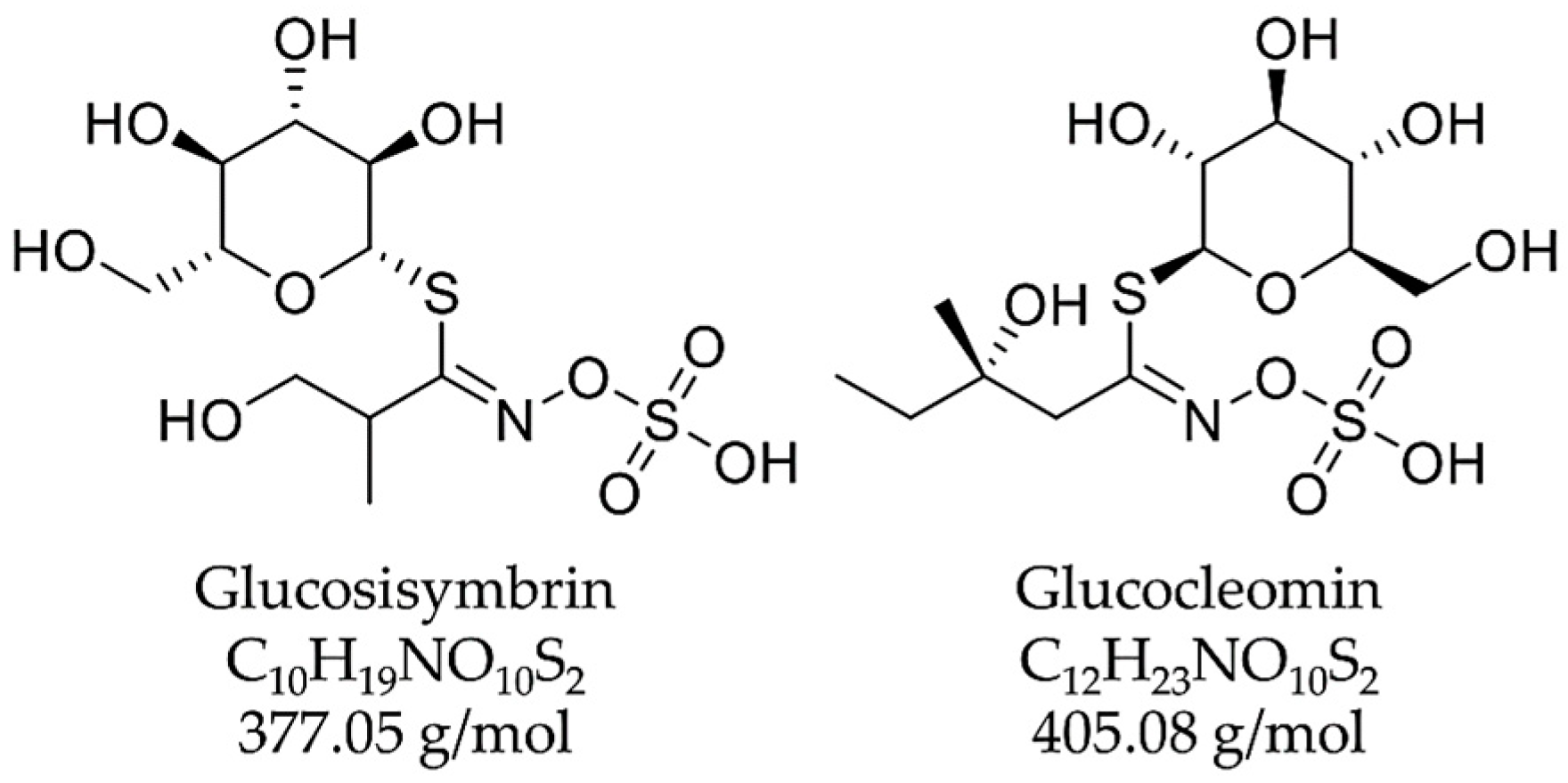
| 2 | 4.18 | 196 | 376.26 [M-H]−, 753.17 [2M-H]−, 1129.94 [3M-H]− | 378.24 [M+H]+, 755.02 [2M+H]+, 1132.05 [3M+H]+ | Glucosisymbrin (glucosinolate) |
| 3 | 6.41 | 230 | 404.31 [M-H]−, 809.20 [2M-H]−, 1214.00 [3M-H]− | 406.25 [M+H]+, 811.07 [2M+H]+, 1216.04 [3M+H]+ | Glucocleomin (glucosinolate) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichenauer, E.; Sykora, C.; Ortmayr, K.; Glasl, S. “Radix Saniculae”: Phytochemical Characterization and Potential Adulteration of an Austrian Traditional Wound-Healing Agent. Plants 2025, 14, 266. https://doi.org/10.3390/plants14020266
Eichenauer E, Sykora C, Ortmayr K, Glasl S. “Radix Saniculae”: Phytochemical Characterization and Potential Adulteration of an Austrian Traditional Wound-Healing Agent. Plants. 2025; 14(2):266. https://doi.org/10.3390/plants14020266
Chicago/Turabian StyleEichenauer, Elisabeth, Christina Sykora, Karin Ortmayr, and Sabine Glasl. 2025. "“Radix Saniculae”: Phytochemical Characterization and Potential Adulteration of an Austrian Traditional Wound-Healing Agent" Plants 14, no. 2: 266. https://doi.org/10.3390/plants14020266
APA StyleEichenauer, E., Sykora, C., Ortmayr, K., & Glasl, S. (2025). “Radix Saniculae”: Phytochemical Characterization and Potential Adulteration of an Austrian Traditional Wound-Healing Agent. Plants, 14(2), 266. https://doi.org/10.3390/plants14020266






