Abstract
Molecular diversity is a key component of overall biodiversity, playing a vital role in evolution. It results from the adaptation of organisms to various habitats, which impacts their survival. The Amaryllidoideae subfamily is a significant group of monocotyledonous plants known for producing an exclusive and still-expanding group of molecules with diverse biological activities. Galanthamine (Gal), the most renowned metabolite from Amaryllidoideae subfamily, has been marketed for the palliative treatment of Alzheimer’s disease since 2001 due to its ability to inhibit the acetylcholinesterase enzyme. Due to the high cost and low yield of its synthesis, pharmaceutical companies extract this drug from Amaryllidoideae plants, such as Narcissus pseudonarcissus cv. Carlton in Europe and Lycoris radiata in China. The aim of this study was to describe the alkaloid profile of fifteen different species of Narcissus L. (commonly known as daffodils) collected in Spain using gas chromatography coupled with mass spectrometry. Fifty-one alkaloids were identified and quantified within these species through our private library of Amaryllidaceae alkaloids (AA) built over the last four decades, while thirty structures remained not identified in thirteen of these species. The highest concentration of these nitrogenate metabolites was quantified in N. confusus, 541 μg Gal·100 mg−1 DW, which also exhibited a notably high concentration of Gal, 301 μg Gal·100 mg−1 DW, which represents about 55% of the alkaloids identified in this species. The species N. bujei was also found to contain a significant quantity of this compound, amounting to 103.2 μg Gal·100 mg−1 DW. The plant N. assoanus harbored a total of seven unidentified compounds, indicating that this species could be a potentially important source of novel alkaloids. In conclusion, this study facilitates a direct comparison of alkaloid profiles for fifteen Narcissus plant species. This serves as a valuable tool for identifying possible new sources of galanthamine, as well as other novel medicinal alkaloids. Finally, this work presents the first alkaloid profile of the species N. minor and N. nevadensis.
1. Introduction
Plants constitute an important pillar of terrestrial ecosystems, providing a vast array of bioresources essential for human nutrition, healthcare, cultural practices, and technological advancements [1]. Plants from the subfamily Amaryllidoideae (Amaryllidaceae) are widely appreciated and cultivated for their ornamental value. This subfamily includes more than 60 genera distributed worldwide, with Narcissus L., commonly known as daffodils, being the most well-known in Europe. This genus, which comprises around 100 species, has a significant center of diversity in the Iberian Peninsula, particularly in Spain [2].
Amaryllidoideae plants also have medicinal value owing to the biological activity of the alkaloids they can biosynthesize. These compounds, known as Amaryllidaceae alkaloids (AA), are isoquinoline-type alkaloids, and they constitute a distinctive chemotaxonomic feature of this subfamily [3]. These molecules have demonstrated extensive biological activities, such as antiviral, antifungal, antibacterial, antimalarial, insecticide, cytotoxic, antitumor, and antifungal activities, as well as the inhibition of acetylcholinesterase (AChE), among others [4,5,6,7].
Over 600 AA have been reported to date, all originating from the precursor norbelladine, which is formed from L-phenylalanine and L-tyrosine. This precursor diverged into different skeleton types through three phenol-oxidative couplings: o-p’ (leading to lycorine and homolycorine types), p-p’ (leading to haemanthamine, crinine, narciclassine, montanine, and tazettine types), and p-o’ (leading to galanthamine-type alkaloids). The final diversity of these alkaloids is then achieved through reactions like reduction, oxidation, hydroxylation, methylation, condensation, and oxide bridge formation [3,8].
Galanthamine, an acetylcholinesterase (AChE) inhibitor, is an Amaryllidaceae alkaloid that has emerged as a valuable therapeutic agent in the palliative management of Alzheimer’s disease (AD) symptoms following its FDA approval in 2001. This drug is able to traverse the blood–brain barrier, and its long-lasting, selective, reversible, and competitive inhibitory properties make it a valuable pharmacological tool in combating AD-associated cognitive decline. This medication is commercialized under the brand names Razadine® in the USA and Reminyl® in Europe [9,10]. Moreover, following patent expiration, it has become available as a generic drug, expanding its accessibility and affordability for AD patients. This alkaloid is obtained from natural sources within the Amaryllidoideae subfamily by pharmaceutical companies. They extract it from plants such as Narcissus pseudonarcissus L. cv. Carlton in Europe and Lycoris radiata (L’Hérit.) Herb in China. However, several problems, including unsuccessful cultivation or slow regeneration, make it difficult to meet increasing pharmaceutical demands. Gal can also be produced by chemical synthesis, but the yield of the total synthesis is still considered too low to be economically feasible [11].
In a recent publication, we described the acetylcholinesterase (AChE)- and butyrylcholinesterase (BuChE)-inhibitory potential of fifteen different Narcissus L. species collected in Spain [12]. In this way, the aim of this current work was to provide the alkaloid profile of these same fifteen species of Narcissus using gas chromatography coupled to mass spectrometry (GC-MS).
2. Results and Discussion
2.1. Identified Alkaloids
Fifty-one alkaloids have been identified and quantified in the leaf extract of fifteen different species of Narcissus (Table 1). Due to limitations in obtaining reference compounds for the absolute quantification of each alkaloid determined in these samples, Gal was used for this purpose. Therefore, the values described in Table 1 represent the alkaloid content expressed as micrograms of Gal equivalents per 100 milligrams of dry weight (DW) of plant material (μg Gal·100 mg−1 DW). The chemical structures of these alkaloids are represented in Figure 1.

Table 1.
Alkaloids identified in Narcissus L. species by GC-MS. Values are expressed in μg Gal·100 mg−1 DW.
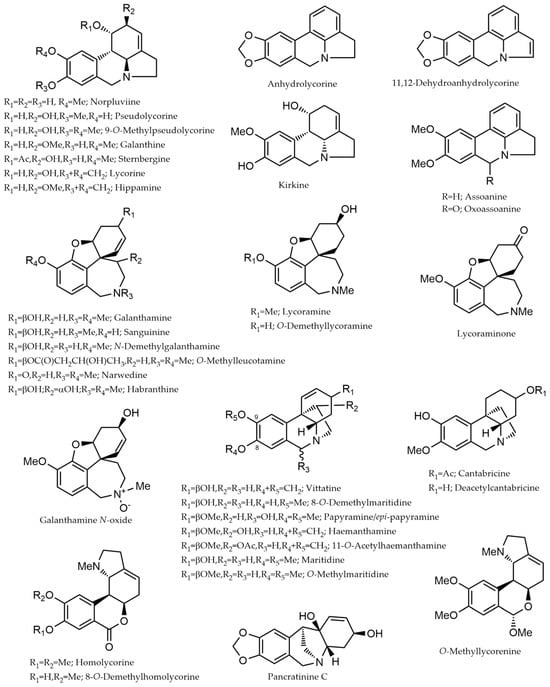
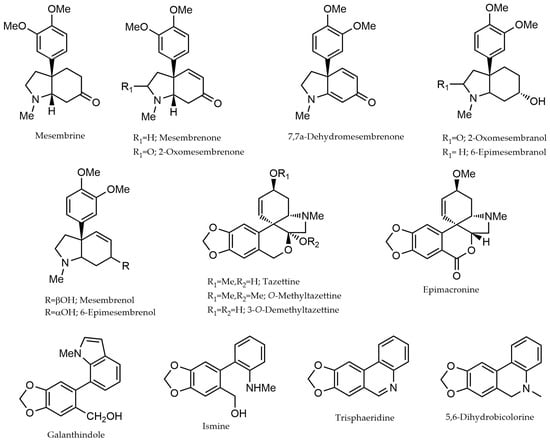
Figure 1.
Chemical structure of the alkaloids identified in the different species of Narcissus L. by GC-MS. The structures were drawn using ChemDraw (version 2023).
Table 1 facilitates the comparison of the alkaloid profiles across the fifteen Narcissus species analyzed herein. Detailed information regarding the yield of each extract is presented by Tallini and co-authors [12]. According to this, N. yepesii exhibited the highest yield (6.14%), significantly exceeding the average yield of 1.32% observed among the remaining species [12].
Evaluating the results expressed in Table 1, the highest quantity of identified alkaloids was observed in the extract of N. confusus (sample E), followed by the species N. bujei (sample I), with a total of 541.0 and 523.2 μg Gal·100 mg−1 DW, respectively. On the other hand, the species N. vasconicus (sample C) and N. minor (sample D) presented the lowest concentration of identified alkaloids, with values of 63.2 and 67.1 μg Gal·100 mg−1 DW, respectively (Table 1).
The species N. confusus stands out among the species tested for showing high quantities of galanthamine-type alkaloids, specially galanthamine, with 301.0 μg Gal·100 mg−1 DW, which represents 55.6% of its total identified alkaloids. This translates to a yield of 0.3% (of DW) of galanthamine from the leaves of this species. The high concentration of galanthamine in this sample is consistent with previously documented levels reported in the literature [13]. As shown in Table 1, alongside galanthamine-type structures, N. confusus also presented alkaloids from haemanthamine-, pretazettine-, homolycorine-, narciclasine-, ismine-, and galanthindole-type alkaloids. In Europe, the bulbs of the species Narcissus pseudonarcissus cv. Carlton are used by pharmaceutical companies to obtain galanthamine, which contains about 0.12–0.15% (of DW) of this drug and significant quantities of haemanthamine and narciclasine [11].
Among all the species evaluated herein, N. bujei (sample I) was the second most representative in galanthamine. This species showed 103.2 μg Gal·100 mg−1 DW of this product, which represents about 20% of the alkaloids content identified in this sample. The presence of structures belonging to other scaffolds have also been observed in this species, represented by high concentrations of the alkaloids haemanthamine and homolycorine, with values of 141.1 and 135.8 μg Gal·100 mg−1 DW, respectively (Table 1).
The sample N. pallidulus (sample J) showed a high diversity of alkaloids from the Sceletium-type group, which are also known as mesembrine-type alkaloids. Six structures with this alkaloid-type skeleton have been identified in this species, totaling 139.9 μg Gal·100 mg−1 DW. Among them, the alkaloid mesembrenone was the most representative, with 71.0 μg Gal·100 mg−1 DW (see Table 1). Previous works also report the presence of different structures from Sceletium-type alkaloids in N. pallidulus [14,15]. This scaffold is the only group of structures that is not exclusively of the monocotiledon subfamily Amaryllidoideae, being typical alkaloids of the genus Sceletium, which belongs to the dicotyledonous family Aizoaceae, with a center of origin in South Africa [14,16]. According to Table 1, three alkaloids from the haemanthamine, pretazettine, and homolycorine groups have also been detected in the leaf extracts of N. pallidulus, but in a very low concentration, totaling 14.6 μg Gal·100 mg−1 DW.
In addition to N. pallidulus, the presence of Sceletium-type alkaloids was also observed in the species N. minor (sample D), specifically mesembrenone and 2-oxomesembrenone, with 2.8 and 3.5 μg Gal·100 mg−1 DW, respectively. This species also showed alkaloids belonging to galanthamine-, homolycorine-, and lycorine-type scaffolds (Table 1). Along with N. nevadensis (sample O), this is the first report about the alkaloid profiling of these two plant species. In N. nevadensis, it was possible to identify lycorine- and homolycorine-type alkaloids, with homolycorine being the most abundant compound at 34.7 μg Gal·100 mg−1 DW (Table 1).
The species N. confusus (sample E) and N. asturiensis (sample F) presented the highest amount of pretazettine-type alkaloids, of which tazettine was the major representative, with 53.2 and 58.7 μg Gal·100 mg−1 DW, respectively (Table 1). This alkaloid is an artifact of pretazettine, which shows no EI mass fragmentation under GC–MS conditions due to its rapid conversion to tazettine in the chromatographic column [17].
Among all the species reported in Table 1, only N. yepesii (sample N) showed the presence of montanine-type alkaloids, represented by pancratinine C (25.1 μg Gal·100 mg−1 DW), which represents around 10% of its total content of identified alkaloids (260.8 μg Gal·100 mg−1 DW). This alkaloid scaffold comprises a limited group of fourteen structures isolated from Amaryllidaceae genera that lack phylogenetic relatedness and is very unusual among Narcissus species [3].
2.2. Not Identified Alkaloids
All the alkaloids quantified in the leaf extracts of the species N. bujei (sample I) and N. yepesii (sample O) have been identified (Table 1). However, it was not possible to identify a total of thirty alkaloids distributed among the other thirteen species evaluated herein (Table 2).

Table 2.
Not identified alkaloids quantified in Narcissus L. species by GC-MS. Values are in μg Gal·100 mg−1 DW.
N. assoanus (sample A) harbored the most diverse range of not identified structures, with a total of seven, followed by N. hedraeanthus (sample G), with five not identified alkaloids. According to Table 2, two species, N. jacetanus (sample B) and N. pallidulus (sample J), exhibited four not identified structures each, while one species, N. minor (sample D), contained three not identified structures. Most of the remaining species had zero-to-two not identified alkaloids in their chemical composition (Table 2).
In addition to showing the greatest diversity of not identified alkaloids, the species N. assoanus also presented the highest quantity of these structures, totaling 76.4 μg Gal·100 mg−1 DW. The second sample with the highest content of not identified alkaloids was N. confusus (sampe E), containing 61.8 μg Gal·100 mg−1 DW of the compound NI-25. Alongside N. confusus, N. alcaracensis (sample H) and N. genesii-lopezii (sample M) also presented high amounts of this structure, which could be a new compound. Consistent with previous reports [18], the presence of a characteristic base peak at m/z 109 in the fragmentation pattern of NI-25 (Table 2) suggests that this molecule belongs to the homolycorine-type skeleton and shows a double bond among C-3 and C-4. The species N. minor and N. nevadensis, whose alkaloid profiles are described here for the first time, each exhibited two not identified alkaloids.
According to the literature, an investigation into the alkaloid profile of 40 ornamental Narcissus taxa was conducted to identify suitable sources of biologically active compounds, such as Gal, lycorine, and haemanthamine, for commercial production [19]. Among all the samples, the authors detected 97 typical AA, of which 49 were not identified [19].
2.3. Alkaloid Type Distribution Among the Species
To represent the distribution of different alkaloid types and not identified alkaloids in each Narcissus L. extract, a doughnut chart was generated for every sample. These charts represent the percentage of identified alkaloid-type scaffolds and not identified structures quantified in the fifteen different species of Narcissus using GC-MS analysis (Figure 2). This type of graphical representation provides a comprehensive overview of each sample. Depicting the relative abundance of identified and not identified alkaloids provides a deeper understanding of the overall alkaloid profile for each species and helps identify species that could be a source of novel bioactive alkaloids.
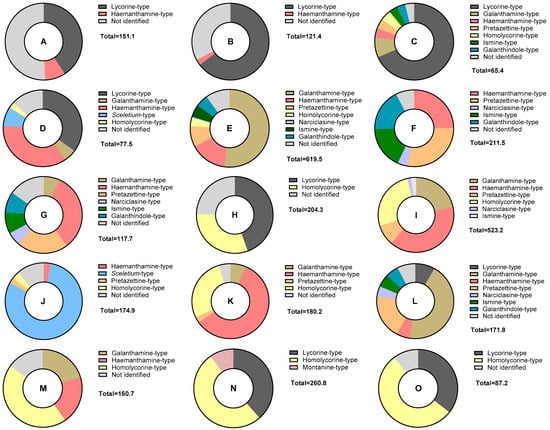
Figure 2.
Doughnut chart representing the percentage of identified alkaloid-type scaffolds and not identified structures quantified in each Narcissus L. extract using GC-MS analysis. Total values are in μg Gal·100 mg−1 DW. A = N. assoanus; B = N. jacetanus; C = N. vasconicus; D = N. minor; E = N. confusus; F = N. asturiensis; G = N. hedraeanthus; H = N. alcaracensis; I = N. bujei; J = N. pallidulus; K = N. tazetta; L = N. jonquilla; M = N. genesii-lopezii; N = N. yepesii; O = N. nevadensis.
In the doughnut chart in Figure 2, it can be observed that the not identified alkaloids (in light gray color) represent an important percentage in the samples A, B, and H (N. assoanus, N. jacetanus, and N. alcaracensis, respectively). On the other hand, this analysis highlights the significant potential of samples E, I, L, and M (N. confusus, N. bujei, N. jonquilla, and N. genesii-lopezii) as a source of galanthamine-type structures, represented by the mustard yellow color.
Samples A and L belong to the section Jonquillae; samples B, C, D, E, F, I, and M to Pseudonarcissus; H, N, and O to Nevadensis; and J, K, and G to Ganymedes, Tazettae, and Bulbocodium, respectively [12]. Taking the sections into account, the species belonging to section Jonquillae, N. assoanus (A) and N. jonquilla (L), did not show high similarity in their alkaloid profiles. In contrast, all species from the Pseudonarcissus section exhibited alkaloids of the haemanthamine type. The species N. nevadensis (O), after which the section Nevadensis was named, contains alkaloids of the lycorine and homolycorine types, similar to other species in the same section, such as N. alcaracensis (H) and N. yepesii (N) (Figure 2).
Based on a comprehensive review of the available literature, our previous publication [12] provided a detailed overview of the alkaloid previously identified in these same fifteen species of Narcissus L. Synthesizing the information previously published, the following structures belonging to the alkaloid-type skeletons, namely, bujeine, ismine, galanthamine, haemanthamine, homolycorine, lycorine, montanine, narciclasine, norbelladine, pretazettine, and Sceletium, have previously been reported among these species [13,15,16,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Detailed information is available in Table 3. No prior information regarding the alkaloid content of the species N. minor (sample D) and N. nevadensis (sample O) has been found in the literature.

Table 3.
Literature information about alkaloid-type scaffolds previously identified in fifteen different species of Narcissus L.
On the other hand, in a recent publication [12], we described the acetylcholinesterase (AChE)- and butyrylcholinesterase (BuChE)-inhibitory potential of the same species of Narcissus reported herein. According to our previous results [12], N. jacetanus exhibited the most potent AChE inhibition, with an IC50 value of 0.75 ± 0.03 μg/mL, while N. jonquilla demonstrated the strongest BuChE inhibition, with an IC50 value of 11.72 ± 1.15 μg/mL [12]. Comprehensive alkaloid profiling of N. jacetanus, as presented in Table 1 and Table 2, identified a range of known alkaloids, including assoanine, oxoassoanine, lycorine, pseudolycorine, 9-O-methylpseudolycorine, kirkine, and cantabricine. Notably, this extract also harbors four not identified structural components. Furthermore, the alkaloids anhydrolycorine, lycorine, galanthamine, lycoraminone, lycoramine, narwedine, haemanthamine, tazettine, epimacronine, 5,6-dihydrobicolorine, ismine, galanthindole, and two unidentified structures have been quantified among the alkaloid profiling of N. jonquilla (Table 1 and Table 2).
In an effort to elucidate the connections between alkaloid biosynthesis and phylogenetic relationships, Berkov and co-authors [16] determined the alkaloid patterns of 22 species and 3 hybrids from 7 main sections of the genus Narcissus, including the species N. confusus, N. genesii-lopezii, N. alcaracensis, N. yepesii, N. hedraeanthus, N. assoanus, N. jonquilla, and N. tazetta. The authors concluded that the chemical diversity within the Narcissus genus suggests that alkaloid biosynthesis, similar to floral morphology, is a dynamic process marked by extensive diversification and convergent evolution. They suggested that the biosynthesis of bioactive compounds such as Gal may take place in different phylogenetic branches. Furthermore, they noted that closely related species, inhabiting confined and neighboring regions, exhibit analogous alkaloid profiles, with variations observed in the proportions of the main alkaloid types [16].
3. Materials and Methods
3.1. Plant Material
In April 2023, a collection of fifteen distinct wild species of Narcissus L. was carried out in the Iberian Narcissus Collection of the Torretes Biological Research Station—Botanical Garden of the UA, in Ibi (Alicante), Spain, during their flowering season. The species were authenticated by a botanist, as previously and extensively described in [12]. The fifteen species evaluated in this work were as follows: N. assoanus Dufour ex Schult. and Schult. f.; N. jacetanus Fern. Casas; N. vasconicus (Fern. Casas) Fern. Casas; N. minor L.; N. confusus Pugsley; N. asturiensis (Jord.) Pugsley; N. hedraeanthus (Webb and Heldr.) Colmeiro; N. alcaracensis S. Ríos, D. Rivera, Alcaraz and Obón; N. bujei (Fern. Casas) Fern. Casas; N. pallidulus Graells; N. tazetta L.; N. jonquilla L.; N. genesii-lopezii Fern. Casas; N. yepesii S. Ríos, D. Rivera, Alcaraz and Obón; and N. nevadensis Pugsley. The leaves of these fifteen different Narcissus L. species were collected, cut into pieces, and dried at 40 °C to preserve the stability of the alkaloids.
3.2. Alkaloid Extraction
The dried material from the various Narcissus L. species was milled into a fine powder using a rotary blade mill (Taurus, Oliana, Spain). For extraction, 1 g of the powder was macerated in methanol for three days at 25 °C. The solvent was refreshed daily with three 50 mL aliquots, and an ultrasonic bath was applied for 20 min, four times a day, to enhance the extraction. The mixture was then strained, and the solvent was evaporated under reduced pressure to obtain crude extracts. The crude extracts were acidified to pH 2 with 30 mL of 2% (v/v) sulfuric acid, followed by a wash with ethyl acetate (3 × 50 mL) to remove neutral materials. The pH of the remaining aqueous phase was subsequently adjusted to 9–10 using a 25% (v/v) ammonium hydroxide solution. Finally, alkaloids were extracted with ethyl acetate (3 × 50 mL), and the solvent was evaporated to yield the dried alkaloid extract (AE).
3.3. Alkaloid Analysis
A total of 2 mg of each sample were dissolved in 1 mL of methanol containing 25 µg·mL−1 of codeine, used as the internal standard, and injected into a gas chromatograph (Agilent Technologies 6890N, Agilent Technologies, Santa Clara, CA, USA) coupled with mass spectrometry (Agilent Technologies 5975, Agilent Technologies, Santa Clara, CA, USA), both of which were obtained from Hewlett Packard, Palo Alto, CA, USA. The equipment was operated using electronic impact ionization (EI) at 70 eV with an autoinjector, 7683B Series (Agilent Technologies, Santa Clara, CA, USA). The column was a Tecknokroma TR- 45232 Sapiens-X5MS (30 m × 0.25 mm, film thickness 0.25 μm), and the splitless injection volume was 1 μL. The temperature gradient was as follows: 12 min at 100 °C, 100–180 °C at 15 °C/min, 1 min of retention at 180 °C, 180–300 °C at 5 °C/min, and 10 min of holding at 300 °C. The injector and detector temperatures were 250 and 280 °C, respectively. The carrier gas (He) flow rate was 1 mL·min−1.
3.4. Alkaloid Identification
The identification of alkaloids present in each Narcissus L. sample involved the comparison of the electron ionization (EI) fragments and the Kovats Retention Indices (RI) obtained for each compound against a reference library of authentic AA. This library was created by the Natural Products Research Group of the Faculty of Pharmacy and Food Sciences at the University of Barcelona, Spain. The structures of these reference compounds have been definitively established using a combination of spectroscopic techniques, including nuclear magnetic resonance (NMR), ultraviolet (UV), circular dichroism (CD), and mass spectrometry (MS). The software AMDIS 2.64 (Automatic Mass Spectral Deconvolution and Identification System) was used to process the mass spectral data. Additionally, the RI values were determined by referencing the compounds to a standard n-hydrocarbon calibration mixture containing n-alkanes from C9 to C30.
3.5. Alkaloid Quantification
A relative quantification of the AA present in each Narcissus L. sample was performed. A galanthamine calibration curve was constructed using concentrations ranging from 10 to 80 µg·mL−1. Codeine, at a fixed concentration of 25 µg·mL−1, was used as the internal standard. Peak areas were determined manually, considering the most abundant ions in the mass spectrum (m/z) for each compound. Typically, the base peak was used, which is m/z 286 for galanthamine and m/z 299 for codeine. For each solution, the ratio between the values obtained for galanthamine and codeine (internal standard) in each solution was plotted against the corresponding concentration of galanthamine. This plot generated the calibration curve and its equation (y = 0.0632x − 0.4557; R2 = 0.9962). All data were normalized to the area of the internal standard (codeine) to account for variations during sample preparation or instrument response. Subsequently, the equation obtained from the galanthamine calibration curve was used to calculate the amount of each alkaloid present in the samples. While this method does not provide the absolute concentrations, it is well-suited for comparing relative amounts of specific alkaloids between different samples. Additionally, this approach allows for comparisons with previously quantified Amaryllidaceae plants analyzed using the same method [35]. The results are expressed as micrograms of galanthamine (Gal) equivalent per 100 milligrams of dry weight (DW) of the plant material (µg GAL·100 mg−1 DW). Data analysis was performed using Microsoft Excel 2016 software.
4. Conclusions
This study reported the alkaloid profile of fifteen different species of Narcissus L. collected in Spain. Notably, the species N. confusus and N. bujei revealed a high content of Gal, while the species N. pallidulus showed a very high concentration of Sceletium-type structures. This is the first alkaloid report for the species N. minor and N. nevadensis, and interestingly, N. minor also showed the presence of Sceletium-type compounds. The species N. assoanus exhibited a high concentration and structural diversity of not identified compounds, highlighting its potential as a novel source of AA.
Author Contributions
Conceptualization L.R.T., L.T.-C. and M.L.R.-E.; methodology, L.R.T. and M.L.R.-E.; software, L.R.T. and M.L.R.-E.; validation, L.R.T. and M.L.R.-E.; formal analysis, L.R.T. and M.L.R.-E.; investigation, L.R.T., L.T.-C., W.d.S.B. and G.E.F.; resources, S.R. and J.B.; writing—original draft preparation, L.R.T., L.T.-C. and M.L.R.-E.; writing—review and editing V.M.-F. and G.E.F.; visualization W.d.S.B. and S.R.; supervision L.R.T. and L.T.-C.; project administration, J.B.; funding acquisition L.R.T., L.T.-C. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Programa Iberoamericano de Ciencia y Tecnologia para el Desarrollo (CYTED, 223RT0140–RENATEC).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank CCiTUB from the University of Barcelona for the technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ahoyo, C.C.; Salako, K.V.; Houéhanou, T.D.; Montcho, I.; Kakaï, R.L.G.; Houinato, M.R.B. Sociodemographic environmental and biological factors affecting uses of plants from open ecosystems: Insights for improved livelihoods and biodiversity conservation. Front. Conserv. Sci. 2023, 4, 1127567. [Google Scholar] [CrossRef]
- Hanks, G.R. (Ed.) Narcissus and Daffodil: The Genus Narcissus; Vol. 21 in the Series: “Medicinal and Aromatic Plants—Industrial Profiles”; Taylor & Francis: London, UK, 2002; pp. 1–428. [Google Scholar]
- Berkov, S.; Osorio, E.; Viladomat, F.; Bastida, J. Chemodiversity, chemotaxonomy and chemoecology of Amaryllidaceae alkaloids. In The Alkaloids: Chemistry and Biology; Knölker, H.-J., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 83, pp. 113–185. [Google Scholar] [CrossRef]
- Sener, B.; Orhan, I.; Satayavivad, J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother. Res. 2003, 17, 1220–1223. [Google Scholar] [CrossRef]
- Nair, J.J.; Wilhelm, A.; Bonnet, S.L.; van Staden, J. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2007, 24, 886–905. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Escobar, M.L.; Tallini, L.R.; Lisa-Molina, J.; Berkov, S.; Viladomat, F.; Meerow, A.; Bastida, J.; Torras-Claveria, L. Chemical and Biological Aspects of Different Species of the Genus Clinanthus Herb. (Amaryllidaceae) from South America. Molecules 2023, 28, 5408. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, S.; Saini, K.S.; Razdan, S. Crinum alkaloids: Their chemistry and biology. Phytochemistry 1985, 24, 2141–2156. [Google Scholar] [CrossRef]
- Maelicke, A.; Samochocki, M.; Jostock, R.; Fehrenbacher, A.; Ludwig, J.; Albuquerque, E.X.; Zerlin, M. Allosteric sensitization of nicotinic receptors by galantamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry 2001, 49, 279–288. [Google Scholar] [CrossRef]
- Heinrich, M.; Teoh, H.L. Galanthamine from snowdrop—The development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004, 92, 147–162. [Google Scholar] [CrossRef]
- Berkov, S.; Georgieva, L.; Sidjimova, B.; Bastida, J. Evaluation of Hippeastrum papilio (Ravenna) Van Scheepen potencial as a new industrial source of galanthamine. Ind. Crop. Prod. 2022, 178, 114619. [Google Scholar] [CrossRef]
- Tallini, L.R.; Manfredini, G.; Rodríguez-Escobar, M.L.; Ríos, S.; Martínez-Francés, V.; Feresin, G.E.; Borges, W.d.S.; Bastida, J.; Viladomat, F.; Torras-Claveria, L. The Anti-Cholinesterase Potential of Fifteen Different Species of Narcissus L. (Amaryllidaceae) Collected in Spain. Life 2024, 14, 536. [Google Scholar] [CrossRef]
- Berkov, S.; Bastida, J.; Viladomat, F.; Codina, C. Development and validation of a GC-MS method for rapid determination of galanthamine in Leucojum aestivum and Narcissus ssp.: A metabolic approach. Talanta 2011, 83, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Pigni, N.B.; Ríos-Ruiz, S.; Martínez-Francés, V.; Nair, J.J.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Narcissus serotinus. J. Nat. Prod. 2012, 75, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Pigni, N.B.; Ríos-Ruiz, S.; Luque, L.; Viladomat, F.; Codina, C.; Bastida, J. Wild daffodils of the section Ganymedes from the Iberian Peninsula as a source of mesembrane alkaloids. Phytochemistry 2013, 95, 384–393. [Google Scholar] [CrossRef]
- Berkov, S.; Martínez-Francés, V.; Bastida, J.; Codina, C.; Ríos, S. Evolution of alkaloid biosynthesis in the genus Narcissus. Phytochemistry 2014, 99, 95–106. [Google Scholar] [CrossRef]
- de Andrade, J.P.; Pigni, N.B.; Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharmaceut. Biomed. 2012, 70, 13–25. [Google Scholar] [CrossRef]
- Berkov, S.; Denev, R.; Sidjimova, B.; Zerev, Y.; Shkondrov, A.; Torras-Claveria, L.; Viladomat, F.; Bastida, J. Gas chromatography-mass spectrometry of some homolycorine-type Amaryllidaceae alkaloids. Rapid Commun. Mass Spectrom. 2023, 37, e9506. [Google Scholar] [CrossRef]
- Breiterová, K.; Ločárek, M.; Kohelová, E.; Talácková, M.; Hulcová, D.; Opletal, L.; Cahlíková, L. Daffodils as Potential Crops of Biologically-active Compounds: Assessment of 40 Ornamental Taxa for their Alkaloid Profile and Cholinesterases Inhibition Activity. Nat. Prod. Commun. 2018, 13, 419–422. [Google Scholar] [CrossRef]
- Llabrés, J.M.; Viladomat, F.; Bastida, J.; Codina, C.; Rubiralta, M. Phenanthridine alkaloids from Narcissus assoanus. Phytochemistry 1986, 25, 2637–2638. [Google Scholar] [CrossRef]
- Viladomat, F.; Llabrés, J.M.; Bastida, J.; Cusidó, R.M.; Codina, C. Ontogenic variations in the alkaloids of Narcissus assoanus. Physiol. Plant. 1986, 68, 657–661. [Google Scholar] [CrossRef]
- Bastida, J.; Viladomat, F.; Llabrés, J.M.; Codina, C.; Rubiralta, M. Alkaloids from Narcissus jacetanus. Planta Med. 1988, 54, 362. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Codina, C.; Viladomat, F. Narcissus alkaloids, XIV. (+)-8-O-Acetylhomolycorine and vasconine, two novel alkaloids from Narcissus vasconicus. J. Nat. Prod. 1992, 55, 122–125. [Google Scholar] [CrossRef]
- López, S.; Bastida, J.; Viladomat, F.; Codina, C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002, 71, 2521–2529. [Google Scholar] [CrossRef]
- Bastida, J.; Viladomat, F.; LLabres, J.M.; Codina, C.; Feliz, M.; Rubiralta, M. Alkaloids from Narcissus confusus. Phytochemistry 1987, 26, 1519–1524. [Google Scholar] [CrossRef]
- Bastida, J.; Llabrés, J.M.; Viladomat, F.; Codina, C. Narcissus alkaloids, III. 9-O-demethylhomolycorine from Narcissus confusus. J. Nat. Prod. 1987, 50, 199–202. [Google Scholar] [CrossRef]
- Viladomat, F.; Sellés, M.; Codina, C.; Bastida, J. Alkaloids from Narcissus asturiensis. Planta Med. 1997, 63, 583. [Google Scholar] [CrossRef]
- Labraña, J.; Choy, G.; Solans, X.; Font-Bardia, M.; de la Fuente, G.; Viladomat, F.; Codina, C.; Bastida, J. Alkaloids from Narcissus bujei (Amaryllidaceae). Phytochemistry 1999, 50, 183–188. [Google Scholar] [CrossRef]
- Seijas, J.A.; Vázquez-Tato, M.P.; Linares, M.T.; Ramil-Rego, P.; Buján, M.I. Mesembrine alkaloids from Narcissus triandrus L. In Proceedings of ECSOC-8, The Eight International Electronic Conference on Synthetic Organic Chemistry; Seijas, J.A., Vázquez Tato, M.P., Eds.; MDPI: Basel, Switzerland, 2004; Available online: https://www.mdpi.org/ecsoc/ecsoc-8/BOCNP/008/index.htm (accessed on 7 February 2024).
- Karakoyun, Ç.; Ünver-Somer, N. Simultaneous quantitative analysis of biologically important Amaryllidaceae alkaloids in Narcissus tazetta L. subsp. tazetta by HPLC/PDA. J. Res. Pharm. 2019, 23, 498–505. [Google Scholar] [CrossRef]
- Karakoyun, Ç.; Bozkurt, B.; Çoban, G.; Masi, M.; Cimmino, A.; Evidente, A.; Somer, N.U. A comprehensive study on Narcissus tazetta subsp. tazetta L.: Chemo-profiling, isolation, anticholinesterase activity and molecular docking of Amaryllidaceae alkaloids. S. Afr. J. Bot. 2020, 130, 148–154. [Google Scholar] [CrossRef]
- Tarakemeh, A.; Azizi, M.; Rowshan, V.; Salehi, H.; Spina, R.; Dupire, F.; Arouie, H.; Laurain-Mattar, D. Screening of Amaryllidaceae alkaloids in bulbs and tissue cultures of Narcissus papyraceus and four varieties of N. tazetta. J. Pharmaceut. Biomed. 2019, 172, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Gotti, R.; Fiori, J.; Bartolini, M.; Cabrini, V. Analysis of Amaryllidaceae alkaloids from Narcissus by GC-MS and capillary electrophoresis. J. Pharmaceut. Biomed. 2006, 42, 17–24. [Google Scholar] [CrossRef]
- Masi, M.; Frolova, L.; Yu, X.; Mathieu, V.; Cimmino, A.; De Carvalho, A.; Kiss, R.; Rogelj, S.; Pertsemlidis, A.; Kornienko, A.; et al. Jonquailine, a new pretazettine-type alkaloid isolated from Narcissus jonquilla quail, with activity against drug-resistant cancer. Fitoterapia 2015, 102, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Torras-Claveria, L.; Berkov, S.; Codina, C.; Viladomat, F.; Bastida, J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind. Crop Prod. 2014, 56, 211–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).