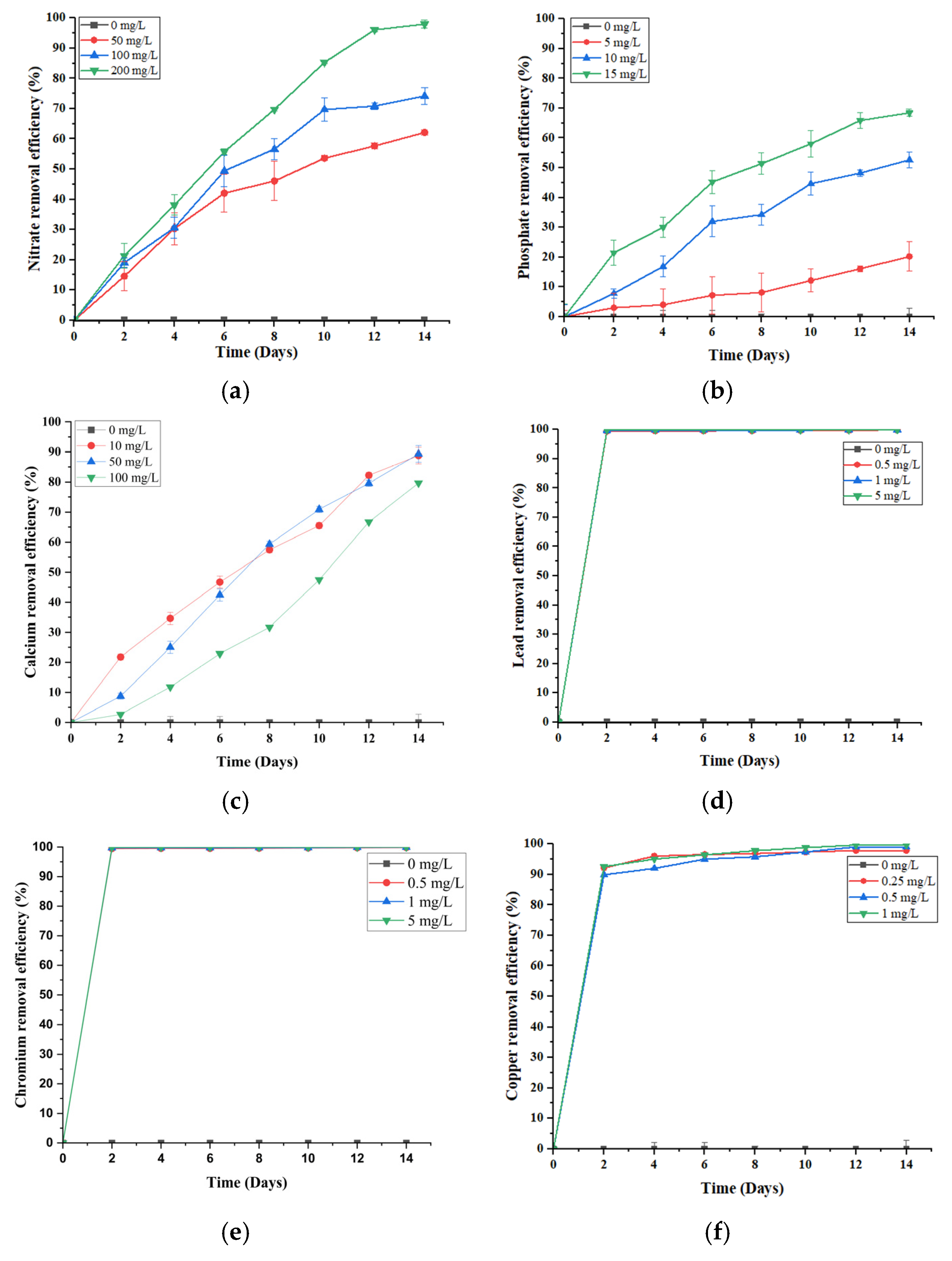
2.1. Removal Efficiency of Nutrients and Heavy Metals by S. brachiata
Pollutant removal capacity remains a primary benchmark for assessing the effectiveness of a phytoremediation system. In this study,
S. brachiata consistently demonstrated high removal efficiencies for nitrate (NO
3−), phosphate (PO
43−), calcium (Ca
2+), lead (Pb
2+), chromium (Cr
6+), and copper (Cu
2+) over a 14-day hydroponic exposure, as illustrated in
Figure 1.
The NO
3− removal efficiency of
S. brachiata increased progressively over time across all concentrations (50, 100, and 200 mg/L), peaking at 96.06 ± 0.79% in the 200 mg/L treatment group by day 12 (
Figure 1a). With a half-life reached by day 6 (55.76% removal from 200 mg/L of initial NO
3− concentration), this rapid uptake suggests the involvement of high-affinity NO
3− transporters operating effectively under moderate to high NO
3− availability. The sustained removal further indicates internal assimilation or compartmentalization into nitrogenous compounds, such as amino acids or osmolytes, contributing to the plant’s salinity tolerance.
Comparable NO
3− uptake efficiency has been well-documented in other
Salicornia species under saline and nutrient-rich conditions. For instance,
Salicornia europaea demonstrated enhanced NO
3− assimilation and biomass accumulation under combined NO
3− and NaCl treatments [
24]. Similarly,
Salicornia persica, used as a biofilter in constructed wetlands treating aquaculture effluents, achieved total nitrogen removal efficiencies of 100% and 81% under surface and subsurface flow systems, respectively, across both high (3.3) and low (0.13) nutrient loading rates (g N/m
2/d) over a six-month period [
25].
The robust NO3− removal capacity demonstrated by S. brachiata in the current study, combined with evidence from related species, highlights the genus Salicornia as a promising candidate for the treatment of nitrogen-enriched saline effluents. Its rapid uptake kinetics, tolerance to salinity and high nutrient loads, and sustained physiological health position it to be well suited for use in phytoremediation strategies where NO3− pollution is a major concern.
PO
43− removal followed a similar concentration-dependent pattern, with efficiency increasing from 20.21% (5 mg/L) to 68.46% (15 mg/L) (
Figure 1b). This enhanced uptake may be driven by stronger concentration gradients, facilitating both passive diffusion and active transport across root membranes, alongside the upregulation of high-affinity PO
43− transporters [
26,
27]. As phosphorus is crucial for ATP production and cellular biosynthesis, moderate enrichment likely stimulates metabolic activity and incorporation into biomass [
27,
28].
Comparable responses have been reported in
Salicornia spp., where PO
43− uptake efficiency increased under moderate to high nutrient loads, particularly in saline conditions [
29]. For instance,
S. persica exhibited significantly higher PO
43− uptake at 1.0 and 1.5 mM compared to 0.1 mM, especially under 0–200 mM NaCl salinity, while uptake declined beyond this threshold, indicating that extreme salinity can hinder membrane transport processes [
30].
Beyond controlled greenhouse experiments,
Salicornia species have also proven to be effective in pilot-scale recirculating aquaculture systems (RAS) [
25]. For example, constructed wetlands planted with
S. europaea achieved nitrogen removal efficiencies up to 98.2 ± 2.2% under ambient dissolved inorganic nitrogen loads (109–383 μmol/L), primarily in the form of ammonium and nitrate [
31]. During routine operations, the same species also demonstrated effective removal of PO
43−, ranging from 36% to 89% [
31]. Although phosphorus uptake is more variable due to its low mobility in saline media, these findings highlight
Salicornia’s dual capacity to manage nitrogen and phosphorus pollution under real-world conditions [
32].
The expansion of aquaculture and agriculture in coastal areas has contributed to elevated nutrient loads—particularly NO
3− and PO
43−—in adjacent water bodies, often leading to eutrophication, algal blooms, hypoxia, and biodiversity loss [
3]. Halophytes such as
Salicornia, with their high salt tolerance, fast growth, and nutrient bioaccumulation capacity, are ideal for use in constructed wetlands and integrated aquaculture systems [
33]. Their dual function as biofilters and biomass crops offers a sustainable approach to mitigating nutrient pollution in saline and brackish environments [
31,
34].
In contrast to the concentration-dependent patterns observed for NO
3− and PO
43−, Ca
2+ removal followed an inverse trend. The highest efficiency (89.3%) was recorded at the lowest initial concentration (50 mg/L), with a decline at 100 mg/L (79.67%) (
Figure 1c). This suggests possible transporter saturation or physiological regulation of Ca
2+ uptake under higher concentrations. Unlike NO
3− and PO
43−, which are actively absorbed and assimilated into key metabolic pathways, Ca
2+ is primarily taken up passively via apoplastic flow and regulated based on cellular demand [
35]. At lower external levels, uptake may be more efficient, supporting structural functions such as cell wall stabilization and ion signaling [
35,
36]. However, excess Ca
2+—especially under saline conditions where Na
+ competes for transport sites—can reduce uptake efficiency by altering membrane integrity or transporter activity [
30,
37].
Given that Ca
2+ overaccumulation—resulting from liming, mineral leaching, and feed inputs—is a recognized concern in coastal aquaculture systems [
11], the ability of
S. brachiata to mitigate excess Ca
2+ highlights its practical phytoremediation potential. Beyond water purification, its biomass offers added value as a gourmet food, forage, or bioenergy source, supporting integrated sustainability in coastal resource management [
38].
In this study, macronutrients—nitrate (NO
3−), phosphate (PO
43−), and calcium (Ca
2+)—displayed a steady, time-dependent increase in removal efficiency over the 14-day period, while the heavy metals—lead (Pb
2+), chromium (Cr
6+), and copper (Cu
2+)—exhibited a distinctly different pattern (
Figure 1d–f). All heavy metals showed a rapid rise in removal efficiency within the first two days, irrespective of the initial concentration. Notably, Pb
2+ (
Figure 1d) and Cr
6+ (
Figure 1e) reached near-complete removal (~99%) by day two, followed by a plateau. Cu
2+ removal (
Figure 1f) was slightly slower, attaining ~90% by day 2 and gradually rising to ~99% by day 12.
This contrast in removal efficiencies over time highlights the divergent uptake mechanisms between macronutrients removal, i.e., nitrate (NO
3−) and phosphate (PO
43−), which is largely driven by active transport and metabolic assimilation into compounds like amino acids and nucleotides [
39], while Ca
2+ a macronutrient is also primarily absorbed through passive apoplastic flow and cation exchange processes; however, its uptake can exhibit a similarly time-dependent trend due to sustained transpiration-driven flow and progressive binding within root tissues [
35]. In contrast, metal removal appears dominated by passive biosorption and surface adsorption.
Salicornia species possess mucilage layers and negatively charged cell walls that facilitate metal ion binding through ion exchange, complexation, and precipitation [
20]. The rapid initial removal of Pb
2+ and Cr
6+ suggests surface-level immobilization without significant internal transport, as reported in other halophytes [
20,
40,
41].
Cu
2+, although initially adsorbed, likely undergoes additional internalization due to its essential role as a micronutrient. It can be taken up via COPT transporters, translocated, and incorporated into metalloproteins or chelated by phytochelatins [
42], suggesting a dual mechanism of uptake and detoxification.
Overall, these findings indicate that S. brachiata employs two complementary strategies: rapid, surface-level exclusion of toxic metals and slower, metabolic assimilation of nutrients. This functional differentiation underscores its suitability for treating saline wastewater contaminated with both nutrient and heavy metal pollutants.
Although previous studies have demonstrated the capacity of the
Salicornia species to accumulate metals such as Zn, Pb, Ni, Cd, and Cu under soil-based conditions [
21,
23,
43,
44], they have largely focused on tissue concentrations rather than removal efficiency from the medium. While
S. brachiata has been reported to absorb Cd
2+, Ni
2+, and As
3+ in hydroponics [
22], few studies have quantified its removal kinetics. This study bridges that gap by providing one of the first detailed evaluations of heavy metal removal efficiency by
S. brachiata under controlled hydroponic conditions.
2.2. Plant Growth Parameters and Biochemical Responses
The growth parameters (fresh weight, shoot length, number of lateral branches, and root length) and biochemical markers (chlorophyll, proline, catalase, superoxide dismutase [SOD], peroxidase [POD], and polyphenol oxidase [PPO]) of
S. brachiata seedlings following 14 days of exposure to the selected excess nutrients and metals are summarized in
Table 1,
Table 2,
Table 3,
Table 4,
Table 5 and
Table 6. Overall, most growth parameters—namely fresh weight, shoot length, number of lateral branches, and root length—did not show consistent positive or negative trends across the full concentration ranges of nutrients and heavy metals. This variability likely reflects the halophytic nature of
S. brachiata, which is known for its ability to buffer short-term physiological stress. However, specific parameters did exhibit significant trends in response to certain treatments.
For instance, fresh weight increased significantly with rising nitrate (NO
3−) concentrations (0, 50, 100, and 200 mg/L;
Table 1), highlighting NO
3−’s central role as a macronutrient that enhances biomass accumulation. In salt-tolerant species such as
Salicornia, moderate NO
3− enrichment can promote photosynthetic activity, osmolyte production, and nitrogen assimilation [
45,
46,
47].
In contrast, shoot length decreased significantly with increasing copper (Cu
2+) concentrations (0, 0.25, 0.5, and 1 mg/L;
Table 6), consistent with Cu-induced toxicity. Excess Cu is known to cause oxidative stress, impair cell elongation, and disrupt hormonal regulation of shoot growth [
42]. In species such as
S. europaea and
S. brachiata, Cu exposure has been associated with reduced apical growth, likely due to interference with auxin transport and suppression of meristematic activity [
48,
49].
In contrast to the variable growth trends, many biochemical parameters—especially antioxidant enzyme activities—showed clearer, dose-dependent responses to pollutant exposure. While chlorophyll content did not consistently follow a single trend, it was generally higher in control treatments across most stress conditions, except under phosphate (PO
43−) exposure. The observed chlorophyll reduction may stem from disrupted uptake of essential cofactors for chlorophyll synthesis, such as Mg
2+, Fe
2+, and Zn
2+—a pattern consistent with findings in halophytes such as
S. europaea, where metal stress led to oxidative damage and impaired nutrient assimilation [
50,
51,
52].
Furthermore, heavy metals can impair chlorophyll synthesis by inhibiting key enzymes such as δ-aminolevulinic acid dehydratase (ALAD), which is essential in the biosynthetic pathway [
53]. Previous studies on
Atriplex halimus and
Suaeda maritima have reported ALAD inhibition and chlorophyll decline under Pb
2+, Zn
2+, and Cd
2+ exposure [
54,
55]. Similar findings in
S. brachiata, and
S. europaea show that metal-induced oxidative stress, ionic toxicity, and ROS-mediated lipid peroxidation disrupt chlorophyll metabolism and reduce pigment levels [
22,
50].
Despite the observed reduction in chlorophyll content,
S. brachiata seedlings remained visibly green throughout the experimental period, with no signs of chlorosis or necrosis. This observation aligns with findings in
Salicornia iranica, where chlorophyll declined under Pb
2+ exposure without visible chlorosis, and in
S. europaea and
A. halimus, which retained green pigmentation under salt and metal stress due to sustained carotenoids and elevated peroxidase activity protecting chloroplast membranes [
54,
56]. Moreover, halophytes often engage in ion compartmentalization—sequestering toxic ions such as Pb
2+, Cu
2+, and Cr
6+ into vacuoles or older tissues—which minimizes their interference with chlorophyll biosynthesis and function in actively photosynthesizing cells [
57]. Such tolerance suggests that
Salicornia species possess robust physiological mechanisms that mitigate the visual and functional symptoms of chlorophyll degradation under heavy metal stress.
Exposure to all tested pollutants—nitrate (NO
3−), phosphate (PO
43−), calcium (Ca
2+), lead (Pb
2+), chromium (Cr
6+), and copper (Cu
2+)—resulted in elevated proline content in
S. brachiata, underscoring the plant’s robust osmotic adjustment capacity under stress. In several treatments, proline levels increased progressively with rising pollutant concentrations, while in others, values remained significantly above the control, regardless of the dose. This response is consistent with findings in other
Salicornia species. For example,
S. persica and
S. brachiata have both shown increased proline levels under salt and heavy metal stress, functioning to stabilize proteins, maintain membrane integrity, and scavenge reactive oxygen species (ROS) [
22,
51]. These results confirm that proline acts as a key biochemical marker of stress resilience in halophytes [
51].
Similarly, the activities of antioxidant enzymes—catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and polyphenol oxidase (PPO)—were upregulated across most pollutant treatments in the present study. These increases were observed either as consistent positive trends across all tested concentrations or as marked enhancements compared to the control. This enzymatic activation is indicative of an oxidative stress response and mirrors earlier reports in
Salicornia species. For instance,
Salicornia fruticosa exposed to Cd
2+ under saline hydroponic conditions exhibited elevated SOD, CAT, and ascorbate peroxidase activities, reinforcing the role of these enzymes in detoxifying ROS generated by metal stress [
44]. Similarly,
S. brachiata demonstrated increased CAT and POD activity under Ni
2+, Cd
2+, and As
3+ exposure, suggesting that antioxidant regulation is a conserved stress-adaptive strategy in this genus [
22].
Interestingly, phosphate (PO
43−) treatment in this study did not induce changes in antioxidant enzyme activity compared to the control (
Table 1). This could be attributed to the essential role of phosphorus as a macronutrient, which is unlikely to provoke oxidative stress at the concentrations tested. Comparable trends have been observed in
S. europaea grown in eutrophic waters, where phosphorus supplementation did not elicit a strong antioxidant response [
31].
Notably, a deviation from the general antioxidant trend was observed in Pb
2+ treatments, where SOD activity was undetectable at higher Pb
2+ concentrations (
Table 4). This suggests that Pb
2+ may directly inhibit SOD synthesis or activity, likely due to its strong affinity for binding enzyme cofactors such as Zn
2+ and Cu
2+, which are essential for SOD function. Similar findings were reported in several non-halophytic plant species exposed to Pb
2+, where higher Pb accumulation was associated with reduced SOD activity and increased lipid peroxidation, highlighting Pb’s disruptive impact on enzymatic antioxidant defenses [
58,
59].
Collectively, these findings demonstrate that S. brachiata responds to a range of pollutant-induced stresses with both osmoprotective (proline) and antioxidative biochemical strategies, consistent with patterns reported in other Salicornia species. However, the magnitude and specificity of responses appear to be pollutant and dose-dependent, revealing the nuanced physiological plasticity of halophytes under complex environmental stress conditions. This integrative response may contribute to the resilience and utility of Salicornia in phytoremediation applications, particularly in saline and pollutant-laden environments.
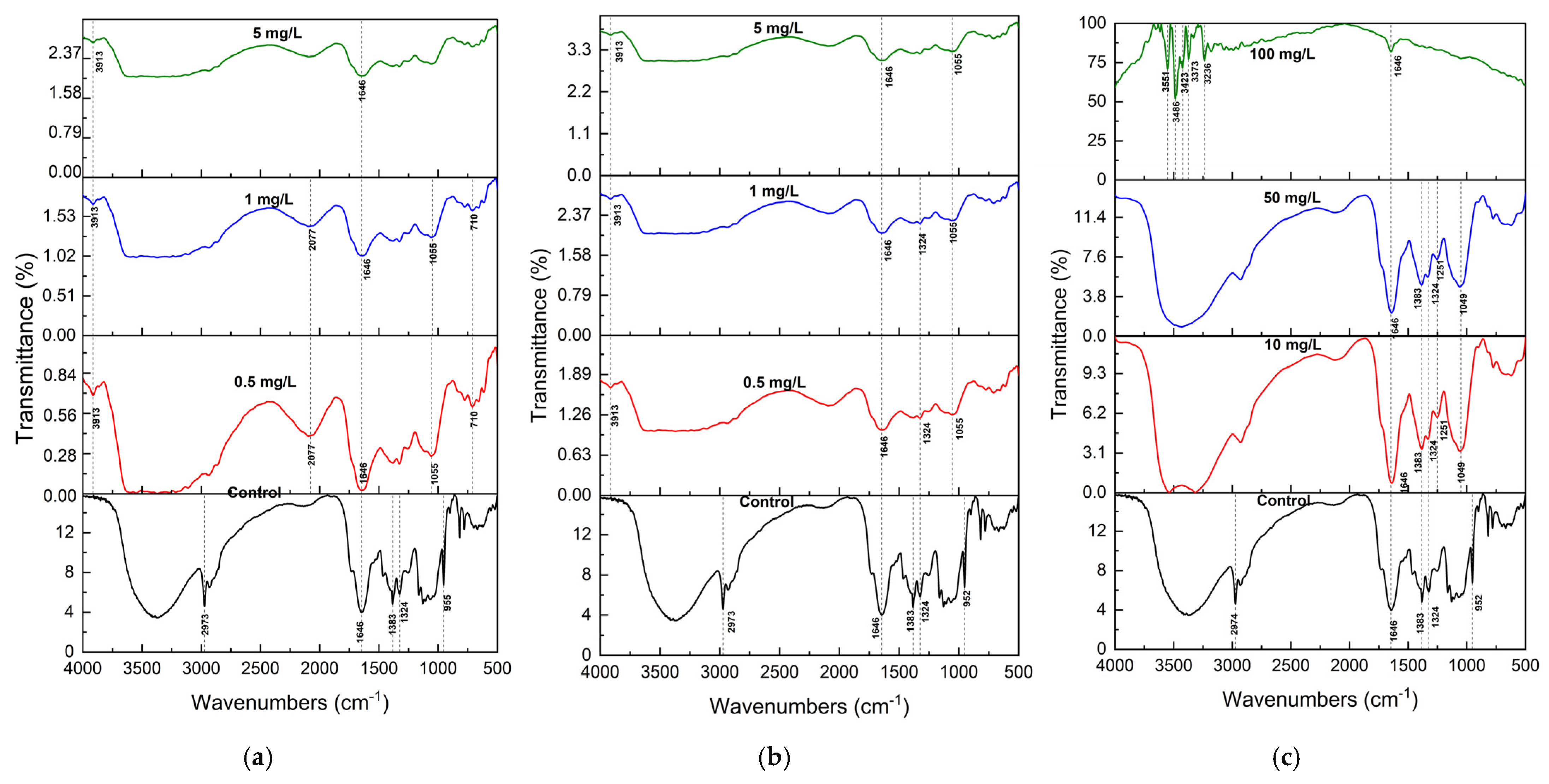
2.3. FTIR Analysis of S. brachiata Plants
Fourier Transform Infrared (FTIR) spectroscopy was employed to analyze the functional group alterations in
S. brachiata tissues before and after 14-day exposure to selected pollutants, such as phosphate (NO
3−), phosphate (PO
43−), chromium (Cr
6+), lead (Pb
2+), copper (Cu
2+), and calcium (Ca
2+) (
Figure 2a and
Figure 3c). The spectral changes provided insights into the molecular interactions underpinning pollutant adsorption and revealed pollutant-specific binding mechanisms.
In control samples, the FTIR spectrum displayed characteristic features of plant biochemical constituents. A broad band centered at 3350 cm
−1 was attributed to O–H and N–H stretching vibrations, indicative of hydroxyl and amine groups present in carbohydrates, proteins, and phenolic compounds [
60]. The peak at 2973 cm
−1 corresponded to asymmetric C–H stretching in lipids [
61], while a prominent band at 1646 cm
−1 represented C=O stretching in the amide I bonds of proteins [
62]. Additional bands at 1400 cm
−1 and 1055 cm
−1 were assigned to symmetric COO
− stretching in carboxylic acids and C–O stretching in alcohols and polysaccharides, respectively [
63]. Bands at 1383, 1324, and 952 cm
−1 were attributed to C=C, C–H, and C–O–C vibrations in polysaccharide structures [
64,
65].
Following exposure to NO
3− and PO
43−, the spectra showed significant broadening and the appearance of new peaks in the 3350 cm
−1 region (
Figure 2a,b). These alterations suggest strong hydrogen bonding and electrostatic interactions between anionic pollutants and protonated amino groups or hydroxyl moieties on the biomass surface. Such binding likely occurs via ion exchange and H-bonding to functional groups such as –NH
3+ and –OH [
66].
Cr
6+ exposure resulted in a more ill-defined FTIR spectrum, with the disappearance of characteristic peaks in the 1300–500 cm
−1 region. These bands are typically associated with alcohols and carboxylic acid groups. At pH ~7.0, Cr
6+ exists primarily as HCrO
4− and CrO
42− [
67], and its uptake may involve not only adsorption but also redox transformations. The loss of O–H-related bands suggests that Cr
6+ may be reduced to Cr
3+ via oxidation of hydroxyl groups, consistent with previous findings in Cr
6+ biosorption systems [
68].
For cationic pollutants, including Pb
2+, Cu
2+, and Ca
2+, FTIR spectra confirmed the involvement of hydroxyl and carboxylate functional groups in metal binding (
Figure 3a–c). These groups are known to form chelates and dicarboxylate complexes with metal ions. The disappearance of the 955 cm
−1 peak across all metal treatments indicates significant perturbation of polysaccharide or alcohol-linked structures. Notably, a new peak at 3913 cm
−1 was observed in Pb
2+- and Cu
2+-treated samples but was absent in the Ca
2+ spectrum, indicating ion-specific interactions, possibly due to differences in hydration behavior and complexation tendency.
Although these metal ions have similar ionic radii (Pb
2+: 0.119 nm; Cu
2+: 0.073 nm; Ca
2+: 0.112 nm), their hydration free energies differ significantly. Pb
2+, with the lowest hydration energy (−1492 kJ/mol), is more readily dehydrated and thus more likely to bind to biomass surfaces than Cu
2+ (−2076 kJ/mol) or Ca
2+ (−1588 kJ/mol) [
69,
70]. This explains the observed thermodynamic preference for Pb
2+ adsorption in
S. brachiata.Overall, FTIR analysis demonstrated pollutant-specific spectral modifications and confirmed that S. brachiata biomass contains a wide array of active functional groups—including hydroxyl, carboxylate, amide, and polysaccharide moieties—that enable adsorption via multiple mechanisms: electrostatic interaction, hydrogen bonding, chelation, and redox reaction. These findings reinforce the multifunctional biosorptive potential of S. brachiata and support its applicability in the phytoremediation of nutrient and heavy metal contaminants in saline environments.