Trait-Based Selection of Seeds Ingested and Dispersed by North American Waterfowl
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Wetland Plant List and Plant Traits
2.3. Data Analysis
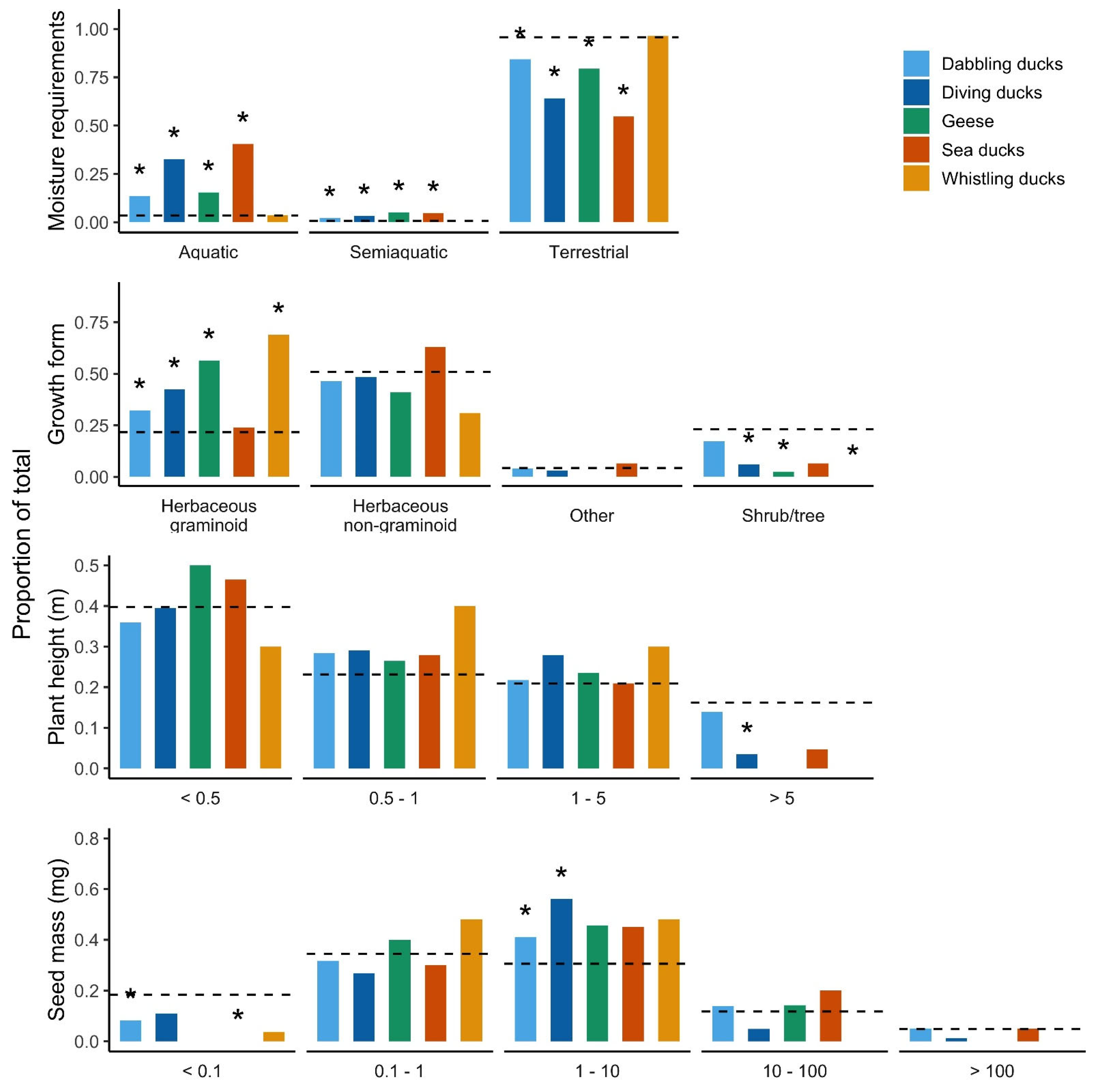
3. Results
4. Discussion
4.1. Terrestrial Species Dominate, but Aquatic Species Are Ingested More Often than Expected
4.2. Growth Form Is Important
4.3. The Tallest Plants Are Avoided by Diving Ducks
4.4. Waterfowl Prefer Medium-Sized Seeds
4.5. Limitations of Our Study
4.6. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Callicut, J.T.; Hagy, H.; Schummer, M. The food preference paradigm: A review of autumn–winter food use by North American dabbling ducks (1900–2009). J. Fish Wildl. Manag. 2011, 2, 29–40. [Google Scholar] [CrossRef]
- Hagy, H.M.; Kaminski, R.M. Winter Waterbird and Food Dynamics in Autumn-Managed Moist-Soil Wetlands in the Mississippi Alluvial Valley. Wildl. Soc. Bull. 2012, 36, 512–523. [Google Scholar] [CrossRef]
- de Vlaming, V.; Proctor, V.W. Dispersal of aquatic organisms: Viability of seeds recovered from the droppings of captive killdeer and mallard ducks. Am. J. Bot. 1968, 55, 20–26. [Google Scholar] [CrossRef]
- Green, A.J.; Brochet, A.L.; Kleyheeg, E.; Soons, M.B. Dispersal of plants by waterbirds. In Why Birds Matter: Avian Ecological Function and Ecosystem Services; Şekercioğlu, C.H., Wenny, D.G., Whelan, C.J., Eds.; University of Chicago Press: Chicago, IL, USA, 2016; pp. 147–195. [Google Scholar]
- Farmer, J.A.; Webb, E.B.; Pierce, R.A.; Bradley, K.W. Evaluating the potential for weed seed dispersal based on waterfowl consumption and seed viability. Pest Manag. Sci. 2017, 73, 2592–2603. [Google Scholar] [CrossRef]
- Costea, M.; El Miari, H.; Laczkó, L.; Fekete, R.; Molnár, A.V.; Lovas-Kiss, Á.; Green, A.J. The effect of gut passage by waterbirds on the seed coat and pericarp of diaspores lacking “external flesh”: Evidence for widespread adaptation to endozoochory in angiosperms. PLoS ONE 2019, 14, e0226551. [Google Scholar] [CrossRef]
- Viana, D.S.; Santamaría, L.; Michot, T.C.; Figuerola, J. Migratory strategies of waterbirds shape the continental-scale dispersal of aquatic organisms. Ecography 2013, 36, 430–438. [Google Scholar] [CrossRef]
- Green, A.J.; Lovas-Kiss, Á.; Reynolds, C.; Sebastián-González, E.; Silva, G.G.; van Leeuwen, C.H.A.; Wilkinson, D.M. Dispersal of aquatic and terrestrial organisms by waterbirds: A review of current knowledge and future priorities. Freshw. Biol. 2023, 68, 173–190. [Google Scholar] [CrossRef]
- Green, A.J.; Elmberg, J. Ecosystem services provided by waterbirds. Biol. Rev. 2014, 89, 105–122. [Google Scholar] [CrossRef]
- Department of the Interior. U.S. Fish and Wildlife Service Waterfowl Population Status, 2023; U.S. Department of the Interior: Washington, DC, USA, 2023. Available online: https://www.fws.gov/media/waterfowl-population-status-2023 (accessed on 3 October 2024).
- Urgyán, R.; Lukács, B.A.; Fekete, R.; Molnár, A.V.; Nagy, A.; Vincze, O.; Green, A.J.; Lovas-Kiss, Á. Plants dispersed by a non-frugivorous migrant change throughout the annual cycle. Glob. Ecol. Biogeogr. 2023, 32, 70–82. [Google Scholar] [CrossRef]
- Jiménez-Martín, I.; Monreal, A.; Martín-Vélez, V.; Navarro-Ramos, M.J.; Fox, A.D.; Lovas-Kiss, Á.; Green, A.J. High levels of seed dispersal by a declining wintering population of migratory geese. Freshw. Biol. 2024, 69, 1857–1870. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Treep, J.; de Jager, M.; Nolet, B.A.; Soons, M.B. Seed dispersal distributions resulting from landscape-dependent daily movement behaviour of a key vector species, Anas platyrhynchos. J. Ecol. 2017, 105, 1279–1289. [Google Scholar] [CrossRef]
- Bullock, J.M.; González, L.M.; Tamme, R.; Götzenberger, L.; White, S.M.; Pärtel, M.; Hooftman, D.A.P.; Rees, M. A synthesis of empirical plant dispersal kernels. J. Ecol. 2017, 105, 6–19. [Google Scholar] [CrossRef]
- Viana, D.S. Can Aquatic Plants Keep Pace with Climate Change? Front. Plant. Sci. 2017, 8, 1906. [Google Scholar] [CrossRef] [PubMed]
- Lovas-Kiss, Á.; Martín-Vélez, V.; Brides, K.; Wilkinson, D.M.; Griffin, L.R.; Green, A.J. Migratory geese allow plants to disperse to cooler latitudes across the ocean. J. Biogeogr. 2023, 50, 1602–1614. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Kelly, T.C.; Davenport, J.; Jansen, M.A.K. Up, up and away: Bird-mediated ectozoochorous dispersal between aquatic environments. Freshw. Biol. 2017, 62, 631–648. [Google Scholar] [CrossRef]
- Green, A.J.; Wilkinson, D.M. Darwin’s digestion myth: Historical and modern perspectives on our understanding of seed dispersal by waterbirds. Seeds 2024, 3, 505–527. [Google Scholar] [CrossRef]
- Howe, H.F.; Smallwood, J. Ecology of Seed Dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Green, A.J.; Baltzinger, C.; Lovas-Kiss, Á. Plant dispersal syndromes are unreliable, especially for predicting zoochory and long-distance dispersal. Oikos 2022, 2022. [Google Scholar] [CrossRef]
- Almeida, B.A.; Silva, G.G.; Costea, M. Complete dataset of seed ingestion by Anatidae in North America. Dataset 2024. [Google Scholar] [CrossRef]
- Almeida, B.A.; Silva, G.G.; Costea, M.; Maltchik, L.; De La Cruz, S.E.W.; Takekawa, J.Y.; Green, A.J. Waterfowl endozoochory: Traits drive plant-bird dispersal interactions in North America. Freshw. Biol. 2025, 70, e70027. [Google Scholar] [CrossRef]
- Kear, J. Bird Families of the World: Ducks, Geese and Swans; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Ducks, Geese, and Waterfowl (Anatidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Navarro-Ramos, M.J.; Green, A.J.; de Vries, R.; van Leeuwen, C.H.A. Float, fly, then sink: Wetland plant seed buoyancy is lost after internal dispersal by waterbirds. Hydrobiologia 2024, 851, 4033–4048. [Google Scholar] [CrossRef]
- Janzen, D.H. Dispersal of small seeds by big herbivores: Foliage is the fruit. Am. Nat. 1984, 123, 338–353. [Google Scholar] [CrossRef]
- Soons, M.B.; Brochet, A.; Kleyheeg, E.; Green, A.J.; Jongejans, E. Seed dispersal by dabbling ducks: An overlooked dispersal pathway for a broad spectrum of plant species. J. Ecol. 2016, 104, 443–455. [Google Scholar] [CrossRef]
- Baldassarre, G.A.; Bolen, E.G. Waterfowl Ecology and Management, 2nd ed.; Krieger: Malabar, FL, USA, 2006. [Google Scholar]
- van Leeuwen, C.H.A.; Lovas-Kiss, Á.; Ovegård, M.; Green, A.J. Great cormorants reveal overlooked secondary dispersal of plants and invertebrates by piscivorous waterbirds. Biol. Lett. 2017, 13, 20170406. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.A.; Soons, M.B.; Vandionant, L.G.V.T.I.; Green, A.J.; Bakker, E.S. Seed dispersal by waterbirds: A mechanistic understanding by simulating avian digestion. Ecography 2023, 2023, e06470. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed dispersal effectiveness revisited: A conceptual review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef]
- van Leeuwen, C.H.A.; Villar, N.; Sagrera, I.M.; Green, A.J.; Bakker, E.S.; Soons, M.B.; Galetti, M.; Jansen, P.A.; Nolet, B.A.; Santamaría, L. A seed dispersal effectiveness framework across the mutualism–antagonism continuum. Oikos 2022, 2022, e09254. [Google Scholar] [CrossRef]
- Gill, F.; Donsker, D.; Rasmussen, P. IOC World Bird List (v11.1); International Ornithological Committee: Baton Rouge, LA, USA, 2021. [Google Scholar]
- U.S. Army Corps of Engineers (USACE). National Wetland Plant List, Version 3.6. 2022. Available online: https://wetland-plants.sec.usace.army.mil/ (accessed on 3 October 2023).
- Reed, P.B., Jr. National List of Plant Species That Occur in Wetlands: Northwest (Region 9). U.S. Fish Wildl. Serv. Biol. Rep. 1988, 88, 89. Available online: https://digitalmedia.fws.gov/digital/collection/document/id/1348 (accessed on 18 November 2024).
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function: Enhanced species-level trait dataset. Sci. Data 2022, 9, 755. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 10 March 2023).
- Ebbert, D. chisq.posthoc.test: A Post Hoc Analysis for Pearson’s Chi-Squared Test for Count Data. R Package, Version 0.1.2. 2019. Available online: https://CRAN.R-project.org/package=chisq.posthoc.test (accessed on 1 March 2023).
- Les, D.H. Aquatic Dicotyledons of North America: Ecology, Life History, and Systematics; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2017. [Google Scholar]
- Les, D.H. Aquatic Monocotyledons of North America: Ecology, Life History, and Systematics; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- González-Varo, J.P.; Rumeu, B.; Bracho-Estévanez, C.A.; Acevedo-Limón, L.; Baltzinger CLovas-Kiss, Á.; Green, A.J. Overlooked seed-dispersal modes and underestimated distances. Glob. Ecol. Biogeogr. 2024, 33, e13835. [Google Scholar] [CrossRef]
- Gurd, D.B. Mechanistic analysis of interspecific competition using foraging trade-offs: Implications for duck assemblages. Ecology 2008, 89, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.A.; Lukács, B.A.; Lovas-Kiss, Á.; Reynolds, C.; Green, A.J. Functional traits drive dispersal interactions between European waterfowl and seeds. Front. Plant Sci. 2022, 12, 795288. [Google Scholar] [CrossRef]
- Birds of the World. Cornell Laboratory of Ornithology; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Birds of the World: Ithaca, NY, USA, 2022; Available online: https://birdsoftheworld.org/bow/home (accessed on 1 December 2024).
- Carlo, T.A.; Messeder, J.V.S.; Espíndola, W.D.; Vizzachero, B.S.; Boyer, B.W.; Hernández-Mejía, J.; Torres-Páucar, E.A.; Fontanella, A.; Pizo, M.A.; Amico, G.; et al. Negative density dependence characterizes mutualistic interactions between birds and fruiting plants across latitudes. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230128. [Google Scholar] [CrossRef]
- Tóth, P.; Green, A.J.; Wilkinson, D.M.; Brides, K.; Lovas-Kiss, Á. Plant traits associated with seed dispersal by ducks and geese in urban and natural habitats. Ecol. Evol. 2023, 13, e10677. [Google Scholar] [CrossRef]
- Lovas-Kiss, A.; Vincze, O.; Kleyheeg, E.; Sramkó, G.; Laczkó, L.; Fekete, R.; Molnár, V.A.; Green, A.J. Seed mass, hardness and phylogeny determine the potential for endozoochory by granivorous waterbirds. Ecol. Evol. 2020, 10, 1413–1424. [Google Scholar] [CrossRef]
- Lovas-Kiss, Á.; Navarro-Ramos, M.J.; Vincze, O.; Löki, V.; Urgyán, R.; Pallér-Kapusi, F.; van Leeuwen, C.H.A.; Green, A.J.; Lukács, B.A. Traits for transport: Alien wetland plants gain an advantage during endozoochorous seed dispersal by waterfowl. Freshw. Biol. 2023, 68, 1703–1715. [Google Scholar] [CrossRef]
- Andersson, K.; Davis, C.A.; Harris, G.; Haukos, D.A.; Romanach, S.S. Changes in waterfowl migration phenologies in central North America: Implications for future waterfowl conservation. PLoS ONE 2022, 17, e0266785. [Google Scholar] [CrossRef]
- Burner, R.C.; Golas, B.D.; Aagaard, K.J.; Lonsdorf, E.V.; Thogmartin, W.E. Marginal value analysis reveals shifting importance of migration habitat for waterfowl under a changing climate. Ecol. Evol. 2023, 13, e10632. [Google Scholar] [CrossRef]
- Cox, A.R.; Frei, B.; Gutowsky, S.E.; Baldwin, F.B.; Bianchini, K.; Roy, C. Sixty-years of community-science data suggest earlier fall migration and short-stopping of waterfowl in North America. Condor 2023, 125, duad041. [Google Scholar] [CrossRef]
| Aquatic or Terrestrial | Growth Form | Plant Height Categories | Seed Mass Categories | |
|---|---|---|---|---|
| Dabbling ducks | 89.72 *** | 24.45 *** | 5.81 | 31.99 *** |
| Diving ducks | 196.18 *** | 31.60 *** | 11.69 ** | 25.90 *** |
| Geese | 25.55 *** | 30.73 *** | 6.65 | 11.19 * |
| Sea ducks | 159.97 *** | 7.38 | 4.41 | 12.53 ** |
| Whistling ducks | 0.21 | 39.46 *** | 6.92 | 11.77 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, B.A.; Costea, M.; Silva, G.G.; Maltchik, L.; De La Cruz, S.E.W.; Takekawa, J.Y.; Green, A.J. Trait-Based Selection of Seeds Ingested and Dispersed by North American Waterfowl. Plants 2025, 14, 1964. https://doi.org/10.3390/plants14131964
Almeida BA, Costea M, Silva GG, Maltchik L, De La Cruz SEW, Takekawa JY, Green AJ. Trait-Based Selection of Seeds Ingested and Dispersed by North American Waterfowl. Plants. 2025; 14(13):1964. https://doi.org/10.3390/plants14131964
Chicago/Turabian StyleAlmeida, Bia A., Mihai Costea, Giliandro G. Silva, Leonardo Maltchik, Susan E. W. De La Cruz, John Y. Takekawa, and Andy J. Green. 2025. "Trait-Based Selection of Seeds Ingested and Dispersed by North American Waterfowl" Plants 14, no. 13: 1964. https://doi.org/10.3390/plants14131964
APA StyleAlmeida, B. A., Costea, M., Silva, G. G., Maltchik, L., De La Cruz, S. E. W., Takekawa, J. Y., & Green, A. J. (2025). Trait-Based Selection of Seeds Ingested and Dispersed by North American Waterfowl. Plants, 14(13), 1964. https://doi.org/10.3390/plants14131964








