Abstract
In the circular economy framework, hydrosols, by-products of the essential oil industry, are gaining attention for their potential in waste reduction and resource reuse. This study analyzed hydrosols from six edible flowers, investigating their chemical composition (VOC-Hyd) and antibacterial properties alongside volatile organic compounds of fresh flowers (VOC-Fs) and essential oils (EOs). Antirrhinum majus exhibited ketones as major VOC-Fs (62.6%) and VOC-Hyd (41.4%), while apocarotenoids dominated its EOs (68.0%). Begonia cucullata showed alkanes (33.7%) and aldehydes (25.7%) as primary VOC-Fs, while alkanes were prevalent in both extracts (65.6% and 91.7% in VOC-Hyd and in EOs, respectively). Calandula officinalis had monoterpenoids in VOC-Fs and VOC-Hyd (89.3% and 49.7%, respectively), while its EOs were rich in sesquiterpenoids (59.7%). Dahlia hortensis displayed monoterpenoid richness in both VOC-Fs and extracts. Monocots species’ VOC-Fs (Polianthes tuberosa, Tulbaghia cominsii) were esters-rich, replaced by monoterpenoids in VOC-Hyd. P. tuberosa EO maintained ester richness, while T. cominsii EOs contained a significant percentage of sulfur compounds (38.1%). Antibacterial assays indicated comparable minimum inhibitory concentration profiles across VOC-Hyd: B. calcullata and P. tuberosa against Staphylococcus aureus and Salmonella enterica ser. typhimurium, T. cominsii against Escherichia coli and S. enterica, A. majus and C. officinalis against S. aureus, and D. hortensis against S. enterica.
1. Introduction
The deep bond between humans and flowers has been reported for millennia. Historically, the latter were protagonists of funeral rituals, feasts, offerings, and songs due to their myriad symbolic meanings. Their undeniable aesthetic beauty has made them highly sought-after for decorative functions, paintings, ceramics, and fabrics [1]. Gardens, in particular, result from skillful combinations of plant species with a multitude of shapes and colors, whose wonderfulness can be fully observed during blooming. Very recently, the concept of a ‘healing garden’ has emerged, specifically designed to promote well-being, comfort, and pain tolerance both in clinical and non-clinical populations, thereby representing a combination of flower beauty and human health [2].
The flowers’ aroma is pivotal, as much as their ornamental value, since it can evoke several emotional responses tied to individual experiences and memories. This is attributed to the varied bouquets of volatile organic compounds (VOCs) emitted by petals, each contributing to the distinctive signature of every flower. Flower extracts as essential oils (EOs) hold particular appeal for industries such as cosmetics, perfume, toiletries, and hygiene [3].
In recent years, the gastronomic and nutritional value of flowers has garnered attention, with several edible flowers (EFs) recognized for their attractiveness, sensory perception [4,5,6,7], nutritional properties [8], and biological activities [9]. EFs are very versatile and suitable for various culinary contexts, such as raw flowers without processing, by minimum processing, or in powdered form [10]. Furthermore, their EOs are gaining more and more interest from the food industry: (1) detailed scientific reports highlighting the antimicrobial, antioxidant, and antiparasitic activities of EFs EOs are increasing; (2) they could be used as safer substitutes for chemical preservatives, thus meeting consumer concerns about the use of artificial and harmful compounds in the food sector [11]. Therefore, the EOs obtained from EFs are the perfect combination for the preservation of food products and food safety.
Hydrodistillation is widely used to extract EOs from flowers [12]. During this process, three main by-products are created: solid residue (plant debris), water residue (non-distilled aqueous phase), and hydrosols (also known as hydrosols or aromatic water). The latter are aqueous aromatic solutions saturated with water-soluble volatile compounds [13]. Hydrosols distillate together with the EOs, retaining a good quantity of dissolved compounds that are, however, in lesser amounts than EOs, both in terms of number and concentration. Despite this, oxygenated components (e.g., oxygenated monoterpenes (OM)) are higher in hydrosols, while EOs are richer in highly hydrophobic compounds such as monoterpene and sesquiterpene hydrocarbons [13]. Currently, aromatic waters have several applications in the food sector, as well as in the aromatherapy, cosmetics, and perfume industries, representing the most commonly used by-product of EO production [14].
Within this framework, the current study meticulously examined the chemical composition of hydrosols derived from six distinctive flowers tailored for culinary purposes, namely, Antirrhinum majus L., Begonia cucullata Willd, Calendula officinalis L., Dahlia hortensis Guillaumin, Polianthes tuberosa L., and Tulbaghia cominsii Vosa. Our investigation extended beyond the hydrosols, encompassing a thorough analysis of the EOs and spontaneous emissions of these botanical specimens. Furthermore, a comprehensive assessment of the antibacterial properties was conducted, involving six bacterial strains. This study scrutinized the primary phytochemicals inherent in these floral extracts, marking a pivotal stride in revealing the transformative potential encapsulated within these often-neglected by-products.
2. Material and Methods
2.1. Plant Material
Plants of Antirrhinum majus L., Begonia cucullata Willd, Calendula officinalis L., Dahlia hortensis Guillaumin, Polianthes tuberosa L., and Tulbaghia cominsii Vosa were kindly provided by CREA Research Centre for Vegetable and Ornamental Crops (CREA, Sanremo, Impe-ria, Italy, GPS: 43.816887, 7.758900) and Chambre d’Agriculture des Alpes-Maritimes (CREAM, Nice, France, GPS: 43.668318 N, 7.204194 E). Detailed information on plant cultivation (propagation, substrate composition, fertilization treatment, and frequency and type of irrigation) has already been published by Drava et al. [15] (A.majus, B. cucullata, D. hortensis—see D. pinnata, and T. cominsii), and Copetta et al. [16] (P. tuberosa). C. officinalis is part of the edible flower collection cultivated at CREA, as reported by Marchioni et al. [17].
Full-opened and flawless flowers were collected at the Department of Pharmacy (University of Pisa) and properly analyzed as follows.
2.2. Essential Oil (EO) and Hydrosol Extraction
The extraction was conducted using 20 g of fresh flowers through a 2 h hydrodistillation process employing a micro-Clevenger-type apparatus. During hydrodistillation, 1 mL of HPLC-grade n-hexane was added to the separator funnel. This precaution was taken due to concerns about the possibility of obtaining an EO yield as low as one drop. The extraction process resulted in obtaining both EO dissolved in hexane and hydrosol, which were then separated based on density difference. The dissolved EO was directly analyzed by GC-MS, while the hydrosol was stored in sealed vials at 4 °C in the dark.
2.3. Headspace Solid-Phase Microextraction (HS-SPME) for VOC Analyses
The HS-SPME procedure, as outlined by Najar et al. [18], involved both spontaneous emissions of fresh EFs (VOC-Fs) and hydrosols (VOC-Hyd). Briefly, 1 g of fresh flower or 1 mL of hydrosol was placed into a 50 mL headspace glass vial, sealed with aluminum foil, and stored at room temperature. HS-SPME extractions utilized a DVB/CAR/PDMS SPME fiber exposed to the headspace for 30 min. Post-extraction, the fiber was introduced into the GC-MS injection system at 220 °C for 3 min for thermal desorption of analytes. All analyses were performed in triplicate (n = 3). The SPME fiber underwent thermal conditioning before use and daily 10 min conditioning before the initial extraction to ensure the absence of carryover effects.
2.4. Phytochemical Analysis: GC-MS Analysis
Chromatographic analyses were conducted on VOC-Fs, EOs, and VOC-Hyd extracted from edible flowers using an Agilent 7890B gas chromatograph system (Agilent Technologies Inc., Santa Clara, CA, USA) equipped with an Agilent HP-5MS capillary column (30 m × 0.25 mm; Santa Clara, CA, USA). Helium served as carrier gas, with a flow rate of 1 mL/min and a column head pressure of 13 psi. The injector temperature was set at 220 °C, with a split ratio of 1:25. The oven temperature programming ranged from 60 to 240 °C at a rate of 3 °C/min. Full scan MS detection was conducted using an Agilent 5977B single quadrupole inert mass selective detector (Agilent Technologies Inc., Santa Clara, CA, USA) with an electron impact (EI) ion energy of 70 eV, and the mass acquisition range was set to 30–300 m/z.
Identification of the constituents was based on a comparison of the retention times (Rt) with those of the authentic samples, comparing their calculated Kovats Index (KI) (determined using the van Den Dool and Kratz equation) to the series of a C8–C22 n-hydrocarbons. Computer matching was also used against commercial [19] and laboratory-developed mass spectra library built up from pure substances and components of commercial essential oils of known composition and MS literature data [20,21,22,23,24]. Analysis was conducted in triplicate for each sample to ensure reliability.
2.5. Preparation and Storage of Bacterial Cultures
Three Gram-positive bacteria, Staphylococcus aureus ATCC 6538, Listeria monocytogenes ATCC 7644, and Enterococcus faecalis V583E, and three Gram-negative bacteria, Escherichia coli ATCC 15325, Pseudomonas aeruginosa ATCC 27853, and Salmonella enterica serovar Typhimurium ATCC 14028, were selected as test organisms. The bacterial cultures were prepared in Tryptone Soy Agar (TSA, pH 7.3 ± 0.2, Oxoid, UK) and stored at −20 °C in BHI broth (pH 7.4 ± 0.2, Merck, Germany) with 20% glycerol as cryoconservant. To revitalize bacterial cultures, frozen stocks were subcultured in BHI broth and incubated at 37 °C for 24 h.
In Vitro Antibacterial Testing of Hydrosols
MIC values for hydrosols against diverse bacterial strains were determined using a modified twofold serial microdilution method in a 96-well microplate. Hydrosols prepared in BHI with DMSO followed a 1:3:4 v/v ratio. Incubation at 37 ± 2 °C for 24 h was performed after bacterial suspension addition. MIC values, evaluated in triplicate, were mode-determined for each isolate. MBC values, indicating the lowest hydrosols concentration preventing bacterial growth, were assessed on TSA plates after 24 h incubation. Results, expressed as v/v, were mode-reported for both MIC and MBC. The entire process was executed in triplicate to ensure reliability.
2.6. Statistical Analysis
Volatile compositions of hydrosols underwent comprehensive multivariate statistical analyses utilizing JMP software (SAS Institute Inc. JMP®. Version 16, SAS Institute Inc., Cary, NC, USA, 2021). Principal Component Analysis (PCA) was performed on a covariance data matrix of 58 × 6 (58 compounds × 6 samples = 348 data). The resulting PCA was plotted by selecting the two principal components with the highest variance explained, obtained by the linear regressions operated on mean-centered, unscaled data. As an unsupervised method, this analysis aimed at reducing the dimensionality of the multivariate data of the matrix whilst preserving most of the variance [25]. Additionally, a two-way Hierarchical Cluster Analysis (HCA) was carried out using Ward’s method, and the squared Euclidean distances were used as a measure of similarity.
3. Results and Discussion
3.1. Phytochemical Insight into the Studied Species
3.1.1. Antirrhinum majus L.
Spontaneous emission of its flowers was characterized by ketones (62.6%), mainly acetophenone (59.9%, (15)) (Table 1), reported for its sweet, pungent odor and taste [26]. This class of constituents was followed by esters (10.8%) and OM (9.2%), of which methyl benzoate (5.4%, (17)) and linalool (8.3%, (18)) were, respectively, the most abundant compounds. In coherence with our results, Suchet et al. [27] reported the presence of acetophenone in the floral scent of wild snapdragon, with a contribution of 69.0% of the absolute emissions.

Table 1.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EO), and hydrosols (VOC-Hyd) derived from Antirrhinum majus.
EO evidenced a lesser number of constituents (13) in comparison with spontaneous emission (18) and VOC-Hyd (21). Sixty-eight percent of the identified portion was represented by apocarotenoids uniquely constituted by hexahydrofarnesylacetone (40). A significant decrease in acetophenone was noticed after hydrodistillation, whose percentage passed from 59.3% in VOC-Fs to 5.7% in EO.
Ketone turned out to be the main class in VOC-Hyd (41.4%), and acetophenone (40.2%) was also confirmed as the main compound by excellence here. Aldehydes constitute more than a quarter of the whole VOC-Hyd composition (26.7%), with nonanal being the main constituent (23.6%, (19)).
3.1.2. Begonia cucullata Willd
The spontaneous emission of begonia pointed out only six compounds, including one alkane (tetradecane (33.7%, (16)), one aldehyde (decanal (25.7%, (14)), and one ester (benzyl acetate (7.6%, (12)), along with three terpenoids, collectively accounting for over 30% of the total identified fraction (Table 2).

Table 2.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EOs), and hydrosols (VOC-Hyds) derived from Begonia cucullata.
The EO yielded eight identified compounds, with five belonging to the alkane class, making up the predominant category at 91.7%.
VOC-Hyd, however, exhibited the highest diversity, featuring 15 identified constituents. Aldehydes prevailed in the composition (65.6%), with nonanal being the chief compound (56.9%, (10)). Notably, both nitrogenous compounds (NCs, 9.8%) and MH (9.9%) were equally represented, with oxime and methoxy phenyl (2) exclusively representing the NCs. Limonene (7.5%, (6)) emerged as the principal MH. As not much research has been performed so far on B. cucullata, making direct comparisons is challenging with only B. reniformis Dryand. Leaf EO was reported in a study by Da Silva et al. [28]. Sesquiterpenoids siliphiperfol-4,7(14)-dine and β-vetispirene were major constituents, constituting 15.7 and 21.0%, respectively. The unique OS compound in the current work was viridiflorol, albeit in a minimal percentage (0.7%, (20)).
3.1.3. Calendula officinalis L.
Spontaneous emissions of C. officinalis were rich in monoterpene hydrocarbons (MH) (49.5%), primarily represented by α-thujene (44.8%, (3)) (Table 3). Additionally, the presence of sesquiterpene hydrocarbons (39.6%) was noted, predominantly represented by δ-cadinene (43) and γ-cadinene (41), comprising 15.3% and 11.1%, respectively (Table 3). α-Thujene was also the main compound found in the SEs of C. arvensis L. [5], which also highlighted the presence of considerable amounts of sesquiterpenes in this species.

Table 3.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EOs), and hydrosols (VOC-Hyds) derived from Calendula officinalis.
The EO contained sesquiterpenes, especially oxygenated ones (42.7% vs. 17.0% hydrocarbons). The main constituents were α-cadinol (18.8%, (49)) and tau-cadinol (16.1%, (47)). It is interesting to note the presence of non-terpenes in significant amounts, representing 29.2% of the identified fraction. α-Cadinol emerged as the predominant constituent identified in Bosnians C. officinalis flowers studied by Ak et al. [29]. Additionally, it is noted as one of the principal compounds in this species, as reported by Dhingara et al. [30].
The by-product of EO extraction was rich in OM (79.9%), primarily represented by eucalyptol (41.4%, (9)) and linalool (12.2%, (14)). However, previous studies on C. arvensis VOC-Hyd have reported a prevalence of oxygenated compounds [31].
3.1.4. Dahlia hortensis Guillaumin
The spontaneous emission of D. hortensis flowers was dominated by terpene compounds, especially monoterpene and sesquiterpene hydrocarbons (61.1% and 38.6%, respectively). Monoterpene hydrocarbons were mainly represented by p-cymene (46.6% (11)) and α-phellandrene (12.1%, (9)) (Table 4). Meanwhile, sesquiterpene hydrocarbons were mainly represented by germacrene D (14.1%, (24)) and β-caryophyllene (11.3%, (19)) (Table 4). This trend was also observed in the EO, with the main classes being the same as those observed in the VOC-Fs, with a slight decrease in their amounts (54.6% and 21.8%, respectively, in MH and SH). It is interesting to note the presence of alkanes in this extract, which represented 15.2% of the identified fraction, mainly n-pentacosane (8.6%, (41)).
Regarding the major compounds found in the EO, we highlighted the presence of p-cymene (18.9%, (11)) and limonene (19.3%, (12)). The EO also showed the largest number of compounds (34) compared to both the molecules that were spontaneously perceived (12) and the VOC-Hyd (13). The latter was dominated by MH (95.5%), and p-cymene (71.3%) was again confirmed to be the molecule par excellence of these flowers, regardless of the type of extract. Besides p-cymene, limonene was also found to have a high percentage of VOC-Hyd (19.2%).
To the best of the authors’ knowledge, no previous paper has been published about the volatilome of this plant. The available literature primarily investigates other species within the same genus, with a predominant focus on EOs. A unique paper investigated D. pinnata Cav. specifically for its VOC-Fs composition using static headspace volatiles extraction and revealed the extract’s richness in myrcene (28.5%), γ-muurolene (27.8%), and (E)-β-ocimene (17.5%) [32]. The method used cannot be directly compared with our extraction technique or our approach to spontaneous emission evaluation. Nevertheless, it is noteworthy that the primary compounds belonged to MH and SH, as observed herein in both VOC-Fs and EO. Flower EO of D. pinnata was also investigated by Wang et al. [33], who reported an EO rich in 4-terpineol (25.7%), methallyl cyanide (14.0%), and D-limonene (10.5%). Only limonene was found in our EO, while the other two compounds were omitted. Within the same genus, the capitulum (flower head) EO of Dahlia imperialis Roezel ex Ortgies was rich in β-pinene (27.7%), α-phellandrene (26.2%), and α-pinene (12.4%). A recent study on flower EO of the same species confirmed its dominance of β-pinene (27.7%), α-phellandrene (26.2%), and α-pinene (12.4%) as major chemicals [34]. Although all these compounds were also present in our studied flowers, they were found in lesser amounts. In a study conducted by Manah et al. [35] on the flowers EO of Dahlia E‘veline’, it was evidenced that more than 80% of the identified fraction was composed of anethole. However, this compound was omitted in the studied species.

Table 4.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EO), and hydrosols (VOC-Hyd) derived from Dahlia hortensis.
Table 4.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EO), and hydrosols (VOC-Hyd) derived from Dahlia hortensis.
| No. | Compounds | Formula | Class | LRI cal | LRI lit | VOC-Fs | EOs | VOC-Hyd |
|---|---|---|---|---|---|---|---|---|
| Relative Abundance (%) | ||||||||
| 1 | hexanal | C6H12O | ADH | 802 | 8008 1 | - | - | 2.2 ± 0.15 |
| 2 | methoxy-phenyl-oxime | C8H9NO2 | NC | 898 | 899 * | - | - | 0.2 ± 0.08 |
| 3 | heptanal | C7H14O | ADH | 901 | 904 1 | - | - | 0.8 ± 0.27 |
| 4 | α-thujene | C10H16 | MH | 933 | 931 1 | - | - | 0.2 ± 0.07 |
| 5 | α-pinene | C10H16 | MH | 941 | 937 1 | - | 0.8 ± 0.16 | - |
| 6 | sabinene | C10H16 | MH | 977 | 976 1 | - | 2.2 ± 0.43 | 1.2 ± 0.08 |
| 7 | β-pinene | C10H16 | MH | 982 | 980 1 | - | 2.0 ± 0.36 | 2.1 ± 0.16 |
| 8 | β-myrcene | C10H16 | MH | 991 | 990 1 | - | 0.5 ± 0.11 | - |
| 9 | α-phellandrene | C10H16 | MH | 1006 | 1007 1 | 12.1 ± 0.66 | 5.6 ± 0.50 | - |
| 10 | α-terpinene | C10H16 | MH | 1020 | 1016 1 | - | - | 0.2 ± 0.04 |
| 11 | p-cymene | C10H14 | MH | 1028 | 1026 1 | 46.6 ± 2.03 | 18.9 ± 0.86 | 71.3 ± 0.21 |
| 12 | limonene | C10H16 | MH | 1029 | 1033 1 | - | 16.3 ± 1.30 | 19.2 ± 0.67 |
| 13 | (E)-β-ocimene | C10H16 | MH | 1052 | 1050 1 | 2.4 ± 0.15 | 8.2 ± 1.50 | 1.0 ± 0.04 |
| 14 | γ-terpinene | C10H16 | MH | 1058 | 1053 1 | - | - | 0.3 ± 0.08 |
| 15 | cosmene | C10H14 | MH | 1131 | 1134 1 | - | 0.1 ± 0.04 | - |
| 16 | 4-terpineol | C10H18O | OM | 1177 | 1171 1 | - | - | 0.2 ± 0.04 |
| 17 | thymol methyl ether | C11H16O | OM | 1235 | 1234 1 | - | 0.1 ± 0.01 | 0.3 ± 0.06 |
| 18 | α-copaene | C15H24 | SH | 1376 | 1372 1 | 3.5 ± 0.29 | 0.5 ± 0.02 | - |
| 19 | β-caryophyllene | C15H24 | SH | 1419 | 1418 1 | 11.3 ± 0.49 | 5.8 ± 0.14 | - |
| 20 | α-humulene | C15H24 | SH | 1455 | 1455 1 | 0.7 ± 0.08 | 0.9 ± 0.03 | - |
| 21 | (E)-β-farnesene | C15H24 | SH | 1458 | 1454 1 | - | 0.6 ± 0.02 | - |
| 22 | cis-muurola-4(14),5-diene | C15H24 | SH | 1463 | 1468 1 | 0.5 ± 0.04 | - | - |
| 23 | γ-muurolene | C15H24 | SH | 1477 | 1477 1 | 0.90.07 | 0.8 ± 0.04 | - |
| 24 | germacrene D | C15H24 | SH | 1481 | 1480 1 | 14.1 ± 1.07 | 8.6 ± 0.64 | - |
| 25 | epi-cubebol | C15H24O | OS | 1493 | 1494 1 | - | 0.5 ± 0.04 | - |
| 26 | bicyclo-germacrene | C15H24 | SH | 1496 | 1494 * | 1.8 ± 0.40 | 0.9 ± 0.06 | - |
| 27 | α-muurolene | C15H24 | SH | 1499 | 1499 1 | 0.4 ± 0.03 | 0.5 ± 0.03 | - |
| 28 | 7-epi-α-selinene | C15H24 | SH | 1517 | 1514 1 | - | 0.5 ± 0.05 | - |
| 29 | δ-cadinene | C15H24 | SH | 1524 | 1524 1 | 5.4 ± 0.61 | 2.7 ± 0.23 | - |
| 30 | germacrene D-4-ol | C15H26O | OS | 1575 | 1578 1 | - | 0.8 ± 0.09 | - |
| 31 | caryophyllene oxide | C15H24O | OS | 1581 | 1582 1 | - | 0.5 ± 0.07 | - |
| 32 | copaborneol | C15H26O | OS | 1600 | 1597 3 | - | 1.1 ± 0.17 | - |
| 33 | 1-epi-cubenol | C15H26O | OS | 1627 | 1623 1 | - | 0.7 ± 0.11 | - |
| 34 | caryophylla-4(14),8(15)-dien-5-ol | C15H24O | OS | 1637 | 1631 1 | - | 0.3 ± 0.06 | - |
| 35 | tau-cadinol | C15H26O | OS | 1641 | 1638 1 | - | 0.1 ± 0.00 | - |
| 36 | ylangenol | C15H24O | OS | 1667 | 1666 1 | - | 0.4 ± 0.07 | - |
| 37 | ent-germacra-4(15),5,10(14)-trien-1-β-ol | C15H24O | OS | 1695 | 1694 1 | - | 2.0 ± 0.44 | - |
| 38 | xanthorrhizol | C15H22O | OS | 1753 | 1754 1 | - | 0.3 ± 0.09 | - |
| 39 | tricosane | C23H48 | ALK | 2300 | 2300 1 | - | 4.4 ± 0.33 | - |
| 40 | n-tetracosane (c24) | C24H50 | ALK | 2400 | 2400 1 | - | 2.2 ± 0.44 | - |
| 41 | n-pentacosane (c25) | C25H52 | ALK | 2500 | 2500 1 | - | 8.6 ± 0.89 | - |
| Number of Identified Compounds | 12 | 34 | 13 | |||||
| Class of Compounds | VOC-Fs | EOs | VOC-Hyd | |||||
| Monoterpene Hydrocarbons (MHs) | 61.1 ± 0.95 | 54.6 ± 0.82 | 95.5 ± 0.22 | |||||
| Oxygenated Monoterpenes (OMs) | - | 0.1 ± 0.01 | 0.5 ± 0.18 | |||||
| Sesquiterpene Hydrocarbons (SHs) | 38.6 ± 0.65 | 21.8 ± 0.18 | - | |||||
| Oxygenated Sesquiterpenes (OSs) | - | 6.7 ± 0.10 | - | |||||
| Aldehydes (ADHs) | - | - | 3.0 ± 0.22 | |||||
| Alkanes (ALKs) | - | 15.2 ± 0.60 | - | |||||
| Nitrogenous Compounds (NCs) | - | - | 0.2 ± 0.08 | |||||
| Non-terpenes | 0.70 ± 0.800 | 15.2 ± 0.60 | 3.2 ± 0.14 | |||||
| Total Identified | 99.7 ± 0.80 | 98.4 ± 0.34 | 99.2 ± 0.19 | |||||
LRI cal: Linear Retention Index calculated LRI lit; Linear Retention Index reported in the literature; 1: NIST 2014 (National Institute of Standards Technology (www.nist.gov) visited 24 February 2024); 3: El-Din et al., 2022 [36]; * Pherobase.com.
3.1.5. Polianthes tuberosa L.
P. tuberosa spontaneous emissions predominantly consisted of esters (76.5%), mirroring its EO composition (90.1%). Methyl benzoate (57.3%, (14)) was the main ester in spontaneous emissions, constituting, together with methyl salicylate (13.0%, (18)), over 70% of the identified portion (Table 5). The presence of lactones (14.4%) was observed, reported uniquely by two compounds: jasminelactone (13.8%, (27)) and δ-decalactone (0.6%, (26)) (Table 5). It is important to highlight that this study partially differs from previous research, which reported the presence of methyl benzoate and methyl salicylate in six out of seven studied cultivars of P. tuberosa [37,38]. Methyl salicylate was present in the studied species. Kutty and Mitra [38] reported the presence of lactones. Even though the identified lactones were different from the ones reported in this work, their presence is similar to what was reported herein.

Table 5.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EO), and hydrosols (VOC-Hyd) derived from Polianthes tuberosa.
On the contrary, the main esters found in the EO were methyl icosanoate (24.4%, (32)) and methyl heneicosanoate (58.4%, (33)). Additionally, chromene compounds (precocene II (28)) were detected, contributing only 4.0% to the overall composition. The EO composition in half-opened and fully-opened flowers was rich in methyl benzoate (37.9 and 28.6%, respectively) [39], a compound also present in our flowers but in a lesser amount (7.3%, (13)).
Analyzing the VOC-Hyd composition revealed that 40.0% of the compounds belonged to the OM class, with eucalyptol being the predominant compound at 38.1% (10). NCs accounted for 22.9%, with oxime, methoxy-phenyl (15.5%, (3)), and 2,4,5-trimethyl oxazole (7.4%, (1)) being the only identified compounds. The high percentage of MH (16.8%) is mainly represented by p-cymene (8.9%, (8)) and limonene (5.7%, (9)).
3.1.6. Tulbaghia cominsii Vosa
Esters (37.2%) and ketones (30.3%) predominate in the chemical composition of T. cominsii’s VOC-Fs. The chief ketone compound was acetoveratrone (28.4%, (45)), while benzyl benzoate (14.5%, (50)) and benzyl acetate (10.6%, (29)) were the principal esters (Table 6). T. simmleri Beauverd VOC-Fs, as investigated by Marchioni et al. [40], showed a different profile, characterized by spontaneous emissions rich in OM (63.8%), primarily represented by eucalyptol (53.1%) and linalool (15.5%), compounds completely absent in the studied species.

Table 6.
Analysis of spontaneous emissions of fresh flowers (VOC-Fs), essential oils (EO), and hydrosols (VOC-Hyd) derived from Tulbaghia cominsii.
Sulfur compounds took precedence as the main class in the EO, constituting 38.1%, with disulfide, methyl (methylothio) methyl (25.8%, (25)) as the major constituent. Additionally, alkanes (22.9%) and OM (18.3%) exhibited high relative abundance represented by n-heneicosane (22.9%, (57)) and thymol (16.3%, (39)).
VOC-Hyd demonstrated a distinct chemical composition, emphasizing a significant amount of monoterpene compounds (46.9% and 22.1% in OM and MH, respectively). Key compounds of these monoterpene constituents include eucalyptol (21.4%, (13)), thymol (19.1%, (39)), and limonene (11.4%, (11)). Furthermore, aldehydes contribute (16.4%) to the overall composition, mostly represented by decanal (9.6%, (34)) and nonanal (5.8%, (22)).
The presence of sulfur compounds was previously reported and is responsible for the characteristic alliaceous smell and taste of Tulbaghia species, of which Tulbaghia violacea Harv. is probably the most studied one so far [41]. Its EO confirmed the presence of sulfur compounds, mainly represented by 2,3,5-trithiahexane and 2,4,5,7-tetrathiaoctane [42]. This is aligned partially with the current result, where only the latter compound was identified with a non-negligible percentage in the EO (10.0%, (44)). The same study also reported the presence of limonene, eucalyptol, and 4-terpineol in both EO and hexane extracts. However, these compounds were only found in the hydrosol of the studied species. (See Table 6).
3.2. Antibacterial Activity of Hydrosols
Due to the limited availability of plant material and consequently very low yields of EOs, the hydrosols, a by-product obtained during the removal of volatile oil through steam distillation [43], underwent antibacterial activity testing. The tests were conducted on six strains, three of which were Gram-positive and three were Gram-negatives.
Among the Gram-positive strains, Staphylococcus aureus exhibited significant susceptibility, displaying a MIC mode value of 1:2 (Table 7) for all tested hydrosols, except for A. majus and D. hortensis. Nonanal and decanal, two aldehydes from green leave volatiles family, were observed in all active hydrosols, except for T. cominsii, where nonanal was completely omitted; these phytochemicals have been granted a ‘generally recognized as safe’ status [44]. The antibacterial efficacy of decanal was assessed against S. aureus strains, including both methicillin-resistant and susceptible strains. However, its effect was less pronounced when compared to the EO of Ducrosia anethifolia Boiss, where decanal was the primary compound [45]. Nonanal, a saturated aldehyde, has been documented to induce notable changes in membrane permeability, functioning as an effective antibacterial agent [46].

Table 7.
Antibacterial activity of flower-derived hydrosols.
Limonene, present in all studied species, exhibited significant inhibition of S. aureus growth [47] and demonstrated activity against isolated methicillin-resistant strains [48]. Additionally, p-cymene, a precursor of carvacrol, present in C. officinalis hydrosols, and γ-terpinene, identified in all active hydrosols (AH), displayed antibacterial and anti-biofilm activities [49]. Furthermore, eucalyptol, found in substantial amounts in the hydrosols of P. tuberosa and C. officinalis, has reported effects on membrane integrity and implications in oxidative stress in methicillin-resistant S. aureus [50].
As regards Gram-negative bacteria, Salmonella enterica emerged as the most sensitive strain, showing susceptibility to the hydrosols of B. cucullata, D. hortensis, P. tuberosa, and T. cominsii. Additionally, the hydrosol of T. cominsii exhibited a MIC value of 1:2 against E. coli. Fenchone, the primary compound detected in T. cominsii, was the focal point of a study evaluating its antibacterial and anti-biofilm properties through in vitro and in silico approaches. In silico predictions revealed interactions with E. coli proteins, which were validated by determining MIC and MBC values [51]. The same work evidenced that fenchone reduced biofilm formation in E. coli. Additionally, the MIC value of this phytochemical against E. coli was 2 [52]. On the other hand, the same hydrosol was rich in viridiflorol, known for its anticancer, antioxidant, anti-inflammatory, and antibacterial activities [52,53,54].
Methoxyphenyl-oxime, found in a high percentage in P. tuberosa but also present in all other hydrosols except for D. hortensis, albeit in lesser abundance, is an alkaloid reported to be isolated from Conocarpus lancifolius Engl. [55]. The authors of this paper demonstrated its antibacterial activity against Gram-negative strains. Further research [56] explored its antiviral effects and confirmed its potential as a potent drug-like compound against capripox viruses, utilizing methanolic extract of Leucas aspera.
3.3. The Multivariate Analysis of Hydrosol and Key Compound Insights
In addition to assessing their antibacterial activity, hydrosols are being explored as valuable by-products, requiring a comprehensive evaluation of their composition. This necessitates the use of advanced analytical tools such as multivariate analysis, including Hierarchical Cluster Analysis (HCA) and Principal Component Analysis (PCA), which provide profound insights into the characteristics of hydrosols. HCA (Figure 1a) analysis revealed two main clusters, with the first one being homogenous, comprising uniquely D. hortensis hydrosol. The second group encompassed the remaining samples, which can be further divided into two subclusters: Subcluster 1, including A. majus and B. cucullata, and the second subcluster comprised the others. According to the PCA plot (Figure 1b), the first axis (PC 1), accounting for over 45% of the variability, clearly distinguished D. hortensis hydrosol from the others.
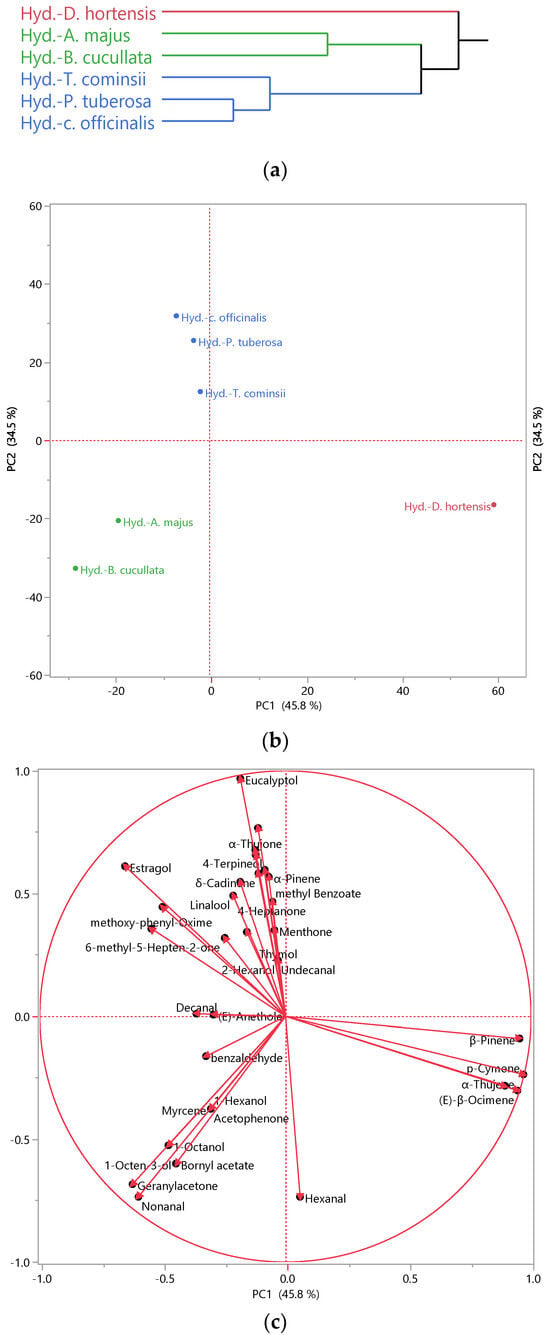
Figure 1.
Hierarchical Cluster Analysis (HCA) (a), Principal Component Analysis (PCA) performed on the hydrosols of the studied species. (b): Score plot; (c): loading plot.
Meanwhile, PC 2 (accounting for 34.5% of the variability) differentiated A. majus and B. cucullata from the rest. These findings were corroborated by the cluster analysis, where the Ward method clustered D. hortensis separately, while A. majus and B. cucullate formed another cluster, and the remaining species grouped, including T. cominsii, P. tuberosa, and C. officinalis.
Examining the biplot of the PCA analysis (Figure 1c), we observed that D. hortensis stood out due to its high percentage in p-cymene, a common compound found in all aromatic waters. p-cymene, an alkyl-substituted aromatic compound, is known for its diverse beneficial activities, such as antioxidant, anti-inflammatory, antiparasitic, antidiabetic, antiviral, antitumor, antibacterial, and antifungal activities [57]. p-Cymene has also been recognized to act as an analgesic, antinociceptive, immunomodulatory, vasorelaxant, neuroprotective agent, and anticancer [57]. Moreover, it found applications in the food industry as a flavor/fragrance agent [58] and served as an intermediate constituent in the chemical syntheses of fragrances [59]. Additionally, sabinene and (E)-β-ocimene were exclusively present in Dahlia aromatic water. These compounds represented 1% of the identified fraction. (E)-β-ocimene was recommended for fragrance use at levels up to 3% and is known as a pheromone involved in social regulation in the honeybee colony [60]. Sabinene was utilized as a perfume additive and possessed anti-fungal activity [61]. Also noticed is the presence of β-pinene reported for broad biological activity, including fungicidal, antimicrobial, and antiviral agents, in addition to its use in the flavor and fragrance industry [62].
Nonanal, a common compound found in both A. majus and B. cucullata, contributed to their clustering in the bottom left quadrant of the PCA analysis. It is renowned for its antifungal properties [63] and is widely used in perfumery products for its green-floral fragrance [64]. Acetophenone, a preliminary compound of A. majus, serves as both an attractant and repellent for blood-sucking insects such as mosquitoes, flies, and ticks, serving as a crucial component in the manipulation of skin microbiota by vector-borne parasites and as an ingredient in trap crops for economically important crop pests [26]. Furthermore, it serves as a flavoring agent and intermediate compound in perfumes and cosmetics [65].
On the other hand, 1-octene-3-ol and bornyl acetate were characteristic compounds of B. cucullata. They exhibited strong antibacterial activity, especially against Gram-positive strains [66,67], which could be one of the reasons why the hydrosols of this species were effective on S. aureus. Bornyl acetate is also documented for its insecticidal activity [68] and anti-inflammatory effect in human chondrocytes [69]. On the contrary, A. majus was characterized by 1-hexanol, a volatile alcohol known to have an effect against food-related Gram-negative bacteria [70].
In the upper left quadrant of PCA analysis, the remaining hydrosols were clustered together due to their high content in eucalyptol, which is known for its pleasant spicy aroma and taste. Eucalyptol found applications as a flavoring agent, fragrance ingredient, and cosmetics additive [71]. Additionally, it is reported to alleviate pain and inflammation associated with monosodium urate [72]. Notably, methoxy-phenyl-oxime constituted at least 5.0% of the identified fraction of these three hydrosols and was found in all the others, imparting a scent reminiscent of fresh shrimp and crabs [73]. This compound also exhibited antibacterial activity [55]. Oximes naturally occur in plants and animals and are known for their anti-inflammatory, antioxidant, and anticancer activities [74]. Thymol, a characteristic constituent of T. cominsii, is versatile and used in therapeutic applications [75] and food [76]. Decanal, another prominent compound in this hydrosol, is utilized in perfume [77]. In contrast, methyl benzoate and 6-methyl-5-hepten-2-one were present in P. tuberosa. Methyl benzoate is suggested as an efficient green pesticide [78]. 6-Methyl-5-hepten-2-one is considered an important chemical intermediate and flavor compound crucial for fruit flavor in tomato, papaya, and guava [79]. C. officinalis evidenced the highest content of linalool, a compound mainly used for its anti-inflammatory, anticancer, antimicrobial, and antioxidant properties, alongside its use in cosmetics, food additives, and perfume [77,80]. Furthermore, this hydrosol is characterized by its high content of camphor and 4-terpineol, both of which are reported for their antibacterial activity [81,82].
Limonene, a shared compound in all hydrosols, although it may not be considered a discriminant compound in PCA due to its presence in significant percentages across all samples, is recognized for its therapeutic effect, including anti-inflammatory, antioxidant, and antiviral effects, besides its use as flavor and fragrance additive owing to its pleasant lemon-like odor [83].
Overall, the studied hydrosols offer various chemical compositions with promising applications across multiple industries, including skincare, fragrance, and therapeutics.
Author Contributions
Conceptualization, B.N. and I.M.; methodology, B.N., B.T., D.V., M.F.B. and F.F.; software, Y.P.; validation, B.N., F.F., L.P. (Laura Pistelli), B.T., L.P. (Luisa Pistelli) and I.M.; formal analysis, B.N., Y.P., B.T., D.V. and M.F.B.; investigation, B.N., F.F., D.V. and B.T.; resources, L.P. (Laura Pistelli), L.P. (Luisa Pistelli), F.F. and B.T.; data curation, B.N., Y.P., M.F.B., B.T., D.V., F.F. and I.M.; writing—original draft preparation, B.N. and I.M.; writing—review and editing, B.N., M.F.B., F.F. and I.M.; visualization, B.N. and Y.P.; supervision, B.N. and I.M.; project administration, B.N. and L.P. (Luisa Pistelli). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the INTERREG-ALCOTRA UE 2014–2020 Project “ANTEA” Attività innovative per lo sviluppo della filiera transfrontaliera del fiore edule (n. 1139), grant number CUP C12F17000080003.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We extend our thanks to the CREA Research Centre for Vegetable and Ornamental Crops (CREA, Sanremo) and Chambre d’Agriculture des Alpes-Maritimes (CREAM, Nice) for providing and cultivating the studied plants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rodrigues, H.; Spence, C. Looking to the Future, by Studying the History of Edible Flowers. Int. J. Gastron. Food Sci. 2023, 34, 100805. [Google Scholar] [CrossRef]
- Harries, B.; Chalmin-Pui, L.S.; Gatersleben, B.; Griffiths, A.; Ratcliffe, E. ‘Designing a Wellbeing Garden’ a Systematic Review of Design Recommendations. Des. Health 2023, 7, 180–201. [Google Scholar] [CrossRef]
- Barrales-Cureño, H.J.; Salgado-Garciglia, R.; López-Valdez, L.G.; Reynoso-López, R.; Herrera-Cabrera, B.E.; Lucho-Constantino, G.G.; Zaragoza-Martínez, F.; Reyes-Reyes, C.; Aftab, T. Use of Secondary Metabolites from Medicinal and Aromatic Plants in the Fragrance Industry. In Medicinal and Aromatic Plants: Healthcare and Industrial Applications; Springer: Cham, Switzerland, 2021; pp. 669–690. [Google Scholar]
- Nicknezhad, S.; Hashemabadi, D.; Allahyari, M.S.; Marzban, S.; Ben Hassen, T.; Surujlal, J. Sensorial Analysis of Factors Influencing Consumers’ Perceptions toward the Consumption of Edible Flowers in Iran. J. Agric. Food Res. 2023, 12, 100580. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Malheiro, R.; Rodrigues, N.; Saraiva, J.A.; Ramalhosa, E. Borage, Calendula, Cosmos, Johnny Jump up, and Pansy Flowers: Volatiles, Bioactive Compounds, and Sensory Perception. Eur. Food Res. Technol. 2019, 245, 593–606. [Google Scholar] [CrossRef]
- Mlcek, J.; Plaskova, A.; Jurikova, T.; Sochor, J.; Baron, M.; Ercisli, S. Chemical, Nutritional and Sensory Characteristics of Six Ornamental Edible Flowers Species. Foods 2021, 10, 2053. [Google Scholar] [CrossRef]
- Marchioni, I.; Taglieri, I.; Dimita, R.; Ruffoni, B.; Zinnai, A.; Venturi, F.; Sanmartin, C.; Pistelli, L. Postharvest Treatments on Sensorial and Biochemical Characteristics of Begonia Cucullata Willd Edible Flowers. Foods 2022, 11, 1481. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Nutraceutical Potential of Dietary Phytochemicals in Edible Flowers—A Review. J. Food Biochem. 2021, 45, e13642. [Google Scholar] [CrossRef] [PubMed]
- Pensamiento-Niño, C.A.; Castañeda-Ovando, A.; Añorve-Morga, J.; Hernández-Fuentes, A.D.; Aguilar-Arteaga, K.; Ojeda Ramírez, D. Edible Flowers and Their Relationship with Human Health: Biological Activities. Food Rev. Int. 2023, 40, 620–639. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Badwaik, L.S.; Annapure, U.; Casanova, F.; Alaskar, K. A Review on the Journey of Edible Flowers from Farm to Consumer’s Plate. Appl. Food Res. 2023, 3, 100312. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, M.; Bhandari, B.; Mujumdar, A.S. Edible Flower Essential Oils: A Review of Chemical Compositions, Bioactivities, Safety and Applications in Food Preservation. Food Res. Int. 2021, 139, 109809. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef] [PubMed]
- Miljanović, A.; Dent, M.; Grbin, D.; Pedisić, S.; Zorić, Z.; Marijanović, Z.; Jerković, I.; Bielen, A. Sage, Rosemary, and Bay Laurel Hydrodistillation By-Products as a Source of Bioactive Compounds. Plants 2023, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Paolini, J.; Leandri, C.; Desjobert, J.-M.; Barboni, T.; Costa, J. Comparison of Liquid–Liquid Extraction with Headspace Methods for the Characterization of Volatile Fractions of Commercial Hydrolats from Typically Mediterranean Species. J. Chromatogr. A 2008, 1193, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Drava, G.; Iobbi, V.; Govaerts, R.; Minganti, V.; Copetta, A.; Ruffoni, B.; Bisio, A. Trace Elements in Edible Flowers from Italy: Further Insights into Health Benefits and Risks to Consumers. Molecules 2020, 25, 2891. [Google Scholar] [CrossRef] [PubMed]
- Copetta, A.; Marchioni, I.; Mascarello, C.; Pistelli, L.; Cambournac, L.; Dimita, R.; Ruffoni, B. Polianthes tuberosa as Edible Flower: In Vitro Propagation and Nutritional Properties. ijfe 2020, 6, 57–62. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Nardi, V.; Cervelli, C.; Mecacci, G.; Mancianti, F.; Ebani, V.V.; Nardoni, S.; Pistelli, L. Volatilome Analyses and In Vitro Antimicrobial Activity of the Essential Oils from Five South African Helichrysum Species. Molecules 2020, 25, 3196. [Google Scholar] [CrossRef] [PubMed]
- NIST NIST/EPA/NIH Mass Spectral Library; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014.
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on Methyl Silicon and Carbowax 20 M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography. J. Chem. Educ. 1981, 58, 12. [Google Scholar] [CrossRef]
- Masada, Y. Analysis of Essential Oils by Gas Chromatography and Mass Spectrometry; John Wiely & Sons, Inc.: New York, NY, USA, 1976. [Google Scholar]
- Stenhagen, E.; Abrahamsson, S.; McLafferty, F.W. Registry of Mass Spectral Data; Wiely & Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Swigar, A.A.; Silverstein, R.M. Monoterpenes; Aldrich Chemical Company: Wisconsin, WI, USA, 1981. [Google Scholar]
- Choi, Y.H.; Kim, H.K.; Hazekamp, A.; Erkelens, C.; Lefeber, A.W.M.; Verpoorte, R. Metabolomic Differentiation of Cannabis sativa Cultivars Using 1H NMR Spectroscopy and Principal Component Analysis. J. Nat. Prod. 2004, 67, 953–957. [Google Scholar] [CrossRef]
- Zubkov, F.I.; Kouznetsov, V.V. Traveling across Life Sciences with Acetophenone—A Simple Ketone That Has Special Multipurpose Missions. Molecules 2023, 28, 370. [Google Scholar] [CrossRef] [PubMed]
- Suchet, C.; Dormont, L.; Schatz, B.; Giurfa, M.; Simon, V.; Raynaud, C.; Chave, J. Floral Scent Variation in Two Antirrhinum majus Subspecies Influences the Choice of Naïve Bumblebees. Behav. Ecol. Sociobiol. 2011, 65, 1015–1027. [Google Scholar] [CrossRef]
- Da Silva, A.G.M.; Silva, M.W.F.; Bezerra, G.B.; Ramos, C.S. The First Report of Chemical and Biological Study of Essential Oil from Begonia reniformis Leaf (Begoniaceae). Eclet. Quim. J. 2017, 42, 60. [Google Scholar] [CrossRef][Green Version]
- Ak, G.; Zengin, G.; Ceylan, R.; Fawzi Mahomoodally, M.; Jugreet, S.; Mollica, A.; Stefanucci, A. Chemical Composition and Biological Activities of Essential Oils from Calendula officinalis L. Flowers and Leaves. Flavour Fragr. J. 2021, 36, 554–563. [Google Scholar] [CrossRef]
- Dhingra, G.; Dhakad, P.; Tanwar, S. Review on Phytochemical Constituents and Pharmacological Activities of Plant Calendula officinalis Linn. Biolsciences 2022, 2, 216–228. [Google Scholar] [CrossRef]
- Belabbes, R.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Tabti, B.; Costa, J.; Muselli, A. Chemical Variability, Antioxidant and Antifungal Activities of Essential Oils and Hydrosol Extract of Calendula arvensis L. from Western Algeria. Chem. Biodivers. 2017, 14, e1600482. [Google Scholar] [CrossRef]
- Bahmanzadegan, A.; Rowshan, V. Static Headspace Analysis and Polyphenol Content of Tagetes erecta, Matthiola incana, Erysimum cheiri, Gaillardia grandiflora and Dahlia pinnata in Iran. Anal. Chem. Lett. 2018, 8, 794–802. [Google Scholar] [CrossRef]
- Wang, D.-C.; Qiu, D.-R.; Shi, L.-N.; Pan, H.-Y.; Li, Y.-W.; Sun, J.-Z.; Xue, Y.-J.; Wei, D.-S.; Li, X.; Zhang, Y.-M.; et al. Identification of Insecticidal Constituents of the Essential Oils of Dahlia pinnata Cav. against Sitophilus zeamais and Sitophilus oryzae. Nat. Prod. Res. 2015, 29, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Cicció, J.F.; Chaverri, C. Chemical Composition of Essential Oils of Dahlia imperialis (Asteraceae) Growing Wild in Costa Rica. J. Mex. Chem. Soc. 2022, 66, 468–479. [Google Scholar] [CrossRef]
- Manan, N.; Ïztelew, B.M.; Azïmbaeva, G.E.; Djïembaev, B.J. Study of Component Composition of Essential Oil Isolated from Dahlia evelines Plants by Chromato-Mass-Spectrometry Method. Bull. Kazakh State Girl’sPedagogical Univ. 2018, 3, 37–45. [Google Scholar]
- Gamal El-Din, M.I.; Youssef, F.S.; Altyar, A.E.; Ashour, M.L. GC/MS Analyses of the Essential Oils Obtained from Different Jatropha Species, teir Discrimination Using Chemometric Analysis and Assessment of their Antibacterial and Anti-Biofilm Activities. Plants 2022, 11, 1268. [Google Scholar] [CrossRef]
- Maiti, S.; Mitra, A. Morphological, Physiological and Ultrastructural Changes in Flowers Explain the Spatio-Temporal Emission of Scent Volatiles in Polianthes tuberosa L. Plant Cell Physiol. 2017, 58, 2095–2111. [Google Scholar] [CrossRef]
- Kutty, N.N.; Mitra, A. Profiling of Volatile and Non-Volatile Metabolites in Polianthes tuberosa L. Flowers Reveals Intraspecific Variation among Cultivars. Phytochemistry 2019, 162, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Ahmadi, N.; Babaei, A.; Naghavi, M.R.; Ayyari, M. Comparison of Volatile Compounds at Various Developmental Stages of Tuberose (Polianthes tuberosa L. Cv. Mahallati) Flower with Different Extraction Methods. J. Essent. Oil Res. 2018, 30, 197–206. [Google Scholar] [CrossRef]
- Marchioni, I.; Najar, B.; Copetta, A.; Ferri, B.; Ruffoni, B.; Pistelli, L.; Pistelli, L. Phytonutritional and Aromatic Profiles of Tulbaghia simmleri Beauv. Edible Flowers during Cold Storage. Adv. Hort. Sci. 2023, 37, 25–32. [Google Scholar] [CrossRef]
- Lyantagaye, S. Ethnopharmacological and Phytochemical Review of Allium Species (Sweet Garlic) and Tulbaghia Species (Wild Garlic) from Southern Africa. Tanzan. J. Sci. 2011, 37, 58–72. [Google Scholar]
- Eid, H.H. The Influence of Extraction Methods on the Composition and Antimicrobial Activity of the Volatile Constituents of Tulbaghia violacea Harv. Cultivated in Egypt. J. Pharmacogn. Phytochem. 2015, 4, 118–125. [Google Scholar]
- Değirmenci, H.; Erkurt, H. Relationship between Volatile Components, Antimicrobial and Antioxidant Properties of the Essential Oil, Hydrosol and Extracts of Citrus aurantium L. Flowers. J. Infect. Public Health 2020, 13, 58–67. [Google Scholar] [CrossRef]
- Goker, G.; Demirtas, A. Preliminary Study on Stimulatory and Inhibitory Effects of Aldehydes from the Green Leaf Volatiles Family on Beneficial and Pathogenic Bacteria from the Intestine. Med. Weter. 2020, 76, 170–175. [Google Scholar] [CrossRef]
- Mahboubi, M.; Feizabadi, M.M. Antimicrobial Activity of Ducrosia anethifolia Essential Oil and Main Component, Decanal Against Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus. J. Essent. Oil Bear. Plants 2009, 12, 574–579. [Google Scholar] [CrossRef]
- Trombetta, D.; Saija, A.; Bisignano, G.; Arena, S.; Caruso, S.; Mazzanti, G.; Uccella, N.; Castelli, F. Study on the Mechanisms of the Antibacterial Action of Some Plant Alpha,Beta-Unsaturated Aldehydes. Lett. Appl. Microbiol. 2002, 35, 285–290. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial Activities and Synergistic Interaction of Citrus Essential Oils and Limonene with Gentamicin against Clinically Isolated Methicillin-Resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef] [PubMed]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic Effect of Eugenol, Carvacrol, Thymol, p-Cymene and γ-Terpinene on Inhibition of Drug Resistance and Biofilm Formation of Oral Bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Merghni, A.; Belmamoun, A.R.; Urcan, A.C.; Bobiş, O.; Lassoued, M.A. 1,8-Cineol (Eucalyptol) Disrupts Membrane Integrity and Induces Oxidative Stress in Methicillin-Resistant Staphylococcus aureus. Antioxidants 2023, 12, 1388. [Google Scholar] [CrossRef]
- Ahmad, W.; Ansari, M.A.; Yusuf, M.; Amir, M.; Wahab, S.; Alam, P.; Alomary, M.N.; Alhuwayri, A.A.; Khan, M.; Ali, A.; et al. Antibacterial, Anticandidal, and Antibiofilm Potential of Fenchone: In Vitro, Molecular Docking and In Silico/ADMET Study. Plants 2022, 11, 2395. [Google Scholar] [CrossRef] [PubMed]
- El Omari, N.; Balahbib, A.; Bakrim, S.; Benali, T.; Ullah, R.; Alotaibi, A.; Naceiri El Mrabti, H.; Goh, B.H.; Ong, S.-K.; Ming, L.C.; et al. Fenchone and Camphor: Main Natural Compounds from Lavandula stoechas L.; Expediting Multiple in Vitro Biological Activities. Heliyon 2023, 9, e21222. [Google Scholar] [CrossRef] [PubMed]
- Asaad, A.Y. In-vitro Antimicrobial Activity of Essential oil Derived from Callistemon viminalis Aerial Part. Al-Kindy Coll. Med. J. 2023, 19, 69–74. [Google Scholar] [CrossRef]
- El Karkouri, J.; Bouhrim, M.; Al Kamaly, O.M.; Mechchate, H.; Kchibale, A.; Adadi, I.; Amine, S.; Alaoui Ismaili, S.; Zair, T. Chemical Composition, Antibacterial and Antifungal Activity of the Essential Oil from Cistus ladanifer L. Plants 2021, 10, 2068. [Google Scholar] [CrossRef]
- Burranboina, K.K.; Kumar, K.M.; Manjunatha Reddy, G.B.; Yogisharadhya, R.; Prashantha, C.N.; Dhulappa, A. GC-MS Analysis, Molecular Docking and Pharmacokinetic Studies of Various Bioactive Compounds from Methanolic Leaf Extracts of Leucas aspera (L.) against Anti—Capripox Viral Activity. Chem. Data Collect. 2022, 39, 100873. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health Beneficial and Pharmacological Properties of p-Cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef]
- Celebioglu, A.; Yildiz, Z.I.; Uyar, T. Electrospun Nanofibers from Cyclodextrin Inclusion Complexes with Cineole and p-Cymene: Enhanced Water Solubility and Thermal Stability. Int. J. Food Sci. Tech. 2018, 53, 112–120. [Google Scholar] [CrossRef]
- Serafini, M.R.; Menezes, P.P.; Costa, L.P.; Lima, C.M.; Quintans, L.J.; Cardoso, J.C.; Matos, J.R.; Soares-Sobrinho, J.L.; Grangeiro, S.; Nunes, P.S.; et al. Interaction of p-Cymene with β-Cyclodextrin. J. Therm. Anal. Calorim. 2012, 109, 951–955. [Google Scholar] [CrossRef]
- Cic, M.Z.; Li, M. Ocimene –A Versatile Floral Ingredient. Perfum. Flavorist 2013, 38, 42–44. [Google Scholar]
- Cao, Y.; Zhang, H.; Liu, H.; Liu, W.; Zhang, R.; Xian, M.; Liu, H. Biosynthesis and Production of Sabinene: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 1535–1544. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Valussi, M.; Morais-Braga, M.; Carneiro, J.; Leal, A.; Coutinho, H.; Vitalini, S.; Kręgiel, D.; Antolak, H.; Sharifi-Rad, M.; et al. Tagetes Spp. Essential Oils and Other Extracts: Chemical Characterization and Biological Activity. Molecules 2018, 23, 2847. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, X.; Xie, Y.; Liang, J. Antifungal Properties and Mechanisms of Three Volatile Aldehydes (Octanal, Nonanal and Decanal) on Aspergillus flavus. Grain Oil Sci. Technol. 2021, 4, 131–140. [Google Scholar] [CrossRef]
- Kim, M.; Sowndhararajan, K.; Choi, H.J.; Park, S.J.; Kim, S. Olfactory Stimulation Effect of Aldehydes, Nonanal, and Decanal on the Human Electroencephalographic Activity, According to Nostril Variation. Biomedicines 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chidananda Varma, P.; Poojitha Reddy, M.; Sreelekha, C.; Chandiramouli, R. Acetophenone and Benzophenone Adsorption Studies on θ-Phosphorene Nanosheets—A DFT Investigation. Comput. Theor. Chem. 2022, 1215, 113808. [Google Scholar] [CrossRef]
- Xiong, C.; Li, Q.; Li, S.; Chen, C.; Chen, Z.; Huang, W. In Vitro Antimicrobial Activities and Mechanism of 1-Octen-3-Ol against Food-Related Bacteria and Pathogenic Fungi. J. Oleo Sci. 2017, 66, 1041–1049. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Ruan, X. Bornyl Acetate: A Promising Agent in Phytomedicine for Inflammation and Immune Modulation. Phytomedicine 2023, 114, 154781. [Google Scholar] [CrossRef]
- Song, H.-J.; Yong, S.-H.; Kim, H.-G.; Kim, D.-H.; Park, K.-B.; Shin, K.-C.; Choi, M.-S. Insecticidal Activity against Myzus persicae of Terpinyl Acetate and Bornyl Acetate in Thuja occidentalis Essential Oil. Horticulturae 2022, 8, 969. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, R.; Chen, H.; Jia, P.; Bao, L.; Tang, H. Bornyl Acetate Has an Anti-inflammatory Effect in Human Chondrocytes via Induction of IL-11. IUBMB Life 2014, 66, 854–859. [Google Scholar] [CrossRef]
- Kyoui, D.; Saito, Y.; Takahashi, A.; Tanaka, G.; Yoshida, R.; Maegaki, Y.; Kawarai, T.; Ogihara, H.; Suzuki, C. Antibacterial Activity of Hexanol Vapor In Vitro and on the Surface of Vegetables. Foods 2023, 12, 3097. [Google Scholar] [CrossRef]
- Bhowal, M.; Gopal, M. Eucalyptol: Safety and Pharmacological Profile. RJPS 2016, 5, 125–131. [Google Scholar] [CrossRef]
- Yin, C.; Liu, B.; Wang, P.; Li, X.; Li, Y.; Zheng, X.; Tai, Y.; Wang, C.; Liu, B. Eucalyptol Alleviates Inflammation and Pain Responses in a Mouse Model of Gout Arthritis. Br. J. Pharmacol. 2020, 177, 2042–2057. [Google Scholar] [CrossRef]
- Chung, M.; Cheng, S.; Lin, C.; Chang, S. Profiling of Volatile Compounds with Characteristic Odors in Bambusa oldhamii Shoots from Taiwan. BioRes 2021, 16, 5901–5914. [Google Scholar] [CrossRef]
- Al-Mussawii, M.A.Y.; Al-Sultan, E.Y.A.; Al-Hamdan, M.A.; Ramadhan, U.H. Antibacterial Activity of Alkaloid Compound Methoxy Phenyl- Oxime (C8H9NO2) Isolated and Purified from Leaf of Conocarpus lancifolius Engl. Teikyo Med. J. 2022, 45, 4971–4981. [Google Scholar]
- Surowiak, A.K.; Lochyński, S.; Strub, D.J. Unsubstituted Oximes as Potential Therapeutic Agents. Symmetry 2020, 12, 575. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Nieto, G. A Review on Applications and Uses of Thymus in the Food Industry. Plants 2020, 9, 961. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and Biological Activities of Decanal, Linalool, Valencene, and Octanal from Sweet Orange Oil. J. Food Sci. 2012, 77, C1156–C1161. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A. A Floral Fragrance, Methyl Benzoate, Is An Efficient Green Pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, X.; Cui, X.; Wang, H. Rapid Determination of 6-Methyl-5-Hepten-2-One in Fruit with LLE-GC-MS. J. Chromatogr. Sci. 2022, 60, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool Bioactive Properties and Potential Applicability in Drug Delivery Systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Poudel, D.K.; Rokaya, A.; Ojha, P.K.; Timsina, S.; Satyal, R.; Dosoky, N.S.; Satyal, P.; Setzer, W.N. The Chemical Profiling of Essential Oils from Different Tissues of Cinnamomum camphora L. and Their Antimicrobial Activities. Molecules 2021, 26, 5132. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Rao, R. A Promising Bioactive Component Terpinen-4-Ol: A REVIEW. Int. J. Pharmacogn. 2016, 3, 336–345. [Google Scholar]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of Innovation in Health and Disease. Chem.-Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).