Dysfunction of Chloroplast Protease Activity Mitigates pgr5 Phenotype in the Green Algae Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Results
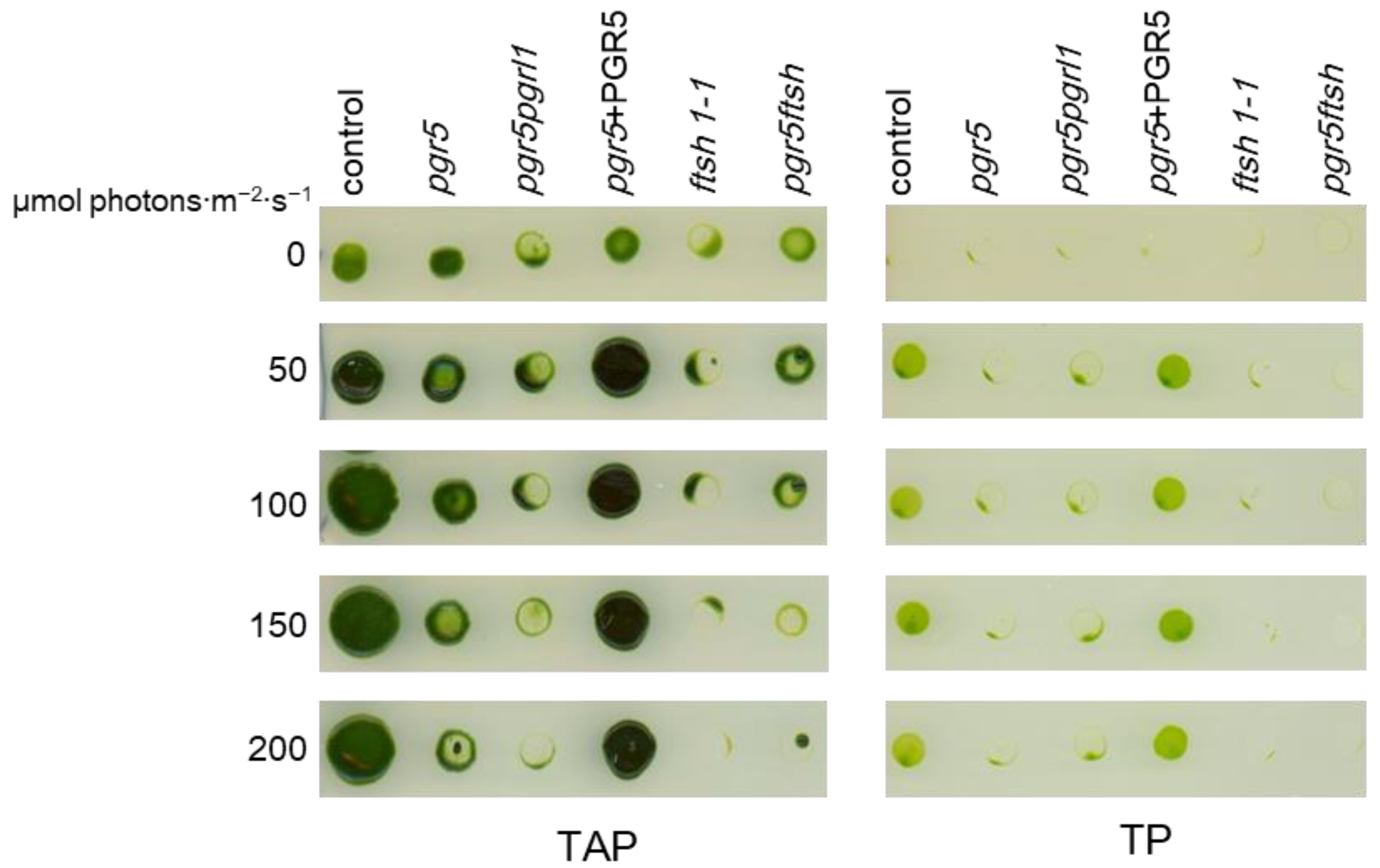
2.1. Growth Check
2.2. Time Course of Fv/Fm
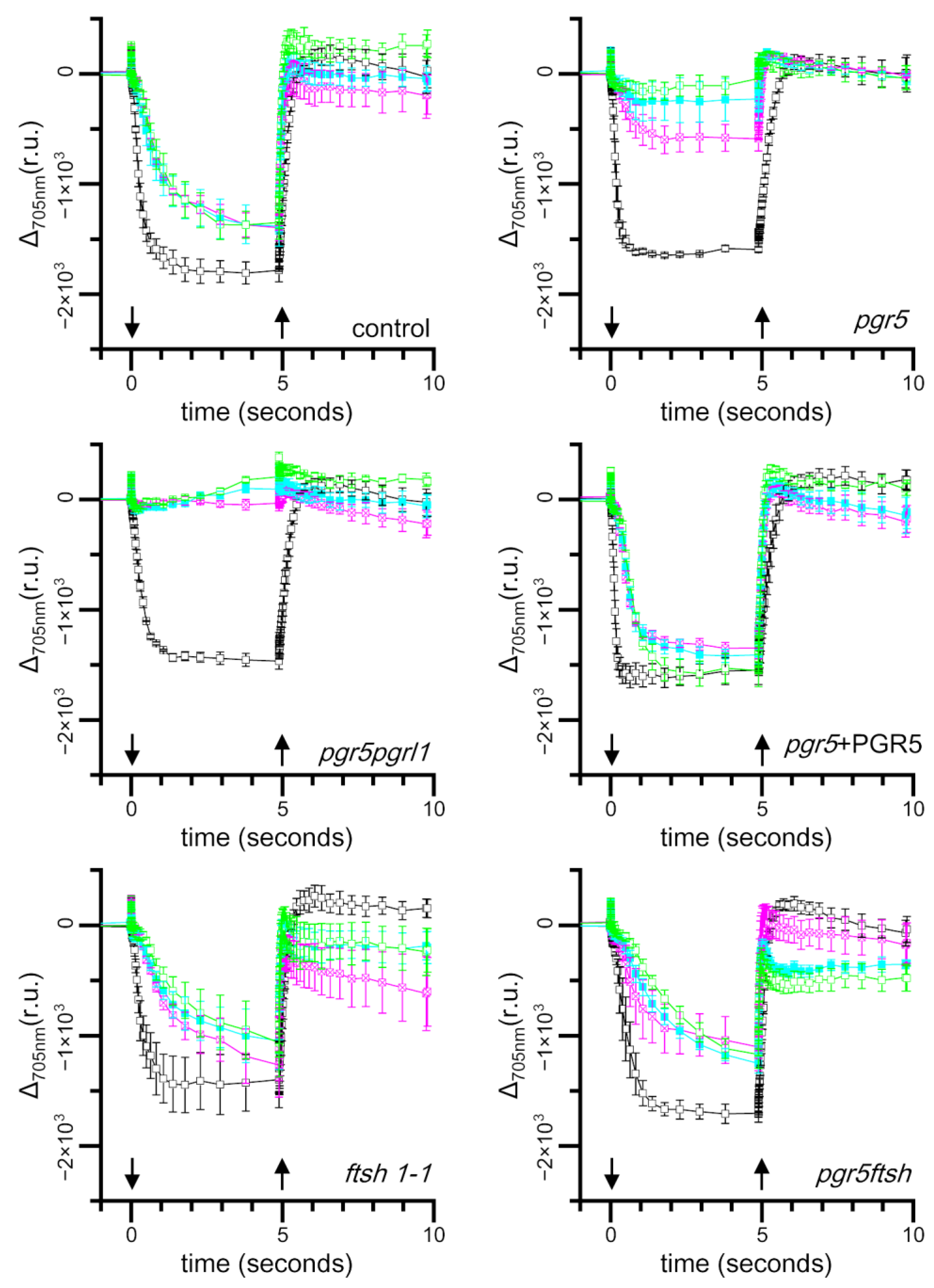
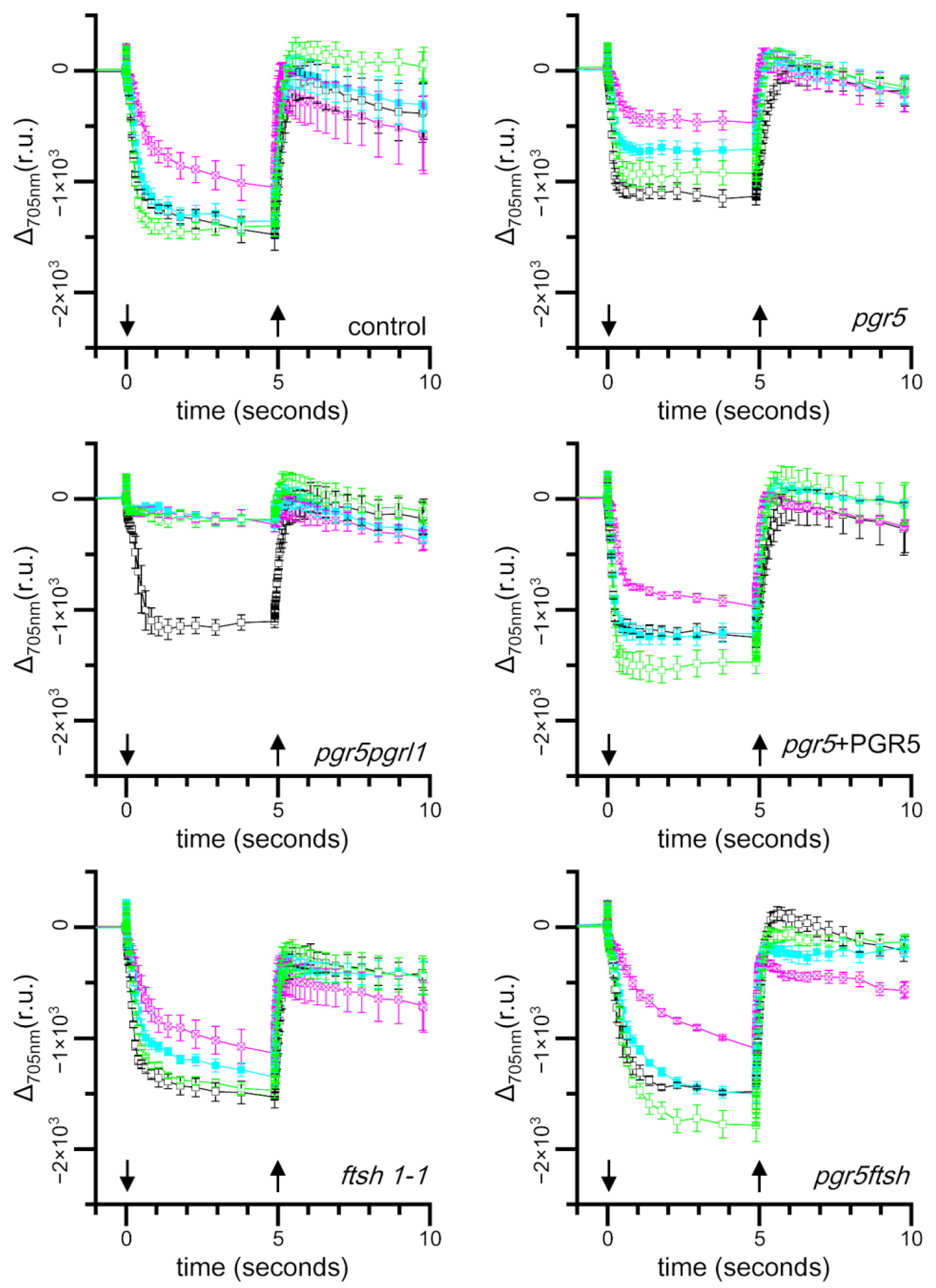
2.3. Time Course of Light-Induced Absorbance Changes at 705 nm
3. Discussion
4. Materials and Methods
4.1. Strains, Growth, Crossing, Spot Test and Immunoblotting
4.2. Light Source
4.3. Chlorophyll Fluorescence Measurement and Absorbance Changes in Cells at 705 nm
4.4. PCR and the Following Restriction Enzyme Digestion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.R. The Structure of Photosystem II and the Mechanism of Water Oxidation in Photosynthesis. Annu. Rev. Plant Biol. 2015, 66, 23–48. [Google Scholar] [CrossRef] [PubMed]
- Sarewicz, M.; Pintscher, S.; Pietras, R.; Borek, A.; Bujnowicz, Ł.; Hanke, G.; Cramer, W.A.; Finazzi, G.; Osyczka, A. Catalytic Reactions and Energy Conservation in the Cytochrome bc(1) and b(6)f Complexes of Energy-Transducing Membranes. Chem. Rev. 2021, 121, 2020–2108. [Google Scholar] [CrossRef] [PubMed]
- Shikanai, T. Cyclic electron transport around photosystem I: Genetic approaches. Annu. Rev. Plant Biol. 2007, 58, 199–217. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kramer, D.M. In vivo modulation of nonphotochemical exciton quenching (NPQ) by regulation of the chloroplast ATP synthase. Proc. Natl. Acad. Sci. USA 2002, 99, 12789–12794. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. FtsH Protease in the Thylakoid Membrane: Physiological Functions and the Regulation of Protease Activity. Front. Plant Sci. 2018, 9, 855. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S. Regulation of Photosynthesis during Abiotic Stress-Induced Photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Baena-González, E.; Aro, E.M. Biogenesis, assembly and turnover of photosystem II units. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1451–1459; discussion 9–60. [Google Scholar] [CrossRef]
- Kato, Y.; Sakamoto, W. Phosphorylation of photosystem II core proteins prevents undesirable cleavage of D1 and contributes to the fine-tuned repair of photosystem II. Plant J. 2014, 79, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Malnoe, A.; Wang, F.; Girard-Bascou, J.; Wollman, F.A.; de Vitry, C. Thylakoid FtsH protease contributes to photosystem II and cytochrome b6f remodeling in Chlamydomonas reinhardtii under stress conditions. Plant Cell 2014, 26, 373–390. [Google Scholar] [CrossRef]
- Kato, Y.; Kuroda, H.; Ozawa, S.I.; Saito, K.; Dogra, V.; Scholz, M.; Zhang, G.; de Vitry, C.; Ishikita, H.; Kim, C.; et al. Characterization of tryptophan oxidation affecting D1 degradation by FtsH in the photosystem II quality control of chloroplasts. eLife 2023, 12, RP88822. [Google Scholar] [CrossRef] [PubMed]
- Kale, R.; Sallans, L.; Frankel, L.K.; Bricker, T.M. Natively oxidized amino acid residues in the spinach PS I-LHC I supercomplex. Photosynth. Res. 2020, 143, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.N.; Zhang, J.; Evans, B.W.; Redding, K. Disassembly and degradation of photosystem I in an in vitro system are multievent, metal-dependent processes. J. Biol. Chem. 2003, 278, 39978–39986. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schunemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef]
- Suorsa, M.; Jarvi, S.; Grieco, M.; Nurmi, M.; Pietrzykowska, M.; Rantala, M.; Kangasjärvi, S.; Paakkarinen, V.; Tikkanen, M.; Jansson, S.; et al. PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 2012, 24, 2934–2948. [Google Scholar] [CrossRef]
- Munekage, Y.; Takeda, S.; Endo, T.; Jahns, P.; Hashimoto, T.; Shikanai, T. Cytochrome b(6)f mutation specifically affects thermal dissipation of absorbed light energy in Arabidopsis. Plant J. 2001, 28, 351–359. [Google Scholar] [CrossRef]
- Jahns, P.; Graf, M.; Munekage, Y.; Shikanai, T. Single point mutation in the Rieske iron-sulfur subunit of cytochrome b6/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis. FEBS Lett. 2002, 519, 99–102. [Google Scholar] [CrossRef]
- Ozawa, S.I.; Buchert, F.; Reuys, R.; Hippler, M.; Takahashi, Y. Algal PETC-Pro171-Leu suppresses electron transfer in cytochrome b6f under acidic lumenal conditions. Plant Physiol. 2023, 191, 1803–1817. [Google Scholar] [CrossRef]
- Alric, J. Cyclic electron flow around photosystem I in unicellular green algae. Photosynth. Res. 2010, 106, 47–56. [Google Scholar] [CrossRef]
- Joliot, P.; Johnson, G.N. Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA 2011, 108, 13317–13322. [Google Scholar] [CrossRef]
- Alric, J. Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii: (II) involvement of the PGR5-PGRL1 pathway under anaerobic conditions. Biochim. Biophys. Acta 2014, 1837, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Schuller, J.M.; Birrell, J.A.; Tanaka, H.; Konuma, T.; Wulfhorst, H.; Cox, N.; Schuller, S.K.; Thiemann, J.; Lubitz, W.; Setif, P.; et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 2019, 363, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Tang, K.; Wang, W.; Wang, C.; Wu, H.; Mao, Z.; An, S.; Chang, S.; Kuang, T.; Shen, J.-R.; et al. Architecture of the chloroplast PSI-NDH supercomplex in Hordeum vulgare. Nature 2022, 601, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Petroutsos, D.; Terauchi, A.M.; Busch, A.; Hirschmann, I.; Merchant, S.S.; Finazzi, G.; Hippler, M. PGRL1 participates in iron-induced remodeling of the photosynthetic apparatus and in energy metabolism in Chlamydomonas reinhardtii. J. Biol. Chem. 2009, 284, 32770–32781. [Google Scholar] [CrossRef] [PubMed]
- Tolleter, D.; Ghysels, B.; Alric, J.; Petroutsos, D.; Tolstygina, I.; Krawietz, D.; Happe, T.; Auroy, P.; Adriano, J.-M.; Beyly, A.; et al. Control of hydrogen photoproduction by the proton gradient generated by cyclic electron flow in Chlamydomonas reinhardtii. Plant Cell 2011, 23, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Hertle, A.P.; Blunder, T.; Wunder, T.; Pesaresi, P.; Pribil, M.; Armbruster, U.; Leister, D. PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol. Cell 2013, 49, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.M.; Allahverdiyeva, Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J. 2018, 94, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Takizawa, K.; Tokutsu, R.; Okamuro, A.; Takahashi, Y.; Minagawa, J. Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 2010, 464, 1210–1213. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- Johnson, X.; Steinbeck, J.; Dent, R.M.; Takahashi, H.; Richaud, P.; Ozawa, S.; Houille-Vernes, L.; Petroutsos, D.; Rappaport, F.; Grossman, A.R.; et al. Proton Gradient Regulation 5-Mediated Cyclic Electron Flow under ATP- or Redox-Limited Conditions: A Study of ΔATPase pgr5 and ΔrbcL pgr5 Mutants in the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 2014, 165, 438–452. [Google Scholar] [CrossRef]
- Buchert, F.; Mosebach, L.; Gabelein, P.; Hippler, M. PGR5 is required for efficient Q cycle in the cytochrome b6f complex during cyclic electron flow. Biochem. J. 2020, 477, 1631–1650. [Google Scholar] [CrossRef]
- Takahashi, S.; Milward, S.E.; Fan, D.Y.; Chow, W.S.; Badger, M.R. How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol. 2009, 149, 1560–1567. [Google Scholar] [CrossRef]
- Wang, F.; Qi, Y.; Malnoe, A.; Choquet, Y.; Wollman, F.A.; de Vitry, C. The High Light Response and Redox Control of Thylakoid FtsH Protease in Chlamydomonas reinhardtii. Mol. Plant 2017, 10, 99–114. [Google Scholar] [CrossRef]
- Kok, B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim. Biophys. Acta 1956, 22, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Kok, B. Absorption Changes induced by the Photochemical Reaction of Photosynthesis. Nature 1957, 179, 583–584. [Google Scholar] [CrossRef]
- Alric, J.; Lavergne, J.; Rappaport, F. Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii (I) aerobic conditions. Biochim. Biophys. Acta 2010, 1797, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Kruk, J.; Trebst, A. Plastoquinol as a singlet oxygen scavenger in photosystem II. Biochim. Biophys. Acta 2008, 1777, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Vass, I. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 2012, 1817, 209–217. [Google Scholar] [CrossRef]
- Yadav, D.K.; Kruk, J.; Sinha, R.K.; Pospisil, P. Singlet oxygen scavenging activity of plastoquinol in photosystem II of higher plants: Electron paramagnetic resonance spin-trapping study. Biochim. Biophys. Acta 2010, 1797, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, B.; Trela-Makowej, A.; Latowski, D.; Strzalka, K.; Szymanska, R. Antioxidant and Signaling Role of Plastid-Derived Isoprenoid Quinones and Chromanols. Int. J. Mol. Sci. 2021, 22, 2950. [Google Scholar] [CrossRef]
- Rastogi, A.; Yadav, D.K.; Szymanska, R.; Kruk, J.; Sedlarova, M.; Pospisil, P. Singlet oxygen scavenging activity of tocopherol and plastochromanol in Arabidopsis thaliana: Relevance to photooxidative stress. Plant Cell Environ. 2014, 37, 392–401. [Google Scholar] [CrossRef]
- Harris, E.H. The Chlamydomonas Sourcebook; Academic Press: San Diego, CA, USA, 1988. [Google Scholar]
- Steinbeck, J.; Nikolova, D.; Weingarten, R.; Johnson, X.; Richaud, P.; Peltier, G.; Hermann, M.; Magneschi, L.; Hippler, M. Deletion of Proton Gradient Regulation 5 (PGR5) and PGR5-Like 1 (PGRL1) proteins promote sustainable light-driven hydrogen production in Chlamydomonas reinhardtii due to increased PSII activity under sulfur deprivation. Front. Plant Sci. 2015, 6, 892. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.I.; Bald, T.; Onishi, T.; Xue, H.; Matsumura, T.; Kubo, R.; Takahashi, H.; Hippler, M.; Takahashi, Y. Configuration of Ten Light-Harvesting Chlorophyll a/b Complex I Subunits in Chlamydomonas reinhardtii Photosystem I. Plant Physiol. 2018, 178, 583–595. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozawa, S.-I.; Zhang, G.; Sakamoto, W. Dysfunction of Chloroplast Protease Activity Mitigates pgr5 Phenotype in the Green Algae Chlamydomonas reinhardtii. Plants 2024, 13, 606. https://doi.org/10.3390/plants13050606
Ozawa S-I, Zhang G, Sakamoto W. Dysfunction of Chloroplast Protease Activity Mitigates pgr5 Phenotype in the Green Algae Chlamydomonas reinhardtii. Plants. 2024; 13(5):606. https://doi.org/10.3390/plants13050606
Chicago/Turabian StyleOzawa, Shin-Ichiro, Guoxian Zhang, and Wataru Sakamoto. 2024. "Dysfunction of Chloroplast Protease Activity Mitigates pgr5 Phenotype in the Green Algae Chlamydomonas reinhardtii" Plants 13, no. 5: 606. https://doi.org/10.3390/plants13050606
APA StyleOzawa, S.-I., Zhang, G., & Sakamoto, W. (2024). Dysfunction of Chloroplast Protease Activity Mitigates pgr5 Phenotype in the Green Algae Chlamydomonas reinhardtii. Plants, 13(5), 606. https://doi.org/10.3390/plants13050606





