Abstract
Acetoin is a volatile organic compound, which is a class of metabolites produced by plant growth-promoting rhizobacteria. The mechanisms underlying plant growth promotion by acetoin and its potential to induce saline stress tolerance in plants are poorly understood. Lettuce (Lactuca sativa L. var. ramosa Hort.) seedlings in hydronics and pots under non-saline or saline conditions were foliar-sprayed with 10 mL of 0 or 1 mg·mL−1 acetoin at 7 and 14 d after transplantation and harvested 7 d after the second spray. Shoots and roots of hydroponic lettuce seedlings were harvested at 6 and 24 h after treatment for RNA sequencing. Seedlings sprayed with acetoin showed more vigorous growth, with higher shoot and root biomass than those of the controls, in both hydronic and pot modes. The transcriptomic analysis revealed acetoin application resulted in 177 differentially expressed genes (39 upregulated and 138 downregulated) in shoots and 397 differentially expressed genes (112 upregulated and 285 downregulated) in roots. These DEGs, mainly involved in plant hormone signal transduction and the mitogen-activated protein kinase, have the potential to trigger plants’ responses to various environmental stimuli, including stress and developmental signals. Under saline conditions, acetoin-treated plants showed increased net leaf photosynthesis and activities of several defense enzymes, indicating that acetoin enhances both fundamental growth and the plant’s stress defenses, especially against salinity. In summary, acetoin appears to act through a complex interplay of genetic and biochemical mechanisms, influencing key signaling pathways and physiological processes that lead to improved growth and stress tolerance in lettuce seedlings.
1. Introduction
Chemical fertilizer application can ensure high crop yield; however, the long-term application of chemical fertilizers can affect the biological and physicochemical health of the soil ecosystem, crop quality, and environment, leading to a decline in agricultural productivity [1,2]. The decreased productivity of crop plants is also influenced by varied environmental factors, such as heat, drought, and salinity [2,3,4]. Among those factors, an increase in soil salinity is one of the main challenges of contemporary agriculture. The global area of salt-affected soils is approximately 1.0×109 ha, accounting for approximately 25% of Earth’s land area [5]. Human activities such as irrigation with saline water, deforestation, and improper agricultural practices involving the excessive use of chemical fertilizers exacerbate soil salinity [6]. Hence, biofertilizers have attracted extensive attention owing to their ability to protect the environment, enhance soil fertility, and improve crop productivity.
As an eco-friendly alternative to chemical fertilizers, plant growth-promoting rhizobacteria (PGPR) are used widely to influence overall plant health by contributing to supporting nutrient acquisition of the host plant and enhancing resistance to biotic and abiotic stress [7]. PGPR employs several mechanisms to provide benefits to crops, including atmospheric nitrogen fixation; solubilization of soil nutrients such as phosphate, zinc, and potassium; and secretion of plant growth-promoting substances, for instance, auxins, ethylene, gibberellins, and volatile organic compounds (VOCs) [8,9,10]. Despite their benefits, the compounds secreted by PGPR are changeable and unstable regarding dynamic environmental factors, which can lead to a failure in maintaining their effectiveness at a consistently positive level.
VOCs, which have a low molecular weight, weak polarity, low boiling point, and high lipophilicity, have been shown to play various roles, including acting as inter- and intra-species signal compounds, stimulating and inhibiting plant growth, and affecting phytopathogens [9,10]. Additionally, VOCs have the potential to promote plant growth and seed germination, increase nutrient uptake [11,12], induce systemic resistance in crops [13], and block the growth of pathogenic bacteria [10,14].
Lettuce (Lactuca sativa L. var. ramosa Hort.), revered for its rich nutritional profile and diverse health benefits, is one of the most important vegetables worldwide, but its moderate sensitivity to salinity seriously restricts yield [4,15]. Studies have proved that its plant physiology is impaired by the osmotic stress response to salinity, resulting in stomatal closure to decrease water loss by transpiration [16]. Furthermore, photosynthesis is affected by Na+ toxicity, where a high cytosolic accumulation of Na+ tends to replace some K+, and consequently, vital enzymatic activities involving K+ are disrupted [17]. Additionally, salinity has been demonstrated to induce the modulation of endogenous phytohormones levels, which in turn affect the signaling pathways (e.g., mitogen-activated protein kinase [MAPK] signaling pathway) involved in the downstream changes in roots, leaves, and cellular structures [18].
Acetoin is a VOC produced by PGPR. Acetoin promotes plant growth, helps prevent plant diseases [19], and enhances plant resistance to biotic and abiotic stressors [9]. However, according to our review of the literature, the effects of exogenous acetoin on any global transcriptome changes in plants have not been investigated. In this study, we investigated the effects of acetoin on lettuce growth under different cultivation modes, including on plants under salt stress. RNA sequencing (RNA-seq) was used to explore the promotion or potential effects of stress tolerance in plants treated with acetoin. We hypothesized that (1) acetoin promotes the growth of lettuce plants by increasing the photosynthetic capacity and expression of functional genes related to plant growth, and (2) acetoin has the potential to induce stress tolerance in lettuce plants by enhancing defense enzyme activities and the expression of defense-related signaling genes. This study presents evidence for acetoin-induced transcriptomic changes in lettuce and their potential to induce stress tolerance.
2. Results
2.1. Phenotypic Response of Hydroponic Lettuce to Acetoin
For identification of the phenotype of lettuce seedlings sprayed by acetoin, the lettuce seedlings were cultured in a hydroponic solution and sprayed with different concentrations of acetoin (0, 0.5, 1, and 2 mg·mL−1) in the hydroponic experiment. Foliar application of acetoin significantly increased the biomass accumulation and root development of lettuce seedlings. Compared to the control treatment (0 mg·mL−1), there was an obvious apparent elongation of roots among the different acetoin-treated seedlings (Figure 1A). The dry weights of shoot and root were significantly increased by the application of 1 mg·mL−1 acetoin, exhibiting 18.08 and 68.68% increases compared to those of the control, respectively (Figure 1B). Both low (0.5 mg·mL−1) and high (2 mg·mL−1) concentrations of acetoin exhibited similar effects. Acetoin in any concentration had no obvious impact on the number of leaves (Figure 1B). However, the leaf-greenness index of seedlings was enhanced with the low concentration of acetoin, and there were no significant changes with higher concentrations (Figure 1D).
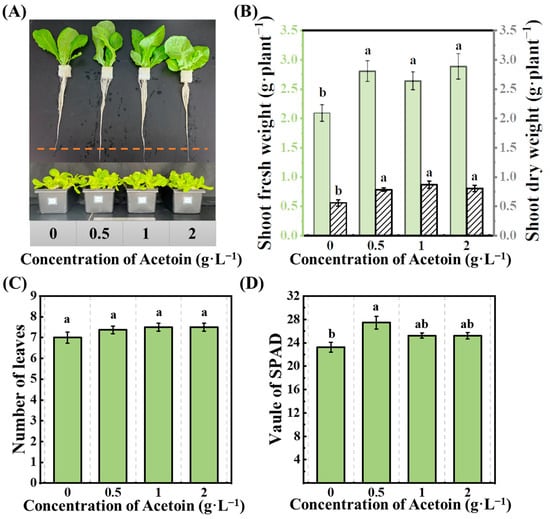
Figure 1.
Effects of acetoin (AT) on phenotypic response of lettuce in the hydroponic experiment. (A) Phenotype of plants, (B) shoot dry weight and root dry weight, (C) number of leaves, and (D) value of SPAD under CK (non-AT control) and AT treatments. Different letters indicate significant differences (p < 0.05) according to an independent sample t-test.
2.2. RNA Sequencing and Analysis of DEGs
In total, 24 samples were used to analyze dynamic gene expression changes in lettuce seedlings after acetoin spraying in the hydroponic experiment. After filtering out adaptor sequences and low-quality reads, 1,057,224,748 clean reads were obtained (Table S1). The Q20 and Q30 values of each sample were greater than 96% and 90%, respectively, and the GC content was approximately 45.6% (Table S1). Subsequently, all clean reads were mapped to the reference genome; the ratio of mapped reads for each sample ranged from 92.32 to 96.23% (Table S2).
Per DEG analysis, 544 genes were differentially expressed, of which 151 were upregulated, and 393 were downregulated (Figure S2). At 6 and 24 h post acetoin treatment, 47 and 130 DEGs were, respectively, observed in the leaves, along with 304 and 93 DEGs in the roots, compared with those in the control group (Figure S2).
2.3. GO Classification Analysis of DEGs
The 544 identified DEGs were divided into biological process, cell component, and molecular function ontologies, and each DEG was assigned at least one GO term. A total of 31 significantly enriched level-2 GO terms were obtained from the 544 DEGs, including 17 biological processes, 10 cellular components, and 4 molecular functions (Figure 2). The largest portion of genes was associated with biological processes and cell components, with relatively few changes in the expression of genes related to molecular function (Figure 2).
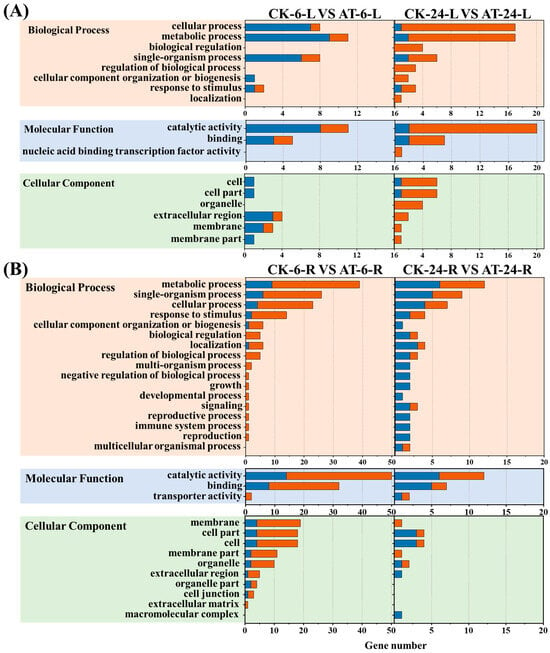
Figure 2.
Numbers of differentially expressed genes associated with biological processes, cellular components, and molecular functions found using Gene Ontology [13] enrichment analysis. (A) GO analysis of DEGs in the shoots of Lettuce. (B) GO analysis of DEGs in the roots of Lettuce. Blue bar represents up-regulated DEGs. Orange bar represents down-regulated DEGs. CK6/24 represents the non-acetoin (AT) control at 6 h (24 h post treatment); AT6/24 represents the AT control at 6 h (24 h post treatment). L and R represent the shoots and roots of lettuce seedlings, respectively.
2.4. KEGG Enrichment Pathway Analysis of DEGs
Among the identified 544 DEGs, 282 were annotated in the KEGG database and divided into 5 KEGG A classes, 15 KEGG B classes, and 68 pathways (Figure 3). In total, there were 12 significantly enriched pathways across all samples from 6 and 24 h post acetoin treatment (Q value ≤ 0.05; Table 1). In the shoot samples, at 6 h post acetoin treatment, genes were significantly enriched in flavonoid biosynthesis, plant hormone signal transduction, and MAPK signaling pathways; at 24 h post acetoin treatment, genes were significantly enriched in the MAPK signaling pathway–plant, plant hormone signal transduction, and plant–pathogen interaction pathways (Table 1). In root samples of plants 6 h post acetoin treatment, the pathways of significant enrichment were biosynthesis of secondary metabolites, phenylpropanoid biosynthesis, fatty acid degradation, metabolic pathways, tyrosine metabolism, and biosynthesis of unsaturated fatty acids compared to those of control samples (Table 1). Plant hormone signal transduction and MAPK signaling pathways involved in relieving saline stress in plants were selected for further analysis.
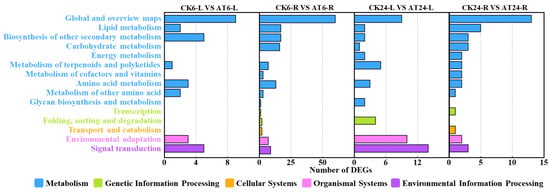
Figure 3.
Kyoto Encyclopedia of Genes and Genomes (KEGG) classification of differentially expressed genes (DEGs). Results are summarized as five main KEGG A Classes: metabolism, genetic information processing, cellular processes, organismal systems, and environmental information processing. CK6/24 represents the non- acetoin (AT) control at 6 h (24 h post treatment); AT6/24 represents the AT control at 6 h (24 h post treatment). L and R represent the shoots and roots of lettuce seedlings, respectively. Numbers indicate the number of DEGs.

Table 1.
Kyoto Encyclopedia of Genes and Genomes enrichment pathways and significantly differentially expressed genes (Q value ≤ 0.05) under acetoin (AT) treatment in lettuce.
2.5. Comparison of DEGs Related to Plant Hormone Signal Transduction Pathways
Plant hormone systems play a crucial regulatory role in plant growth; the ethylene signal transduction pathway is one such pathway. Among the metabolic genes of the ethylene signal transduction pathway, the genes encoding EIN3-binding F-box protein 1 (EBF1) (ncbi_111917430, 1.5-fold) and EIN3-binding F-box protein 2 (EBF2) (ncbi_111921717, 1.2-fold) were downregulated at 6 h after acetoin treatment, and the genes encoding serine/threonine-protein kinase CTR1 (CTR1) (ncbi_111906571, 1.3-fold) and EBF2 (ncbi_111921717, 1.1-fold) were downregulated at 24 h after acetoin treatment compared to that in controls (Figure 4 and Table S3). In the ABA signaling pathway, the gene encoding ABA receptor PYR1-like protein (PYL4) (ncbi_111879857, 1.6-fold) was upregulated at 6 h after acetoin treatment, and the gene encoding protein phosphatases 2C (PP2CA) (ncbi_111910618, 1.0-fold) was downregulated at 24 h after acetoin treatment compared to those in controls (Figure 4 and Table S3).
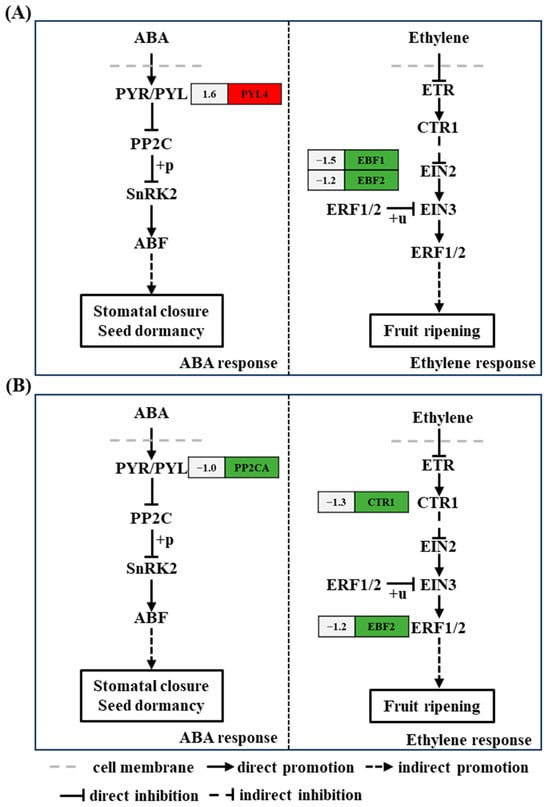
Figure 4.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes (DEGs) related to plant hormones and signal transduction at 6 and 24 h post acetoin treatment. (A) KEGG pathway analysis of DEGs related to plant hormones and signal transduction at 6 h. (B) and at 24 h. Red and green indicate up- and downregulated genes, respectively and the numbers on the left represent the differential expression of genes. ABA represents abscisic acid; PYR/PYL, PP2C, SnRK2, ABF, ETR, CTR1, EIN2, EIN3, and ERF1/2 represent the genes encoding abscisic acid receptor PYR/PYL family, protein phosphatases 2C, SNF1-related type 2 protein kinase, ABA-response element binding factors, ethylene receptor, serine/threonine-protein kinase CTR1, ethylene-insensitive protein 2, ethylene-insensitive protein 3, and ethylene-responsive transcription factor 1/2, respectively.
2.6. Comparison of DEGs Related to the MAPK Signaling Pathway–Plant
The MAPK signaling pathway is a key element in plant defense reactions. Genes such as EBF1 (ncbi_111917430, 1.5-fold) and EBF2 (ncbi_111921717, 1.2-fold) related to the ethylene signal transduction pathway were downregulated, and PYL4 (ncbi_111879857, 1.6-fold), related to the ABA signaling pathway, was upregulated at 6 h after acetoin treatment (Figure 5 and Table S4). CTR1 (ncbi_111906571, 1.3-fold), mitogen-activated protein kinase kinase 9 (MKK9) (ncbi_111915142, 1.3-fold), and EBF2 (ncbi_111921717, 1.1-fold) related to ethylene signal transduction were downregulated; PP2CA (ncbi_111910618, 1.0-fold) related to ABA signaling pathway was downregulated; and CP1 (ncbi_111914172, 1.2-fold) and CML46 (ncbi_111920042, 1.2-fold) encoding calmodulin (CALM) and calmodulin-like protein (CML) were downregulated at 24 h after acetoin treatment (Figure 5 and Table S5).
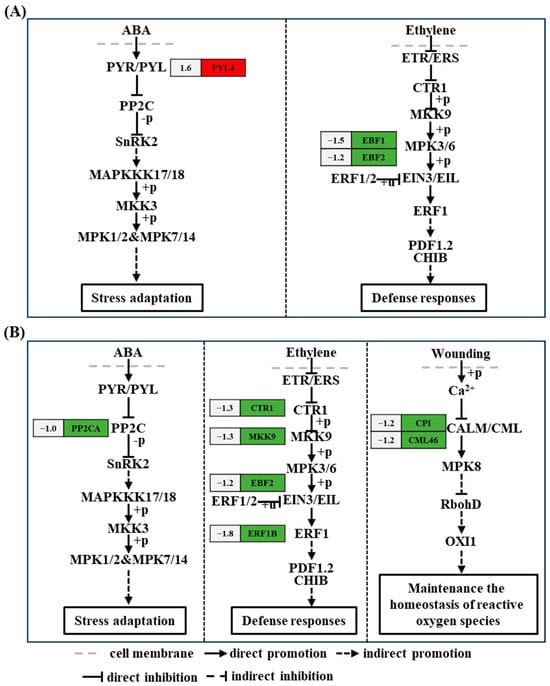
Figure 5.
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis of differentially expressed genes (DEGs) related to MAPK signaling pathway–plant at 6 and 24 h post acetoin treatment. KEGG pathway analysis of DEGs related to MAPK signaling pathway–plant at 6 (A) and 24 h (B). Red and green indicate up- and downregulated genes, respectively and the numbers on the left represent the differential expression of genes. ABA represents abscisic acid; PYR/PYL, PP2C, SnRK2, MAPKKK17/18, MKK3/9, ETR/ERS, CTR1, EIN3/EIL, ERF1, PDF1.2, CHIB, CALM/CML, RbohD, and OXI1 represent the genes encoding abscisic acid receptor PYR/PYL family, protein phosphatases 2C, SNF1-related type 2 protein kinase, mitogen-activated protein kinase kinase kinase 17/18, mitogen-activated protein kinase 3/9, ethylene receptor, serine/threonine-protein kinase CTR1, ethylene-insensitive protein 3, ethylene-responsive transcription factor 1, defensin-like protein 16, basic endochitinase B, calmodulin/calmodulin-like protein, respiratory burst oxidase homologous protein D, and serine/threonine-protein kinase OXI1, respectively. MPK1/2, MPK7/14, MPK3/6, and MPK8 represents the genes encoding mitogen-activated protein kinase 1/2, 7/14, 3/6, and 8, respectively.
2.7. Validation of RNA-seq Data by Using qRT-PCR
The amplification efficiency of all tested primers was between 90% and 105%, and the dissolution curves were all single peaks, indicating that these primers could be used for qRT-PCR. No significant differences were observed between the three internal reference genes across all treatments. The relative expression of eight of the genes was consistent with the expression levels obtained from RNA-seq, and two genes (CML46 and PP2CA) showed the same expression tendencies (Figure 6 and Table S6), indicating that the transcriptome sequencing results were reliable.
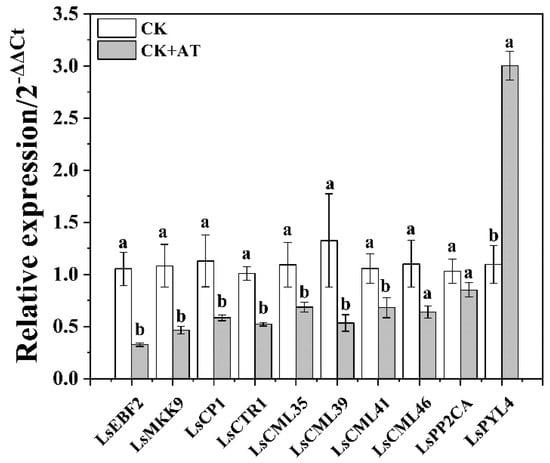
Figure 6.
Relative expression levels of 10 differentially expressed genes were measured using qRT-PCR (2−ΔΔCt). CK represents the non-acetoin (AT) control. According to independent sample t-test, different letters indicate significant differences (p < 0.05). Ls represents lettuce; EBF2, MKK9, CP1, CTR1, CML35/39/41/46, PP2CA, and PYL4 represent the genes encoding the EIN3-binding F-box protein 2, mitogen-activated protein kinase 9, calmodulin, serine/threonine-protein kinase, calmodulin-like protein 35/39/41/46, protein phosphatases 2C, and ABA receptor PYR1-like protein, respectively.
2.8. Effects of Acetoin on Growth and Alleviation of Saline Stress
Saline stress inhibited the growth of lettuce seedlings, and acetoin treatment promoted growth and relieved saline stress in lettuce seedlings in the pot experiment (Figure 7). Compared with those in CK group, the fresh and dry shoot weights of acetoin-treated lettuce seedlings increased significantly (p < 0.05) by 24.8% and 26.5%, respectively (Figure 7). Under saline conditions, plant height, fresh and dry shoot weights, and fresh root weight of lettuce seedlings in the acetoin group increased significantly (p < 0.05) by 15.5%, 29.0%, 26.5%, and 40.9%, respectively, compared to those in the CK group (Figure 2).
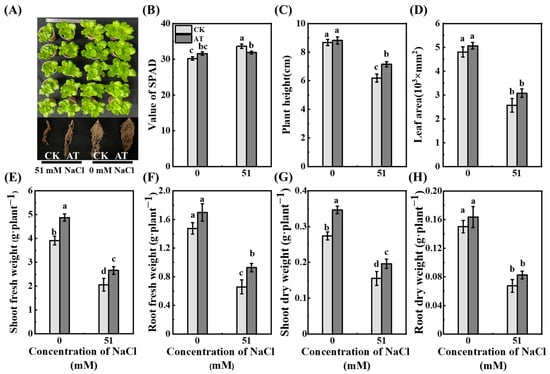
Figure 7.
Effects of acetoin (AT) on the growth of lettuce under non-saline and saline conditions in the pot experiment. (A) Phenotype of shoots, (B) value of SPAD, (C) plant height, (D) leaf area, (E) shoot fresh weight, (F) root fresh weight, (G) shoot dry weight, and (H) root dry weight under CK (non-AT control) and AT treatments. According to independent sample t-test, different letters indicate significant differences (p < 0.05).
Compared to those of the control seedlings, Mg2+ concentrations in leaves of lettuce were significantly decreased by saline stress, but K+ and Na+ concentrations and the K+/Na+ ratio of lettuce seedlings were increased significantly. Under non-saline conditions, Ca2+ and Mg2+ concentrations in the roots were significantly (p < 0.05) increased in acetoin-treated seedlings compared to those in CK seedlings (Table 2). Under saline conditions, acetoin-treated seedlings showed significantly (p < 0.05) increased Ca2+ concentrations and significantly (p < 0.05) decreased Na+ concentrations compared to controls (Table 2).

Table 2.
Variation in ionic elements on lettuce sprayed by acetoin for 21 d after transplantation under non-saline and saline conditions in the pot experiment.
2.9. Effects of Acetoin on Leaf Photosynthetic Index and Defense Enzyme Activity
Acetoin had a favorable effect on the photosynthetic competence of lettuce seedlings. Within 7 d, the Pn, E, and gsw of lettuce leaves in the NaCl group were significantly lower (p < 0.05) than those in the CK group (Figure 8A–C), and Ci was not significantly changed (p < 0.05) (Figure 8D. After acetoin treatment in saline conditions, the photosynthetic indices (Pn, E, and gsw) of seedlings were consistently higher than those of untreated seedlings (Figure 8A–C).
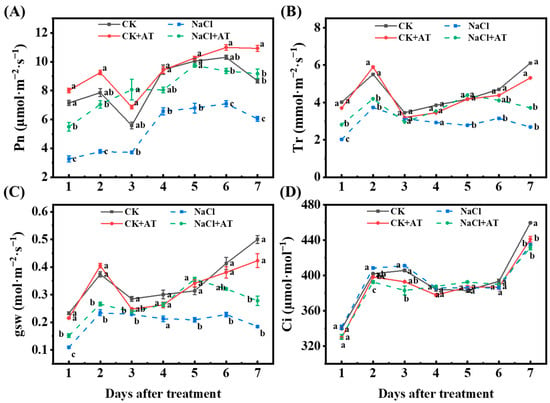
Figure 8.
Effects of acetoin (AT) on the photosynthetic parameters of lettuce seedlings under non-saline and saline conditions in the pot experiment. (A) Net photosynthetic rate (Pn), (B) transpiration rate (Tr), (C) stomatal conductance (gsw), and (D) intercellular CO2 concentration (Ci) under CK (non-AT and non-NaCl treatment control), CK + AT, NaCl (3 g NaCl per kg of soil), and NaCl + AT treatments. According to independent sample t-test, different letters indicate significant differences (p < 0.05).
Under non-saline conditions, SOD and MDA activities and soluble protein content were not significantly different (p < 0.05) between the CK and CK+ acetoin groups (Figure 9). SOD activity significantly increased (p < 0.05) in NaCl lettuce leaves compared to that in CK seedlings. On days 1, 2, and 3 after acetoin treatment, SOD activity in the NaCl + acetoin group was significantly higher (p < 0.05) than that in the NaCl group (Figure 9A). Compared with that in the CK group, MDA activity significantly increased (p < 0.05) in the NaCl group and significantly decreased (p < 0.05) with the application of acetoin at similar levels as those in the CK and CK+ acetoin groups (Figure 9B). Soluble protein content significantly decreased (p < 0.05) in the NaCl group compared to that in the CK group and was restored after acetoin treatment (Figure 9C). POD and CAT activities across the groups were not significantly different (Figure S1).
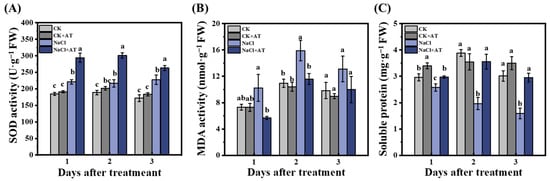
Figure 9.
Dynamic changes in the defense enzyme activities in leaves of lettuce seedlings under non-saline and saline conditions in the pot experiment. (A) Superoxide dismutase (SOD) activity; (B) malondialdehyde (MDA) activity; and (C) soluble protein content under CK (non-AT and non-NaCl treatment control), CK + AT, NaCl (3 g NaCl per kg of soil), and NaCl + AT treatments. According to independent sample t-test, different letters indicate significant differences (p < 0.05).
3. Discussion
3.1. Acetoin Promotes Plant Growth and Alleviates Saline Stress
Acetoin is an active signaling molecule that promotes plant growth and stress resistance. Studies have shown that acetoin effectively promotes various growth parameters in lettuce [19]. In this study, we investigated the effects of acetoin on lettuce under hydroponic and soil conditions. We observed a significant increase in dry weights of lettuce shoots and roots due to acetoin (1 mg·mL−1) (Figure 1). Moreover, the descending growth parameters of seedlings under saline stress, such as plant height, shoot weight, and root weight, were significantly alleviated by acetoin (Figure 7). Thses findings are consistent with those of existing studies showing that acetoin can induce plants to develop systemic resistance to salinity and effectively inhibit external stress and promote plant growth [20]. Additionally, acetoin has potential to enhance the symbiotic relationship between microbes and plants [21]. However, further research should explore the dosage, usage time, and promotion mechanisms of acetoin on different crops on the basis of differences in physiological habits and cultivation environment.
Photosynthesis is the basis of plant growth [22]. Studies have shown that when plants are exposed to salt stress, photosynthesis levels decrease, hindering the normal growth of plants [23]. We found that the Pn, E, and Ci of lettuce decreased significantly under saline conditions. After applying acetoin, the photosynthetic index of lettuce significantly increased, potentially promoting favorable regulation of lettuce growth and nutrient accumulation (Figure 8A–C).
The defense response to environmental stress can also affect plant growth. Salt stress is one abiotic stressor affecting plant growth and yield, and the Na+/K+ ratio is a critical indicator to measure the degree of salt stress. Maintaining an appropriate Na+/K+ ratio is essential to alleviating salt stress [24]. The main effect of salt stress is that the constant accumulation of Na+ can lead to a decrease in K+ content. In our study, under saline conditions, the Na+ content of seedlings increased significantly. After acetoin treatment, the K+ shoot content significantly increased, significantly increasing the K+/Na+ ratio, effectively alleviating the harmful effects of salt stress (Table 2).
Salt stress in plants can also cause the accumulation of reactive oxygen species [25] such as hydrogen peroxide (H2O2) and superoxide free radicals (O2−), resulting in an imbalance of cellular active oxygen metabolism and affecting plant growth and development [23]. Plants can accelerate the removal of reactive oxygen species (ROS), repair damage, maintain active oxygen metabolism, and reduce damage to the plant caused by salt stress by increasing the activity of antioxidant enzymes such as SOD and POD [26]. Our results showed that under saline conditions, SOD activity significantly increased after the application of acetoin compared to in controls (Figure 9A). Furthermore, MDA activity was significantly (p < 0.05) higher, and the soluble protein content was significantly (p < 0.05) lower in the NaCl control group than in the CK group, and the contents of both were restored with the application of acetoin (Figure 9B,C). These results indicate that acetoin can alleviate the hazardous effects of ROS in lettuce and contribute to its growth and development.
We conducted transcriptome analysis to understand the growth mechanisms of plants under salt stress with acetoin treatment. RNA-sequencing technology is increasingly used to characterize genomes and transcriptomes and has considerably increased the speed and efficiency of gene discovery. This sequencing technology can be used to determine global gene expression differences between populations or species, phenotypes, and responses to environmental stress [27]. In this study, whole-transcriptome analysis of lettuce leaves and roots in response to acetoin treatment was performed using RNA-seq. In total, 544 DEGs were identified in lettuce leaves and roots at 6 and 24 h post acetoin treatment when compared to that in the control groups (Figure S2). The GO and KEGG pathway enrichments for identified DEGs were analyzed. Significant GO enrichment was observed for metabolic processes, cellular processes, single biological processes, catalytic activity, and binding activity (Figure 2). Additionally, the notable KEGG pathways enriched in acetoin-treated lettuce were “plant hormones and signal transduction” and “MAPK signaling pathway–plant” in leaves at 6 and 24 h (Table 1). Many genes related to growth and defense response were activated, indicating that acetoin may enhance plant growth and resistance to salinity.
3.2. Expression of Genes Related to Plant Hormones and Signal Transduction
Plant hormones control plant growth and almost all other relevant developmental processes. In addition to its role in plant growth and development, ethylene is involved in regulating plant defense responses to biotic and abiotic stressors [26]. CTR1 functions downstream of the ethylene receptor as a negative regulator of ethylene signaling, and EBF1 and EBF2 degrade transcription factors related to the ethylene signaling pathway [28]. ERF1 is an upstream component of ABA signaling. Evidence suggests that ERF1 plays an active role in salt stress tolerance via ethylene signaling [29]. Our study showed that genes related to ethylene metabolism (CTR1, EBF1, and EBF2) were downregulated in the leaves at 6 and 24 h after acetoin treatment (Figure 3 and Table S3). This possibly affected the transcriptional regulators ethylene-insensitive protein 3 (EIN3) and the downstream of ethylene-responsive transcription factor 1/2 (ERF1/2), completing the ethylene signal output. Decreased expression of ERF1 may have also affected ABA signaling, which plays an important role in plant growth and defense response.
ABA, a phytohormone, has been shown to play an essential role in regulating plant growth and response to abiotic stresses such as heat, drought, and salinity [8,30]. ABA receptors include regulatory components of ABA receptors, pyrabactin resistance 1, and PYR1-like proteins (PYLs) [30]. The PYL family of ABA receptors plays an active role in plant growth, development, and stress resistance [30], and PP2C acts as a negative regulator, impairing plant growth and activation of stress response during ABA metabolism. Acetoin treatment caused the overexpression of PYL in leaves after 6 h and decreased the expression of PP2CA in leaves after 24 h (Figure 3 and Table S3); both responses may improve plant growth and stress adaptation. Moreover, the inhibited phosphatase activity of PP2C could lead to the release of SNF1-related type 2 protein kinase (SnRK2) from the PP2C-SnRK2 complex and increase the activity of SnRK2 phosphokinase [31]. SnRK2 phosphorylates downstream target proteins, further activating basic leucine zipper transcription factors such as ABA-response elements and ABA-response element binding factors [32].
3.3. Expression of Genes Related to MAPK Signaling Pathway–Plant
The MAPK cascade is a major downstream pathway in eukaryotes that converts extracellular stimuli into sensors and receptors for intracellular responses [33]. The MAPK cascade is a relevant signal transduction pathway involved in plant cell differentiation and development [34] and responses to various biotic and abiotic stressors, including drought, low temperature, salinity, and hormones.
The mitogen-activated protein kinase in the MAPK cascade is activated by the core ABA signaling pathway composed of PYL and RCAR-PP2C-SnRK2, contributing to the enhancement of stress resistance in crops [33]. The PYL and PP2C family proteins, members of the ABA signaling pathway, play several roles in the regulation of plant MAPK pathways and are effectively activated during the stress response [35]. With acetoin treatment, the activity of SnRK2 phosphokinase was enhanced by the upregulation of PYL4 and downregulation of PP2CA (Figure 4 and Table S4), which enabled plants to have the potential to resist stress. Moreover, CTR1, a unique MAPKKK and important negative regulator in ethylene signaling appears to control branching cascades and antagonize MAPK cascades that target the same key nuclear transcription factors in ethylene signaling, allowing plants to regulate and adapt to the external environment [36]. In this study, the expression of CTR1 was downregulated in acetoin-treated seedlings (Figure 4 and Table S4), which likely had a positive effect on defense response.
Additionally, the binding of CALM/CML to Ca2+ was shown to affect MAPK signal transduction [37], further activating mitogen-activated protein kinase 8 (MPK8) that negatively regulates ROS production by controlling the expression of respiratory burst oxidase homologous protein D (RbohD) [38]. ROS play a key role in plant development and adaptability to salt stress [39]. In our study, CALM/CML expression was downregulated in leaves after acetoin treatment (Figure 4 and Table S4), which may have led to the upregulation of ROS signaling. Additionally, we demonstrated that the removal of ROS was possibly caused by the increasing activity of SOD under salt stress. In general, these regulatory genes induced by acetoin were associated with signal transduction under adversity stress, which indirectly implies its capacity to alleviate the effects of stressors such as heat, drought, and salinity.
4. Materials and Methods
4.1. Experimental Materials
Lettuce seeds were purchased from Woshu Seed Industry Co. Ltd, Nanjing, China. Acetoin was purchased from Sigma-Aldrich Chemical Company, USA. Hoagland nutrient solution was purchased from Qingdao Hope Bio-Technology Co., Ltd., China. Soil was collected from a long-term, traditional, open vegetable plot in Yixing, Jiangsu Province, China (N 30°12′23″, E 119°52′89″). The soil was air-dried and sieved through a 2 -mm mesh. The saline soil was obtained by adding 51 mM of NaCl, and then mixed thoroughly. The physicochemical characteristics of the soil were reported in existing research [40], as follows: 11.0 g kg−1 organic matter, 29.7 mg kg−1 alkali-hydrolyzed nitrogen, 68.2 mg kg−1 available P, 271.2 mg kg−1 available K, and pH 7.08.
4.2. Greenhouse Hydroponic and Pot Experiments
Hydroponic experiments were performed using four spray solutions (0, 0.5, 1, and 2 mg·mL−1 acetoin), conducted in a humidified plant growth chamber (MLR-352H; Panasonic Healthcare Co., Ltd., Oizumi-Machi, Japan) controlled at 25 ± 1 °C, with 70% relative humidity, under a 14/10 h (light/dark) photoperiod. The seeds were surface-sterilized by immersion in 2% (w/v) sodium hypochlorite for 5–10 min and then rinsed 8–10 times with sterile distilled water. Surface-sterilized seeds were germinated for 7 d in a hydroponic system containing one-fifth of Hoagland solution [41,42]. Uniform germinated seedlings were transferred to a new Hoagland’s hydroponic system and grown for 21 d. The lettuce seedlings were sprayed with acetoin at 7 and 14 d after transplantation. The Hoagland solution was changed every 2–3 d. The growth parameters of lettuce seedlings were determined at 28 d after sowing.
The pot experiments were performed using two soil types (0 or 3 g NaCl kg−1 soil) and two spray solutions (0 or 1 mg·mL−1 acetoin), conducted in a greenhouse controlled at 25 ± 2 °C, 50–70% relative humidity, with a 14 h/10 h (light/dark) photoperiod. Surface-sterilized seeds were sown and germinated in sieved soil. The uniform seedlings within two pairs of true leaves were selected and transplanted into 360 mL paper pots containing 320 g of fresh soil, with two seedlings in each pot. Each treatment group contained five pots, with two seedlings in one pot serving as a single replication. The lettuce seedlings were sprayed with 10 mL of 0 or 1 mg·mL−1 acetoin at 7 and 14 d after transplantation. The position of the paper pots within the greenhouse was changed daily to ensure randomization, and the seedlings were irrigated every two days. The seedlings were harvested 21 d after transplantation.
4.3. Growth Parameters and Nutrient Elements Analysis
The leaf chlorophyll content was measured using a SPAD-502 chlorophyll meter (Minolta Corporation, Ltd., Osaka, Japan). We randomly selected eight fully expanded leaves from each treatment group to ensure reliable results. These leaves were scanned using a Microtek ScanMaker i800 Plus system (Wseen, Hangzhou, China). Leaf area was calculated using LA-S Leaf Area Analysis software (Version number 2.2.3.8, Wseen, Hangzhou, China). Shoot and root fresh weights were measured immediately after collection. Dry weights were measured after oven drying at 65 °C to a constant weight. The dried materials were ground to a powder, and 0.1 g of the pulverized samples were digested with nitric acid for the determination of Mg2+, Ca2+, Na+, and K+ contents. The concentrations of Mg2+, Ca2+, Na+, and K+ were determined using a flame atomic absorption spectrophotometer (PinAAcle 900T; PerkinElmer, Inc., Waltham, MA, USA).
4.4. Leaf Photosynthetic Index Determination and Defense Enzyme Activity Assays
The net photosynthesis rate (Pn), transpiration rate (Tr), stomatal conductance (gsw), and intercellular CO2 concentration (Ci) were measured for the third leaf from the top of each lettuce seedling using an Li-6800 portable photosynthesis system (LI-COR, Lincoln, NE, USA). All measurements were performed between 8:30 a.m. and 12:00 a.m. within 7 d of acetoin treatment. Gas exchange measurements were performed using an artificial light source in the leaf chamber (light intensity, 800 μmol·m−2·s−1; CO2 concentration, 400 μmol·mol−1; temperature, 25 °C; relative humidity, 50%).
On days 1, 2, and 3 after acetoin treatment, leaf samples of approximately 0.1 g were collected, frozen in liquid nitrogen for 10 min, and stored at −80 °C until enzyme extraction. The levels of superoxide dismutase (SOD; Cat, No. SOD-1-Y), peroxidase (POD; Cat, No. POD-1-Y), catalase (CAT; Cat, No. CAT-1-Y), malondialdehyde (MDA; Cat, No. MDA-1-Y), and soluble proteins (Cat, No. KMSP-1-W) in the lettuce leaves were measured using antioxidant kits, following the kit instruction manuals (Suzhou Keming Biotechnology Co., Ltd., Suzhou, China).
4.5. Preparation of Samples and Transcriptome Sequencing
The hydroponic seedlings were selected for RNA-seq. Uniform seedlings were sprayed with distilled H2O (CK group) or 1 mg·mL−1 acetoin (AT group). Shoots and roots were sampled 6 and 24 h after treatment, respectively. Each sample group included three biological replicates, and the shoots and roots of the six seedlings in each treatment were combined into one biological replicate. A total of 24 samples (two treatments × two tissues × three biological replicates × two time points) were used for RNA extraction, library preparation, and sequencing.
Total RNA was extracted using a TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA quality assessment, cDNA preparation, and library sequencing were performed as previously described [40]. Briefly, RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and verified using an RNase-free agarose gel electrophoresis. After extraction of total RNA, the eukaryotic mRNA was enriched using Oligo [43] beads, and prokaryotic mRNA was enriched by removing rRNA via the Ribo-ZeroTM Magnetic Kit (Epicenter, Madison, WI, USA). Next, the enriched mRNA was fragmented using a fragmentation buffer and reverse-transcribed into cDNA with random primers. Second-strand cDNA was synthesized using DNA polymerase I, RNase H, and dNTPs in a buffer. Subsequently, the cDNA fragments were purified using the QiaQuick PCR extraction kit (Qiagen, Venlo, The Netherlands) and end-repaired; poly(A) was added, and the fragments were ligated to Illumina sequencing adapters. The ligation products were size-selected via agarose gel electrophoresis, PCR-amplified, and sequenced using Illumina HiSeq2500 by Gene Denovo Biotechnology Co. (Guangzhou, China).
4.6. Screening and Analysis of Differentially Expressed Genes
The DESeq2 package was used to identify differentially expressed genes (DEGs) between groups, and the edgeR package was used to identify DEGs between samples [40]. Genes with a false-discovery rate below 0.05 and an absolute fold change ≥ 2 were considered DEGs, as previously described [40]. The function of DEGs was investigated using Gene Ontology (GO) [13] enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses (https://www.omicsmart.com/).
4.7. Quantitative Real-Time PCR
To verify the reliability of the expression trends of DEGs, 10 DEGs from hydroponic samples collected at 6 and 24 h after treatment were randomly selected and verified using quantitative real-time PCR (qRT-PCR) (Table 3). cDNA was synthesized using a PrimeScript™ RT Master Mix kit (Takara Biotech, Dalian, China) according to the manufacturer’s instructions. qRT-PCR amplification was performed using a TB Green® Premix Ex Taq™ II kit (Tli RNaseH Plus, Takara Biotech, Dalian, China) with a CFX96 RT-PCR Detection System (Bio-Rad Company, Pleasanton, CA, USA). LsGAPC, LsActin, and LsTublin were selected as internal reference genes. The relative expression level of genes was determined using the 2−ΔΔCt method [44], with two technical replicates and three independent biological replicates per gene.

Table 3.
Primer sequences used for qRT-PCR.
4.8. Statistical Analysis
IBM SPSS (version 26.0; IBM Corp., Armonk, NY, USA) for Windows was used for the statistical analyses. All data are expressed as the mean ± standard error. Significant differences were calculated using independent sample t-test. One-way analysis of variance was performed to determine differences in the relative expression levels of the genes determined using qRT-PCR. Results were considered significant at a threshold of p < 0.05.
5. Conclusions
This study investigated the mechanisms underlying plant growth and stress alleviation after acetoin spraying (Figure 10). Acetoin treatment was found to promote shoot and root growth, enhance net leaf photosynthesis, and increase the activity of several defense enzymes in lettuce seedlings grown under saline conditions. In addition, under non-saline conditions, ethylene and ABA signal transduction and MAPK signaling pathway cascades were regulated by acetoin. These pathways are crucial for plants to adapt and respond to various biotic and abiotic stresses, and the modulation by acetoin indicates its potential in fine-tuning the plant’s stress-response mechanisms. The insights gained from this research into the molecular mechanisms of how acetoin impacts plant growth and combats salt stress are not only scientifically significant but also hold substantial promise for agricultural applications. These parsing processes can develop strategies for better bio-fertilizer performance, leading to more resilient and productive crops, especially in saline environments. The prospect of acetoin as a tool for enhancing plant resilience and productivity is particularly exciting given the growing challenges of climate change and the need for sustainable agricultural practices. However, since this study was conducted under laboratory conditions, further testing under field conditions is necessary to verify the potential of acetoin to promote growth and alleviate environmental stress, ensuring its full integration into agricultural practices.
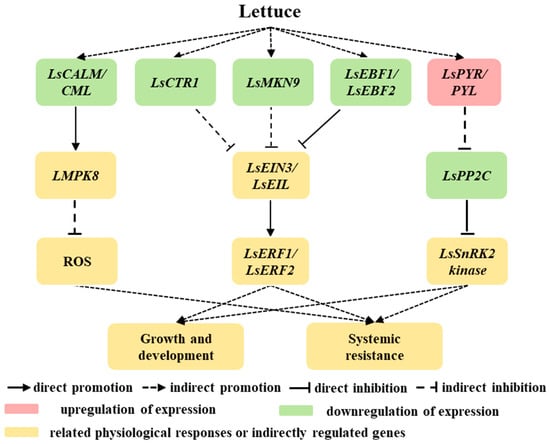
Figure 10.
Schematic of growth-promotion mechanisms in lettuce influenced by acetoin. Red modules represent upregulated genes or positive promotion. Green modules represent downregulated genes. Yellow modules represents related physiological responses or indirectly regulated genes. Ls represents lettuce; CALM/CML, CTR1, MKK9, EBF1/2, PYR/PYL, MPK8, EIN3/EIL, PP2C, ERF1/2, and SnRK2 represent the gene encoding calmodulin/calmodulin-like protein, serine/threonine-protein kinase CTR1, mitogen-activated protein kinase kinase 9, EIN3-binding F-box protein 1/2, abscisic acid receptor PYR/PYL family, mitogen-activated protein kinase 8, ethylene-insensitive protein 3, protein phosphatases 2C, ethylene-responsive transcription factor 1/2, and SNF1-related type 2 protein kinase, respectively; ROS represents reactive oxygen species.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13233312/s1, Table S1. Summary of sequencing data quality. Table S2. Comparison and matching analysis of RNA seq. Table S3. KEGG pathway enrichment of differentially expressed genes (DEGs). Table S4. The level of gene expression (up/down) related to plant hormone under the acetoin (AT) treatment in lettuce. Table S5. Cascade pathway of MAPK and plant hormone signal as well as significant differentially expressed genes. Table S6. Relative expression level of 10 genes with response to acetoin (AT) application measured using qRT-PCR (2−ΔΔCt). Figure S1. Dynamic changes of the defense enzyme activities in leaves of lettuce seedlings under non-saline and saline conditions. (a) Peroxidase (POD) activity, and (b) catalase (CAT) activity under CK (non-AT and non-NaCl treatment control), CK + AT, NaCl (3 g NaCl per kg of soil), and NaCl + AT treatments. Different letters indicate significant difference (p < 0.05) according to independent sample t-test. Figure S2. Numbers of upregulated and downregulated genes induced by acetoin. CK6/24 represents the non-AT control at 6 h (24 h post-treatment) and AT6/24 represents the AT control at 6 h (24 h post-treatment); L and R represent the shoots and roots of lettuce seedlings, respectively; and DEGs represents differentially expressed genes.
Author Contributions
C.Z., C.M. and N.G. contributed to the study conception and design; C.Z., H.S., S.Y., C.M. and Y.S. conceived the study and proposed the experimental approaches; C.Z., H.S. and C.M. conducted the experiments; C.Z., H.S., S.Y. and C.M. contributed to data processing and analysis; S.Y., Y.S. and J.L. contributed for discussion; C.Z., C.M. and N.G. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Foundation of China (32172673) and the Jiangsu Synergetic Innovation Center for Advanced Bio-Manufacture, China (XTB2202).
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABA | abscisic acid |
| AT | acetoin |
| CALM | calmodulin |
| CAT | catalase |
| Ci | intercellular CO2 concentration |
| CTR1 | serine/threonine-protein kinase CTR1 |
| DEG | differentially expressed gene |
| EIN3 | ethylene-insensitive protein 3 |
| EBF1/ EBF2 | EIN3-binding F-box protein 1/2 |
| ERF1/2 | ethylene-responsive transcription factor 1/2 |
| GO | Gene Ontology |
| gsw | stomatal conductance |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPKKK | mitogen-activated protein kinase kinase kinase |
| MAPK (MPK) | mitogen-activated protein kinase |
| MDA | malondialdehyde |
| MKK | mitogen-activated protein kinase kinase |
| PGPR | plant growth promoting rhizobacteria |
| Pn | net photosynthesis rate |
| POD | peroxidase |
| PP2C | protein phosphatases 2C |
| PYL | PYR1-like proteins |
| PYR/PYL | abscisic acid receptor PYR/PYL family |
| RbohD | respiratory burst oxidase homologous protein D |
| ROS | reactive oxygen species |
| SnRK2 | SNF1-related type 2 protein kinase |
| SOD | superoxide dismutase |
| Tr | transpiration rate |
| VOC | volatile organic compound |
References
- Zainuddin, N.; Keni, M.F.; Ibrahim, S.A.S.; Masri, M.M.M. Effect of integrated biofertilizers with chemical fertilizers on the oil palm growth and soil microbial diversity. Biocatal. Agric. Biotechnol. 2022, 39, 102237. [Google Scholar] [CrossRef]
- Shen, W.S.; Hu, M.C.; Qian, D.; Xue, H.W.; Gao, N.; Lin, X.G. Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 2021, 463, 1–18. [Google Scholar] [CrossRef]
- Thessalia, T.; Ioannis, I. Effects of sand dune, desert and field arbuscular mycorrhizae on lettuce (Lactuca sativa, L.) growth in a natural saline soil. Sci. Hortic.-Amst. 2020, 264, 109191. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Cartes, P.; Vidal, G.; Cornejo, P. Aquaporins and cation transporters are differentially regulated by two arbuscular mycorrhizal fungi strains in lettuce cultivars growing under salinity conditions. Plant Physiol. Biochem. 2021, 158, 396–409. [Google Scholar] [CrossRef]
- Yang, S.; Hao, X.; Xu, Y.; Yang, J.; Su, D. Meta-Analysis of the Effect of Saline-Alkali Land Improvement and Utilization on Soil Organic Carbon. Life 2022, 12, 1870. [Google Scholar] [CrossRef]
- Tarolli, P.; Luo, J.; Park, E.; Barcaccia, G.; Masin, R. Soil salinization in agriculture: Mitigation and adaptation strategies combining nature-based solutions and bioengineering. iScience 2024, 27, 108830. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.; Reddy, M.; El Enshasy, H. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: Recent developments, constraints, and prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Parveen, A.; Ahmar, S.; Kamran, M.; Malik, Z.; Ali, A.; Riaz, M.; Abbasi, G.H.; Khan, M.; Sohail, A.B.; Rizwan, M. Abscisic acid signaling reduced transpiration flow, regulated Na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum L.) seedlings. Environ. Technol. Innov. 2021, 24, 101808. [Google Scholar] [CrossRef]
- Peng, G.; Zhao, X.; Li, Y.; Wang, R.; Huang, Y.; Qi, G. Engineering Bacillus velezensis with high production of acetoin primes strong induced systemic resistance in Arabidopsis thaliana. Microbiol. Res. 2019, 227, 126297. [Google Scholar] [CrossRef]
- Syed-Ab-Rahman, S.F.; Carvalhais, L.C.; Chua, E.T.; Chung, F.Y.; Moyle, P.M.; Eltanahy, E.G.; Schenk, P.M. Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth. Sci. Total Environ. 2019, 692, 267–280. [Google Scholar] [CrossRef]
- Fincheira, P.; Parada, M.; Quiroz, A. Volatile organic compounds stimulate plant growing and seed germination of Lactuca sativa. J. Soil Sci. Plant Nutr. 2017, 17, 853–867. [Google Scholar] [CrossRef]
- Jiang, C.H.; Xie, Y.S.; Zhu, K.; Wang, N.; Li, Z.J.; Yu, G.J.; Guo, J.H. Volatile organic compounds emitted by Bacillus sp. JC03 promote plant growth through the action of auxin and strigolactone. Plant Growth Regul. 2019, 87, 317–328. [Google Scholar] [CrossRef]
- Rath, M.; Mitchell, T.R.; Gold, S.E. Volatiles produced by Bacillus mojavensis RRC101 act as plant growth modulators and are strongly culture-dependent. Microbiol. Res. 2018, 208, 76–84. [Google Scholar] [CrossRef]
- Marzouk, T.; Chaouachi, M.; Sharma, A.; Jallouli, S.; Mhamdi, R.; Kaushik, N.; Djébali, N. Biocontrol of Rhizoctonia solani using volatile organic compounds of solanaceae seed-borne endophytic bacteria. Postharvest Biol. Technol. 2021, 181, 111655. [Google Scholar] [CrossRef]
- Bilias, F.; Tsolis, V.; Zafeiriou, I.; Koukounaras, A.; Kalderis, D.; Chlouveraki, E.; Gasparatos, D. Effects of Sewage Sludge Biochar and a Seaweed Extract-Based Biostimulant on Soil Properties, Nutritional Status and Antioxidant Capacity of Lettuce Plants in a Saline Soil with the Risk of Alkalinization. J. Soil Sci. Plant Nutr. 2024. [Google Scholar] [CrossRef]
- Ali, M.A.A.; Nasser, M.A.; Abdelhamid, A.N.; Ali, I.A.A.; Saudy, H.S.; Hassan, K.M. Melatonin as a Key Factor for Regulating and Relieving Abiotic Stresses in Harmony with Phytohormones in Horticultural Plants—A Review. J. Soil Sci. Plant Nutr. 2024, 24, 54–73. [Google Scholar] [CrossRef]
- Atero-Calvo, S.; Magro, F.; Masetti, G.; Navarro-León, E.; Blasco, B.; Ruiz, J.M. Salinity stress mitigation by radicular and foliar humic substances application in lettuce plants. Plant Growth Regul. 2024, 104, 151–167. [Google Scholar] [CrossRef]
- Monterisi, S.; Zhang, L.; Garcia-Perez, P.; Alzate Zuluaga, M.Y.; Ciriello, M.; El-Nakhel, C.; Buffagni, V.; Cardarelli, M.; Colla, G.; Rouphael, Y.; et al. Integrated multi-omic approach reveals the effect of a Graminaceae-derived biostimulant and its lighter fraction on salt-stressed lettuce plants. Sci. Rep. 2024, 14, 10710. [Google Scholar] [CrossRef]
- Fincheira, P.; Venthur, H.; Mutis, A.; Parada, M.; Quiroz, A. Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species. Microbiol. Res. 2016, 193, 39–47. [Google Scholar] [CrossRef]
- Cappellari, L.d.R.; Banchio, E. Microbial Volatile Organic Compounds Produced by Bacillus amyloliquefaciens GB03 Ameliorate the Effects of Salt Stress in Mentha piperita Principally Through Acetoin Emission. J. Plant Growth Regul. 2020, 39, 764–775. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Liu, X.; Zhou, Y.; Huang, W.; Guan, X.; Zhang, X.; Xie, Z. The volatile organic compound acetoin enhances the colonization of Azorhizobium caulinodans ORS571 on Sesbania rostrata. Sci. Total Environ. 2024, 912, 169006. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xie, J.; Wang, Q.; Shi, D.; Jia, L.; Feng, H. Effect of Alternative Respiratory Pathway on Chlorophyll Content and Chlorophyll Fluorescence Characteristics under NaCl Stress. Acta Bot. Boreali-Occident. Sin. 2017, 37, 1175–1181. [Google Scholar]
- Zhao, L.F.; Xu, Y.J.; Shao, X.; Yang, J.Y. Two endophytic Bacillus strains from soybean nodules affect superoxide dismutase and peroxidase activities in soybean seedlings under salt stress Microbiol. China 2022, 49, 1664–1677. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Ali, B.; Ren, X.; Chen, X.; Li, Q.; Saqib, M.; Ahmad, N. Recent progress in understanding salinity tolerance in plants: Story of Na+/K+ balance and beyond. Plant Physiol. Biochem. 2021, 160, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Garabello, C.; Contartese, V.; Ferrante, A. Effect of exogenous application of salt stress and glutamic acid on lettuce (Lactuca sativa L.). Sci. Hortic.-Amst. 2022, 299, 111027. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Han, C.; Wang, S.; Bai, M.; Song, C. Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef]
- Wei, X.; Rahim, M.A.; Zhao, Y.; Yang, S.; Wang, Z.; Su, H.; Li, L.; Niu, L.; Rashid, M.H.-U.; Yuan, Y. Comparative Transcriptome Analysis of Early-and Late-Bolting Traits in Chinese Cabbage (Brassica rapa). Front. Genet. 2021, 12, 119. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The plant hormone ethylene restricts Arabidopsis growth via the epidermis. Proc. Natl. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, J.; Li, Z.; Qiao, J.; Quan, R.; Wang, J.; Huang, R.; Qin, H. SALT AND ABA RESPONSE ERF1 improves seed germination and salt tolerance by repressing ABA signaling in rice. Plant Physiol. 2022, 189, 1110–1127. [Google Scholar] [CrossRef]
- Lei, P.; Wei, X.; Gao, R.; Huo, F.; Nie, X.; Tong, W.; Song, W. Genome-wide identification of PYL gene family in wheat: Evolution, expression and 3D structure analysis. Genomics 2021, 113, 854–866. [Google Scholar] [CrossRef]
- Wang, B.; Li, L.; Peng, D.; Liu, M.; Wei, A.; Li, X. TaFDL2-1A interacts with TabZIP8-7A protein to cope with drought stress via the abscisic acid signaling pathway. Plant Sci. 2021, 311, 111022. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Li, Y.; Wang, Y.; Liu, X.; Ma, L.; Zhang, Z.; Mu, C.; Zhang, Y.; Peng, L.; Xie, S.; et al. Initiation and amplification of SnRK2 activation in abscisic acid signaling. Nat. Commun. 2021, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Ghorbel, M.; Zaidi, I.; Ebel, C.; Brini, F.; Hanin, M. The wheat Mitogen Activated Protein Kinase TMPK3 plays a positive role in salt and osmotic stress response. Acta Physiol. Plant. 2023, 45, 71. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, H.; Qi, Y.; Zhao, Y.; Duan, C.; Wang, Y.; Meng, Z.; Zhang, Q. Genome-wide identification of PYL/PYR-PP2C (A)-SnRK2 genes in Eutrema and their co-expression analysis in response to ABA and abiotic stresses. Int. J. Biol. Macromol. 2023, 253, 126701. [Google Scholar] [CrossRef]
- Park, H.L.; Seo, D.H.; Lee, H.Y.; Bakshi, A.; Park, C.; Chien, Y.-C.; Kieber, J.J.; Binder, B.M.; Yoon, G.M. Ethylene-triggered subcellular trafficking of CTR1 enhances the response to ethylene gas. Nat. Commun. 2023, 14, 365. [Google Scholar] [CrossRef]
- Parvathaneni, S.; Li, Z.; Sacks, D.B. Calmodulin influences MAPK signaling by binding KSR1. J. Biol. Chem. 2021, 296, 100577. [Google Scholar] [CrossRef]
- Takahashi, F.; Mizoguchi, T.; Yoshida, R.; Ichimura, K.; Shinozaki, K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 2011, 41, 649–660. [Google Scholar] [CrossRef]
- Tavanti, T.R.; de Melo, A.A.R.; Moreira, L.D.K.; Sanchez, D.E.J.; dos Santos Silva, R.; da Silva, R.M.; Dos Reis, A.R. Micronutrient fertilization enhances ROS scavenging system for alleviation of abiotic stresses in plants. Plant Physiol. Biochem. 2021, 160, 386–396. [Google Scholar] [CrossRef]
- Zhou, C.; Li, S.; Zheng, Y.; Lei, P.; Chen, Y.; Ying, H.; Gao, N. An Energy-Rich Phosphate Compound Enhances the Growth of Lettuce Through the Activation of Photosynthesis, Growth, and Induced Systemic Resistance-Related Processes. J. Soil Sci. Plant Nutr. 2022, 22, 1955–1969. [Google Scholar] [CrossRef]
- Fedeli, R.; Loppi, S.; Cruz, C.; Munzi, S. Evaluating Seawater and Wood Distillate for Sustainable Hydroponic Cultivation: Implications for Crop Growth and Nutritional Quality. Sustainability 2024, 16, 7186. [Google Scholar] [CrossRef]
- Fedeli, R.; Cruz, C.; Loppi, S.; Munzi, S. Hormetic Effect of Wood Distillate on Hydroponically Grown Lettuce. Plants 2024, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Danquah, A.; de Zélicourt, A.; Boudsocq, M.; Neubauer, J.; Frei dit Frey, N.; Leonhardt, N.; Pateyron, S.; Gwinner, F.; Tamby, J.P.; Ortiz-Masia, D. Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J. 2015, 82, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).