Abstract
This study aimed to investigate the growth characteristics of Sageretia thea and analyze the correlations between soil physicochemical properties and microbial communities in its native habitats. Soil physicochemical properties were characterized by organic matter (0.37–36.43%), available phosphate (57.96–315.90 mg/kg), potassium (0.11–1.17 cmol+kg−1), calcium (1.23–25.97 cmol+kg−1), magnesium (0.43–15.01 cmol+kg−1), sodium (0.04–6.16 cmol+kg−1), and pH (4.68–7.05), indicating slightly acidic to neutral conditions. S. thea exhibited variable growth characteristics across habitats; leaf length and width were largest in Jangnam-ri and Hacka-ri, respectively, whereas Docheong-ri promoted higher fruit growth. The soil microbial community composition was dominated by Proteobacteria, Actinobacteria, and Acidobacteria at the phylum level (76.09%) and by Alphaproteobacteria, Actinobacteria_c, and Vicinamibacter_c at the class level (40%). Soil physicochemical properties were significantly correlated with Actinobacteria, Acidobacteria, and Chloroflexi at the phylum level, and all microbial groups except Spartobacteria at the class level. Furthermore, growth characteristics were significantly correlated with all microbial communities except Acidobacteria and Firmicutes at the phylum level, and Acidobacteria, Thermoleophilia, and Rubrobacteria at the class level. These findings provide a foundation for developing efficient cultivation techniques for S. thea based on its soil microbiome and habitat conditions.
1. Introduction
Bacteria represent the most abundant microorganisms in soil, with over 100 million individuals across 104–106 species per gram of soil, and the total number of microorganisms, including bacteria, is estimated to exceed 108 species per gram of soil [1]. Microorganisms play a pivotal ecological role in regulating biogeochemical cycles and influence plant growth [2]. Their abundance and diversity are regulated by various physical and chemical properties of the soil, such as pH, organic matter, P and K content, and meteorological conditions, such as temperature, precipitation, and light [3,4]. Microbial communities are sensitive to environmental changes and serve as indicators of soil conditions [5]. Therefore, studies have been conducted to screen microbial communities and analyze the soil microbiome of native or cultivated lands for optimizing growing conditions for crops, identifying the causes of crop damage in monocropping systems [6,7,8,9].
(Osback) M. C. Johnst., a perennial shrub belonging to the family Rhamnaceae, is represented by a single genus and species in Korea [10]. This species is distributed along the southern coast and Jeju island. The extracts derived from this plant have diverse medicinal potential; its branch and leaf extracts reduce the viability of human colorectal cancer cells, inducing cyclin D1 proteasomal degradation and HO-1 expression [11], silver nanoparticles synthesized from its root extract exhibit antioxidant effects [12], and flavonoids and phenolic acids extracted from its above-ground parts contribute to the improvement and prevention of degenerative diseases [13]. Additionally, fruit extracts of this plant have antioxidant and immune response-modulation effects [14,15], while callus extracts are used in cosmetics to inhibit reactive oxygen species and IL-8 production induced by fine dust [16]. Related species such as S. gracilis are popular as ornamental plants for bonsai gardening in China [17]. Notably, despite its substantial potential, S. thea is primarily collected from natural environments with a lack of established cultivation techniques. Therefore, there is a need to cultivate S. thea, a wild plant, because its anti-cancer, anti-inflammatory, and immune response-modulating properties are expected to be useful and profitable, and there is a lack of research on its cultivation.
Most plants on Earth are associated with soil microorganisms throughout their life cycle [18]. For example, the genera Pseudomonas and Bacillus dissolve insoluble phosphate compounds into an absorbable form, supplying plants with essential nitrogen and phosphorus for their growth [19]. Microorganisms such as Glomus intraradices, G. claroideum, Gigaspora margarita, and others enhance plant growth by improving water and nutrient uptake [20].
In addition, Bacillus species are antagonistic to plant pathogens, such as Botrytis cinerea, Rhizoctonia solani, and Sclerotinia minor, and have been shown to promote plant growth [21]. In particular, orchids are closely related to soil microbes; for non-photosynthetic orchids, interactions with orchid mycorrhizal fungi are crucial for seed germination, early chlorophyll production, and meeting their nutritional requirements [22,23]. Collectively, various soil microorganisms exert profound effects on plant growth, and analyzing their interactions with plants is imperative for the stable production and efficient management of plants.
In this study, we aimed to analyze the native soil microbiome of S. thea, which has substantial medicinal, cosmetic, and ornamental potential. We examined correlations between the soil microbiome, soil physicochemical properties, and growth characteristics to establish data that will inform the development of efficient cultivation models.
2. Results
2.1. Soil Physicochemical Properties
The soil physicochemical properties of S. thea habitats were analyzed and found to be slightly acidic to neutral, with a pH of 4.68–7.05 in sandy loam (BS, CD, MS), loamy loam (DC, ND), and loamy sand (DH, HG, JN, JJ, HY). The organic matter (OM) ranged from 0.37% in BS to 36.43% in HY, total nitrogen ranged from 0.03% in BS to 1.39% in HY, available phosphate (Avai. P) ranged from 57.96 mg/kg in the BS to 315.90 mg/kg in the ND group. In addition, potassium (K) was observed as 0.11–1.17 cmol+kg−1, calcium (Ca) as 1.23–25.97 cmol+kg−1, magnesium (Mg) as 0.43–15.01 cmol+kg−1, and sodium (Na) as 0.04–6.16 cmol+kg−1. Furthermore, cation exchange capacity (CEC) was observed as 4.15–50.88 cmol+kg−1, and electrical conductivity (EC) as 0.11–1.78 dS m−1 (Table 1).

Table 1.
Soil physicochemical properties of 10 S. thea habitats.
2.2. Growth Characteristics
Table 2 presents the growth characteristics of S. thea. S. thea showed regional differences in growth characteristics; leaf length was 38.15 mm in JN and width was 22.55 mm in HG. The fruit growth characteristics were the highest in DC, with a 7.87 mm length, 8.95 mm width, and 0.41 g fresh weight compared to other regions. The sweetness was 19.81 °Brix in MS, and the hardness was 1.56 N in ND, which was the highest value measured.

Table 2.
Growth characteristics of S. thea among 10 habitats.
2.3. Soil Microbial Community
The analysis of the relative abundance of microbial communities in the soil of S. thea habitats demonstrated that Proteobacteria dominated at the phylum level and Alphaproteobacteria dominated at the class level in all habitats (Figure 1). In terms of phylum, Proteobacteria was identified as the most dominant phylum, with an average 34.83%, with the highest value for DH (45.83%) and the lowest for HY (29.74%). Actinobacteria had an average of 21.31% and Acidobacteria had an average of 19.95%; three microbial phyla accounted for three-quarters of the total (76.09%) of all microbial phyla. And Verrucomicrobia accounted for 6.41%, followed by Firmicutes (4.48%), Chloroflexi (4.25%), and Bacterioidetes (3.91%), which represented 3–4% of the total; Gemmatimonadetes (2.22%), Planctomycetes (2.08%), Nitrospirae (1.48%), Cyanobacteria (1.40%), and Laterscibacteria_WS3 (1.11%) showed an average of 1–2% (Figure 1A).
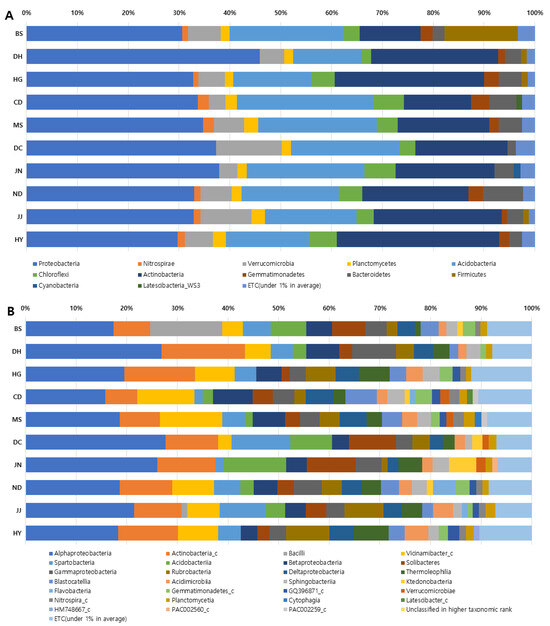
Figure 1.
Clustering and relative abundance of microbial community in 10 different S. thea habitats. (A). Phylum; (B) classes; BS., Baeksan-ri, Sinan-gun; DH., Dohang-ri, Jindo-gun; HG., Hakga-ri, Haenam-gun; CD., Chungdo-ri, Wando-gun; MS., Mangseok-ri, Wando-gun; DC., Docheong-ri, Wando-gun; JN., Jangnam-ri, Goheung-gun; ND., Nangdo-ri, Yeosu-si; JJ., Jeoji-ri, Jeju-si; HY., Haye-dong, Seogwipo-si.
At the class level, Alphaproteobacteria had the highest value, with an average of 21.02%, followed by Actinobacteria_c, with an average of 10.54%, and Vicinamibacter_c, with an average of 7.31%, resulting in three microbial classes accounting for 38.87%. This was followed by Spartobacteria (5.22%). Acidobacteria (5.06%), and Betaproteobacteria (5.02%), which accounted for 5%, and Solibacteres (4.65%), Gammaproteobacteria (4.47%), and Rubrobacteria (4.26%) accounted for 4%; Deltaproteobacteria (3.96%), Themoleophilia (3.79%), Blastocatellia (3.43%) accounted for 3%, and others accounted for 1–2% (Figure 1B).
2.4. Correlation Between Soil Microbial Community and Soil Properties
Duncan’s correlation analysis between soil microbial communities and soil properties was performed to determine the effect of soil properties on soil microbial communities (Tables S1 and S2 and Figure 2). At the phylum level, microbial communities showed significant correlations, except for Chloroflexi and Firmicutes, which showed different behaviors between Acidobacteria and Actinobacteria. Acidobacteria exhibited a negative correlation with all soil properties, showing statistical significance for all except P (r = −0.247, p = 0.094), Na (r = −0.251, p = 0.090), and CEC (r = −0.037, p = 0.423). In contrast, Actinobacteria demonstrated a positive correlation with most soil heterogeneities, being significant for all except P (r = 0.265, p = 0.078) and CEC (r = 0.041, p = 0.415).
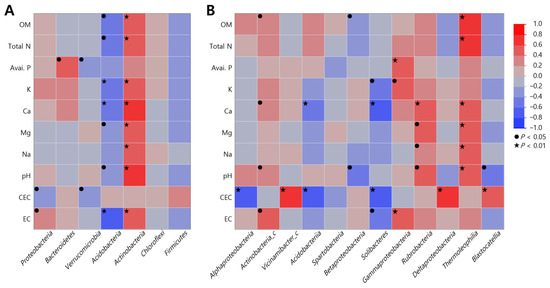
Figure 2.
Duncan’s correlation analysis between soil physicochemical properties and microbial communities of S. thea habitats. (A) Phylum; (B) classes. Values in brackets indicate p value (● p < 0.05, ★ p < 0.01).
When correlations were analyzed at the class level, Alphaproteobacteria demonstrated a significant negative correlation with CEC (r = −0.622, p = 0.000), while Actinobacteria_c was significantly negatively correlated with Ca (r = 0. 398, p = 0.029), pH (r = 0.378, p = 0.040), and EC (r = 0.430, p = 0.018), and Vicinamibacter_c showed a significant and positive correlation with CEC (r = 0.631, p = 0.000). Acidobacteria were negatively correlated with Ca (r = −0.574, p = 0.008) and pH (r = −0.619, p = 0.004), Betaproteobacteria were negatively correlated with OM (r = −0.386, p = 0.035) and pH (r = −0.408, p = 0.025), and Solibacteres were negatively correlated with K (r = −0. 396, p = 0.033), Ca (r = −0.626, p = 0.001), CEC (r = −0.643, p = 0.000), and EC (r = −0.400, p = 0.031). In contrast, Gammaproteobacteria and Thermoleophilia showed a strong positive correlation with organic matter, total nitrogen, and pH, including trace elements such as calcium and magnesium, which is in contrast to Acidobacteria, Betaproteobacteria, and Solibacteres.
2.5. Correlation Between Soil Microbial Community and Growth Characteristics
Duncan’s correlation analysis between the growth characteristics of S. thea and microbial communities in the soil revealed that at the phylum level, all communities, except Acidobacteria and Firmicutes, were significantly correlated (Tables S3 and S4 and Figure 3). Proteobacteria was positively correlated with leaf width (r = 0.344, p = 0.031) and fruit weight (r = 0.321, p = 0.042), Verrucomicrobia with fruit width (r = 0.382, p = 0.019), and Actinobacteria with sweetness (r = 0.361, p = 0.025); Bacteroidetes was negatively correlated with leaf length (r = −0.373, p = 0.025) and width (r = −0.319, p = 0.049), and Chloroflexi demonstrated a significant negative correlation with fruit growth characteristics [length (r = −0.359, p = 0.028), width (r = 0.470, p = 0.005), and weight (r = 0.476, p = 0.004)] and a significant positive correlation with hardness (r = 0.330, p = 0.040). At the class level, we found a positive correlation between Acidobacteria and leaf length (r = 0.447, p = 0.048), Thermoleophilia and sweetness (r = 0.380, p = 0.042), and Rubrobacteria was negatively correlated with fruit weight (r = −0.377, p = 0.044).
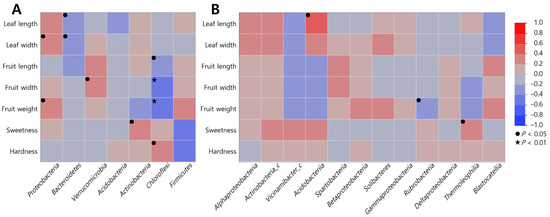
Figure 3.
Duncan’s correlation analysis between growth characteristics of S. thea and soil microbial communities. (A) Phylum; (B) classes. Correlation coefficients (r) indicate significant correlations between variables compared. Negative values denote negative correlation and positive values denote positive correlation. Values in brackets indicate p value (● p < 0.05, ★ p < 0.01).
3. Discussion
3.1. Soil Physicochemical Properties of S. thea Habitats
S. thea is a plant resource native to Korea, China, Japan, India, Taiwan, and Vietnam, and is expected to be utilized for medicinal and ornamental purposes [24]. Therefore, this study was conducted to obtain basic data for developing efficient cultivation technology. A previous study on the soils of S. thea habitats found that sandy loam soils, characterized by a high percentage of sand, were the most prevalent in these areas [25]. This study also confirmed similar results to the previous study’s report of a high proportion of sand and a low proportion of silt and clay, which differed from the average forest soil in Korea (41.7% sand, 41.5% silt, and 16.8% clay) [26]. In addition, it was found that there were differences in trace elements such as Ca and Mg, including OM, among the sites, especially of Na, which was 6.16 cmol+kg−1 in HY compared to 0.04–0.51 cmol+kg−1 in most sites. HY is located on the coast of Jeju Island, South Korea, and is a representative island region with a subtropical oceanic climate [27]. In other words, compared with other sites, it is believed that the high Na values are due to geographical characteristics that are directly affected by strong winds and waves caused by the oceanic climate.
3.2. Growth Characteristics of S. thea Across Different Habitats
In this study, we identified differences in S. thea growth among sites. Plant growth is not driven by a single factor but by complex interactions between many environmental factors, including weather conditions, soil chemistry, and microbial communities [28,29,30]. This study was conducted at various native sites of S. thea from Jeolla Province to Jeju Island, Korea. In other words, there are differences in latitude (HY: 33°14′–BS: 34°52′) and longitude (BS: 126°01′–ND: 128°32′) of each study site, and consequently differences in meteorological characteristics such as average annual temperature (2.2 °C) and precipitation (400 mm) due to geographical characteristics [31]. In addition, the interaction of various environmental factors, such as the soil characteristics at each site identified earlier, may have contributed to the differences in S. thea growth examined in this study.
3.3. Abundance of Soil Microbial Communities in S. thea Habitats
Soil microorganisms have a general influence on the life of plants, particularly the uptake of inorganic elements (Mg, Na, Ca, etc.) required for growth from the soil [30]. Therefore, soil microbial community analysis was conducted for each study site, and it was found that Proteobacteria dominated at the phylum level, Alphaproteobacteria dominated at the class level, and the sum of the abundances of Proteobacteria, Acidobacteria, and Actinobacteria phyla accounted for 76.09% at the phylum level. Proteobacteria, Acidobacteria, and Actinobacteria are the dominant phyla of microorganisms in most soils and are highly abundant in coastal areas of Korea and most coastal areas of East Asian countries, such as China and Japan [32,33,34,35], and Alphaproteobacteria are also the most abundant class of microorganisms in the distribution of marine microorganisms [6,36]. It is known that the above microbial communities show a close correlation with the metallic composition of soils (Fe, Cu, As, etc.) and are highly abundant in most soils, especially in low-pH and polluted environments [37,38,39,40]. These characteristics imply a high adaptation and resilience of the microbial community to the surrounding environment and may also explain the native environment of S, thea, which is distributed in coastal areas in slightly acidic to neutral soils with a low pH.
3.4. Effect of Soil Physicochemical Properties on Microbial Communities
The soil environment affects the distribution and abundance of microorganisms, as has been recognized in various studies [41]. Therefore, in this study, we analyzed the correlation between soil physicochemical properties and microbial communities, and identified different patterns of Acidobacteria and Actinobacteria at the phylum level. Acidobacteria were negatively correlated with most soil chemistries, whereas Actinobacteria were positively correlated. Both Acidobacteria and Actinobacteria are major microbial communities present in the soil, and each has its own characteristics and roles in the ecosystem. Acidobacteria can live over a wide range of soil pH but are particularly adaptable to acidic environments [42]. They are primarily responsible for cycling organic matter in the soil and enhancing the availability of various nutrients [43]. Studies involving Acidobacteria have shown that their abundance is strongly and negatively correlated with soil organic carbon [44]. These are not all subgroups within this phylum, but many microbes are oligotrophic groups that thrive in low-nutrient environments [43]. Actinobacteria, on the other hand, are predominantly found in neutral or slightly alkaline soils [45] and perform ecological functions, such as antibiotic action and pathogen inhibition [46]. They also show a strong positive correlation with soil moisture and temperature, including available nitrogen and organic carbon [47]. The two microbial communities exhibit differences in their ecological roles and environmental adaptations. However, both taxa play important roles in the soil, such as organic matter cycling and antibiotic activity, and their distribution in the soil is strongly correlated with soil chemistry, such as with pH, organic matter content, and nutrient concentration. This suggests that the distribution and activity of both communities are regulated by the soil properties and play important roles in soil ecosystems. At the class level, CEC was strongly correlated with diverse microbial communities (Alphaproteobacteria, Vicinamibacteria_c, Acidobacteriia, Solibacteres, Deltaproteobacteria, and Blastocatellia). CEC is an indicator of the ability of soil to absorb and exchange cations (Ca, Mg, K, etc.), and soils with a higher CEC have an increased ability to absorb and retain nutrients [48,49]. In other words, CEC affects the distribution and abundance of soil microbial communities, which has been shown in various previous studies [50,51,52], and our results confirmed this through a correlation analysis between soil physicochemical properties and microbial communities.
3.5. Effect of Microbial Communities on Growth Characteristics of S. thea
The soil microbial community is influenced by a variety of environmental factors and interacts closely with plant growth. In this study, we characterized the growth and interactions of S. thea within the microbial community phyla Proteobacteria and Chloroflexi. Proteobacteria abundance was significantly and positively correlated with leaf width and fruit weight. Proteobacteria are found in soils under various conditions and are involved in the decomposition of soil OM and the biogeochemical cycling of carbon, nitrogen, and sulfur [53]. This microbial community is called plant growth-promoting rhizobacteria and is closely related to plant growth [54]. The soil microbiota plays a crucial role in plant growth through interactions that influence nutrient absorption, disease resistance, and environmental stress tolerance. Soil microorganisms such as plant growth-promoting rhizobacteria (PGPR) and mycorrhizal fungi can alter soil structure and the availability of essential nutrients like nitrogen (N) and phosphorus (P), promote root development, and enhance resistance to pathogens, thereby facilitating nutrient uptake [43]. In addition, studies on Arabidopsis have confirmed its ability to increase plant growth by enhancing the availability of phosphorus (P), which is involved in cell membrane formation and energy-dependent metabolic processes, and that of iron (Fe), which is involved in cellular redox functions such as photosynthesis and respiration [55,56,57]. In other words, the microorganisms in Proteobacteria transform nutrients in the soil needed for plant growth into a form that can be absorbed by plant roots, which in turn affects plant growth. This is why they are positively correlated with leaf and fruit growth characteristics.
In general, Chloroflexi can improve soil fertility through ecological roles, such as the decomposition of organic matter in the soil and nitrogen fixation, and can provide the nutrients (nitrogen, phosphorus, etc.) necessary for fruit growth [33,58]. However, in this study, Chloroflexi was negatively correlated with overall fruit growth characteristics (length, width, and weight). Although studies have been conducted on the effects of different soil microbial communities on plant fruit growth, a clear correlation between Chloroflexi and fruit growth is not known [59,60,61]. In addition, plant growth can be affected by many variables, such as environment, soil type, and plant species [62]. Therefore, it is necessary to study the effects of Chloroflexi on plant fruit growth. Once the specific mechanisms are identified, we will have a clearer understanding of how these microbial communities affect fruit growth.
4. Materials and Methods
4.1. Study Habitat Site Selection and Collecting Soil Samples
A total of 10 S. thea sites were selected in Jeolla Province and Jeju Island, Republic of Korea (Table 3, Figure 4), and 100 g of rhizosphere soil samples from 10 to 30 cm of soil core was collected in May 2023 after removing the soil topsoil to collect soil samples from the study sites. The soil samples were divided into those used for soil physicochemical properties and those for microbial community analysis. Soil samples for the soil physicochemical properties’ analysis were air-dried in a cool place and debris was removed using a 2 mm mesh sieve; soil microbial community analysis was conducted in separate 50 mL conical tubes before these were dried and frozen at −20 °C before analysis.

Table 3.
Collection of S. thea accessions from different locations in Korea.
Figure 4.
Map of collected sites of S. thea in Korea. A. BS (Baeksan-ri, Sinan-gun); B. DH (Dohang-ri, Jindo-gun); C. HG (Hakga-ri, Haenam-gun); D. CD (Chungdo-ri, Wando-gun); E. MS (Mangseok-ri, Wando-gun); F. DC (Docheong-ri, Wando-gun); G. JN (Jangnam-ri, Goheung-gun); H. ND (Nangdo-ri, Yeosu-si); I. JJ (Jeoji-ri, Jeju-si); J. HY (Haye-dong, Seogwipo-si).
4.2. Soil Physicochemical Property Analysis
Soil samples were air-dried and then sieved through a 2 mm mesh; gravel content was determined by weighing particles retained on the sieve, while the portion passing through the sieve was used for soil physicochemical property analysis. Soil texture analysis, which can measure the content of sand, mass, and clay in soil, was measured based on Stokes’s law [63]. The soil was analyzed according to the soil physicochemical analysis method proposed by the Rural Development Administration. Organic matter (OM) content was determined by the Walkley–Black method [64], and available phosphate (Av.P2O5) by the Lancaster leaching method with 1-amino-2-naphtol-4-sulfanic acid. The cation exchange capacity (CEC) was measured by the Kjeldahl distillation method, where NH4+ is substituted in the soil after leaching with 1-N ammonium acetate solution [65]. The cation content of Ca, K, Mg, and Na were measured using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) [66], and the acidity (pH) and electrical conductivity (EC) of the soil were measured using a pH meter and an EC meter, respectively, after diluting dry soil and distilled water 1:5.
4.3. Growth Characteristics of S. thea
To investigate the growth characteristics of S. thea, seven growth characteristics of leaves (length, width) and fruits (length, width, weight, sweetness, and hardness) were measured for each study site. The length and width of leaves and fruits were measured to the nearest 0.01 mm using a digital caliper (CD-200 APX, Mitutoyo Cp., Kanagawa, Japan), and the weight of fruits was measured to the nearest 0.01 g using a high-precision analytical balance (PAG214C, Ohaus Co., NJ, USA). The sweetness of the fruits was measured using a digital saccharimeter (PR-101α, Atago Co. LTD., Tokyo, Japan) to determine the soluble solid content of the fruit juice, and the hardness was measured using a physical property tester (CR-3000EX-S, Sun Scientific CO., Tokyo, Japan) with a probe diameter of 2 mm and a depth of 4 mm.
4.4. Soil DNA Extraction and PCR Amplification
The total DNA of each rhizosphere soil sample was extracted using the DNeasy Power Soil kit (QIAGEN, Hilden, Germany) following manufacturer instructions. After extraction, the quantification and quality of DNA were measured by PicoGreen and Nanodrop (Thermo Scientific, Rockford, IL, USA). Each sequenced sample was prepared according to the Illumina 16S Metagenomics Sequencing Library protocols (Macrogen, Seoul, Republic of Korea). In amplicon PCR, the V3-V4 region of the 16S rRNA gene of bacteria was targeted using the 16S V3-V4 primers [67]. The 16S V3-V4 primer sequences are as follows: 16S amplicon PCR forward primer, 5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3’; 16S amplicon PCR reverse primer, 5’-GTCTCGTGGGCTCGGAGATGTGTATAAGA GACAGGACTACHVGGGTATCTAATCC-3’. Input gDNA was amplified with 16S V3-V4 primers, and a subsequent limited-cycle amplification step was performed to add multiplexing indices and Illumina sequencing adapters. The conditions for amplicon PCR were as follows. First PCR: initial denaturation at 95 °C for 3 min, followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The condition for the index PCR was as follows. Second PCR: initial denaturation at 95 °C for 3 min, followed by 8 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The final products were normalized and pooled using PicoGreen, and the size of the libraries were verified using the TapeStation DNA screentape D1000 (Agilent, Santa Clara, CA, USA).
4.5. Pyrosequencing and Data Processing
Bacterial DNA sequencing was performed using the Illumina MiSeq™ sequencing system (Illumina Inc., San Diego, CA, USA) according to the manufacturer’s instructions. Raw sequences of bacterial DNA were processed using Mothur pipeline (version 1.43.0, The University of Michigan, Ann Arbor, MI, USA) [68]. The forward and reverse reads obtained from the Illumina platform were assembled, and sequences with a quality score <20 and ambiguous nucleotides were discarded before performing downstream analysis. The resulting sequences spanning the V3-V4 region were checked for the presence of chimeras using the function chimera.uchime. Taxonomic classification was performed using the “Greengenes reference database” for bacteria. Greengenes was used as it was reported to provide the best combination of speed and quality [69]. The sequences were clustered into operational taxonomic units (OTUs) at a 97% similarity level using a distance-based greedy clustering method (DGC) in Mothur. OTUs with less than 10 sequences were discarded to reduce false diversity.
4.6. Data Analysis
Data analysis was performed using SPSS software (Statistical Package for Social Sciences, Version 26, IBM SPSS statistics, Chicago, IL, USA), and data were expressed as means. The data of the results underwent one-way analysis of variance (ANOVA) and Duncan’s test, with statistical significance set at p < 0.05. And correlation coefficient analysis between soil physicochemical properties, growth characteristics, and soil microbial communities were analyzed using Pearson’s correlation.
5. Conclusions
This study analyzed the correlation between soil geochemistry, growth characteristics, and soil microbial communities of S. thea habitats. The study revealed significant correlations between soil chemistry and Acidobacteria and Actinobacteria phyla, and between S. thea growth characteristics and the Chloroflexi phylum. These results can provide a wealth of information about the optimal growing conditions for S. thea. And it is believed that with continued research, we will be able to establish more definitive cultivation techniques.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants13233310/s1, Table S1: Duncan’s correlation coefficient between soil physicochemical properties and microbial communities (phylum) of S. thea habitats; Table S2: Duncan’s correlation coefficient between soil physicochemical properties and microbial communities (classes) of S. thea habitats; Table S3: Duncan’s correlation coefficient between growth characteristics of S. thea and microbial communities (phylum) in habitats; Table S4: Duncan’s correlation coefficient between growth characteristics of S. thea and microbial communities (classes) in habitats.
Author Contributions
Involved in every stage of this study, and in manuscript writing and data analysis, D.-H.J. and H.-J.S.; conducted field survey of S. thea, D.-H.J., Y.-B.Y., J.K., and H.-J.S.; writing, review and editing, D.-H.J. and Y.-B.Y.; data curation, J.-H.S., J.K., Y.U., and H.-J.S.; project administration and funding acquisition, J.K. and J.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Forest Science (NIFoS), Korea (grant number FP0802-2023-01-2023).
Data Availability Statement
The data presented in this study are available under permission from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sylvia, D.M.; Hartel, P.G.; Fuhrmann, J.J.; Zuberer, D.A. Principles and Applications of Soil Microbiology; Pearson Education Inc.: London, UK, 2005; pp. 3–20. [Google Scholar]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent understanding of soil Acidobacteria and their ecological significance: A critical review. Front. Microbiol. 2020, 11, 5800214. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Serala, H.; Putten, W.H.; Wall, D.H. Ecological Linkages Between Aboveground and Belowground Biota. Seience 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.K.; Tayung, K.; Rath, C.C.; Parida, D. Occurrence of culturable soil fungi in a tropical moist deciduous forest Similipal Biosphere Reserve, Odisha, India. Braz. J. Microbiol. 2015, 46, 85–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, Y.; Wang, M.; Zhang, W.; Ni, Z.; Hashidoko, Y.; Shen, W. Ammonium nitrogen content is a dominant predictor of bacterial community composition in an acidic forest soil with exogenous nitrogen enrichment. Sci Total Environ. 2018, 624, 407–415. [Google Scholar] [CrossRef]
- Gazulla, C.R.; Auladell, A.; Ruiz-Gonzalez, C.; Junger, P.C.; Royo-Llonch, M.; Duarte, C.M.; Gasol, J.M.; Sanchez, O.; Ferrera, I. Global diversity and distribution of aerobic anoxygenic phototrophs in the tropical and subtropical oceans. Environ. Microbiol. 2022, 24, 2222–2238. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Huang, L.; Shan, Q.; Hu, A.; Yan, D.; Zhang, J.; Long, X.E. Soil profile rather than reclamation time drives the mudflat soil microbial community in the wheat-maize rotation system of Nantong, China. J. Soil. Sediment. 2021, 21, 1672–1687. [Google Scholar] [CrossRef]
- Kim, K.Y.; Um, Y.; Jeong, D.H.; Kim, H.J.; Kim, M.J.; Jeon, K.S. Study on the correlation between the soil bacterial community and growth characteristics of wild-simulated ginseng (Panax ginseng C.A. Meyer). Korean J. Environ. Biol. 2019, 37, 380–388. [Google Scholar] [CrossRef]
- Kim, K.Y.; Han, K.M.; Kim, H.J.; Jeon, K.S.; Kim, C.W.; Jung, C.R. The study of soil chemical properties and soil bacterial communities on the cultivation systems of Cnidium officinale Makino. Korean J. Environ. Agric. 2020, 39, 1–9. [Google Scholar] [CrossRef]
- Lee, T.B. Coloured Flora of Korea; Hayangmunsa: Seoul, Republic of Korea, 2003; Volume 1, pp. 713–720. [Google Scholar]
- Kim, H.N.; Park, G.H.; Park, S.B.; Kim, J.E.; Eo, H.J.; Son, H.J.; Song, J.H.; Jeong, J.B. Extracts from Sageretia thea reduce cell viability through inducing cyclin D1 proteasomal degradation and HO-1 expression in human colorectal cancer cells. BMC Complement. Altern. Med. 2019, 19, 43. [Google Scholar] [CrossRef]
- Shah, S.; Din, S.; Khan, A.; Rehmanullah; Shah, S.A. Green Synthesis and Antioxidant Study of Silver Nanoparticles of Root Extract of Sageretia thea and Its Role in Oxidation Protection Technology. J. Polym. Environ. 2018, 26, 2323–2332. [Google Scholar] [CrossRef]
- Pyo, S.J.; Le, Y.J.; Park, S.I.; Lee, C.I.; Park, J.Y.; Sohn, H.Y. Evaluation of the Anti-thrombosis Activities of the Aerial Parts of Sageretia thea. J. Life Sci. 2020, 30, 443–451. [Google Scholar]
- Eo, H.J.; Kim, D.S.; Kang, Y.G.; Kim, K.Y.; Park, Y.K.; Park, G.H. Antioxidant and immunoregulatory effects of Korean Rhamnaceae. J. Plant Biotechnol. 2020, 47, 254–259. [Google Scholar] [CrossRef]
- Ko, G.A.; Hoh, S.Y.; Ryu, J.Y.; Kim, S.M. Comparison of proximate compositions, antioxidant, and antiproliferative activities between blueberry and Sageretia thea (Osbeck) M.C.Johnst fruit produced in Jeju Island. J. Appl. Biol. Chem. 2017, 60, 161–171. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.Y.; Jung, T.K. Skin Physiological Activities from Sageretia thea Callus Extract. J. Investig. Cosmetol. 2022, 18, 451–456. [Google Scholar]
- Yang, Y.; Peng, H.; Sun, H. Taxonomic revision of Sageretia (Rhamnaceae) from China I: Identities of S. lucida, S. thea var. cordiformis and S. yunlongensis, with the description of a new species S. ellipsoidea. PhytoKeys 2021, 179, 13–28. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal associations and other means of nutrition of vascular plants: Understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Lendenmann, M.; Thonar, C.; Barnard, R.L.; Salmon, Y.; Werner, R.A.; Frossard, E.; Jansa, J. Symbiont identity matters: Carbon and phosphorus fluxes between Medicago truncatula and different arbuscular mycorrhizal fungi. Mycorrhiza 2011, 21, 689–702. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, S.W.; Kim, Y.S.; Lamsal, K.; Lee, Y.S. Inhibition effects against plant pathogenic pungi and plant growth promotion by beneficial microorganisms. Korean J. Mycol. 2013, 41, 118–126. [Google Scholar] [CrossRef]
- Lee, B.H.; Han, H.K.; Kwon, H.J.; Eom, A.H. Diversity of Endophytic Fungi Isolated from Roots of Cypripedium japonicum and C. macranthum in Korea. Korean J. Mycol. 2015, 43, 20–25. [Google Scholar] [CrossRef]
- Leake, J.R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Raven, P.H.; Hong, D. (Eds.) Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 12, pp. 133–139. [Google Scholar]
- Son, Y.H.; Son, H.G.; Park, G.H.; Lee, D.H.; Cho, H.J.; Lee, S.Y.; Kim, H.J. Growing environment characteristics and vegetational structure of Sageretia thea, medicinal plant. Korean J. Plant Res. 2020, 35, 594–606. [Google Scholar]
- National Geographic Information Institute. The National Atlas Korea II; National Geographic Information Institute: Suwon, Republic of Korea, 2020; pp. 52–73. [Google Scholar]
- Choi, G.Y. Spatio-temporal patterns and long-term trends of apparent temperature in Jeju island, Korea. JAKG 2018, 7, 29–41. [Google Scholar] [CrossRef]
- Tredennick, A.T.; Teller, B.J.; Adler, P.B.; Hooker, G.; Ellner, S.P. Size-by-environment interactions: A neglected dimension of species’ responses to environmental variation. Ecol. Lett. 2018, 21, 1757–1770. [Google Scholar] [CrossRef]
- Winn, A.A. Adaptation to fine-grained environmental variation: An analysis of within-individual leaf variation in an annual plant. Evolution 1996, 50, 1111–1118. [Google Scholar]
- Williams, A.; Sinanaj, B.; Hoysted, G.A. Plant-microbe interactions through a lens: Tales from the mycorrhizosphere. Ann. Bot. 2024, 133, 399–412. [Google Scholar] [CrossRef]
- Moon, J.G.; Shim, C.S.; Jung, O.J.; Hong, J.W.; Han, J.H.; Song, Y.I. Characteristics in regional climate change over South Korea for regional climate policy measures: Base on long-term observations. J. Clim. Change Res. 2020, 11, 755–770. [Google Scholar] [CrossRef]
- Eom, T.Y.; Khang, Y.H. Changes in the microbiome of Haeundae beach in summer. Korean J. Microbiol. 2021, 57, 243–248. [Google Scholar]
- Fierer, N.; Bradford, M.A.; Jackson, R.B. Toward an ecological classification of soil bacteria. Ecology 2007, 88, 1354–1364. [Google Scholar] [CrossRef]
- Inagaki, F.; Suzuki, M.; Takai, K.; Oida, H.; Sakamoto, T.; Aoki, K.; Nealson, K.H.; Horikoshi, K. Microbial communities associated with geological horizons in coastal subseafloor sediments from the sea of Okhotsk. Appl. Environ. Microbiol. 2003, 69, 7224–7235. [Google Scholar] [CrossRef]
- Du, J.; Xiao, K.; Li, L.; Ding, X.; Liu, H.; Lu, Y.J.; Zhou, S. Temporal and spatial diversity of bacterial communities in coastal waters of the South China sea. PLoS ONE 2013, 8, e66968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, H.; Csuros, M.; Hughes, A.L.; Moran, M.A. Evolution of divergent life history strategies in marine Alphaproteobacteria. MBio 2013, 4, e00373-13. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Su, W.; Chen, H.; Barberan, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases vacterial diversity and favors the growth of Actiovacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, S.H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Soung, S.H.; Kim, K.H.; Hwang, S.J.; Oh, Y.T.; Park, S.H.; Lee, S.G.; Jeong, H.I.; Han, S.I. Molecular genetic analysis of Actinobacterial odorous substances (geosmin, 2-MIB) in North Han River watershed. Korean J. Microbiol. 2022, 58, 245–254. [Google Scholar]
- Zheng, Q.; Hua, Y.; Zhang, S.; Noll, L.; Böckle, T.; Dietrich, M.; Herbold, C.W.; Eichorst, S.A.; Woebken, D.; Richter, D.; et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity. Soil Biol. Biochem. 2019, 136, 107521. [Google Scholar] [CrossRef]
- Sait, M.; Davis, K.E.R.; Janssen, P.H. Effect of pH on isolation and distribution of members of subdivision 1 of the Phylum Acidobacteria occurring in soil. Appl. Environ. Microbiol. 2006, 72, 1852–1857. [Google Scholar] [CrossRef]
- Chauhan, P.; Sharma, N.; Tapwal, A.; Kumar, A.; Verma, G.S.; Meena, M.; Seth, C.S.; Swapnil, P. Soil microbiome: Diversity, benefits and interactions with plants. Sustainability 2023, 15, 14643. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Venturini, A.M.; Meyer, K.M.; Klein, A.M.; Tiedje, J.M.; Bohannan, B.J.M.; Nüsslein, K.; Siu, M.; Tsai, S.M.; Rodrigues, J.L.M. Differential response of Acidobacteria subgroups to forest-to-pasture conversion and their biogeographic patterns in the Western Brazilian Amazon. Front. Microbiol. 2015, 6, 1443. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhou, X.; Guo, D.; Zhao, J.H.; Yan, L.; Feng, G.Z.; Gao, Q.; Yu, H.; Zhao, L.P. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Lee, Y.; Kwak, Y.S. Synergistic inhibitory effect of Actinomycetes and Bactericides against fire blight pathogen. Korean J. Pestic. Sci. 2024, 28, 13–21. [Google Scholar] [CrossRef]
- Liu, X.; Cong, J.; Lu, H.; Xue, Y.; Wang, X.; Li, D.; Zhang, Y. Community structure and elevational distribution pattern of soil Actinobacteria in alpine grasslands. Acta Ecol. Sin. 2017, 37, 213–218. [Google Scholar] [CrossRef]
- Yoo, M.G.B.G.B.N.L.; Choi, H.J.; Lee, M.S.; Lee, S.Y. Measurement of Properties of Domestic Bentonite for a Buffer of an HLW Repository. J. Nucl. Fuel Cycle Waste Technol. 2016, 14, 135–147. [Google Scholar]
- Kim, G.Y.; Lee, Y.J.; Cho, E.J.; Lee, J.I.; Im, E.C.; Hwang, H.; Kim, S.Y.; Hong, S.C.; Kim, J.H.; Park, S.J. Investigation of factors influencing on ammonia emission from soils in agricultural land. J. Korean Soc. Environ. Eng. 2022, 44, 444–452. [Google Scholar] [CrossRef]
- Lynn, T.M.; Liu, Q.; Hu, Y.; Yuan, H.; Wu, X.; Khai, A.A.; Wu, J.; Ge, T. Infuence of land use on bacterial and archaeal diversity and community structures in three natural ecosystems and one agricultural soil. Arch. Microbiol. 2017, 199, 711–721. [Google Scholar] [CrossRef]
- Catania, V.; Bueno, R.S.; Alduina, R.; Grilli, E.; Mantia, T.L.; Castaldi, S.; Quatrini, P. Soil microbial biomass and bacterial diversity in southern European regions vulnerable to desertification. Ecol. Indic. 2022, 145, 109725. [Google Scholar] [CrossRef]
- Kim, K.; Kim, H.J.; Jeong, D.H.; Huh, J.H.; Jeon, K.S.; Um, Y. Correlation between soil bacterial community structure and soil properties in cultivation sites of 13-Year-old wild-simulated ginseng (Panax ginseng C.A. Meyer). Appl. Sci. 2021, 11, 937. [Google Scholar] [CrossRef]
- Spain, A.M.; Krumholz, L.R.; Elshahed, M.S. Abundance, composition, diversity and novelty of soil Proteobacteria. ISME J. 2009, 8, 992–1000. [Google Scholar] [CrossRef]
- Bruto, M.; Prigent-Combatet, C.; Muller, D.; Moenne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related Proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef]
- Poirier, Y.; Jaskolowski, A.; Clúa, J. Phosphate acquisition and metabolism in plants. Curr. Biol. 2022, 32, R623–R629. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Rosillo, A.C.; Peralta-Alvarez, C.A.; Ojeda-Rivera, J.O.; Arzate-Mejía, R.G.; Recillas-Targa, F.; Herrera-Estrella, L. GGenome accessibility dynamics in response to phosphate limitation is controlled by the PHR1 family of transcription factors in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 33, 118. [Google Scholar]
- Orellana, D.; Machuca, D.; Ibeas, M.A.; Estevez, J.M.; Poupin, M.J. Plant-growth promotion by proteobacteria strains depends on the availability of phosphorus and iron in Arabidopsis thaliana plants. Front. Mocrobiol. 2022, 13, 1083270. [Google Scholar]
- Wei, M.; Liu, X.; He, Y.; Xu, X.; Wu, Z.; Yu, K.; Zheng, X. Biochar inoculated with Pseudomonas putida improves grape (Vitis vinifera L.) fruit quality and alters bacterial diversity. Rhizosphere 2020, 16, 100261. [Google Scholar]
- Zhang, D.; Yan, D.; Fang, W.; Huang, B.; Wang, X.; Wang, X.; Zhu, J.; Liu, J.; Ouyang, C.; Li, Y.; et al. Chloropicrin alternated with biofumigation increases crop yield and modifies soil bacterial and fungal communities in strawberry production. Sci. Total Environ. 2019, 675, 615–622. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Z.; Zhu, Y.; Wang, J.; Liu, B. Efects of a microbial restoration substrate on plant growth and rhizosphere bacterial community in a continuous tomato cropping greenhouse. Sci. Rep. 2020, 10, 13729. [Google Scholar] [CrossRef]
- Che, J.; Wu, Y.; Yang, H.; Wang, S.; Wu, W.; Lyu, L.; Li, W. Long-term cultivation drives dynamic changes in the rhizosphere microbial community of blueberry. Front. Plant Sci. 2022, 13, 962759. [Google Scholar] [CrossRef]
- Jeong, D.H.; Kwon, H.Y.; Kim, Y.K. Phenotypical characteristics investigation and selection of superior individuals from natural habitats of Sageretia thea in South Korea. Korean J. Plant Res. 2024, 37, 214–224. [Google Scholar]
- Sparks, D.L. Environmental Soil Chemistry; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–37. [Google Scholar] [CrossRef]
- Konen, M.E.; Jacobs, P.M.; Burras, C.L.; Talaga, B.J.; Mason, J.A. Equations for predicting soil organic carbon using loss-on-ignition for north central U.S. Soil Science. Soil Sci. Soc. Am. J. 2002, 66, 1878–1881. [Google Scholar] [CrossRef]
- Sumner, M.E.; Miller, W.P. Cation exchange capacity and exchange coefficients. In Methods of Soil Analysis. Part 3: Chemical Methods, 3rd ed.; Sparks, D.L., Ed.; Soil Science Society of America Book Series No. 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Canfora, L.; Vendramin, E.; Felici, B.; Tarricone, L.; Florio, A.; Benedetti, A. Vineyard microbiome variations during different fertilization practices revealed by 16S rRNA gene sequencing. Appl. Soil Ecol. 2018, 123, 71–80. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.K.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Opensource, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS ONE 2009, 4, e8230. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).