Salt Tolerance of Sea Flax (Linum maritimum L.), a Rare Species with Conservation Interest in Eastern Spain
Abstract
1. Introduction
2. Results
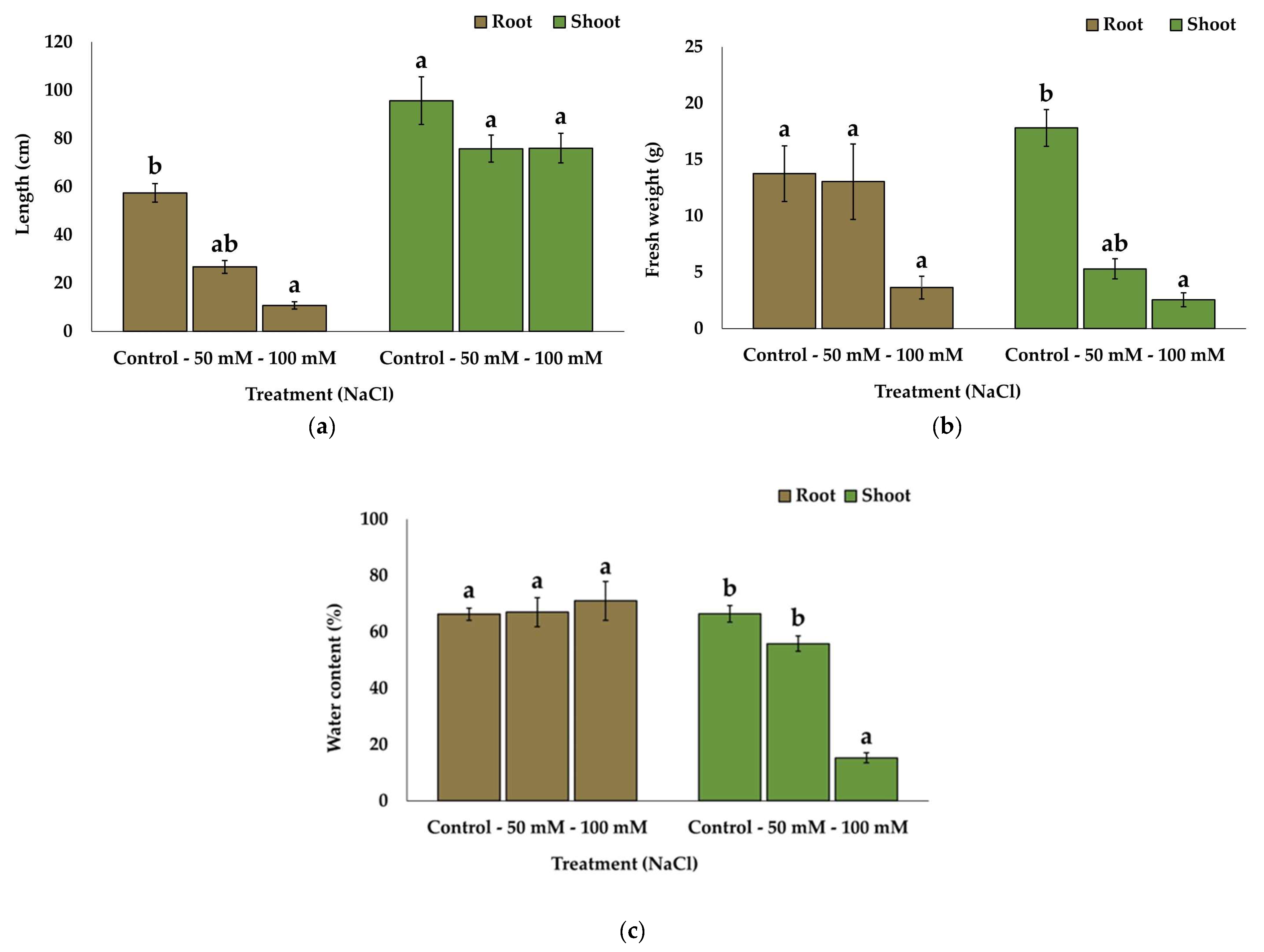
2.1. Substrate Analysis and Growth Parameters
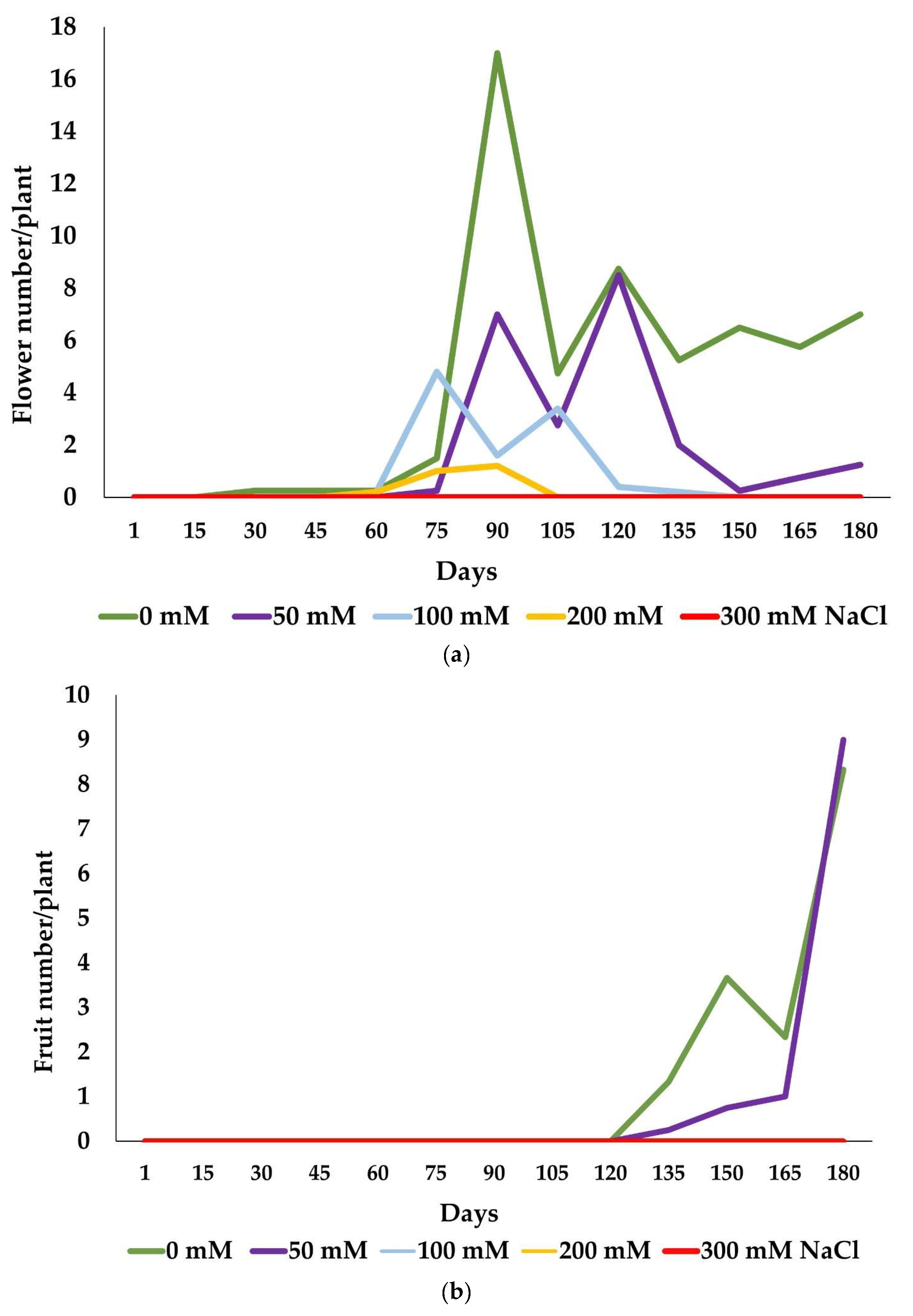
2.2. Reproductive Success
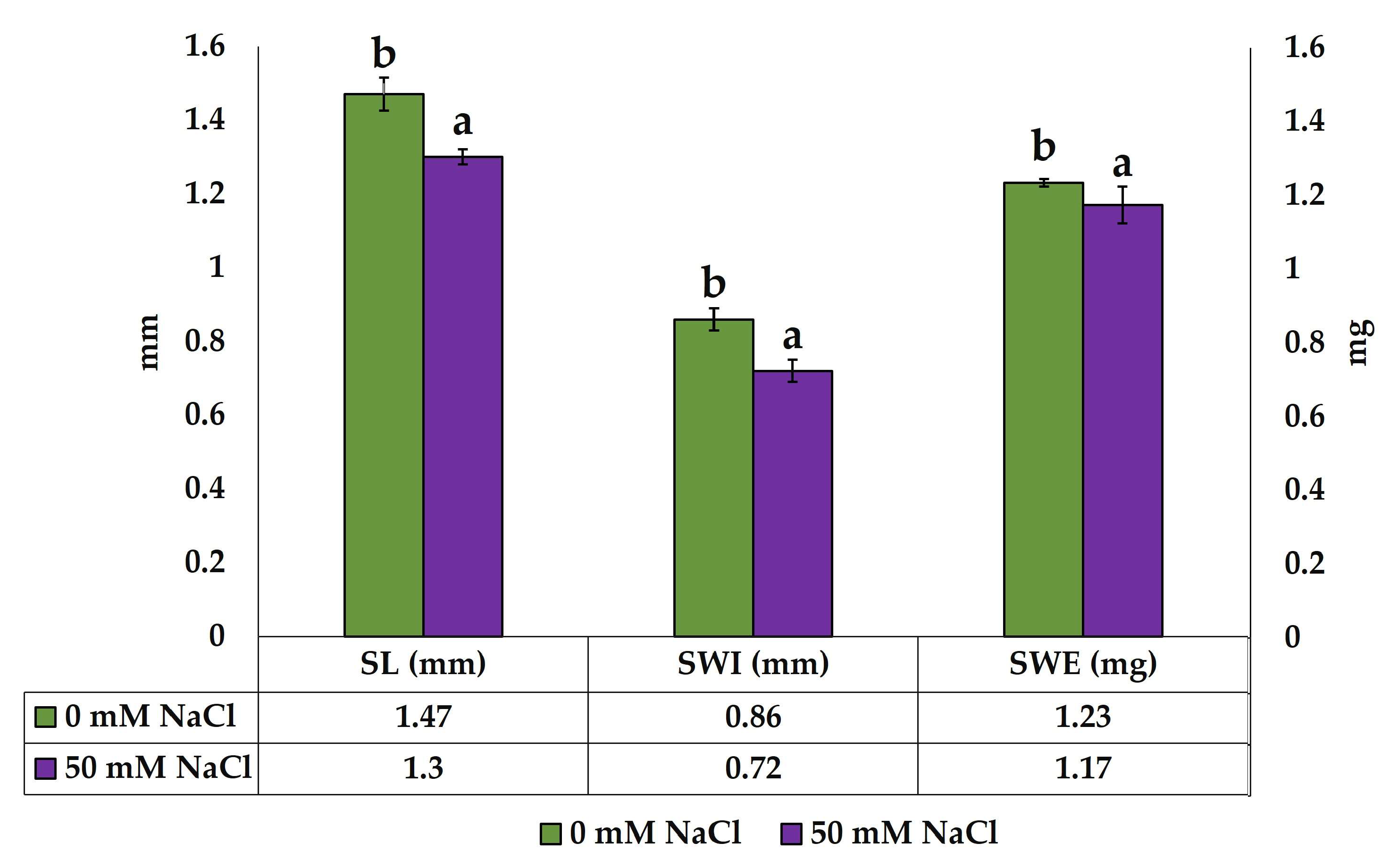
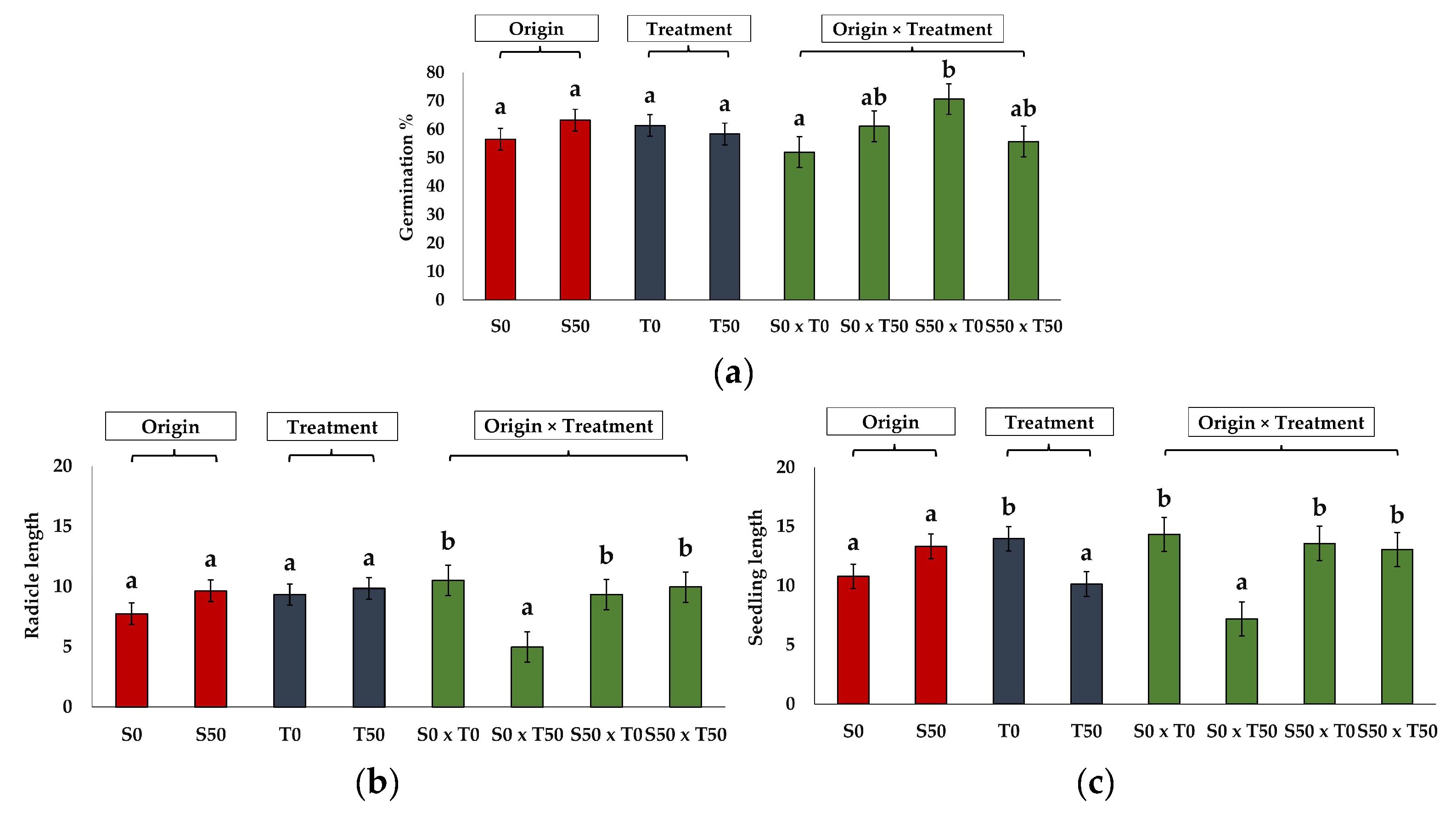
2.3. Germination of Seeds Produced by Control and Salt-Treated Plants
2.4. Analysis of Biochemical Parameters
2.5. Ion Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Stress Treatments in the Greenhouse
4.2. Substrate Analysis
4.3. Plant Growth and Reproductive Parameters
4.4. Seed Germination Tests
germinated seeds on the last day of germination)] × 100
4.5. Biochemical Analyses
4.5.1. Photosynthetic Pigments
4.5.2. Quantification of Osmolytes
4.5.3. Determination of Oxidative Stress Markers and Antioxidant Compounds
4.6. Quantification of Ions
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2013—The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauel, A., Xia, Y., Midgley, P., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Shehu, J.; Mullaj, A.; Ibraliu, A. Salt marshes plant diversity of coastal zone in Albania. In Proceedings of the BALWOIS, Ohrid, North Macedonia, 25–29 May 2010. [Google Scholar]
- Boorman, L.A. Salt marshes—Present functioning and future change. Mangroves Salt Marshes 1999, 3, 227–241. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Wu, H.; Bose, J. Salt stress sensing and early signalling events in plant roots: Current knowledge and hypothesis. Plant Sci. 2015, 4, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Marcum, K.B.; Murdoch, C.L. Salt tolerance of the coastal salt marsh grass, Sporobolus virginicus (L.) Kunth. New Phytol. 1992, 120, 281–288. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Weigelt, B.; Santos-Guerra, A.; Caujapé-Castells, J.; Fernández-Palacios, J.M.; Conti, E. Surviving in isolation: Genetic variation, bottlenecks and reproductive strategies in the Canarian endemic Limonium macrophyllum (Plumbaginaceae). Genetica 2017, 145, 91–104. [Google Scholar] [CrossRef]
- Sorce, C.; Bottega, S.; Spanò, C. Seasonal and microclimatic influences on the ecophysiology of Mediterranean coastal dune plants. Estuar. Coast. Shelf Sci. 2019, 219, 317–327. [Google Scholar] [CrossRef]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone, J.A.; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. USA 2007, 104, 198–202. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.A.; Zavaleta, E.S.; Selmants, P.C. Flowering phenology shifts in response to biodiversity loss. Proc. Natl. Acad. Sci. USA 2017, 114, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Ungar, I.A. Halophyte Seed Germination. Bot. Rev. 1978, 44, 233–264. [Google Scholar] [CrossRef]
- Ungar, I.A. Seed germination and seed-bank ecology in halophytes. In Seed Development and Germination; Kigel, J., Galili, G., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 599–628. [Google Scholar]
- Gul, B.; Ansari, R.; Flowers, T.; Khan, M. Germination strategies of halophyte seeds under salinity. Environ. Exp. Bot. 2012, 92, 4–18. [Google Scholar] [CrossRef]
- Lombardi, T.; Bedini, S. Seed germination strategies of Mediterranean halophytes under saline condition. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Vromans, J. Molecular Genetic Studies in Flax (Linum usitatissimum L.). Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2006. [Google Scholar]
- Hickey, M. 100 Families of Flowering Plants; Cambridge University Press: Cambridge, UK, 1988; p. 624. [Google Scholar]
- Heywood, V.H. Flowering Plants of the World; Oxford University Press: Oxford, UK, 1993; p. 335. [Google Scholar]
- McDill, J.R.; Repplinger, M.; Simpson, B.B.; Kadereit, J.W. The phylogeny of Linum and Linaceae subfamily Linoideae, with implications for their systematics, biogeography, and evolution of heterostyly. Syst. Bot. 2009, 34, 386–405. [Google Scholar] [CrossRef]
- McDill, J.R.; Simpson, B.B. Molecular phylogenetics of Linaceae with complete generic sampling and data from two plastid genes. Bot. J. Linn. Soc. 2011, 165, 64–83. [Google Scholar] [CrossRef]
- Winkler, H. Linaceae. In Die Natürlichen Pflanzenfamilien; Engler, H.G.A., Prantl, K.A.E., Eds.; Engelmann: Leipzig, Germany, 1931; Volume 19, pp. 82–130. [Google Scholar]
- Rogers, C.M. The systematics of Linum sect. Linopsis (Linaceae). Plant Syst. Evol. 1982, 140, 225–234. [Google Scholar] [CrossRef]
- Diederichsen, A.; Richards, K. Cultivated flax and the genus Linum L.: Taxonomy and germplasm conservation. In Flax: The Genus Linum; Muir, A.D., Westcott, N.D., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 22–54. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book: A Portable Dictionary of Plants, Their Classification and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; 1019p. [Google Scholar]
- Greuter, W.; Burdet, H.M.; Long, G. (Eds.) Med-Checklist: A Critical Inventory of Vascular Plants of the Circum-Mediterranean Countries; Dicotyledones (Lauraceae-Rhamnaceae); Conservatoire et Jardin Botanique Ville de Genève, Med-Checklist Trust of Optima: Geneva, Switzerland; Berlin, Germany, 1989; Volume 4, 458p. [Google Scholar]
- Baytop, T. Therapy with Medicinal Plants in Turkey (Past and Present), 2nd ed.; Nobel Tıp Kitabevi: Istanbul, Turkey, 1999; 163p. [Google Scholar]
- Pengilly, N.L. Traditional food and medicinal uses of flaxseed. In Flax: The Genus Linum; Muir, A.D., Westcott, N.D., Eds.; Taylor and Francis: London, UK, 2003; pp. 252–267. [Google Scholar]
- Ockendon, D.J.; Walters, S.M. Linaceae. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1968; Volume 2, pp. 589–603. [Google Scholar]
- Laguna, E.; Fos, S.; Ferrando-Pardo, I.; Ferrer-Gallego, P.P. Endangered Halophytes and Their Conservation. In Handbook of Halophytes; Springer: Cham, Switzerland, 2021. [Google Scholar]
- Mansanet, J. Flora y Vegetación de la Dehesa de La Albufera [Flora and Vegetation of the Dehesa de La Albufera]; Departamento de Botánica, Facultad de Ciencias, Universidad de Valencia: Valencia, Spain, 1979. [Google Scholar]
- González-Orenga, S.; Trif, C.; Donat-Torres, M.P.; Llinares, J.V.; Collado, F.; Ferrer-Gallego, P.P.; Laguna, E.; Boscaiu, M.; Vicente, O. Responses to increased salinity and severe drought in the eastern Iberian endemic species Thalictrum maritimum (Ranunculaceae), threatened by climate change. Plants 2020, 9, 1251. [Google Scholar] [CrossRef]
- Mateo, G.; Vizcaíno, A. Flora y vegetación del Parque Natural de l’Albufera. In Monografías de Botánica Ibérica; No. 28; Jolube: Jaca, Spain, 2023. [Google Scholar]
- Ferrer-Gallego, P.P. Tipificación de dos nombres linneanos del género Linum L.: L. maritimum y L. narbonense (LInaceae). Boletín Real Soc. Española Hist. Nat. Sección Biol. 2014, 108, 103–106. [Google Scholar]
- Martínez Labarga, J.M.; Muñoz Garmendia, F. Linum L. In Flora Iberica; Real Jardín Botánico, CSIC: Madrid, Spain, 2015; Volume 9, pp. 174–266. [Google Scholar]
- POWO. Linum maritimum. Plant of the World. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:544571-1 (accessed on 1 November 2023).
- Care Mediflora—Sóller Botanical Garden Foundation, Balearic Islands. Available online: http://www.care-mediflora.eu/en/outputs/in_situ (accessed on 3 October 2023).
- Laguna, E.; Deltoro, V.; Fos, S.; Pérez-Rovira, P.; Ballester, G.; Olivares, A.; Serra, L.; Pérez-Botella, J. Priority Habitats of the Valencian Community; Generalitat Valenciana: Valencia, Spain, 2003. [Google Scholar]
- González-Orenga, S.; Ferrer-Gallego, P.P.; Laguna, E.; López-Gresa, M.P.; Donat-Torres, M.P.; Verdeguer, M.; Vicente, O.; Boscaiu, M. Insights on salt tolerance of two endemic Limonium species from Spain. Metabolites 2019, 9, 294. [Google Scholar] [CrossRef]
- Forte Gil, J.A.; Yabor, L.; Bellido Nadal, A.; Collado, F.; Ferrer-Gallego, P.; Vicente, O.; Boscaiu, M. A Methodological Approach for Testing the Viability of Seeds Stored in Short-Term Seed Banks. Not. Sci. Biol. 2017, 9, 563–570. [Google Scholar] [CrossRef]
- eHALOPH. Available online: http://www.sussex.ac.uk/affiliates/halophytes/ (accessed on 1 November 2023).
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Grigore, M.N. Definition and classification of halophytes as an ecological group of plants. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Cham, Switzerland, 2021; pp. 3–50. [Google Scholar]
- European Commission. DG Evironment 2013: Interpreation Manual of European Union Habitats; European Commission: Brussels, Belgium, 2013. [Google Scholar]
- Mercadal i Corominas, G. Caracterización geobotánica de algunos sintaxones de prados mesófilos (all. Brachypodio-Centaureion nemoralis) del sudoeste de Europa. Acta Bot. Malacit. 2021, 46, 45–55. [Google Scholar] [CrossRef]
- Boira, H. Edaphic characteristics of salt meadow vegetation in the eastern regions of Spain. Ecol Medit. 1995, 21, 1–11. [Google Scholar] [CrossRef]
- Kargas, G.; Londra, P.; Sotirakoglou, K. The effect of soil texture on the conversion factor of 1:5 soil/water extract electrical conductivity (EC1:5) to soil saturated paste extract electrical conductivity (ECe). Water 2022, 14, 642. [Google Scholar] [CrossRef]
- Bech i Borràs, J.; Hernández, A.M. Estudios sobre suelos y vegetación del delta del Llobregat. Coll. Bot. 1976, 10, 31–105. [Google Scholar]
- Guo, J.; Li, Y.; Han, G.; Song, J.; Wang, B.S. NaCl markedly improved the reproductive capacity of the euhalophyte Suaeda salsa. Funct. Plant Biol. 2018, 44, 350–361. [Google Scholar] [CrossRef]
- Yuan, F.; Guo, J.; Shabala, S.; Wang, B. Reproductive physiology of halophytes: Current Standing. Front. Plant Sci. 2019, 9, 1954. [Google Scholar] [CrossRef]
- Rogers, C.M. Pollen dimorphism in distylous species of Linum sect. Linastrum (Linaceae). Grana 1980, 19, 19–20. [Google Scholar] [CrossRef]
- Murray, B. Floral biology and self-incompatibility in Linum. Bot. Gaz. 1986, 147, 327–333. [Google Scholar] [CrossRef]
- Van Zandt, P.A.; Mopper, S. The effects of maternal salinity and seed environment on germination and growth in Iris hexagona. Evol. Ecol. Res. 2004, 6, 813–832. [Google Scholar]
- Shah, S.Z.; Rasheed, A.; Gul, B. Maternal salinity improves yield, size and stress tolerance of Suaeda fruticosa seeds. J. Arid Land 2020, 12, 283–293. [Google Scholar] [CrossRef]
- Mohamed, E.; Kasem, A.M.; Gobouri, A.A.; Elkelish, A.; Azab, E. Influence of maternal habitat on salt tolerance during germination and growth in Zygophyllum coccineum. Plants 2020, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Schaun Harter, F.; Holbig Harter, L.S.; Meneghello, G.E. Rice seed production under conditions of salinity stress. Cienc. Rural 2018, 48, e20170057. [Google Scholar] [CrossRef]
- Pezo, C.; Valdebenito, S.; Flores, M.F. Impact of mother plant saline stress on the agronomical quality of pepper seeds. J. Soil Sci. Plant Nutr. 2020, 20, 2600–2605. [Google Scholar] [CrossRef]
- Nguyen, C.D.; Chen, J.; Clark, D.; Perez, H.; Huo, H. Effects of maternal environment on seed germination and seedling vigor of Petunia × hybrida under different abiotic stresses. Plants 2021, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Bhargava, A.; Fuentes, F.; Shukla, S.; Srivastava, S. Effect of salinity stress on yield and quality parameters in flax (Linum usitatissimum L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 954–966. [Google Scholar] [CrossRef]
- Abdullah, M.A.; Bibi, S.; Naqve, M.; Javaid, M.M.; Zia, M.A.; Jabbar, A.; Ud-Din, W.; Attia, K.A.; Khan, N.; Al-Doss, A.A.; et al. Physiological, biochemical, and yield responses of linseed (Linum usitatissimum L.) in α-Tocopherol-mediated alleviation of salinity stress. Front. Plant Sci. 2022, 13, 867172. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signaling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Alaei, Y.; Kazemitabar, S.; Zadeh, M.; Zarini, H.; Kiani, G. A study on the salinity stress effects on the biochemical traits of seedlings and its relationship with resistance toward sensitive and tolerant flax genotypes. J. Appl. Biol. 2020, 8, 47–52. [Google Scholar] [CrossRef]
- Pervaiz, S.; Gul, H.; Rauf, M. Screening of Linum usitatissimum lines using growth attributes, biochemical parameters, and ionomics under salinity stress. Gesunde Pflanz. 2023, 75, 2591–2609. [Google Scholar] [CrossRef]
- Patil, N.M.; Datir, S.S.; Shash, P.V. Salt-induced physiological and biochemical changes in two varieties of Linum usitatissimum L. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 296–304. [Google Scholar]
- Abido, W.A.E.; Zsombik, L. Effect of salinity on germination characters and seedlings parameters of Egyptian flax cultivars growing in Nyiregyhaza. Acta Ecol. Sin. 2019, 39, 102–108. [Google Scholar] [CrossRef]
- Qayyum, M.A.; Akhtar, J.; Bashir, F.; Naz, T.; Iqbal, M.M.; Farooq, O.; Atique-ur, R.; Zafar, M.I.; Ali, M.; Imtiaz, M.; et al. Physiological and biochemical characterization of linseed genotypes under salinity stress. Int. J. Agric. Biol. 2020, 23, 630–636. [Google Scholar] [CrossRef]
- Kiani, R.; Nazeri, V.; Shokrpour, M.; Hano, C. Morphological, physiological, and biochemical impacts of different levels of long-term water deficit stress on Linum album Ky. ex Boiss. accessions. Agronomy 2020, 10, 1966. [Google Scholar] [CrossRef]
- Calone, R.; Mircea, D.-M.; González-Orenga, S.; Boscaiu, M.; Lambertini, C.; Barbanti, L.; Vicente, O. Recovery from salinity and drought stress in the perennial Sarcocornia fruticosa vs. the annual Salicornia europaea and S. veneta. Plants 2022, 11, 1058. [Google Scholar] [CrossRef]
- Giri, J. Glycinebetaine and abiotic stress tolerance in plants. Plant Signal Behav. 2011, 6, 1746–1751. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, W.; Wang, C.; Meng, Q.; Li, G.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycinebetaine leads to alleviate salt-induced potassium efflux and enhances salt tolerance in tomato plants. Plant Sci. 2017, 257, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gil, R.; Boscaiu, M.; Lull, C.; Bautista, I.; Lidón, A.; Vicente, O. Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 2013, 40, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Pellegrini, E.; Cotrozzi, L.; Neri, L.; Baraldi, R.; Carrari, E.; Nali, C.; Lorenzini, G.; Paoletti, E.; Hoshika, Y. Stress markers and physiochemical responses of the Mediterranean shrub Phillyrea angustifolia under current and future drought and ozone scenarios. Environ. Res. 2021, 201, 111615. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Qiu, C.; Ye, Y.; Guo, X.; Chen, G.; Li, T.; Wang, Y.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem. 2017, 214, 227–233. [Google Scholar] [CrossRef]
- Khan, M.; Siddiqui, M.; Firoz, M.; Naeem, M.; Khan, M. M Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol. Plant 2009, 32, 121–132. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The Role of Na+ and K+ Transporters in Salt Stress Adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Greenway, H.; Munns, R. Mechanisms of salt tolerance in nonhalophytes. Annu. Rev. Plant Physiol 1980, 31, 149–190. [Google Scholar] [CrossRef]
- Sun, Y.; Lindberg, S.; Shabala, L.; Morgan, S.; Shabala, S.; Jacobsen, S.E. A comparative analysis of cytosolic Na+ changes under salinity between halophyte quinoa (Chenopodium quinoa) and glycophyte pea (Pisum sativum). Environ. Exp. Bot. 2017, 141, 154–160. [Google Scholar] [CrossRef]
- Al Hassan, M.; Pacurar, A.; López-Gresa, M.P.; Donat-Torres, M.P.; Llinares, J.V.; Boscaiu, M.; Vicente, O. Effects of salt stress on three ecologically distinct Plantago species. PLoS ONE 2016, 11, e0160236. [Google Scholar] [CrossRef]
- Al Hassan, M.; López-Gresa, M.P.; Boscaiu, M.; Vicente, O. Stress tolerance mechanisms in Juncus: Responses to salinity and drought in three Juncus species adapted to different natural environments. Funct. Plant Biol. 2016, 43, 949–960. [Google Scholar] [CrossRef] [PubMed]
- González Orenga, S.; Leandro, M.E.; Tortajada, L.; Grigore, M.; Llorens, J.; Ferrer-Gallego, P.P.; Laguna, E.; Boscaiu, M.; Vicente, O. Comparative studies on the stress responses of two Bupleurum (Apiaceae) species in support of conservation programmes. Environ. Exp. Bot. 2021, 191, 104616. [Google Scholar] [CrossRef]
- Ellis, R.A.; Roberts, E.H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kader, M. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. NSW 2005, 138, 65–75. [Google Scholar] [CrossRef]
- Islam, A.K.M.A.; Anuar, N.; Yaakob, Z. Effect of genotypes and pre-sowing treatments on seed germination behavior of Jatropha. Asian J. Plant Sci. 2009, 8, 433–439. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Grieve, C.M.; Grattan, S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Valadez-Bustos, M.G.; Aguado-Santacruz, G.A.; Tiessen-Favier, A.; Robledo-Paz, A.; Muñoz-Orozco, A.; Rascón-Cruz, Q.; Santacruz-Varela, A. A reliable method for spectrophotometric determination of glycine betaine in cell suspension and other systems. Anal. Biochem. 2016, 498, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Taulavuori, E.; Hellström, E.; Taulavuori, K.; Laine, K. Comparison of two methods used to analyse lipid peroxidation from Vaccinium myrtillus (L.) during snow removal, reacclimation and cold acclimation. J. Exp. Bot. 2002, 52, 2375–2380. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Blainski, A.; Lopes, G.; Mello, J. Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Weimberg, R. Solute adjustments in leaves of two species of wheat at two different stages of growth in response to salinity. Physiol. Plant 1987, 70, 381–388. [Google Scholar] [CrossRef]

| Treatment | Control | 50 mM NaCl | 100 mM NaCl | 200 mM NaCl | 300 mM NaCl |
|---|---|---|---|---|---|
| EC1:5 (dS m−1) | 1.50 ± 0.2 a | 3.37 ± 0.4 b | 4.03 ± 0.7 b | 9.04 ± 1.2 c | 11.06 ± 1.5 c |
| Survival rate (%) | 100 | 100 | 60 | 0 | 0 |
| Treatment | Control | 50 mM NaCl | 100 mM NaCl | 200 mM NaCl | 300 mM NaCl |
|---|---|---|---|---|---|
| Flower number | 146.65 ± 23.9 c | 51.01 ± 1.5 b | 39.20 ± 16.9 ab | 19.80 ± 3.8 a | 0 |
| Fruit number | 17.68 ± 2.6 a | 11.50 ± 2.3 a | 0 | 0 | 0 |
| Source of Seeds | Control | 50 mM NaCl | ||
|---|---|---|---|---|
| Germination Parameters | Control | 50 mM NaCl | Control | 50 mM NaCl |
| GP | 52.00 ± 8.0 a | 61.00 ± 5.1 ab | 70.70 ± 11.0 b | 55.70 ± 10.9 ab |
| MGT | 7.95 ± 0.9 a | 11.71 ± 1.2 b | 7.83 ± 1.3 a | 13.22 ± 1.1 b |
| FGD | 4.00 ± 0.0 ab | 4.00 ± 0.60 ab | 2.80 ± 0.2 a | 4.40 ± 0.5 b |
| LGD | 14.8 ± 3.8 a | 25.40 ± 1.0 b | 15.00 ± 4.9 a | 26.00 ± 0.5 b |
| TSG | 10.80 ± 3.8 a | 21.40 ± 1.5 b | 12.20± 5.0 ab | 21.60 ± 0.8 b |
| GI | 0.87 ± 0.1 a | 0.77 ± 0.2 a | 0.99 ± 0.1 a | 0.56 ± 0.1 a |
| SE | 29.95 ± 3.8 a | 39.33 ± 8.9 a | 24.24 ± 3.3 a | 31.67 ± 5.5 a |
| SVI | 7.60 ± 1.5 ab | 4.50 ± 0.7 a | 9.70± 1.6 b | 7.72 ± 2.7 ab |
| Hyp L (mm) | 3.82 ± 0.5 a | 2.22 ± 0.3 a | 4.24 ± 0.3 b | 3.10 ± 0.5 b |
| Rad L (mm) | 10.52 ± 1.4 ab | 4.99 ± 0.7 a | 9.34 ± 0.9 b | 9.96 ± 1.6 ab |
| Seedling length (mm) | 14.35 ± 1.6 b | 7.21 ± 0.9 a | 13.59 ± 0.6 b | 13.10 ± 2.0 b |
| Parameter | Origin | Treatment | Origin × Treatment |
|---|---|---|---|
| GP | 1.54 ns | 0.30 ns | 4.99 * |
| MGT | 0.32 ns | 14.62 ** | 0.47 ns |
| FGD | 0.91 ns | 3.66 ns | 3.66 ns |
| LGD | 0.02 ns | 11.58 ** | 0.00 ns |
| TSG | 0.06 ns | 9.28 ** | 0.03 ns |
| SVI | 4.09 ns | 3.72 ns | 0.21 ns |
| GI | 0.13 ns | 3.07 ns | 1.04 ns |
| SE | 1.31 ns | 2.06 ns | 0.03 ns |
| Hyp L | 2.01 ns | 8.94 ** | 0.26 ns |
| Rad L | 2.26 ns | 3.79 ns | 5.95 * |
| Seedling length | 3.10 ns | 7.00 * | 5.24 * |
| Treatment | Control | 50 mM NaCl |
|---|---|---|
| Chl a (mg g−1 DW) | 1.27 ± 0.2 a | 0.99 ± 0.3 a |
| Chl b (mg g−1 DW) | 0.63 ± 0.0 a | 0.35 ± 0.2 a |
| Caro (mg g−1 DW) | 0.34 ± 0.0 a | 0.29 ± 0.0 a |
| Pro (µmol g−1 DW) | 1.33 ± 0.6 a | 1.56 ± 0.7 a |
| TSS (mg glucose g−1 DW) | 17.62 ± 2.5 a | 18.98 ± 1.7 a |
| GB (µmol g−1 DW) | 33.21 ± 4.2 a | 93.22 ± 12.8 b |
| MDA (nmol g−1 DW) | 41.61 ± 5.2 a | 127.67 ± 1.7 b |
| H2O2 (µmol g−1 DW) | 1.61 ± 0.3 a | 5.36 ± 0.3 b |
| TPC (mg eq. GA g−1 DW) | 6.59 ± 0.4 a | 13.61 ±1.3 b |
| TF (mg eq. C g−1 DW) | 0.79 ± 0.2 a | 2.33 ± 0.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mircea, D.M.; Ferrer-Gallego, P.P.; Ferrando-Pardo, I.; Vicente, O.; Mir, R.; Boscaiu, M. Salt Tolerance of Sea Flax (Linum maritimum L.), a Rare Species with Conservation Interest in Eastern Spain. Plants 2024, 13, 305. https://doi.org/10.3390/plants13020305
Mircea DM, Ferrer-Gallego PP, Ferrando-Pardo I, Vicente O, Mir R, Boscaiu M. Salt Tolerance of Sea Flax (Linum maritimum L.), a Rare Species with Conservation Interest in Eastern Spain. Plants. 2024; 13(2):305. https://doi.org/10.3390/plants13020305
Chicago/Turabian StyleMircea, Diana M., P. Pablo Ferrer-Gallego, Inmaculada Ferrando-Pardo, Oscar Vicente, Ricardo Mir, and Monica Boscaiu. 2024. "Salt Tolerance of Sea Flax (Linum maritimum L.), a Rare Species with Conservation Interest in Eastern Spain" Plants 13, no. 2: 305. https://doi.org/10.3390/plants13020305
APA StyleMircea, D. M., Ferrer-Gallego, P. P., Ferrando-Pardo, I., Vicente, O., Mir, R., & Boscaiu, M. (2024). Salt Tolerance of Sea Flax (Linum maritimum L.), a Rare Species with Conservation Interest in Eastern Spain. Plants, 13(2), 305. https://doi.org/10.3390/plants13020305











