Abstract
The genus Phlomis is one of the largest genera in the Lamiaceae family and includes species used since ancient times in traditional medicine, as flavoring for food and as fragrance in cosmetics. The secretory structures (represented by glandular trichomes) as well as the essential oils produced by them constitute the subject of this review. While representatives of this genus are not typically regarded as large producers of essential oils compared to other species of the Lamiaceae family, the components identified in their essential oils and their biological properties necessitate more investigation of this genus. A comprehensive analysis of the specialized literature was conducted for each of the 93 currently accepted species to identify all the results obtained by researchers regarding the secretory structures and essential oils of this genus up to the present time. Glandular trichomes, still insufficiently studied, present morphological peculiarities that differentiate this genus within the family: they are of two categories: capitate (with a wide distribution in this genus) and dendroid. The peltate trichomes, characteristic of many species of this family, are absent. The essential oils from the species of the genus Phlomis have been much more widely studied than the secretory structures. They show considerable variability depending on the species and the environmental conditions.
1. Introduction
Glandular trichomes, which are found in almost one-third of plant species [1], play an important role in the life of plants by serving as specialized structures for the production and storage of various substances, including essential oils and other secondary metabolites [2]. These substances can act as a defense mechanism against biotic and abiotic stresses [3], thereby helping plants to survive and thrive in their natural habitats. Additionally, glandular trichomes can also attract pollinators [4] and deter pests [5], contributing to the reproductive success and overall fitness of the plant species.
The genus Phlomis (family Lamiaceae) includes 93 species (excluding hybrids and subspecies) accepted today and spread over three continents, Asia, Europe and Africa, in temperate or subtropical climates. This is according to the current data on Plants of the World Online (POWO) [6] (https://powo.science.kew.org; accessed on 18 February 2024), administered by the Royal Botanic Gardens, Kew, UK and World Flora Online (WFO) [7] (https://about.worldfloraonline.org; accessed at 18 February 2024), These databases were considered in carrying out the investigations in this paper.
From a taxonomic point of view, the genus Phlomis belongs to the family Lamiaceae (Lamiales) [8], the subfamily Lamioideae and the tribe Phlomideae (which includes the genera Phlomis and Phlomoides) [6,9]. The genus is monophyletic [10], including perennial species [11]. The phylogenetic studies carried out in the last two decades led to the inclusion of a significant number of species from the genus Phlomis (which now contains only sub-shrubs or shrubs) in the genus Phlomoides (with herbaceous species) (today, they are accepted as distinct genera [12], after numerous controversies that followed their separation by Moench in 1794) [11]. Azizian and Cutler [13] found anatomical and morphological affinities between the species belonging to the genera Phlomis and Eremostachys. But later, most of the species of this genus were included in the genus Phlomoides [11]. Relevant is the example of the species Phlomoides tuberosa Moench, removed by Moench in 1794 [11] from the genus Phlomis, reintroduced by Bunge in 1830 [14] in the genus Phlomis (P. tuberosa L.) and resettled by Mathisen et al. [11] in the genus Phlomoides.
The species of plants from the Lamiaceae family have been used since ancient times by people in medicine, food, hygiene, cosmetics and agriculture due to their secondary metabolites and, primarily, due to their essential oils [15,16]. They are found in large quantities and with varied compositions in most of the species belonging to this family [2]. The essential oils from the Phlomis species have antibacterial activity: for example, the oil of Phlomis lanata [17] and P. salicifolia [18] acts especially on the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa, the oil of P. fruticosa [19] and P. olivieri [20] showed an antibacterial effect against both Gram-positive and Gram-negative bacteria (Bacillus subtilis and E. coli) and the oil of P. rigida was active against the Gram-positive bacteria Staphylococcus aureus [21]. The antifungal effects of oils from various Phlomis species have also been investigated; those from P. cretica, P. samia [22], P. lanata [17] and P. rigida [21] have been proven to exert antifungal action on some pathogenic species of Candida sp.
Essential oils extracted from certain species of the Phlomis genus have demonstrated potent antioxidant properties. For instance, the oil derived from P. bourgaei [23] and P. pungens var. pungens [24] displayed notable metal chelation activity, while the oil of P. armeniaca exhibited a significant reducing capacity in the presence of ferric and cupric ions [24]. The effect of the inhibition of α-amylase enzyme activity by P. nissolii essential oil and of α-glucosidase by P. armeniaca oil [24] can be associated with the use of these species in the treatment of diabetes [25].
Glandular trichomes are considered true “natural biofactories” [2] essential oils but are also for other secondary metabolism compounds, their secretion being polymorphic, depending on the species and their structure [26]. Although there are numerous studies on the morphology and histochemistry of glandular trichomes in species of the Lamiaceae family [27,28,29,30,31,32], the number of research works that refer to the Phlomis genus remains quite limited.
According to the data available in the literature, trichomes from the species of the Lamiaceae family are of two major types: peltate and capitate [1,33,34,35]. Peltate trichomes are formed by a basal cell, a foot cell and a variable number of secretory cells, arranged in a single plane [36]. They are usually specialized in the secretion of essential oils, which they store in the subcuticular space. Capitate trichomes have a basal cell, one or more stalk cells (of variable lengths) and one to four (rarely more) glandular cells [37]. Often, their secretion is mixed, with a variable structure. Sometimes, it can consist exclusively of hydrophilic compounds (as, for example, in some capitate trichomes from Salvia officinalis [28]), but in some cases (especially in species that do not present peltate trichomes), they can mainly secrete essential oils [38]. Besides the biological role of producing essential oils, glandular trichomes [39,40] (along with non-glandular ones) [41] have an important role in the taxonomic delimitation of species and genera from the Lamiaceae family.
The purpose of this review is to provide up-to-date information on the state of research on glandular trichomes and the composition of essential oils from all currently recognized Phlomis species. The research concerned querying the Web of Science, Scopus and Google Academic databases with keywords consisting of the scientific names of the 93 Phlomis species accepted (as well as their synonyms, used in the past), plus “glandular trichomes”, “glandular hairs”, “secretory hairs” and “essential oil”, in order to identify papers that contain information about these aspects. In the case of essential oils, since the chemical composition of the product extracted from the leaves or aerial parts at anthesis is analyzed in most cases, this was considered in the present study; for each case, the first five compounds of the essential oil, in descending order of concentration, were mentioned in the synthetic table.
2. Results
2.1. Glandular Trichomes in the Phlomis Genus
The secretory trichomes of species from the Lamiaceae family have been investigated from morphological, histochemical and ultrastructural points of view [27,28,29,30] in an attempt to understand as precisely as possible the mechanisms of synthesis of the secondary metabolites elaborated by them. But for the Phlomis genus, compared to the total number of species currently accepted, the number of species for which there are data (complete or partial) in the literature remains small (13 species out of 93 in total).
2.1.1. Structure of the Glandular Trichomes
The data available in the literature regarding the morphology of glandular trichomes in the Phlomis species are synthesized in Table 1 and Figure 1. Considering the morphological characters described by various authors [13,39,42,43,44,45,46,47,48,49], we grouped the capitate trichomes into five categories (C1–C5) and the dendroid trichomes also into six categories (D1–D6). There is still variability in relation to these categories; the classification was made to simplify the description. In establishing the subtypes, the number of secretory cells, the relative size of the stalk, the number of component cells and the positioning and density of the branches (in the case of dendroid trichomes) were taken into consideration.

Table 1.
Types of glandular trichomes described in some species of the genus Phlomis.
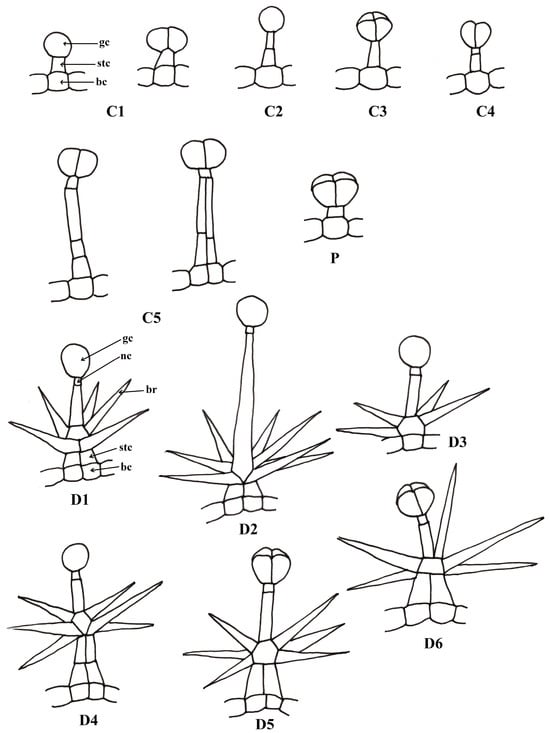
Figure 1.
Types of glandular trichomes described in some species of the genus Phlomis: bc—base cell, stc—stalk cell, gc—glandular cell, br—branch, nc—neck cell.
The following types have been described: capitate glandular trichomes—C1: 1 basal cell + 1 stalk cell + 1–2 glandular cells; C2: 1 basal cell + 2–3 stalk cells + 1 glandular cell; C3: 1 basal cell + 1 stalk cells + 4 glandular cells; C4: basal cell + 1–2 stalk cells + 2 glandular cells; C5: uni- or biseriate stalk with 4–5 cells + 2 glandular cells; dendroid glandular trichomes—D1: 4–10 branches; D2: 7–9 branches, long stalk cell; D3: branches inserted at the base + 1–2 glandular cells; D4: branches inserted in the median area + 1–2 glandular cells; D5: branches inserted in the median area + 4 glanduar cells; D6: mixed + 4 glandular cells and non-glandular branching at the top; P: glandular trichome described as a peltate, reclassified in the capitate trichome category with 4 glandular cells (C3).
Azizian and Cutler [13] describe four forms of capitate trichomes, which correspond to C1 (form 1, found in many of the studied species: P. aurea, P. chimerae, P. lanata, P. samia), and C4 (form 2: P. chimerae) and C5 (form 3, 4: P. crinita). Type C2 is described by El-Banhawy and Al-Juhani [42] in P. aurea (distinct from type C1, consisting of short trichomes, but also from C3 or C4, because it has a single large glandular cell). Type C3 is described in P. aurea [42], P. herva-venti [46] and P. fruticosa [39] and has four large glandular cells.
Dendroid glandular trichomes are very rarely described in the literature, but they are not found only in Lamiaceae species; Gangaram et al. [50] describe a similar type of trichome in Barleria albostellata C.B. Clarke and Ahmad [51] describe them in Dyschoriste vagans (Wight) Kuntze, from the Acanthaceae family, a family related to the Lamiaceae, both belonging to the Lamiales order [52]. The term “dendroid glandular trichome” was used by Nikolakaki and Christodoulakis [44], Yetişen [48], El-Banhawy, Al-Juhani [42] and Gostin [46]. These trichomes were also described as “compound glandular hairs” by Azizian and Cutler [13], “stellate type with glandular arms” by Çalı [49] and “branched stalked” by Giuliani [39]. We chose to use this terminology due to the fact that the non-secretory part of the trichome is clearly dendroid, an accepted term for non-glandular trichomes in the same category [53].
El-Banhawy and Al-Juhani [42] describe two subtypes of dendroid trichomes, which correspond to D1 and D2 (in P. aurea). Azizian and Cutler [13] describe a type of dendroid trichome branched directly from the base (D3) in P. brevilabris, also found sporadically in P. herba-venti [46]. D4 is the most frequent type of dendroid trichome (long-legged, multiseriate) (P. crinita, P. fruticosa and P. monocephala) [43,44,48]. The last two types, D5 and D6, which present four glandular cells, were described in P. oliveri and P herba-venti [46,48].
Peltate glandular trichomes were reported in two species of the genus Phlomis–P. oliveri and P russeliana. In the literature, peltate trichomes are defined as having a basal cell, a short leg and a secretory head consisting of 4–12 cells that are covered by a common cuticle [54]. In the absence of clear characters that differentiate the peltate trichomes from the capitate ones from a morphological point of view, their classification in one category or another by some authors remains controversial. Werker [33] leaves the possibility of being included in the category of capitate trichomes and those with four secretory cells, of different sizes; peltate trichomes (also called “glandular scales”) should have secretory cells flattened in a horizontal plane. Muravnik [35] describes the petaloid trichomes, characteristic of the Lamiaceae species, as having “a disk-shaped head”. The research of Azizian and Cutler [13] on the trichomes of Phlomis lanata and P. chimerae place trichomes with four secretory cells in the “capitate” category, not the “peltate” category. Therefore, they investigate the species P. russeliana and do not mention the “peltate trichomes” category related to it.
Referring to these observations, and taking into account that in the case of the other species of Phlomis investigated regarding the presence of secretory trichomes, the “peltate trichomes” category is not described, we consider that future investigations (micromorphological, anatomical and histochemical) are necessary to clarify the presence or absence of peltate trichomes in P. oliveri and P. russeliana.
One of the problems encountered in the inventory of the categories of glandular trichomes was the illustrations in some analyzed papers (which were insufficient or of poor quality); this did not always allow an objective evaluation of the type of trichome described by the authors, it being necessary to take into account the authors’ description. Without sufficient photo documentation, these data must be considered cautiously, as a reevaluation of the species is necessary from this point of view.
2.1.2. Secretion of Glandular Trichomes
Although they are the most widespread within the Lamiaceae family and responsible for the production of significant amounts of essential oils, peltate trichomes are absent in species of the Phlomis genus. Their role in the secretion of volatile oils has long been considered major within the family, some authors [33] considering species lacking peltate trichomes as not belonging to the category of aromatic plant species (for example, Prasium majus L.). However, the progress of research on the histochemistry of glandular trichomes indicated the presence of secretion consisting of essential oils in capitate and dendroid trichomes [39,46].
The genus Phlomis, along with other genera like Sideritis, Lycopus, Micromeria [55], Marubium and Balota [40], are not known for being the most abundant producers of essential oils within the Lamiaceae family. However, despite their relatively lower quantity of essential oils compared to some other Lamiaceae species, the essential oils derived from these genera are recognized for their valuable bioactive compounds. Phlomis species also present other secondary metabolites (besides the essential oil components) for which these species are utilized for medicinal purposes, including iridoid glucosides, flavonoids, phenylethanoid glycoside [25,56], phenylpropanoids and phenolic acids [57]. These compounds have been utilized in traditional medicine practices for centuries, indicating their historical significance and therapeutic potential [58,59]. Despite their long history of use, the full range of their medicinal properties and applications has yet to be fully explored and exploited in modern medicine and pharmacology.
The classification made by Werker [33], depending on the timing of secretion, which groups the secretory trichomes into short-term glandular hairs (capitate) and long-term glandular hairs (peltate), cannot be applied to the species of the genus Phlomis. Being devoid of peltate trichomes, the synthesis of essential oils is localized at the level of capitate and dendroid trichomes, which show continuous activity also on mature leaves [38,44,48] (where they are found also in the secretory phase and not only the post-secretory one).
Unlike the peltate trichomes, the capitate ones present much more varied secretion products. In Phlomis herba-venti, in C2-type capitate trichomes, visible drops’ essential oils were identified (by staining with the NADI reagent and Sudan III) [46], while C1-type trichomes have a mixed secretion containing, besides lipids, phenolic compounds and polysaccharides (identified by staining with toluidine blue and Ruthenium Red with the PAS reagent) [46]. The capitate trichomes of type C1 from P. fruticosa show only hydrophilic secretion (polysaccharides and mucopolysaccharides), being positive when stained with Ruthenium red and Alcian blue [39]. At the same time, C4-type trichomes accumulate terpenes, polyphenols and flavonoids, showing strong positive reactions to Fluoral Yellow-088, NADI reagent and aluminum trichloride. A similar reaction was shown by the dendroid trichomes (D4 type) described in this species [39].
Positive reactions for compound terpenes and phenolics were also observed in type C1 and C4 trichomes from the Phlomis fruticosa species [44]. The essential oil accumulates as droplets in the space between the cuticle and the external wall of the glandular cells [44]; this space stores phytotoxic compounds, serving as a primary defense mechanism at the plant’s surface [60]. The subcuticular space was observed primarily in capitate trichomes, while the extrusion of secretion products in dendroid trichomes typically occurs through the cell wall and the cuticle, into the external environment [46].
Dendroid trichomes also present mixed secretions, with lower amounts of essential oils than capitate ones; in Phlomis herba-venti, the glandular cells of these trichomes showed positive reactions to phenolic compounds, sesquiterpenes, polysaccharides and lipids [46].
Neck cells were observed in all dendroid glandular trichomes, often recording the same positive histochemical reactions as with secretory cells or secretory products [44]. They represent a special structure, with properties different from those of ordinary stalk cells, compared to which they are considerably shorter. The neck cells are involved in the secretion process, and there is communication with the neighboring cells, as shown by the ultrastructural studies performed on the capitate trichomes from Stachys heraclea All. [61]. Their lower transverse walls are cutinized [35], the structure being similar to Casparyan strips from the root or stem. In this way, the flow of substances is controlled (especially secondary metabolites secreted by the cell/glandular cell + neck cell complex), preventing the reflux towards the rest of the stalk cells [46] and the dissipation of the active substances in the trichome body. This structural peculiarity was also observed in secretory trichomes from other species of Lamiaceae [62].
The branch cells of dendroid trichomes are alive at their full development; in confocal microscopy observations, viable chloroplasts were observed in all these cells in P. herba-venti [46]. Non-glandular trichomes from Lamiaceae do not represent inert structures, with only a protective role against physical factors, but produce various categories of substances (proteins, lipids, terpenes, alkaloids, phenolic compounds and polysaccharides) that modulate the interactions between plants and other species from ecosystem. They complement the active protective role that glandular trichomes have for the plant [41].
The main role of glandular trichomes is to protect plants against herbivores: as a result, species that present trichomes (both glandular and protective) are less consumed by herbivores or attacked by parasites, a fact observed by researchers in individuals of the same species that present polymorphism for trichome production [63]. Moreover, Phlomis species are known to be little consumed by herbivores or attacked by phytophagous insects [64].
Second, trichomes located in the reproductive sphere (on sepals, petals or even ovaries) can play a role in attracting pollinators through the volatile substances they eliminate [65]; among the components found in the essential oil of Phlomis species, 1,8-cineole, linalool and (E, E)-α-farnesene have been proven to be attractive to various species of pollinating insects (Hymenoptera) [65] and bicyclogermacrene for some Diptera species [66].
2.2. Essential Oils from Phlomis Species
Species belonging to the Phlomis genus generally produce lower amounts of essential oils than other species from the Lamiaceae family [9]. However, the diversity of the component elements with valuable therapeutic properties or with the potential to be used in agriculture and industry makes a more careful evaluation of them necessary. The biological activity was observed both at the level of the essential oils as a whole and in the case of their components investigated separately [16]; many times, their effect was manifested synergistically [5].
Table 2 presents the existing data in the specialized literature regarding the composition of essential oils from all 93 recognized species. Because the geographical origin of the analyzed species is important, the area from which they are native and the predominant biome were indicated for each species (cf. POWO) [6]. Among the 93 species, complete or partial information on the composition of the essential oils was found for 48 (51.61%). The identification of the components of the essential oils was achieved by gas chromatography coupled to mass spectrometry (GC–MS) techniques. The lack of information for the other species leaves open a significant area of research in this field to cover the “white spots”.

Table 2.
The chemical composition of essential oils from species of the Phlomis genus (a synthesis). The first five components of the essential oil extracted from the leaves, in descending order of concentration (where available), were considered. (A dash ‘—’ in the table means that there is no information available about these species; for Phlomis fruticosa L., collected fromBar, Montenegro: locality A is exposed to the sun and locality B is in the forest).
The increased variability of the composition of essential oils is well known not only in the representatives of the Lamiaceae family [16] but also in those of other botanical families such as Asteraceae [122] and Lauraceae [123]. This fact is due both to genotypic variations (between individuals of the same species, which belong to different populations) as well as to environmental conditions or agrotechnical factors in the case of cultivated species [124,125]. Also, in the Phlomis genus, there is a high, well-known variability between the composition of the essential oils produced by the glandular trichomes on the leaves compared to those in the floral sphere [68,77], as well as between the oils produced in different stages of the ontogenetic development of plants [105].
The presence of different compounds in essential oils is part of a wider register of the modulation of interrelationships in ecosystems, between plants (immobile organisms) and various animal species (especially insects); mobile individuals have the role of performing various “services” for those in the first category, or, on the contrary, immobile plants must defend themselves against them, using biochemical signals because physical movement is impossible.
Analyzing the main compounds of the essential oils from 25 species of the genus Phlomis (of which 4 were reclassified in the genus Phlomoides: P. younghunsbandii, P. szechuanensis, P. megalantha and P. umbrosa), Amor [56] classifies them into four chemotypes: 1—which contains predominantly sesquiterpenes, 2—which contains both monoterpenes and sesquiterpenes, 3—which contains fatty acids, aliphatic compounds and alcohol and 4—which contains terpenes, fatty acids, aliphatic compounds and alcohol. Chemotype 3 includes only three species of those currently found in the genus Phlomoides (P. younghunsbandii, P. szechuanensis and P. umbrosa) [6].
2.2.1. Sesquiterpenes from Essential Oils
Among the components of the essential oils extracted from the leaves of species belonging to the genus Phlomis, sesquiterpenes comprise the largest share (Figure 2). Germacrene-D is one of the main components found in 31 out of the 47 species for which data are available in the literature, while β-caryophyllene is present in 25 species.
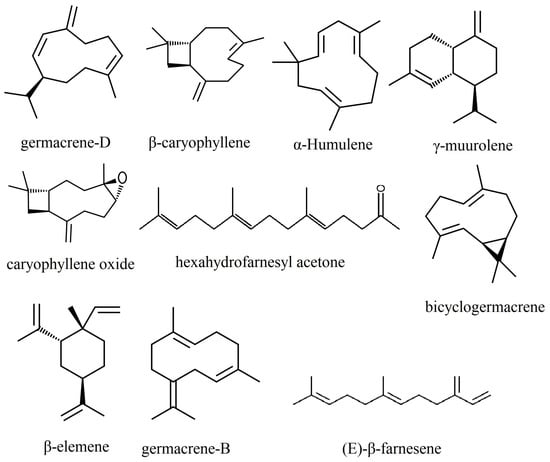
Figure 2.
The main sesquiterpenes in the composition of essential oils of species of the Phlomis genus.
Germacrene-D was identified in the highest quantities in the species Phlomis anisodonta (65.0%) [58], P. bruguieri (60.05%) [78], P. kurdica (55.4%) [97] and P. aurea (51.56%) [74]. This compound was not among the first five components of essential oils in the species P. brevibracteata [77], P. bucharica [81], P. cashmeriana [85], P. elliptica [89], P. lanata [17], P. lurestanica [104], P. platystegia [76], P. regelii [115], P. salicifolia [81] and P. thapsoides [120]. Most of these species have, as their main component, a monoterpene or a fatty acid, which confirms the existence of some chemotypes within the species of this genus, as was pointed out by Amor [56].
β-caryophyllene and its oxidized form, caryophyllene oxide, are present among the first five components of essential oils from the majority of Phlomis species, with the exception of P. aurea [74], P. brachyodon [76], P. bucharica [81], P. cashmeriana [85], P. lurestanica [104], P. monocephala [67], P. platystegia [76], P. salicifolia [81] and P. thapsoides [120]. We notice that in six species of Phlomis, both germacrene-D and β-caryophyllene are missing from the main components of the essential oils. In these, the chemotypes are mainly based on monoterpenes and fatty acids.
Large amounts of β-caryophyllene are found in the species Phlomis aucheri (27.0%) [64], P. bourgaei (37.37%) [23], P. chimerae (31.6%) [86], P. cypria (37.4%) [77] and P. rigida (31.2%) [116], and large amounts of caryophyllene oxide are found in P. aucheri (33.5%) [64].
Some of the main constituents identified in the essential oil from various species of Phlomis—germacrene D and β-caryophyllene—are substances with a well-known deterrent role, which protects plants against herbivores [126]. β-farnesene is the main constituent of the essential oil of Phlomis elliptica (28.9%) [89] and P. samia (20.7%) [22], being part of the first 5 constituents of 15 other species of Phlomis. (E)-β-farnesene has an interesting biological role, being an alarm pheromone for insects from the Aphididae family [127]. It is emitted by aphids when they are attacked by enemies to warn individuals from the same group [128]. For this reason, (E)-β-farnesene acts as a repellent against these harmful insects, which avoid plants whose oil contains this compound. However, the repellent effect does not manifest equally against all insect species: Mumm and Hilker [129] showed that (E)-β-farnesene has an attractive effect against the wasp Chrysonotomyia ruforum Krausse (Hymenoptera, Eulophidae), an oophagous parasitoid for Diprion pini L. (Hymenoptera, Diprionidae).
2.2.2. Monoterpenes from Essential Oils
Monoterpenes (Figure 3) are found less often and in smaller quantities in essential oils from the Phlomis species; however, the oils from some species proved to be richer in monoterpenes than in sesquiterpenes. Monoterpenes and their derivatives give flavor and aroma to the essential oils in which they are found [130].
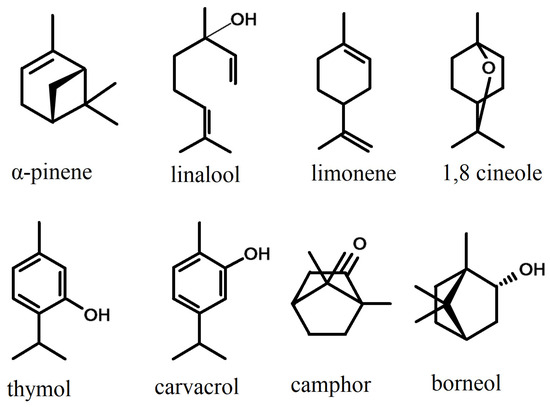
Figure 3.
The main monoterpenes in the composition of essential oils of the species of the Phlomis genus.
Linalool is part of the group of acyclic monoterpenoids and represents an important component in essential oils for its pharmacological effects. Research has highlighted its antidepressant [131], immunomodulator and antimicrobial roles. It was indicated as the main component in the essential oil of Phlomis leucophracta (36.4%) [98], being also found in important quantities in the oils of P. fruticosa (8.0%) [22], P. nissolii (11.3%) [24], P. cretica (7.5%) [22] and P. platystegia (7.72%) [76].
Limonene is the main component of Phlomis leucophracta oil (14.56–27.86%), refs [70,99] observed this in two other populations distinct from the one investigated by Sarikurkcu et al. [98]. The essential oil from P. leucophracta possesses very strong antioxidant activity, similar to that of ascorbic acid, which denotes the increased and still unexploited potential of these species for use in the pharmaceutical and food industries [98,132].
Other monoterpenes found in large quantities in the essential oils extracted from the leaves are 1–8 cineole (15.9%) in Phlomis regelii [115], camphor (14.46%) in P. bucharica [81] and thymol in P. bucharica (20.41%) and P. sewerzowii (35.76%) [81]. In the last species, thymol together with carvacrol (8.9%) represent almost half of the components identified in the essential oil. Among the monoterpenes found in Phlomis species oils, α-limonene, pinene, camphor, linalool and borneol represent the compounds with the most significant aromatic properties [125].
1–8 cineole (also known as eucalyptol) is frequently found in the oil from different species of Lamiaceae: populations of Lavandula angustifolia from Brazil and L. x intermedia from Iran or Mexico make up between 31.6% and 47.94% of the composition of the essential oil from this compound [133]. In the Phlomis species, it is found in larger quantities in P. bucharica (13.69%) [81] and in P. regelii (15.9%) [115], both species being part of the predominant chemotype with monoterpenes. 1–8 cineoles have a strong anti-inflammatory and antioxidant effect [134], as well as an insecticide effect [135].
Thymol and its isomer, carvacrol [136], have phytotoxic, cytotoxic and genotoxic properties, being able to be used as selective bioherbicides [137]; they also have important antibacterial effects [138], being recommended even in the case of bacteria resistant to classic antibiotics.
Recent studies are increasingly highlighting the anticancer action of some monoterpenes; among those found in the composition of the oil of the Phlomis species, linalool shows cytotoxic, apoptotic and antiproliferative properties on breast cancer cells [139], α-pinene induces apoptosis in vitro on the human gastric adenocarcinoma cell-line (AGS) [140] and limonene acts on receptors involved in the chemoresistance of cancer cells [141].
2.2.3. Other Compounds from Essential Oils
Hexadecanoic acid (palmitic acid) is the main component of Phlomis essential oils, apart from monoterpenes and sesquiterpenes. This is the dominant component from P. herba-venti (68.1%) [24], P. cancellata (17.13%) [84] and P. elliptica (19.1%) [64]. It is also found, among the main components, in P. armeniaca (4.9%) [67], P. kurdica (8.4%) [97], P. lunariifolia (9.7%) [103] and P. tenorei (12.8%) [119]. Hexadecanoic acid has an antibacterial and antifungal effect and can be used therapeutically in patients with asthma [142].
Methyl palmitate (the main component of Phlomis salicifolia oil) [81] has an effect similar to that of brood pheromone in honeybees [143]; these hormones are produced by larvae and trigger feeding instincts in nurse insects, including by increasing the amount of pollen collected from various species. This species, endemic to Central Asia, grows in a semi-arid habitat [81], where the pollinating insects are few in number, and the plant species have to make considerable “efforts” to attract them. Hexadecane (8.97%) is the main component of the essential oil from Phlomis lurestanica, an endemic species from the mountainous areas of Iran [144].
The presence of different compounds in essential oils is part of a wider register of the modulation of interrelationships in ecosystems, between plants (immobile organisms) and various animal species (especially insects); mobile individuals have the role of performing various “services” for those in the first category, or, on the contrary, immobile plants must defend themselves against them, using biochemical signals because physical movement is impossible.
3. Conclusions
The review of specialized literature aimed at identifying the results of the research conducted so far on the secretory structures and volatile oils from species of the Phlomis genus, which has highlighted the fact that the level of knowledge is still insufficient. If, in terms of the chemical composition of essential oils, 51.61% of the taxonomically accepted species have had their component elements described (even partially), the knowledge regarding glandular trichomes is limited to only 13 species (13.97%).
Although there is a substantial amount of information available regarding the essential oils from species of the genus Phlomis, future studies are needed to fully understand their composition. There are still 45 species whose essential oils remain completely unknown, and they may represent a potential source of biologically active compounds.
The genus Phlomis is unique among the genera of the Lamiaceae family because of the presence of a rare type of glandular trichomes, namely, dendroid glandular trichomes. They have from one to four secretory cells arranged on a stalk of a trichome morphologically similar to the non-glandular ones, with which it coexists in the indumentum on the vegetative or reproductive organs. But the current data on their morphology and structure are still very limited. Based on the data available so far, peltate trichomes are absent in species of the genus Phlomis. Investigations regarding the histochemistry of glandular trichomes (carried out in order to locate secretion products) are rare, and those regarding their ultrastructure are completely missing. Considering that trichomes, both glandular and non-glandular, serve as taxonomically significant traits for plants, it is imperative to conduct further investigations into species within the Phlomis genus in order to help clarify some classification and phylogenetic problems that exist in this taxon.
Author Contributions
Conceptualization, I.N.G.; methodology, I.N.G. and C.F.B.; formal analysis, I.N.G. and C.F.B.; investigation, I.N.G. and C.F.B.; data curation, I.N.G.; writing—original draft preparation, I.N.G. and C.F.B. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Scientific Research Budget of Oradea University.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fahn, A. Secretory Tissues in Vascular Plants. New Phytol. 1988, 108, 229–257. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.S.; Maddock, S.; Adal, A.M. Isoprenoid Metabolism and Engineering in Glandular Trichomes of Lamiaceae. Front. Plant Sci. 2021, 12, 699157. [Google Scholar] [CrossRef] [PubMed]
- Glas, J.; Schimmel, B.; Alba, J.; Escobar-Bravo, R.; Schuurink, R.; Kant, M. Plant Glandular Trichomes as Targets for Breeding or Engineering of Resistance to Herbivores. Int. J. Mol. Sci. 2012, 13, 17077–17103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus proximus Serg. Essential Oil and Its Major Constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef] [PubMed]
- Gostin, I.N.; Popescu, I.E. Evaluation of the Essential Oils Used in the Production of Biopesticides: Assessing Their Toxicity toward Both Arthropod Target Species and Beneficial Pollinators. Agriculture 2023, 14, 81. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, 2024, Kew. Published on the Internet. Available online: http://www.plantsoftheworldonline.org/ (accessed on 18 February 2024).
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 18 February 2024).
- The Angiosperm Phylogeny Group. An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Çalış, İ.; Başer, K.H.C. Review of Studies on Phlomis and Eremostachys Species (Lamiaceae) with Emphasis on Iridoids, Phenylethanoid Glycosides, and Essential Oils. Planta Med. 2021, 87, 1128–1151. [Google Scholar] [CrossRef] [PubMed]
- Eyvazadeh Khosroshahi, E.; Salmaki, Y. Evolution of Trichome Types and Its Systematic Significance in the Genus Phlomoides (Lamioideae–Lamiaceae). Nord. J. Bot. 2019, 37, njb.02132. [Google Scholar] [CrossRef]
- Mathiesen, C.; Scheen, A.-C.; Lindqvist, C. Phylogeny and Biogeography of the Lamioid Genus Phlomis (Lamiaceae). Kew Bull. 2011, 66, 83–99. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, L.; Yang, Y.; Wang, Y.; Yang, L.; Wei, C.; Guo, J.; Yan, K.; Chen, H.; Yang, Z.; et al. Complete Chloroplast Genome Sequences of Phlomis fruticosa and Phlomoides strigosa and Comparative Analysis of the Genus Phlomis Sensu Lato (Lamiaceae). Front. Plant Sci. 2022, 13, 1022273. [Google Scholar] [CrossRef]
- Azizian, D.; Cutler, D.F. Anatomical, Cytological and Phytochemical Studies on Phlomis L. and Eremostachys Bunge (Labiatae). Bot. J. Linn. Soc. 1982, 85, 249–281. [Google Scholar] [CrossRef]
- Ryding, O. Pericarp structure and phylogeny of the Phlomis group (Lamiaceae subfam. Lamioideae). Bot. Jahrbücher 2008, 130, 299. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Ramos Da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; De Jesus Pereira Franco, C.; Oliveira Dos Anjos, T.; Cascaes, M.M.; Almeida Da Costa, W.; Helena De Aguiar Andrade, E.; Santana De Oliveira, M. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. J. Evid. Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef] [PubMed]
- Couladis, M.; Tanimanidis, A.; Tzakou, O.; Chinou, I.B.; Harvala, C. Essential Oil of Phlomis lanata Growing in Greece: Chemical Composition and Antimicrobial Activity. Planta Med. 2000, 66, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Mamadalieva, N.Z.; Akramov, D.K.; Böhmdorfer, S.; Azimova, S.S.; Rosenau, T. Extractives and Biological Activities of Lamiaceae Species Growing in Uzbekistan. Holzforschung 2020, 74, 96–115. [Google Scholar] [CrossRef]
- Ristíc, M.D.; Duletić-Lausević, S.; Knezević-Vukcević, J.; Marin, P.D.; Simić, D.; Vukojević, J.; Janaćković, P.; Vajs, V. Antimicrobial Activity of Essential Oils and Ethanol Extract of Phlomis fruticosa L. (Lamiaceae). Phytother. Res. 2000, 14, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Variation in Chemical Composition and Antibacterial Activity of the Essential Oil of Wild Populations of Phlomis olivieri. Chem. Biodivers. 2017, 14, e1600444. [Google Scholar] [CrossRef] [PubMed]
- Nikan, M.; Saeidnia, S.; Manayi, A.; Saadatmand, S. Essential Oils of Four Phlomis Species Growing in Iran: Chemical Composition, Antimicrobial and Antifungal Activity. Progr. Nutr. 2017, 19, 75–79. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Kyriakopoulou, I.; Mitaku, S.; Chinou, I.B. Essential Oils of Phlomis Species Growing in Greece: Chemical Composition and Antimicrobial Activity. Flavour Fragr. J. 2004, 19, 320–324. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Sabih Ozer, M.; Cakir, A.; Eskici, M.; Mete, E. GC/MS Evaluation and In Vitro Antioxidant Activity of Essential Oil and Solvent Extracts of an Endemic Plant Used as Folk Remedy in Turkey: Phlomis bourgaei Boiss. J. Evid. Based Complement. Altern. Med. 2013, 2013, 293080. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Uren, M.C.; Kocak, M.S.; Cengiz, M.; Tepe, B. Chemical Composition, Antioxidant, and Enzyme Inhibitory Activities of the Essential Oils of Three Phlomis Species as Well as Their Fatty Acid Compositions. Food Sci. Biotechnol. 2016, 25, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Eruygur, N.; Kirci, D.; Ayaz, F.; Doğu, S.; Bağci, Y. Biological Activities of Three Phlomis Species. J. Res. Pharm. 2022, 26, 255–262. [Google Scholar] [CrossRef]
- Feng, Z.; Bartholomew, E.S.; Liu, Z.; Cui, Y.; Dong, Y.; Li, S.; Wu, H.; Ren, H.; Liu, X. Glandular Trichomes: New Focus on Horticultural Crops. Hortic. Res. 2021, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Ascensão, L.; Pais, M.S. The Leaf Capitate Trichomes of Leonotis leonurus: Histochemistry, Ultrastructure and Secretion. Ann. Bot. 1998, 81, 263–271. [Google Scholar] [CrossRef]
- Corsi, G.; Bottega, S. Glandular Hairs of Salvia officinalis: New Data on Morphology, Localization and Histochemistry in Relation to Function. Ann. Bot. 1999, 84, 657–664. [Google Scholar] [CrossRef]
- Moon, H.-K.; Hong, S.-P.; Smets, E.; Huysmans, S. Phylogenetic Significance of Leaf Micromorphology and Anatomy in the Tribe Mentheae (Nepetoideae: Lamiaceae). Bot. J. Linn. Soc. 2009, 160, 211–231. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.; Padurariu, C.; Trută, E.; Gostin, I. The Composition and Antibacterial Activity of Essential Oils in Three Ocimum Species Growing in Romania. Cent. Eur. J. Biol. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Tozin, L.R.D.S.; Rodrigues, T.M. Morphology and Histochemistry of Glandular Trichomes in Hyptis villosa Pohl Ex Benth. (Lamiaceae) and Differential Labeling of Cytoskeletal Elements. Acta Bot. Bras. 2016, 31, 330–343. [Google Scholar] [CrossRef]
- Naidoo, Y.; Dladla, T.; Dewir, Y.H.; Gangaram, S.; Naidoo, C.M.; Rihan, H.Z. The Micromorphology and Histochemistry of Foliar Mixed Indumentum of Leucas lavandulaefolia (Lamiaceae). Plants 2021, 10, 1767. [Google Scholar] [CrossRef]
- Werker, E. Function of Essential Oil-secreting Glandular Hairs in Aromatic Plans of Lamiaceae—A Review. Flavour Fragr. J. 1993, 8, 249–255. [Google Scholar] [CrossRef]
- Ascensão, L. Glandular Trichomes on Vegetative and Reproductive Organs of Leonotis leonurus (Lamiaceæ). Ann. Bot. 1995, 75, 619–626. [Google Scholar] [CrossRef]
- Muravnik, L.E. The Structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–35. ISBN 978-3-030-11253-0. [Google Scholar]
- Haratym, W.; Weryszko-Chmielewska, E. Ultrastructural and Histochemical Analysis of Glandular Trichomes of Marrubium vulgare L. (Lamiaceae). Flora 2017, 231, 11–20. [Google Scholar] [CrossRef]
- Baran, P.; Aktaş, K.; Özdemir, C. Structural Investigation of the Glandular Trichomes of Endemic Salvia smyrnea L. S. Afr. J. Bot. 2010, 76, 572–578. [Google Scholar] [CrossRef]
- Maleci Bini, L.; Giuliani, C. The Glandular Trichomes of The Labiatae. A Review. Acta Hortic. 2006, 723, 85–90. [Google Scholar] [CrossRef]
- Giuliani, C.; Bottoni, M.; Spada, A.; Falsini, S.; Santagostini, L.; Pieracci, Y.; Flamini, G.; Milani, F.; Fico, G. Micromorphological and Phytochemical Insights on Phlomis fruticosa L. Cultivated at the G.E. Ghirardi Botanical Garden (Lombardy, Northern Italy). Flora 2024, 314, 152505. [Google Scholar] [CrossRef]
- Siadati, S.; Salmaki, Y.; Bräuchler, C. Trichome Morphology Provides Phylogenetically Informative Signal for Generic Delimitation in Tribe Marrubieae (Lamiaceae). Flora 2020, 273, 151720. [Google Scholar] [CrossRef]
- Tozin, L.R.S.; De Melo Silva, S.C.; Rodrigues, T.M. Non-Glandular Trichomes in Lamiaceae and Verbenaceae Species: Morphological and Histochemical Features Indicate More than Physical Protection. N. Z. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- El-Banhawy, A.; Al-Juhani, W. DNA Barcoding and Phylogeny of Phlomis aurea (Lamiaceae) Endemic to Sinai Peninsula, Egypt. Pak. J. Bot. 2019, 51, 1263–1271. [Google Scholar] [CrossRef]
- Albaladejo, R.G.; Aparicio, A.; Silvestre, S. Variation patterns in the Phlomis × composita (Lamiaceae) hybrid complex in the Iberian Peninsula. Bot. J. Linn. Soc. 2004, 145, 97–108. [Google Scholar] [CrossRef][Green Version]
- Nikolakaki, A.; Christodoulakis, N.S. Secretory Structures and Cytochemical Investigation of the Leaf of Phlomis fruticosa, a Seasonally Dimorphic Subshrub. Secreting Activity of the Leaf-Originating Calluses. Flora 2007, 202, 429–436. [Google Scholar] [CrossRef]
- Saeidnia, S.; Nikan, M.; Mirnezami, T.; Gohari, A.R.; Manayi, A. Micromorphological characterizations and phytochemicals contents of some Phlomis species from Iran. Int. J. Pharm. Sci. 2015, 8, 157–161. [Google Scholar]
- Gostin, I.N. Glandular and Non-Glandular Trichomes from Phlomis herba-venti subsp. pungens Leaves: Light, Confocal, and Scanning Electron Microscopy and Histochemistry of the Secretory Products. Plants 2023, 12, 2423. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Amirahmadi, A.; Atri, M.; Naderi, R. An investigation of the anatomy, palynology and trichome types of Phlomis olivieri (Lamiaceae). Taxon. Biosyst. 2014, 21, 59–70. [Google Scholar]
- Yetişen, K. Morphological and Anatomical Study of the Endemic Species Phlomis monocephala (Lamiaceae). Phytol. Balc. 2014, 20, 49–55. [Google Scholar]
- Çalı, I.Ö. Anatomy and trichome characteristics of endemic taxon Phlomis russeliana (Sims.) Bentham and their systematic implications. Bangladesh J. Bot. 2016, 45, 297–304. [Google Scholar]
- Gangaram, S.; Naidoo, Y.; Dewir, Y.H. Foliar Micromorphology, Ultrastructure, and Histochemical Analysis of Barleria albostellata C.B. Clarke. S. Afr. J. Bot. 2020, 135, 212–224. [Google Scholar] [CrossRef]
- Ahmad, K.J. Epidermal hairs of Acanthaceae. Blumea 1978, 24, 101–117. [Google Scholar]
- Refulio-Rodriguez, N.F.; Olmstead, R.G. Phylogeny of Lamiidae. Am. J. Bot. 2014, 101, 287–299. [Google Scholar] [CrossRef]
- Xiang, C.-L.; Dong, Z.-H.; Peng, H.; Liu, Z.-W. Trichome Micromorphology of the East Asiatic Genus Chelonopsis (Lamiaceae) and Its Systematic Implications. Flora 2010, 205, 434–441. [Google Scholar] [CrossRef]
- Atalay, Z.; Celep, F.; Bara, F.; Doğan, M. Systematic Significance of Anatomy and Trichome Morphology in Lamium (Lamioideae; Lamiaceae). Flora 2016, 225, 60–75. [Google Scholar] [CrossRef]
- Kokkini, S.; Karousou, R.; Hanlidou, E. Herbs of the Labiatae. In Encyclopedia of Food Sciences and Nutrition; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2003; pp. 3082–3090. [Google Scholar]
- Amor, I.L.-B.; Boubaker, J.; Sgaier, M.B.; Skandrani, I.; Bhouri, W.; Neffati, A.; Kilani, S.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Phytochemistry and Biological Activities of Phlomis Species. J. Ethnopharmacol. 2009, 125, 183–202. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, M.K.; Tsiftsoglou, O.S.; Mašković, P.Z.; Lazari, D.; Katerinopoulos, H.E. Chemical Constituents and Anticancer Activities of the Extracts from Phlomis × commixta Rech. f. (P. cretica × P. lanata). Int. J. Mol. Sci. 2024, 25, 816. [Google Scholar] [CrossRef]
- Sarkhail, P.; Amin, G.; Shafiee, A. Composition of the Essential Oil of Phlomis persica Boiss and Phlomis chorassanica Bunge from Iran. Flavour Fragr. J. 2004, 19, 538–540. [Google Scholar] [CrossRef]
- Heydari, F.; Ghafarzadegan, R.; Mofasseri, M.; Ghasemi, S.V.; Kashefi, M. Phytochemical Analysis and Biological Activities of Essential Oil and Extract of Phlomis rigida Labill. J. Med. Plant Res. 2021, 20, 13–22. [Google Scholar] [CrossRef]
- Wagner, G.J. Secreting Glandular Trichomes: More than Just Hairs. Plant Physiol. 1991, 96, 675–679. [Google Scholar] [CrossRef]
- Giuliani, C.; Maleci Bini, L. Insight into the Structure and Chemistry of Glandular Trichomes of Labiatae, with Emphasis on Subfamily Lamioideae. Plant Syst. Evol. 2008, 276, 199–208. [Google Scholar] [CrossRef]
- Bruni, A.; Modenesi, P. Development, Oil Storage and Dehiscence of Peltate Trichomes in Thymus vulgaris (Lamiaceae). Nord. J. Bot. 1983, 3, 245–251. [Google Scholar] [CrossRef]
- Løe, G.; Toräng, P.; Gaudeul, M.; Ågren, J. Trichome Production and Spatiotemporal Variation in Herbivory in the Perennial Herb Arabidopsis lyrata. Oikos 2007, 116, 134–142. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R.; Soltani, M.; Khosravi, A.R. Essential Oil Composition of Two Species of Phlomis L. (Phlomis aucheri Boiss. and Phlomis elliptica Benth.) (Lamiaceae) from Iran. J. Essent. Oil Res. 2010, 22, 314–317. [Google Scholar] [CrossRef]
- Giuliani, C.; Ascrizzi, R.; Lupi, D.; Tassera, G.; Santagostini, L.; Giovanetti, M.; Flamini, G.; Fico, G. Salvia verticillata: Linking Glandular Trichomes, Volatiles and Pollinators. Phytochemistry 2018, 155, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Yafuso, M. Pollination of Alocasia cucullata (Araceae) by Two Colocasiomyia Flies Known to Be Specific Pollinators for Alocasia odora. Plant Species Biol. 2005, 20, 201–208. [Google Scholar] [CrossRef]
- Demirci, B.; Toyota, M.; Demirci, F.; Dadandi, M.Y.; Can Baser, K.H. Anticandidal Pimaradiene Diterpene from Phlomis Essential Oils. C. R. Chim. 2009, 12, 612–621. [Google Scholar] [CrossRef]
- Amiri, H.; Ghiasvand, A.R. Changes in Composition and Antioxidant Activities of Essential Oils in Phlomis anisodonta (Lamiaceae) at Different Stages of Maturity. Progress Biol. Sci. 2016, 6, 205–212. [Google Scholar] [CrossRef]
- Yasar, S.; Fakir, H.; Erbas, S. Gas chromatographic (GC-GC/MS) analysis of essential oil of Phlomis armeniaca Willd. from mediterranean region in Turkey. Asian J. Chem. 2010, 22, 2887. [Google Scholar]
- Sarikaya, A.G.; Fakİr, H. The Effect of Reaping Times on Volatile Components of Natural Phlomis L. (Lamiaceae) Taxa In The Lakes District of Turkey. Appl. Ecol. Environ. Res. 2016, 14, 753–772. [Google Scholar] [CrossRef]
- Taherkahni, M.; Masoudi, S.; Karaminia, M.; Rustaiyan, A. Chemical Composition, Antimicrobial Activity, Antioxidant and Total Phenolic Content of the Essential Oil of Phlomis aucheri Boiss Growing Wild in Iran. Nashrieh Shimi Mohandesi Shimi Iran 2014, 33, 11–17. [Google Scholar]
- Karamian, R.; Azizi, A.; Asadbegy, M.; Pakzad, R. Essential Oil Composition and Antioxidant Activity of the Methanol Extracts of Three Phlomis Species from Iran. J. Biol. Act. Prod. Nat. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Masoudi, S. Volatile Constituents from Different Parts of Three Lamiacea Herbs from Iran. Iran J. Pharm. Res. 2018, 17, 365–376. [Google Scholar]
- Torky, Z.A.; Moussa, A.Y.; Abdelghffar, E.A.; Abdel-Hameed, U.K.; Eldahshan, O.A. Chemical Profiling, Antiviral and Antiproliferative Activities of the Essential Oil of Phlomis aurea Decne Grown in Egypt. Food Funct. 2021, 12, 4630–4643. [Google Scholar] [CrossRef]
- Liolios, C.; Laouer, H.; Boulaacheb, N.; Gortzi, O.; Chinou, I. Chemical Composition and Antimicrobial Activity of the Essential Oil of Algerian Phlomis bovei De Noé subsp. bovei. Molecules 2007, 12, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Obeidat, S.M.; Saleh, A.M.; El-Oqlah, A.A.; Al-Masaeed, E.; Al-Jaber, H.I.; Abu Orabi, S.T. Volatile Components Analysis, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Phlomis Species Collected from Jordan. J. Essent. Oil-Bear. Plants 2018, 21, 583–599. [Google Scholar] [CrossRef]
- Hanoğlu, A.; Yiğit Hanoğlu, D.; Demirci, B.; Özkum Yavuz, D.; Başer, K.H.C.; Çaliş, İ. Essential Oil Composition of Leaves and Flowers of Two Endemic Phlomis L. Species (Phlomis cypria Post and Phlomis brevibracteata Turrill) from Northern Cyprus. J. Essent. Oil Res. 2019, 31, 196–202. [Google Scholar] [CrossRef]
- Sarkhail, P.; Amin, G.; Surmaghi, M.H.S.; Shafiee, A. Composition of the Volatile Oils of Phlomis lanceolata Boiss. & Hohen., Phlomis anisodonta Boiss. and Phlomis bruguieri Desf. from Iran. Flavour Fragr. J. 2005, 20, 327–329. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M. The Essential Oil Composition of Phlomis bruguieri Desf. from Iran. Flavour Fragr. J. 2005, 20, 344–346. [Google Scholar] [CrossRef]
- Akman, F.; Demirpolat, A.; Kazachenko, A.S.; Kazachenko, A.S.; Issaoui, N.; Al-Dossary, O. Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules 2023, 28, 2684. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Ashour, M.L.; Akramov, D.K.; Sasmakov, S.A.; Ramazonov, N.S.; Azimova, S.S. A Comparative Study on Chemical Composition and Antimicrobial Activity of Essential Oils from Three Phlomis Species from Uzbekistan. Nat. Prod. Res. 2021, 35, 696–701. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Moshiri, K.; Akbarzadeh, M. The Essential Oil Composition of Phlomis cancellata Bunge. J. Essent. Oil Res. 2006, 18, 672–673. [Google Scholar] [CrossRef]
- Aghakhani, F.; Kharazian, N.; Lori Gooini, Z. Flavonoid Constituents of Phlomis (Lamiaceae) Species Using Liquid Chromatography Mass Spectrometry. Phytochem. Anal. 2018, 29, 180–195. [Google Scholar] [CrossRef]
- Deylamsalehi, M.; Mahdavi, M.; Motavalizadehkakhky, A.; Akbarzadeh, M.; Mahmudi, J.; Mirahmadi, S.F.; Ebrahimi, Z.; Abedi, F. Chemical Compositions and Antimicrobial Activity of Essential Oil of Phlomis cancellata Bunge. from Mazandaran, Iran. J. Essent. Oil-Bear. Plants 2013, 16, 555–562. [Google Scholar] [CrossRef]
- Ullah, R.; Abd EI-Salam, N.M.; Hussain, I.; Ahmad, S. Essential oil composition of the medicinal plant Phlomis cashmeriana. Life Sci. J. 2013, 10, 905–906. [Google Scholar]
- Celik, S.; Suleyman Gokturk, R.; Flamini, G.; Luigi Cioni, P.; Morelli, I. Essential Oils of Phlomis leucophracta, Phlomis chimerae and Phlomis grandiflora var. grandiflora from Turkey. Biochem. Syst. Ecol. 2005, 33, 617–623. [Google Scholar] [CrossRef]
- Basta, A.; Tzakou, O.; Couladis, M. The Essential Oil Composition of Phlomis cretica C. Presl. Flavour Fragr. J. 2006, 21, 795–797. [Google Scholar] [CrossRef]
- Amor, I.L.-B.; Neffati, A.; Ben Sgaier, M.; Bhouri, W.; Boubaker, J.; Skandrani, I.; Bouhlel, I.; Kilani, S.; Ben Ammar, R.; Chraief, I.; et al. Antimicrobial Activity of Essential Oils Isolated from Phlomis crinita Cav. ssp. Mauritanica Munby. J. Am. Oil Chem. Soc. 2008, 85, 845–849. [Google Scholar] [CrossRef]
- Akramian, M.; Hadian, J.; Joharchi, M.R.; Asghari, B.; Mumivand, H. Volatile Constituents of Phlomis elliptica Benth., A Rare Plant Endemic to Iran. J. Essent. Oil-Bear. Plants 2010, 13, 747–752. [Google Scholar] [CrossRef]
- El Mokni, R.; Majdoub, S.; Chaieb, I.; Jlassi, I.; Joshi, R.K.; Hammami, S. Chromatographic Analysis, Antimicrobial and Insecticidal Activities of the Essential Oil of Phlomis floccosa D. Don. Biomed. Chromatogr. 2019, 33, e4603. [Google Scholar] [CrossRef] [PubMed]
- Özcan, M.M.; Chalchat, J.C.; Bagci, Y.; Dural, H.; Figueredo, G.; Savran, A. Chemical Composition of Essential Oils of Phlomis grandiflora H.S. Thompson var. grandiflora Flowers and Leaves of Turkish Origin. J. Food Biochem. 2011, 35, 125–132. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Azadbakht, M.; Goodarzi, A. The Essential Oils Composition of Phlomis herba-venti L. Leaves and Flowers of Iranian Origin. Flavour Fragr. J. 2004, 19, 29–31. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Tajbakhsh, M.; Rineh, A. Study of the Essential Oils Composition of Leaves and Flowers of Two Subspecies Phlomis herba-venti (pungens and lenkoranica) from Iran. J. Essent. Oil Res. 2008, 20, 46–48. [Google Scholar] [CrossRef]
- Masoudi, S.; Rustaiyan, A.; Azar, P.A.; Larijani, K. Composition of the Essential Oils of Cyclotrichium straussii (Bornm.) Rech. f. and Phlomis pungens Willd. from Iran. J. Essent. Oil Res. 2006, 18, 16–18. [Google Scholar] [CrossRef]
- Delnavazi, M.R.; Baba-Ali, F.; Soufiabadi, S.; Sherafatmand, M.; Ghahremani, F.; Tavakoli, S.; Yassa, N. Essential Oil Composition, Antioxidant Activity and Total Phenolic Content of Some Lamiaceae Taxa Growing in Northwest of Iran. Pharm. Sci. 2014, 20, 22–28. [Google Scholar]
- Başer, K.H.C.; Demirci, B.; Yüzbaşıoğlu, E.; Dadandı, M.Y. Essential oils of Phlomis species of Turkey. In Proceedings of the 37th International Symposium on Essential Oils (37th ISEO), Grasse-Opio, France, 10–13 September 2006. [Google Scholar]
- Karadağ, A.E.; Demirci, B.; Kültür, Ş.; Demirci, F.; Başer, K.H.C. Antimicrobial, Anticholinesterase Evaluation and Chemical Characterization of Essential Oil Phlomis kurdica Rech. Fil. Growing in Turkey. J. Essent. Oil Res. 2020, 32, 242–246. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ćavar Zeljković, S. Chemical Composition and Antioxidant Activity of Phlomis leucophracta, an Endemic Species from Turkey. Nat. Prod. Res. 2020, 34, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Türkmenoğlu, G.; Sarikaya, A.G.; Fakir, H. Phlomis grandiflora H. S. Thompson var. grandiflora ve Phlomis leucophracta P. H. Davis & Hub.-Mor. Taksonlarının Farklı Toplama Zamanlarına Ait Uçucu Bileşenleri. Eur. J. Eng. Sci. Tech. 2019, 17, 145–151. [Google Scholar] [CrossRef]
- Demirci, B.; Dadandı, M.Y.; Paper, D.H.; Franz, G.; Başer, K.H.C. Chemical Composition of the Essential Oil of Phlomis linearis Boiss. & Bal., and Biological Effects on the CAM-Assay: A Safety Evaluation. Z. für Naturforschung C 2003, 58, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Göger, G.; Türkyolu, Ü.; Gürşen, E.N.; Yur, S.; Karaduman, A.B.; Göger, F.; Tekİn, M.; Özek, G. Phytochemical Characterisation of Phlomis linearis Boiss. & Bal and Screening for Anticholinesterase, Antiamylase, Antimicrobial, and Cytotoxic Properties. Turk. J. Chem. 2021, 45, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Asil, H. Chemical Components in the Essential Oils and Ultrasonic Extraction of Amanus Sage Pholomis longifolia var. longifolia Boiss. & Bl. Bangladesh J. Bot. 2023, 52, 799–803. [Google Scholar] [CrossRef]
- Demirci, F.; Guven, K.; Demirci, B.; Dadandi, M.Y.; Baser, K.H.C. Antibacterial Activity of Two Phlomis Essential Oils against Food Pathogens. Food Control 2008, 19, 1159–1164. [Google Scholar] [CrossRef]
- Hemmati Hassan Gavyar, P.; Amiri, H. Chemical Composition of Essential Oil and Antioxidant Activity of Leaves and Stems of Phlomis lurestanica. Int. J. Food Prop. 2018, 21, 1414–1422. [Google Scholar] [CrossRef]
- Sarikaya, A.G.; Fakir, H. Effects of Reaping Time On Volatile Components of Natural Phlomis rigida Labill. And Phlomis monocephala P.H.Davis in Turkey. Ecol. Env. Res. 2019, 17, 1923–1928. [Google Scholar] [CrossRef]
- Kirimer, N.; Başer, K.H.C.; Kürkcüoglu, M. Composition of the Essential Oil of Phlomis nissolii L. J. Essent. Oil Res. 2006, 18, 600–601. [Google Scholar] [CrossRef]
- Sarıkaya, A.G.; Fakir, H. Göller Yöresi Doğal Phlomis L. (Lamiaceae) Taksonlarının Morfolojik ve Yayılış Alanı Özellikleri. Turk. J. For. 2016, 17, 85. [Google Scholar] [CrossRef][Green Version]
- Salehi, M.; Kalvandi, R. New Insights into the Chemical Composition of Essential Oils from Phlomis olivieri Benth. Shoots. Biochem. Syst. Ecol. 2023, 108, 104642. [Google Scholar] [CrossRef]
- Mirza, M.; Nik, Z.B. Volatile Constituents of Phlomis olivieri Benth. from Iran. Flavour Fragr. J. 2003, 18, 131–132. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Rustaiyan, A.; Masoudi, S.; Tajbakhsh, M. Essential Oils of Phlomis persica Boiss. and Phlomis olivieri Benth. from Iran. J. Essent. Oil Res. 2005, 17, 624–625. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Rineh, A.; Khalilzadeh, M.A. Chemical Composition of the Essential Oils from Leaves, Flowers, Stem and Root of Phlomis olivieri Benth. J. Essent. Oil Res. 2007, 19, 501–503. [Google Scholar] [CrossRef]
- Jamzad, M.; Jamzad, Z.; Mokhber, F.; Ziareh, S. Essential Oil Composition of the Leaves and Flowers of Phlomis persica Boiss. and Phlomis olivieri Benth. from Iran. J. Essent. Oil-Bear. Plants 2013, 16, 451–455. [Google Scholar] [CrossRef]
- Mohammadifar, F.; Delnavazi, M.-R.; Yassa, N. Chemical Analysis and Toxicity Screening of Phlomis olivieri Benth. and Phlomis persica Boiss. Essential Oils. J. Pharm. Sci. 2015, 21, 12–17. [Google Scholar] [CrossRef]
- Ghavam, M. Phytochemical Analysis and Antibacterial/Antifungal Activity of the Essential Oil of Phlomis olivieri Benth in Iran. Inflammopharmacology 2023, 31, 2493–2504. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Youssef, F.S.; Ashour, M.L.; Sasmakov, S.A.; Tiezzi, A.; Azimova, S.S. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oils of Three Uzbek Lamiaceae Species. Nat. Prod. Res. 2019, 33, 2394–2397. [Google Scholar] [CrossRef]
- Demirci, B.; Baser, K.H.C.; Dadandi, M.Y. Composition of the Essential Oils of Phlomis rigida Labill. and P. samia L. J. Essent. Oil Res. 2006, 18, 328–331. [Google Scholar] [CrossRef]
- Azami, A.; Vahdat, S.M.; Ariayi, P.; Tavakoli, R. Chemical Composition of Essential Oil And Antioxidant Activity of Extract of Phlomis russeliana From North of Iran. In Proceedings of the 4th National Congress on Medicinal Plants, Tehran, Iran, 12–13 May 2015; p. 664. [Google Scholar]
- Ozdemir, F.A.; Kilic, O.; Yildirimli, S. Essential Oil Composition and Antimicrobial Activity of Endemic Phlomis sieheana Rech. From Bingol (Turkey). J. Essent. Oil-Bear. Plants 2017, 20, 516–523. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Bruno, M.; Bellone, G. Chemical Composition and Antimicrobial Activity of the Essential Oil of Phlomis ferruginea Ten. (Lamiaceae) Growing Wild in Southern Italy. Flavour Fragr. J. 2006, 21, 848–851. [Google Scholar] [CrossRef]
- Sobeh, M.; Mamadalieva, N.Z.; Mohamed, T.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Azimova, S.S.; Wink, M. Chemical Profiling of Phlomis thapsoides (Lamiaceae) and in Vitro Testing of Its Biological Activities. Med. Chem. Res. 2016, 25, 2304–2315. [Google Scholar] [CrossRef]
- Karaman, S.; Cömlekcioğolu, N. Essential oil of Nepeta cilicia Boiss. Apud Bentham and Phlomis viscosa Poiret from Turkey. Int. J. Bot. 2007, 3, 122–124. [Google Scholar] [CrossRef][Green Version]
- Németh-Zámboriné, É. Natural variability of essential oil components. In Handbook of Essential Oils, 2nd ed.; Başer, K.H.C., Buchbauer, G., Eds.; CRC Press-Taylor and Francis Group LLC: Boca Raton, FL, USA, 2016; p. 95. [Google Scholar]
- Zaini, N.N.M.; Salleh, W.M.N.H.W.; Salihu, A.S.; Shaharudin, S.M. Assessment of Variability of Essential Oil Components in the Genus Lindera (Lauraceae) by Multivariate Analysis. Malays. J. Chem. 2024, 26, 271–280. [Google Scholar] [CrossRef]
- Barra, A. Factors Affecting Chemical Variability of Essential Oils: A Review of Recent Developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Chizzola, R. Regular Monoterpenes and Sesquiterpenes (Essential Oils). In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008. ISBN 978-3-642-22143-9. [Google Scholar]
- Noge, K.; Becerra, J. Germacrene D, A Common Sesquiterpene in the Genus Bursera (Burseraceae). Molecules 2009, 14, 5289–5297. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.E.; Nault, L.R. Comparative response of aphids to the alarm pheromone, (E)-ß-Farnesen. Entomol. Exp. Appl. 1977, 22, 236–242. [Google Scholar] [CrossRef]
- Satyal, P.; Shrestha, S.; Setzer, W.N. Composition and Bioactivities of an (E)-β-Farnesene Chemotype of Chamomile (Matricaria chamomilla) Essential Oil from Nepal. Nat. Prod. Commun. 2015, 10, 1453–1457. [Google Scholar] [CrossRef]
- Mumm, R.; Hilker, M. The Significance of Background Odour for an Egg Parasitoid to Detect Plants with Host Eggs. Chem. Senses 2005, 30, 337–343. [Google Scholar] [CrossRef][Green Version]
- Loza-Tavera, H. Monoterpenes in Essential Oils. In Chemicals via Higher Plant Bioengineering; Shahidi, F., Kolodziejczyk, P., Whitaker, J.R., Munguia, A.L., Fuller, G., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1999; Volume 464, pp. 49–62. ISBN 978-1-4613-7143-4. [Google Scholar]
- Dos Santos, É.R.Q.; Maia, J.G.S.; Fontes-Júnior, E.A.; Do Socorro Ferraz Maia, C. Linalool as a Therapeutic and Medicinal Tool in Depression Treatment: A Review. Curr. Neuropharmacol. 2022, 20, 1073–1092. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Cengiz, M.; Kucukyumru, A.; Zengin, G. Determination of antioxidant activities of solvent extracts from an endemic plant: Phlomis leucophracta. Marmara Pharm. J. 2018, 22, 86–90. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential Oils of Lavandula Genus: A Systematic Review of Their Chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-Cineole: A Review of Source, Biological Activities, and Application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Tripathi, A.K.; Prajapati, V.; Kumar, S. Toxicity of 1,8-Cineole towards Three Species of Stored Product Coleopterans. Int. J. Trop. Insect Sci. 2001, 21, 155–160. [Google Scholar] [CrossRef]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol Bioactivity: A Review Focusing on Practical Applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- De Assis Alves, T.; Fontes Pinheiro, P.; Miranda Praça-Fontes, M.; Fonseca Andrade-Vieira, L.; Barelo Corrêa, K.; De Assis Alves, T.; Da Cruz, F.A.; Lacerda Júnior, V.; Ferreira, A.; Bastos Soares, T.C. Toxicity of Thymol, Carvacrol and Their Respective Phenoxyacetic Acids in Lactuca sativa and Sorghum bicolor. Ind. Crops. Prod. 2018, 114, 59–67. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants Belonging to the Genus Thymus as Antibacterial Agents: From Farm to Pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Elbe, H.; Ozturk, F.; Yigitturk, G.; Baygar, T.; Cavusoglu, T. Anticancer Activity of Linalool: Comparative Investigation of Ultrastructural Changes and Apoptosis in Breast Cancer Cells. Ultrastruct. Pathol. 2022, 46, 348–358. [Google Scholar] [CrossRef]
- Han, E.-J.; Choi, E.-Y.; Jeon, S.-J.; Moon, J.-M.; Lee, S.-W.; Lee, J.-H.; Jung, G.-H.; Han, S.-H.; Jung, S.-H.; Yang, M.-S.; et al. Anticancer Effects of α-Pinene in AGS Gastric Cancer Cells. J. Med. Food 2024, 27, jmf.2023.K.0267. [Google Scholar] [CrossRef]
- Araújo-Filho, H.G.D.; Dos Santos, J.F.; Carvalho, M.T.B.; Picot, L.; Fruitier-Arnaudin, I.; Groult, H.; Quintans-Júnior, L.J.; Quintans, J.S.S. Anticancer Activity of Limonene: A Systematic Review of Target Signaling Pathways. Phytother. Res. 2021, 35, 4957–4970. [Google Scholar] [CrossRef] [PubMed]
- Ogunlesi, M.; Okiei, W.; Ofor, E.; Osibote, A.E. Analysis of the essential oil from the dried leaves of Euphorbia hirta Linn (Euphorbiaceae), a potential medication for asthma. Afr. J. Biotechnol. 2009, 8, 7042–7050. [Google Scholar]
- Francke, W.; Schulz, S. Pheromones. In Comprehensive Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 1999; pp. 197–261. ISBN 978-0-08-091283-7. [Google Scholar]
- Mehrnia, M.; Jamzad, Z.; Jalili, A. The conservation status of Phlomis lurestanica. J. Iran. Nat. 2020, 5, 122860. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).