Sulfated Nutrition Modifies Nutrient Content and Photosynthetic Pigment Concentration in Cabbage under Salt Stress
Abstract
1. Introduction
2. Results
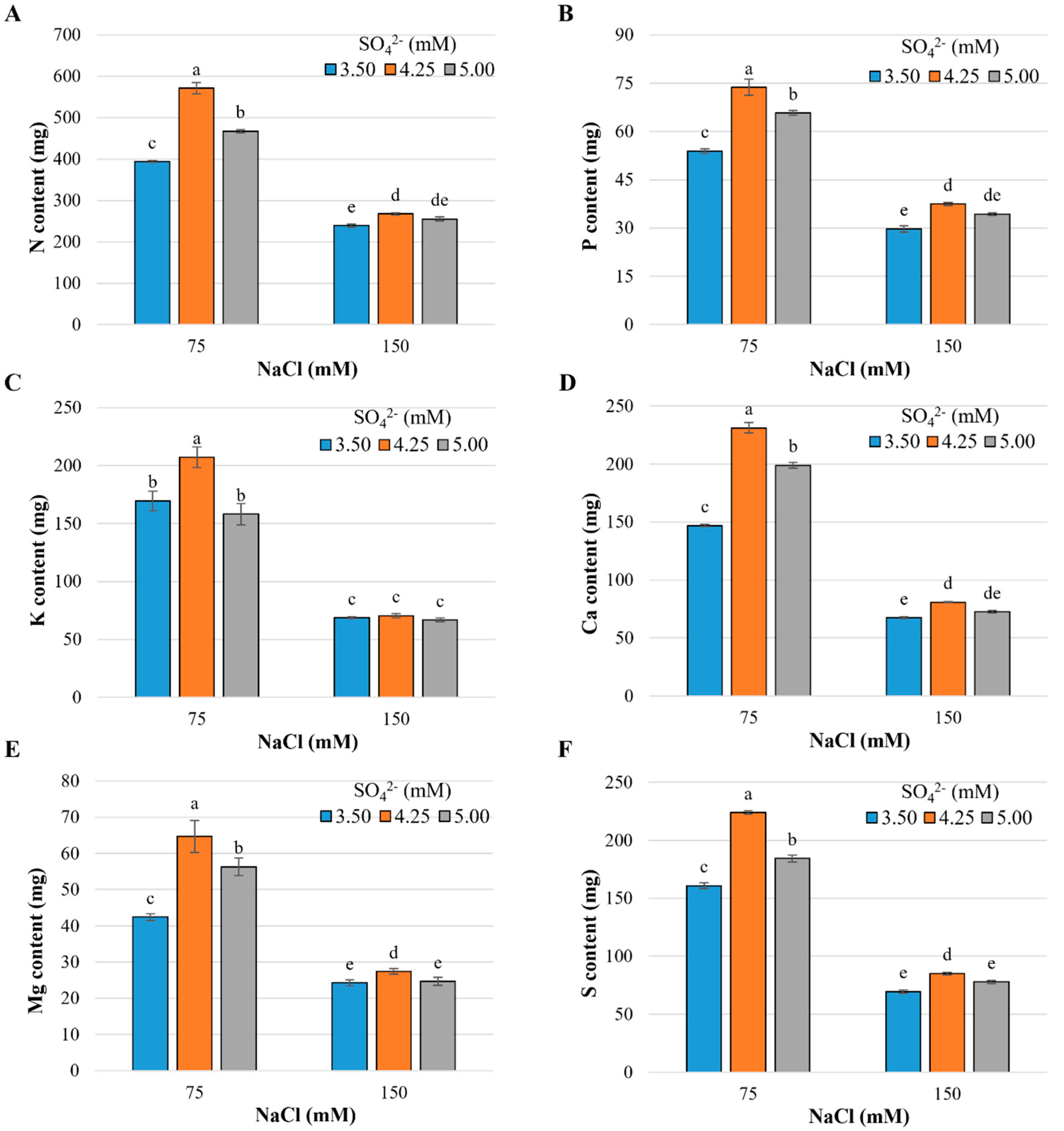
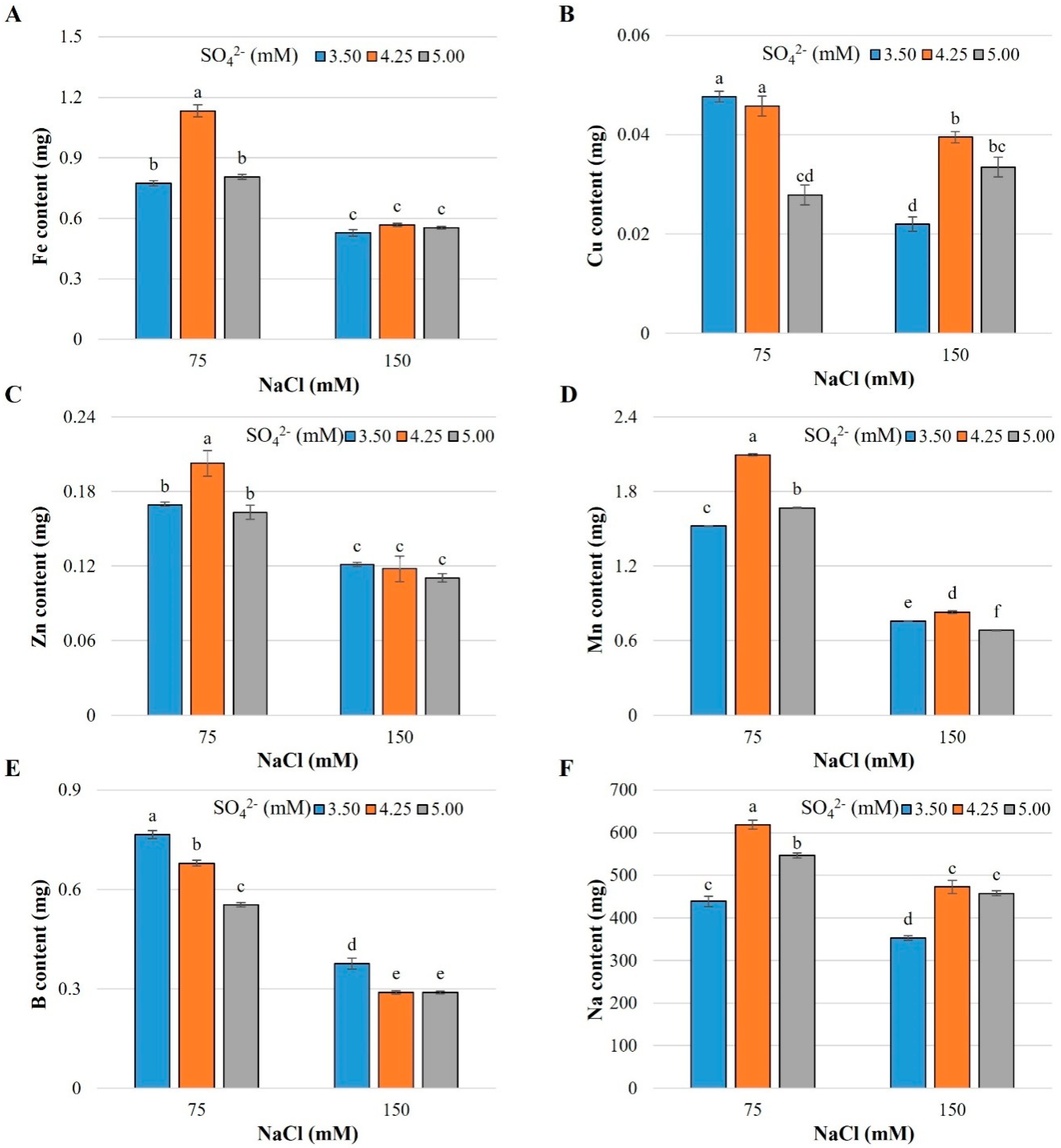
2.1. Nutrient and Sodium Content
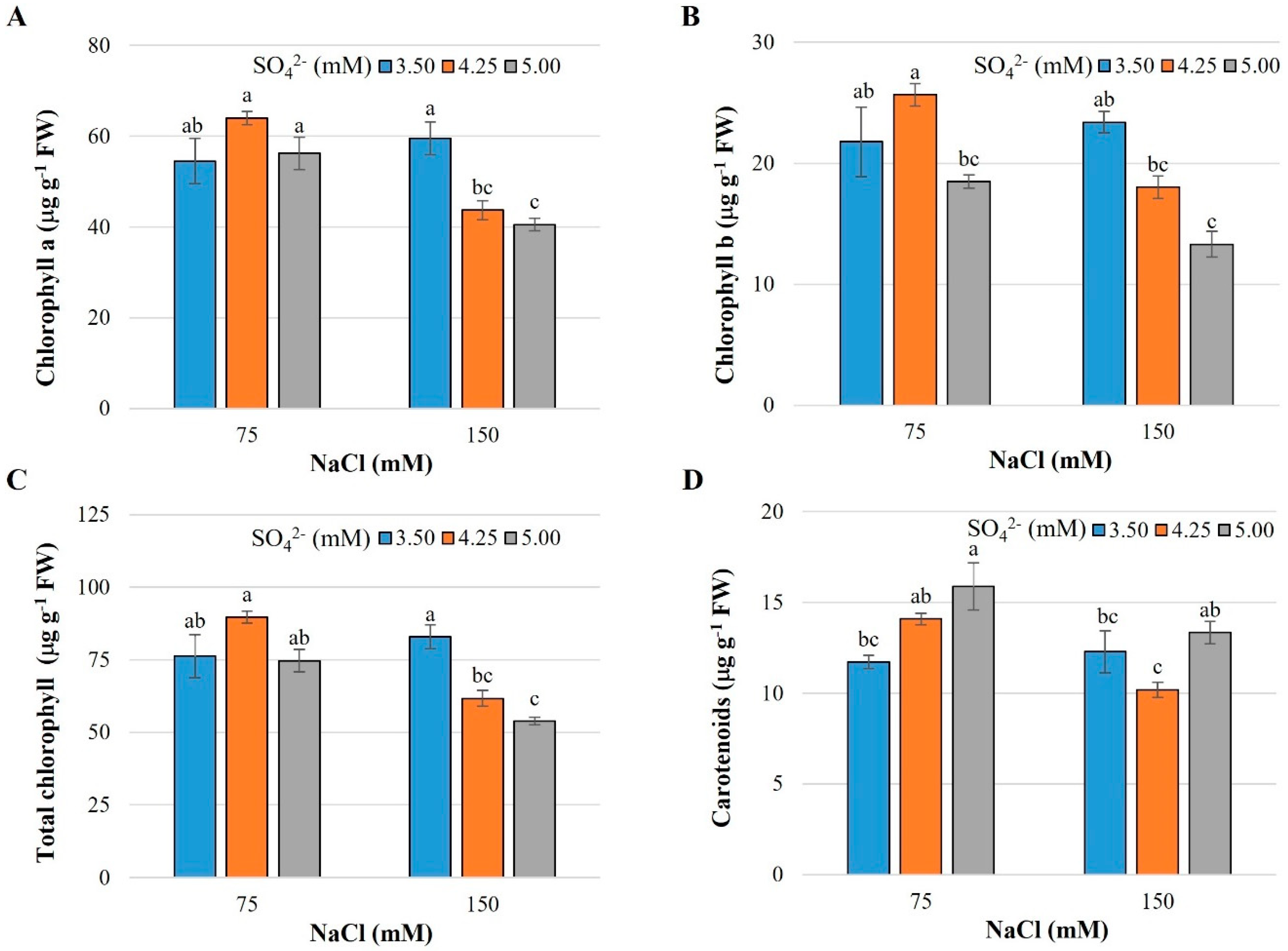
2.2. Photosynthetic Pigments
3. Discussion
4. Materials and Methods
4.1. Experiment Location and Experimental Conditions
4.2. Plant Material
4.3. Treatments and Experimental Design
4.4. Evaluated Variables
4.4.1. Nutrient and Sodium Content
4.4.2. Photosynthetic Pigments
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Blasco, B.; Martos, V. Combating Salinity through Natural Plant Extracts Based Biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Atta, K.; Mondal, S.; Gorai, S.; Singh, A.P.; Kumari, A.; Ghosh, T.; Roy, A.; Hembram, S.; Gaikwad, D.J.; Mondal, S.; et al. Impacts of Salinity Stress on Crop Plants: Improving Salt Tolerance Through Genetic and Molecular Dissection. Front. Plant Sci. 2023, 14, 1241736. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Bilal, C.M.; Ahsin, A.M.; Rizwan, A.M.; Soufan, W.; Hassan, M.U.; Rahman, M.A.; et al. Mitigation of Salinity-Induced Oxidative Damage, Growth, and Yield Reduction in Fine Rice by Sugarcane Press Mud Application. Front. Plant Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Sulaiman, M.A.H.; Bilal, M.H.; Reham, A.; Wahid, A.; Siddque, K.H.M.; Farooq, M. Regulation of Photosynthesis under Salt Stress and Associated Tolerance Mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Chakraborty, K. Brassicaceae plants response and tolerance to salinity. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 203–228. [Google Scholar] [CrossRef]
- Yan, S.; Chong, P.; Zhao, M. Effect of Salt Stress on the Photosynthetic Characteristics and Endogenous Hormones, and: A Comprehensive Evaluation of Salt Tolerance in Reaumuria soongorica Seedlings. Plant Signal. Behav. 2022, 17, 2031782. [Google Scholar] [CrossRef]
- Zhang, X.; He, P.; Guo, R.; Huang, K.; Huang, X. Effects of Salt Stress on Root Morphology, Carbon and Nitrogen Metabolism, and Yield of Tartary Buckwheat. Sci. Rep. 2023, 13, 12483. [Google Scholar] [CrossRef] [PubMed]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium Signaling and Salt Tolerance are Diversely Entwined in Plants. Plant Signal. Behav. 2019, 14, e166545. [Google Scholar] [CrossRef]
- Guo, J.; Shan, C.; Zhang, Y.; Wang, X.; Tian, H.; Han, G.; Zhang, Y.; Wang, B. Mechanisms of Salt Tolerance and Molecular Breeding of Salt-Tolerant Ornamental Plants. Front. Plant Sci. 2022, 13, 854116. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ Response Mechanisms to Salinity Stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive Oxygen Species (ROS) and Response of Antioxidants as ROS-Scavengers during Environmental Stress in Plants. Front. Environ. Sci. 2014, 2, 00053. [Google Scholar] [CrossRef]
- Shahzad, B.; Rehman, A.; Tanveer, M.; Wang, L.; Park, S.K.; Ali, A. Salt Stress in Brassica: Effects, Tolerance Mechanisms, and Management. J. Plant Growth Regul. 2022, 41, 781–795. [Google Scholar] [CrossRef]
- Essoh, A.P.; Monteiro, F.; Peña, A.R.; País, M.S.; Moura, M.; Romeiras, M.M. Exploring Glucosinolates Diversity in Brassicaceae: A Genomic and Chemical Assessment for Deciphering Abiotic Stress Tolerance. Plant Physiol. Biochem. 2020, 150, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Asgher, M.; Iqbal, N.; Khan, N.A. Potentiality of Sulphur-Containing Compounds in Salt Stress Tolerance. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 443–472. [Google Scholar] [CrossRef]
- Saad-Allah, K.M.; Ragab, G.A. Sulfur Nanoparticles Mediated Improvement of Salt Tolerance in Wheat Relates to Decreasing Oxidative Stress and Regulating Metabolic Activity. Physiol. Mol. Biol. Plants 2020, 26, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.V.; Banerjee, P.; Verma, V.C.; Sukumaran, S.; Chandran, M.A.S.; Gopinath, K.A.; Venkatesh, G.; Yadav, S.K.; Singh, V.K.; Awasthi, N.K. Plant Nutrition: An Effective Way to Alleviate Abiotic Stress in Agricultural Crops. Int. J. Mol. Sci. 2022, 23, 8519. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Khalid, M.F.; Hussain, M.; Ali, M.A.; Nawaz, A.; Zakir, I.; Fátima, Z.; Ahmad, S. Role of Micronutrients in Salt Stress Tolerance to Plants. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 297–324. [Google Scholar] [CrossRef]
- Nazir, F.; Mahajan, M.; Khatoon, S.; Albaqami, M.; Ashfaque, F.; Chhillar, H.; Chopra, P.; Khan, M. Sustaining Nitrogen Dynamics: A Critical Aspect for Improving Salt Tolerance in Plants. Front. Plant Sci. 2023, 14, 1087946. [Google Scholar] [CrossRef] [PubMed]
- Sikder, R.K.; Wang, X.; Zhang, H.; Gui, H.; Dong, Q.; Jin, D.; Song, M. Nitrogen Enhances Salt Tolerance by Modulating the Antioxidant Defense System and Osmoregulation Substance Content in Gossypium hirsutum. Plants 2020, 9, 450. [Google Scholar] [CrossRef]
- Carillo, P.; Rouphael, Y. Nitrate Uptake and Use Efficiency: Pros and Cons of Chloride Interference in the Vegetable Crops. Front. Plant Sci. 2022, 13, 899522. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Churchill, K.A.; Sze, H. Anion-Sensitive, H+-Pumping ATPase of Oat Roots. Direct Effects of Cl−NO3−, and a Disulfonic Stilbene. Plant Physiol. 1984, 76, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Franco-Navarro, J.D.; Brumos, J.; Rosales, M.A.; Cubero-Font, P.; Talon, M.; Colmenero-Flores, J.M. Chloride Regulates Leaf Cell Size and Water Relations in Tobacco Plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G. High Concentrations of Na+ and Cl− Ions in Soil Solution Have Simultaneous Detrimental Effects on Growth of Faba Bean under Salinity Stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.A.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M. Salinity Effects on Nitrogen Metabolism in Plants—Focusing on the Activities of Nitrogen Metabolizing Enzymes: A Review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Böckmann, R.A.; Hac, A.; Heimburg, T.; Grubmüller, H. Effect of Sodium Chloride on a Lipid Bilayer. Biophys. J. 2003, 85, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A. Asymmetry of Lipid Bilayers Induced by Monovalent Salt: Atomistic Molecular-Dynamics Study. J. Chem. Phys. 2005, 122, 244902. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Vattulainen, I. Effect of NaCl and KCl on Phosphatidylcholine and Phosphatidylethanolamine Lipid Membranes: Insight from Atomic-Scale Simulations for Understanding Salt-Induced Effects in the Plasma Membrane. J. Phys. Chem. 2008, 112, 1953–1962. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Rupasinghe, T.; Roessner, U.; Barkla, B. Salt Stress Alters Membrane Lipid Content and Lipid Biosynthesis Pathways in the Plasma Membrane and Tonoplast. Plant Physiol. 2022, 189, 805–826. [Google Scholar] [CrossRef]
- Islam, M.M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous Application of Calcium Ameliorates Salinity Stress Tolerance of Tomato (Solanum lycopersicum L.) and Enhances Fruit Quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous Calcium: Its Mechanisms and Research Advances Involved in Plant Stress Tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of Sodium Transport in Plants-Progresses and Challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Ahmad, S.; Hussain, S.; Hussain, M. Nutrient Homeostasis and Salt Stress Tolerance. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 391–413. [Google Scholar] [CrossRef]
- Mehmood, M.Z.; Afzal, O.; Ahmed, M.; Qadir, G.; Kheir, A.M.S.; Askam, M.A.; Din, A.M.U.; Khan, I.; Hassan, M.J.; Meraj, T.A.; et al. Can Sulphur Improve the Nutrient Uptake Partitioning, and Seed Yield of Sesame? Arab. J. Geosci. 2021, 14, 865. [Google Scholar] [CrossRef]
- Khan, N.A.; Khan, M.I.R.; Asgher, M.; Farma, M.; Masood, A.; Syeed, S. Salinity Stress Tolerance in Plants: Revisiting the role of sulfur metabolites. J. Plant Biochem. Physiol. 2014, 2, 1000120. [Google Scholar] [CrossRef]
- Cao, M.J.; Wang, Z.; Zhao, Q.; Mao, J.L.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.K.; Xiang, C.B. Sulfate Availability Affects ABA Levels and Germination Response to ABA and Salt Stress in Arabidopsis thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef]
- Reich, M.; Aghajanzadeh, T.; Helm, J.; Parmar, S.; Hawkesford, M.J.; Kok, L. Chloride and Sulfate Salinity Differently Affect Biomass, Mineral Nutrient Composition and Expression of Sulfate Transport and Assimilation Genes in Brassica rapa. Plant Soil 2017, 411, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, K.; Courty, P.E.; Le Signor, C.; Wipf, D.; Vernoud, V. Sulfate Transporters in the Plant’s Response to Drought and Salinity: Regulation and Possible Functions. Front. Plant Sci. 2014, 5, 580. [Google Scholar] [CrossRef]
- Rabhi, M.; Barhoumi, Z.; Ksouri, R.; Abdelly, C.; Gharsalli, M. Interactive Effects of Salinity and Iron Deficiency in Medicago ciliaris. Comptes Rendus Biol. 2007, 330, 779–788. [Google Scholar] [CrossRef]
- Farooq, M.; Asif, S.; Jang, Y.H.; Park, J.R.; Zhao, D.D.; Kim, E.G.; Kim, K.M. Effect of Different Salts on Nutrients Uptake, Gene Expression, Antioxidant, and Growth Pattern of Selected Rice Genotypes. Front. Plant Sci. 2022, 13, 895282. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.; Shukla, L.M. Effect of Boron–Sulphur Interaction on Their Uptake and Quality Parameters of Mustard (Brassica juncea L.) and Sunflower (Helianthus annuus L.). Indian Soc. Soil Sci. 2008, 56, 225–230. [Google Scholar]
- Turan, S.; Tripathy, B.C. Salt-Stress Induced Modulation of Chlorophyll Biosynthesis during De-Etiolation of Rice Seedlings. Physiol. Plant. 2015, 153, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Björn, L.O.; Papageorgiou, G.C.; Blankenship, R.E. A Viewpoint: Why Chlorophyll a? Photosynth. Res. 2009, 99, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits Through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shokramraji, Z.; Tavakkoli, S.; Mihaylova, D.; Lante, A. Chlorophylls as Natural Bioactive Compounds Existing in Food By-Products: A Critical Review. Plants 2023, 12, 1533. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under Stressful Environments: An Overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Torres-Neto, A.; Campostrini, E.; De Oliverira, J.G.; Bressan-Smith, R.E. Photosynthetic Pigments, Nitrogen, Chlorophyll a Fluorescence and SPAD-502 Readings in Coffee Leaves. Sci. Hortic. 2005, 104, 199–209. [Google Scholar] [CrossRef]
- Chauhan, J.; Prathibha, M.D.; Sing, P.; Choyal, P.; Mishra, U.N.; Saha, D.; Kumar, R.; Anuragi, H.; Pandey, S.; Bose, B.; et al. Plant Photosynthesis under Abiotic Stresses: Damages, Adaptive, and Signaling Mechanisms. Plant Stress 2023, 10, 100296. [Google Scholar] [CrossRef]
- Nenko, N.; Kiseleva, G.; Ilina, I.; Sokolova, V.; Zaporozhets, N. Grapes Adaptive Resistance to Summer Stresses in the Conditions of Climate Change. In Proceedings of the International Scientific and Practical Conference “Fundamental and Applied Research in Biology and Agriculture, Orel, Russia, 24–25 February 2021. [Google Scholar] [CrossRef]
- Dietz, K.J. Thiol-Based Peroxidases and Ascorbate Peroxidases: Why Plants Rely on Multiple Peroxidase Systems in the Photosynthesizing Chloroplast? Mol. Cell. 2016, 39, 20–25. [Google Scholar] [CrossRef]
- Lunde, C.; Zygadlo, A.; Toft-Simonsen, H.; Nielsen, P.; Blennow, A.; Haldrup, A. Sulfur Starvation in Rice: The Effect on Photosynthesis, Carbohydrate Metabolism, and Oxidative Stress Protective Pathways. Physiol. Plant. 2008, 134, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Linić, I.; Salopek-Sondi, B. Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae. Agronomy 2021, 11, 361. [Google Scholar] [CrossRef]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.; Pateiro, M.; Lorenzo, J.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Leiva-Ampuero, A.; Agurto, M.; Matus, J.T.; Hoppe, G.; Huidobro, C.; Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Stange, C.; Canessa, P.; Vega, A. Salinity Impairs Photosynthetic Capacity and Enhances Carotenoid-Related Gene Expression and Biosynthesis in Tomato (Solanum lycopersicum L. cv. Micro-Tom). PeerJ 2020, 17, e9742. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Hossain, M.S.; Bhuyan, M.H.M.B.; Al Mahmud, J.; Nahar, K.; Fujita, M. The Role of Sulfur in Plant Abiotic Stress Tolerance: Molecular Interactions and Defense Mechanisms. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Oku, H., Nahar, K., Hawrylak-Nowak, B., Eds.; Springer: Singapore, 2018; pp. 221–252. [Google Scholar] [CrossRef]
- Steiner, A.A. The Universal Nutrient Solution. In Proceedings of the 6th International Congress on Soilless Culture, ISOSC, Wageningen, The Netherlands, 29 April–5 May 1984; pp. 633–649. [Google Scholar]
- Bremner, M.J. Total Nitrogen. In Methods of Soil Analysis; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Alcántar-González, G.; Sandoval-Villa, M. Handbook of Chemical Analyses of Plant Tissues; Mexican Society of Soil Science: Chapingo, Mexico, 1999; p. 156. [Google Scholar]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric Analysis of Chlorophylls and Carotenoids from Commonly Grown Fern Species by Using Various Extracting Solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar]
| NaCl (mM) | SO42− (mM) | Chlorophyll a/ Chlorophyll b Ratio | Total Chlorophyll/ Carotenoid Ratio |
|---|---|---|---|
| 75 | 3.50 | 2.505 | 6.514 |
| 4.25 | 2.500 | 6.364 | |
| 5.00 | 3.043 | 4.709 | |
| 150 | 3.50 | 2.543 | 6.751 |
| 4.25 | 2.426 | 6.057 | |
| 5.00 | 3.048 | 4.040 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco-Sangerman, F.; Gómez-Merino, F.C.; Peralta-Sánchez, M.G.; Trejo-Téllez, L.I. Sulfated Nutrition Modifies Nutrient Content and Photosynthetic Pigment Concentration in Cabbage under Salt Stress. Plants 2024, 13, 1337. https://doi.org/10.3390/plants13101337
Pacheco-Sangerman F, Gómez-Merino FC, Peralta-Sánchez MG, Trejo-Téllez LI. Sulfated Nutrition Modifies Nutrient Content and Photosynthetic Pigment Concentration in Cabbage under Salt Stress. Plants. 2024; 13(10):1337. https://doi.org/10.3390/plants13101337
Chicago/Turabian StylePacheco-Sangerman, Fresia, Fernando Carlos Gómez-Merino, María Guadalupe Peralta-Sánchez, and Libia I. Trejo-Téllez. 2024. "Sulfated Nutrition Modifies Nutrient Content and Photosynthetic Pigment Concentration in Cabbage under Salt Stress" Plants 13, no. 10: 1337. https://doi.org/10.3390/plants13101337
APA StylePacheco-Sangerman, F., Gómez-Merino, F. C., Peralta-Sánchez, M. G., & Trejo-Téllez, L. I. (2024). Sulfated Nutrition Modifies Nutrient Content and Photosynthetic Pigment Concentration in Cabbage under Salt Stress. Plants, 13(10), 1337. https://doi.org/10.3390/plants13101337






