Abstract
Driven by a surge in global interest in natural products, macroalgae or seaweed, has emerged as a prime source for nutraceuticals and pharmaceutical applications. Characterized by remarkable genetic diversity and a crucial role in marine ecosystems, these organisms offer not only substantial nutritional value in proteins, fibers, vitamins, and minerals, but also a diverse array of bioactive molecules with promising pharmaceutical properties. Furthermore, macroalgae produce approximately 80% of the oxygen in the atmosphere, highlighting their ecological significance. The unique combination of nutritional and bioactive attributes positions macroalgae as an ideal resource for food and medicine in various regions worldwide. This comprehensive review consolidates the latest advancements in the field, elucidating the potential applications of macroalgae in developing nutraceuticals and therapeutics. The review emphasizes the pivotal role of omics approaches in deepening our understanding of macroalgae’s physiological and molecular characteristics. By highlighting the importance of omics, this review also advocates for continued exploration and utilization of these extraordinary marine organisms in diverse domains, including drug discovery, functional foods, and other industrial applications. The multifaceted potential of macroalgae warrants further research and development to unlock their full benefits and contribute to advancing global health and sustainable industries.
1. Introduction
The world is witnessing a surge in the popularity of natural products (NPs) due to their incredible therapeutic potential and positive impact on human health. Notably, around 40% of FDA-approved drugs between 1981 and 2014 were derived from NPs [1]. The marine ecosystem has piqued the interest of researchers as a potential source of NPs for various industrial applications, including food, pharmaceuticals, and cosmetics. Among the marine species, algae occupy a prominent position [2,3,4]. Algae, the primary producers of the marine ecosystem [5], have been under study for more than half a century. However, the emergence of new diseases and global malnutrition has made the scrutiny of these tiny marine species even more critical. Algae are being investigated as a potential source of various products such as antibiotics [6], proteins [7], metabolites [8], antioxidants [9], and dietary supplements [10]. Some species of algae can be mass cultivated using photobioreactors, and their short generation time reduces the overexploitation of marine resources [11].
Algae are genetically diverse and can be classified into macroalgae and microalgae [12]. Macroalgae, also known as seaweed, contain various nutrients essential for the human diet, including proteins, fibers, vitamins, fats, and minerals [13]. These macroalgae are capable of growth without relying on land areas and grow faster when compared to terrestrial plants [14]. Similar to their terrestrial counterparts, seaweeds also perform crucial ecological roles, such as serving as bioindicators of water quality and participating in bioaccumulation and bioremediation processes, thereby helping to maintain aquatic environments. In addition to their contributions to marine ecosystems, seaweeds are responsible for producing about 80% of the atmospheric oxygen, which is then used by land organisms for respiration [3]. Furthermore, due to their balanced nutrient composition, macroalgae are a promising food source and are currently utilized in various countries [15]. Seaweed contains a wealth of bioactive metabolites that have the potential to treat a variety of diseases [16]. Recent research has highlighted the pharmaceutical potential of seaweed as a complementary medicine as gelling and thickening agents in various industries [17]. Consequently, macroalgae have emerged as an alternative source of food and medicine in many countries [18]. China, the Philippines, and Indonesia are the largest seaweed-producing countries in the world [19].
As the exploration and utilization of macroalgae in therapeutic applications continues to grow, it is increasingly important to have a thorough understanding of their physiological and molecular characteristics. Omics approaches are an invaluable tool for achieving this goal. Rapid advances in omics technologies have ushered in a new era in biotechnology, extending beyond genomics. This review focuses on macroalgae as a potential source for nutraceutical and therapeutic applications and consolidates the latest advancements in this field using omics approaches.
2. Macroalgae—A Brief Overview
Macroalgae are a diverse group of multicellular marine organisms with a long history of cultivation and traditional use in different regions of the world. They are classified into three major phyla based on their photosynthetic pigments: red algae (Rhodophyta), green algae (Chlorophyta), and brown algae (Ochrophyta) [20]. Each of these phyla has unique properties and bioactive compounds. Red algae are rich in phycobiliproteins, pigments, phycolectins, mycosporine, unsaturated fatty acids, polysaccharides, minerals, and vitamins [21]. Both red and green algae are abundant sources of bromophenols, phenolic acids, and flavonoids, while brown algae are high in phlorotannin content [22]. Some properties of these three phyla are compared and tabulated in Table 1.

Table 1.
Comparison of Key Properties among Three Phyla of Macroalgae.
The most cultivated macroalgae are Pyropia spp., Kappaphycus spp., Undaria spp., Gracilaria spp., and Eucheuma spp. [19]. Lonicera japonica, Undaria pinnatifida, and Hizikia fusiforme are some of the notable commercially important seaweeds [30]. In brief, these marine organisms with diverse metabolites can be used as an alternative source for synthetic ingredients in pharmaceuticals and nutraceuticals.
2.1. Nutraceutical Potentials of Macroalgae
Seaweeds are an important source of high-quality proteins in the marine environment, which are essential in nutraceutical formulations [31]. Macroalgae contain various amino acids, including threonine, alanine, arginine, glutamic acid, and aspartic acid [32]. In addition, these organisms also contain micronutrients such as Manganese (Mn), Copper (Cu), Zinc (Zn), Iron (Fe), Cadmium (Cd), Lead (Pb) [33], and macronutrients such as phlorotannins, catechol, and quercetin [34]. Macroalgal species and their nutrients are listed in Table 2.

Table 2.
List of nutrients present in macroalgae.
A nutraceutical is a substance that has biological effects or can protect against some chronic diseases. This can be used to improve health, delay senility, increase life expectancy, or improve the body’s metabolism. The major reason macroalgae are necessary is their size, which provides an increased surface area for greater biosorption of hazardous chemicals even at low concentrations. A few nutraceutical applications are depicted in Figure 1a and the macroalgal ingredients are given in Figure 1b.
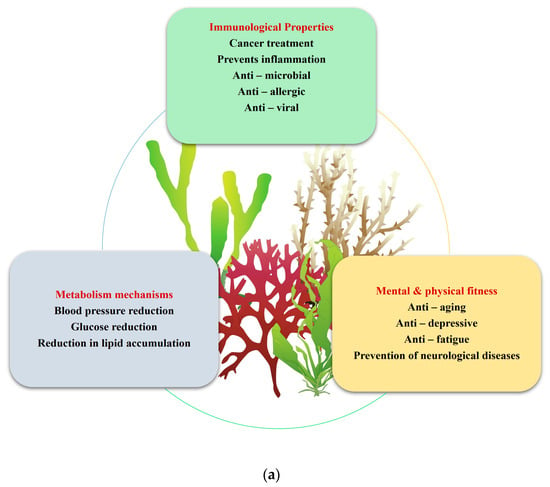
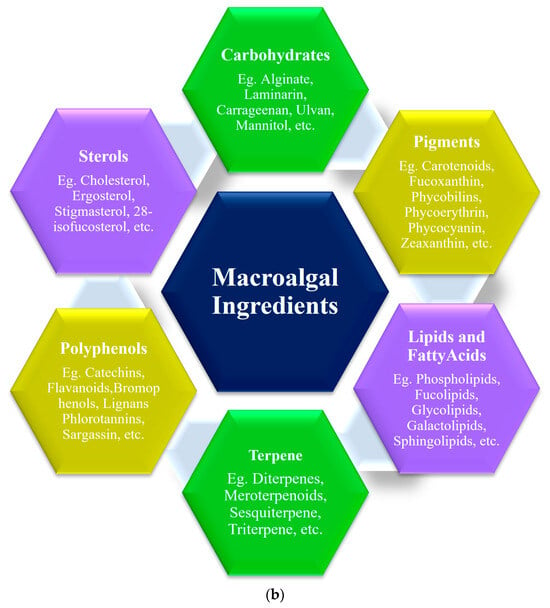
Figure 1.
(a) Nutraceutical applications of macroalgae, (b) Macroalgal active ingredients.
A few representative macroalgae derived compounds and their nutraceutical and therapeutic applications are given in the Table 3.

Table 3.
Macroalgal Nutraceutical and Therapeutic applications.
The studies show the efficiency of the nutrients derived from the marine macroalgae, which gain its importance in medical, food, cosmetics, and other industrial segments to list on.
2.2. Pharmaceutical Potentials of Macroalgae
Seaweeds are a rich source of bioactive compounds, particularly brown algae, which contain over 700 biochemicals with various therapeutic properties, such as anti-cancer, anti-inflammatory, antioxidant, anti-coagulant, anti-HIV, anti-diabetic, and anti-allergic activities [14]. For example, fucoxanthin, found in the brown algae U. pinnatifida has been shown to have significant anti-cancer, anti-inflammatory, and anti-obesity effects [104]. Fucoidan, synthesized by Saccharina japonica, has antiviral, anticancer, and anti-inflammatory effects [105]. Ulva spp. produces eicosapentaenoic and docosahexaenoic acids, which have anti-inflammatory and antioxidant activities [106]. Other important bioactive compounds present in seaweeds include phytocoerythrobilin, neoxanthin, chlorophyll-a, heparins, carrageenan, fucans, and galactans [107].
The diversity of macroalgae is a potential source of bioactive substances with biotechnological and medicinal applications. The investigation into marine natural products began in 1951 when Bergman and Feeney extracted spongothymidine and spongouridine from the sponge Cryptotethya crypta Laubenfels. This led to the discovery of arabinosyl cytosine (Ara-C), an anti-cancer compound with marine origins used to treat many types of leukaemia [108]. According to the existence of colors, macroalgae can be categorized into three groups: Rhodophyceae (red algae), Phaeophyceae (brown algae), and Chlorophyceae (green algae). Chlorophylls a and b are responsible for the color of Chlorophyta, while fucoxanthin, chlorophylls a, b, and c, are thought to be responsible for the greenish-brown color of Phaeophyta [109]. The pigments that give Rhodophyta its color include phycobilins such as phycoerythrin and phycocyanin. These are great sources of the vitamins A, Bl, B12, C, D, and E, riboflavin, niacin, pantothenic acid, and folic acid, as well as the minerals calcium, phosphorus, sodium, and potassium. They are also considered a source of bioactive chemicals because they can form a range of secondary metabolites [108]. In fact, macroalgae, like photosynthesizing plants, have antioxidant defense mechanisms. They also include polysaccharides and glycoproteins that contain chemicals with anti-cancer, antioxidant, anti-inflammatory, anti-HIV, anti-coagulant, anti-allergic, and anti-diabetic properties. This review focuses on the medicinal qualities of macroalgae, and the bioactive substances used to cure diseases [109].
Brown seaweed is widely used as nutritional supplements, herbal medications, and traditional treatments because they contain substantial amounts of the antioxidant fucoxanthin. Several cell culture models and animal investigations have shown that fucoxanthin has great antioxidant capacity and may be involved in controlling the Nrf2/ARE pathway. Fucoxanthin has an anti-inflammatory action, and their molecular methods of prevention are defined by the suppression of NF-B-related pathways [110]. Fucoxanthin has been shown to have anticancer properties in the MDA-MB-231 human breast cancer cell line, K562 and TK6 human leukemia cell lines, and the mice cancer model [111]. Hitoe and Shimoda, conducted a double-blind placebo-controlled trial in which capsules containing 1 and 3 mg fucoxanthin were given to moderately obese males and females for 4 weeks, resulted in decreased body weight, BMI, fat area and mass [112]. Padina tetrastromatica extract, which is fucoxanthin-rich, reduced the effects of high-calorie diet-induced obesity in c57bl/6j mice by inhibiting adipocytic lipogenesis, causing fat mass reduction, and lowering intracellular lipid content, adipocyte size, and adipose weight Fucoxanthin, interestingly, has anti-obesity benefits through modulating gut microbiota [112,113]. It also serves as a neurotrophic factor-like chemical that provides CNS neurons with neuroprotection and neurite outgrowth. In addition, fucoxanthin exhibits antibacterial potential and can treat disorders of the metabolic, hepatic, renal, cardiovascular, bone, ophthalmic, skin, and respiratory types [113,114]. Red and green seaweed contain the highest levels of phenolic substances, such as flavonoids, phenolic acids, and bromophenols [115]. The long-chain saturated fatty acids found in Jania rubens, such as n-hexadecanoic acid and docosanoic acid 1,2,3-propanetriyl ester and hexanedioic acid, as well as dioctyl ester, exhibited antimicrobial activity against 32 isolates of multidrug-resistant bacteria [116].
Red alga Sphaerococcus coronopifolius produces brominated cyclic diterpenes that function as antibacterial action against methicillin-resistant Staphylococcus aureus strains [117]. From Kappaphycus alvarezii, phenolic compounds, glycosides, and carbohydrates have been shown to have antibacterial effects on a variety of human pathogens [115]. Human pathogenic fungi, such as Aspergillus and Candida, showed resistance to the antifungal effects of methanolic extracts from Corallina mediterranea, Hypnea musciformis, and Laurencia papillosa [118]. A diterpene known as ‘Sphaerodactylomelol fraction 2′, recently discovered in S. coronopifolius, exhibited the most potent antifungal properties against Candida albicans [116]. Notably, the antifungal effectiveness of methanolic extracts from Acanthaphora spicifra was comparable to that of over-the-counter medications, such as ciprofloxacin and amphotericin [118]. Carrageenan from Solieria chordalis displayed antiviral activity toward HSV1 and had a protective influence against various sexually transmitted viruses, including genital warts and herpes simplex virus (HSV), thereby contributing to limiting the spread of HIV [119]. Additionally, the Mexican red seaweed, S. filiformis produces polyphenol-rich extracts with significant virucidal properties against the measles virus and prevent virus spread in vitro. For instance, residents in China have utilized crude extracts from the brown seaweed Sargassum naozhouense to cure fever, infections, laryngitis, and other illnesses. Vietnamese physicians have also employed species from the Kappaphycus and Eucheuma genera to lessen the likelihood of tumors, ulcers, and headaches. Furthermore, seaweed, such as sargassum, has been used to treat iodine deficient illnesses, including Goitre [120].
2.2.1. Antioxidant Activity
Many scientists are actively working on natural antioxidants as safe alternatives to synthetic counterparts. As an antioxidant, R-phycoerythrin from Palmaria palmata and Polysiphonia urceolata was discovered [121]. In contrast, some synthetic antioxidants, such as butylated hydroxyl anisole (BHA), butylated hydroxyl toluene (BHT), tocopherol, and propyl gallate, have been found to cause liver damage and induce cancer [119]. Mycosporine rich seaweed has antioxidant properties. Despite having low macroalgal lipid content, it is rich in polyunsaturated fatty acids (PUFA), particularly arachidonic, eicosapentaenoic, linoleic, and octadecatetraenoic acids. These PUFAs exhibit antibacterial, antiviral, and antioxidant properties and are also known to prevent cardiovascular diseases and diabetes [122].
2.2.2. Anticancer and Antiproliferative Activities
Recently, a new anticancer medication was developed using green seaweed Cladophoropsis sp. and red seaweed Gracilaria foliifera [123]. Carrageenan oligosaccharides from different red seaweed species have demonstrated anticarcinogenic activity with reduced cytotoxicity. They also exhibit synergistic effects when combined with conventional medications as well as improving the immunocompetence of cells killed by these medications [124]. Furthermore, the organic extract from the red seaweed Rhodomela confervoides contains 3-bromo-4,5-dihydroxybenzaldehyde bromophenols and 3-bromo-4,5-dihydroxy benzoic acid methyl ester. These compounds have shown high efficiency against the KB and A549 cancer cell lines, including Bel-7402 (associated with endocervical adenocarcinoma linked to human papillomavirus) [125].
2.2.3. Anti-Inflammatory Activity
By preventing histamine release, neutrophil migration, and vascular permeability, a sulfated polysaccharide fraction from the cornea of the Gracilaria exhibits anti-inflammatory properties [126]. Nitric oxide (NO) production is reduced, and NF-B activation in mouse macrophages of RAW264.7 cells is hampered by Porphyrans derived from Porphyra sp., which exhibits anti-inflammatory effects in humans [127]. Plant extracts are classified as AChEI inhibitors (>50% inhibition), moderate inhibitors (30–50% inhibition), and weak inhibitors (30% inhibition) based on their level of potency [124]. Red algae extract from H. musciformis (7.21%) and Pterocladia capillacea (5.38%) had a minimal impact, while Ochtodes secundiramea extracts exhibited a moderate potency (48.59%) [128]. Antipyretics lower the increased body temperature. For example, flavonoids like baicalin, showed an antipyretic impact by inhibiting tumor necrosis factor (TNF) and preventing arachidonic acid peroxidation, which decreased the prostaglandin ratio and lessened fever and discomfort [129]. In comparison to normal paracetamol, the methanolic extract of H. musciformis and Gracilaria dura had a stronger antipyretic effect on albino mice and reduced body temperature for up to 4 h after administration [129].
2.2.4. Anticoagulant
Galactans from red seaweed may serve as an alternate source for brand-new anticoagulant medications [130]. Seaweed has an anticoagulant property that may be related to the composition, molecular weight, sulfate concentration, and location of its polysaccharides, such as uronic acids, which carry a negative charge and have the ability to bind calcium ions, preventing the development of a clot [131]. The direct influence of algae on thrombin and the enhancement of antithrombin III may be the cause of the algal anticoagulation mechanism. Algal polysaccharides also prolonged crucial pathway-dependent coagulating durations, reduced platelet aggregation, and delayed activated partial thromboplastin time (APTT), suggesting the obstruction of intrinsic factors. Galactans from red seaweed are frequently potential alternate sources of intriguing anticoagulant medications [132]. Heparin-like anticoagulant effectiveness was demonstrated by sulfated galactans from Grateloupia indica [133]. The -O-SO3H group found in the red species carrageenans is crucial in preventing blood clots by blocking platelet aggregation [131]. About one-fifth of the activity of heparin was produced by carrageenans. Due to its increased sulfate concentration, λ-carrageenan demonstrated improved anticoagulant ability compared to κ- -carrageenan [131]. The anticoagulant action was boosted by depolymerizing agars obtained from Porphyra yezoensis and Gracilaria birdiae with the use of ultrasound [131].
2.2.5. Macroalgae in Skincare
Macroalgae metabolites also improved skin’s brightness, remineralization, hydration, and firmness while also reducing the appearance of redness and blemishes, as well as UV damage [134]. The seaweeds Asparagopsis armata, Gelidium corneum, and Corallina officinalis have extracts that can be used in skincare products such as creams, oils, soaps, masks, and lotions to restore skin elasticity, softness, and whitening/lightening effects [3]. Certain anti-aging creams contain an amino acid that was isolated from A. armata. Mycosporine from several Rhodophyta species serves as a photoprotective ingredient [135].
3. Advancements in Algal Research
Marine macroalgae are generally safe to consume and contain numerous bioactive compounds, but direct consumption alone is insufficient for therapeutic applications. Further formulation is necessary, considering digestibility and biochemical profiling, to develop algae-based drugs and food products [136]. However, conventional formulation methods, such as bioassays, can prove to be time-consuming and challenging. Fortunately, omics approaches have emerged as valuable tools for researchers seeking to manipulate and construct new algal metabolisms for use as potential therapeutic candidates [137]. Omics approaches are accelerating the development of algae-based therapeutics and food products (Figure 2). These approaches include genomics, proteomics, metabolomics, metagenomics, and other omics toolsets.
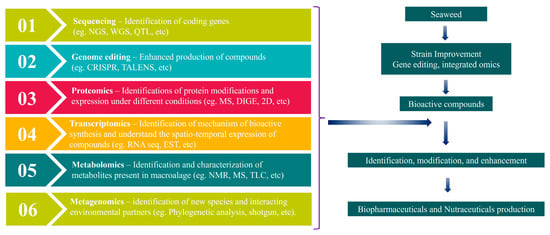
Figure 2.
Omics approaches in macroalgal research.
By comparing genomes across diverse macroalgae species, we gain insights into their unique adaptations, informing selection for cultivation. Transcriptomic and metabolic analyses pinpoint genes and metabolites responsible for valuable bioactive compounds like pigments, antioxidants, and polysaccharides, guiding strain selection and production optimization. CRISPR-Cas9 and gene silencing techniques enable the engineering of desirable traits like growth rate, stress tolerance, and compound production. Studying the macroalgal microbiome reveals its role in nutrient cycling, pathogen defense, and bioactive production, fostering beneficial interactions and improving cultivation practices. Genomics further guides the development of sustainable, closed-loop cultivation systems, maximizing resource efficiency and minimizing environmental impact. Omics technology, therefore, stands poised to revolutionize macroalgal research by unlocking their full potential for diverse applications.
3.1. Genomics
Genomics is a powerful omics technology that can reveal the metabolic and biosynthetic capabilities of organisms, enabling their manipulation for bioproduction and genetic engineering. Genomic tools not only help to identify the macroalgal compounds, but also help to characterize the identified bioactives [138].
3.1.1. Genome Sequencing and Gene Mining
The number of available macroalgal genome sequences is still relatively insignificant compared to that of microalgae, due to the complexity of macroalgal genomes [139]. However, advanced next-generation sequencing technologies such as Hi-Seq, Mi-Seq, real-time sequencing, and pyro sequencing, have made whole-genome sequencing more efficient and accurate [140]. These technologies have been used to sequence a large number of marine macroalgae, with Ectocarpus siliculosus is the first marine macroalga that sequenced in 2010 [141]. By analyzing the genomic information, researchers can use gene mining to identify novel bioactive compounds with potential therapeutic or industrial applications [142]. For example, genomic analysis of the brown macroalga Saccharina japonica led to the discovery of a novel fucoxanthin synthase gene that can be used to produce fucoxanthin, a carotenoid with anti-cancer and anti-obesity properties [143].
3.1.2. Genomic Analysis of Metabolic Pathways
Genomic analysis of macroalgae also provides insight into intracellular metabolic pathways, which are important for bioproduction [144]. For example, genomic analysis of the red macroalga Chondrus crispus has been used to identify genes involved in the production of carrageenan, a polysaccharide with a wide range of industrial applications [145].
3.1.3. Web Resources for Genomic Analysis
In the era of genomics, a considerable number of web resources have been developed to support genomic analysis of marine macroalgae. These resources include:
- AlgaePath: A platform for predicting metabolic pathways in algae [146].
- pico-PLAZA: A database of genome information for algae [147].
- Joint Genome Institute (JGI) Portal: A portal that provides access to genome data and analysis tools from the JGI [148].
- Symbiodiniaceae and Algal Genomic Resource Database (SAGER): A database of genome data for Symbiodiniaceae algae [149].
- Organelle Genome Database for Algae (OGDA): A database of organelle genome data for algae [150].
- BioSyc: A database of metabolic pathways for algae [151].
- GOLD database: A database of genome data for over 8000 organisms (https://gold.jgi.doe.gov/ (accessed on 23 July 2023)).
However, an integrated database or web server specifically for macroalgae is yet to be developed.
3.1.4. Genetic Engineering and DNA Barcoding
Genetic information from algae provides information on protein-coding genes, functional RNAs, enhancers, and regulatory elements [152]. This information can also be used for variety of applications, including:
- Phylogenetic analyses: To study the evolutionary relationships between distinct species of algae;
- DNA barcoding: To identify and classify algae species using short DNA sequences which are important for commercial and industrial applications. Genetic information can also be used for genetic engineering. Techniques such as RAPD, RFLP, hybridization, and AFLP are commonly used for barcoding [153]. Genetic engineering technologies such as TALENs, ZFNs, and CRISPR can also be used to alter the genetic material of macroalgae, enhancing the expression of genes with medicinal applications [137]. For example, CRISPR has been used to enhance the production of fucoxanthin in Saccharina japonica [154].
Nuclear genetic transformation has been established in the red microalgae Cyanidioschyzon merolae using homologous recombination technology, but these tools have yet to be used to alter macroalgal genomes [155]. Notably, recent efforts have seen improvements in the annotation of the giant kelp genome, aiming to establish it as a universal reference for genomic projects [156].
Overall, genomics is a powerful tool for marine macroalgae bioproduction and genetic engineering. By analyzing the genomic information, researchers can identify novel bioactive compounds, develop new bioproduction methods, and engineer macroalgae for improved traits. Also, genomics also acts as an important foundation through which macroalgal compounds can be utilized for industrial and biopharmaceutical purposes [157].
3.2. Transcriptomics
Transcriptomic profiling of macroalgae enables the identification of biochemical pathways involved in the production of medically significant compounds, aiding in the elucidation of these compounds [139]. For example, transcriptomic analyses revealed the response to blue light in S. japonica, revealing the overexpression of 7808 genes [158]. With advancements in technology, transcriptomic techniques have evolved from Expression Sequencing Tags (ESTs) to high-throughput methods, such as RNA-Sequencing and Biochips [159]. Transcriptomic studies focus on enhancing metabolites under specific growth conditions [160], and several transcriptome sequencing projects are available for this purpose. The Marine Microbial Eukaryotic Transcriptome Sequencing Project (MMETSP) contains more than 650 transcriptomes of diverse taxa [161], which can be used for transcriptomic studies.
In addition to identifying biochemical pathways, transcriptomic analysis can also identify the regulatory mechanisms involved in the accumulation of various metabolites [162]. For example, the characterization of Laminaria digitata identified the genes involved in alginate synthesis, while transcriptome analysis of Ectocarpus siliculosus revealed the upregulation of genes coding for chlorophyll a and c binding proteins under saline and oxidative stress conditions [163]. More recently, transcriptomic studies were utilized to identify the potential gene, to enhance the eicosapntemacnioc acid (EPA) production, which plays a key role in fluidity and organization of membrane [164]. Also, transcriptomic analysis can be helpful in identifying the differentially expressed compounds under varying environment conditions [165].
Overall, transcriptomic profiling is a powerful tool for marine macroalgae bioprospection. By analyzing the transcriptome data, researchers can identify genes and their expression associated with novel bioactive compounds, develop new bioproduction methods, and engineer macroalgae for improved traits.
3.3. Metagenomics
Metagenomics is a valuable tool for identifying algal strains and activating silent cryptic gene clusters involved in the synthesis of bioactive compounds [166]. Shotgun metagenomics is a high-throughput technique that enables the identification of multiple microbial communities [167]. However, studies on the algal microbiome and its associated community are still limited. Prokaryotes play a crucial role in enhancing the level of Vitamin B12 in algal species by strongly associating with the surface of macroalgae [168]. Metagenomic profiling of these prokaryotes identifies these organisms and helps to understand the interactions of macroalgae with other organisms. For example, metagenomic profiling has been used to identify the microbiome of Porphyra and Pyropia spp., which can enhance studies on the macroalgal microbiome [169]. Metagenomic analysis has also provided detailed information on the influence of the environment on bacterial communities [170].
Furthermore, Metagenomics is also a significant tool for bioprospecting, which can be employed for metabolic engineering of various algal strains. For example, metagenomic analysis of the microbiome of L. digitata revealed that algal polysaccharides provide a potential carbon source for bacteria, which can utilize them and produce numerous enzymes with biotechnological importance [171].
Overall, metagenomics is a powerful tool for marine macroalgae bioprospection. By analyzing the metagenome data, researchers can:
- Identify novel algal strains with potential for bioproduction of valuable compounds.
- Activate silent cryptic gene clusters in known algal strains to produce new bioactive compounds.
- Understand the interactions between macroalgae and their associated microbiome.
- Develop new bioprospection strategies for the discovery of novel enzymes and other biotechnologically important molecules.
3.4. Proteomics
Marine macroalgae exhibit high adaptability to their environment and produce various chemicals under different conditions [172,173]. Comparative proteomic analysis of macroalgae under varying environmental conditions can help identify differential protein expression within an algal system [174].
Several proteomic techniques can be employed to identify proteins in algal species, including:
- One-dimensional gel electrophoresis;
- Peptide fingerprinting;
- Sequencing;
- Two-dimensional electrophoresis;
- Mass spectrometry.
For example, proteomic analysis of the macroalga Gracilaria changii using two-dimensional electrophoresis and mass spectrometry has identified several novel proteins [175]. A deeper understanding of proteomics can aid in the redesign of the metabolic pathways of macroalgae, leading to increased production and accumulation of metabolites [176]. This can be used to develop new bioproduction methods for valuable compounds, such as drugs, nutraceuticals, and biofuels. However, proteomic studies on macroalgae, especially related to stress, are still limited compared to other plant species. One recent study used liquid chromatography-mass spectrometry (LC-MS/MS) and de novo sequencing to identify the increase in production of phycobiliproteins and superoxide dismutase in Pyropis orbicularis under desiccation conditions and the increase in phosphomannomutase and glyceraldehyde-3-phosphate in Scytosiphon gracilis in the presence of high concentrations of copper [177]. This study demonstrates the potential of proteomics to identify proteins that are involved in stress responses and other important metabolic pathways in macroalgae. Moreover, the combination of proteomic techniques including LC-MS, proteome analysis coupled with transcriptomic techniques can be used to evaluate and validate the potentials of macroalgal bioactive compounds [178].
Overall, proteomics is a powerful tool for marine macroalgae bioprospection. By analyzing the proteome data, researchers can:
- Identify novel proteins with potential for bioproduction of valuable compounds;
- Understand the molecular mechanisms of stress responses and other important metabolic pathways in macroalgae;
- Develop new bioprospection strategies for the discovery of new drugs, enzymes, and other biotechnologically important molecules.
3.5. Metabolomics
Metabolomics is the study of the complete set of metabolites produced by an organism. Marine macroalgae are known to produce a wide range of bioactive metabolites with potential applications in drug discovery, nutraceutics, and other industrial sectors. For example, macroalgae-derived metabolites have been shown to be effective against cancer [179], Alzheimer’s and other neurological disorders [180], and various infections [181]. Metabolomics is a valuable tool for assessing the quantity and quality of these metabolites in algae under natural and induced conditions since metabolites often vary based on environmental cues [182]. This information can be used to identify novel bioactive compounds with potential for therapeutic or industrial applications, as well as to develop new bioproduction methods for these compounds. Metabolomics plays a crucial role in drug discovery by profiling algal metabolites [181].
Various techniques are employed for metabolite analysis [183], including:
- Mass spectrometry (MS);
- Gas chromatography (GC);
- Nuclear magnetic resonance (NMR);
- Thin layer chromatography (TLC).
For example, NMR was used to perform microbial profiles of L. digitata and Gracilaria conferta [184], while GC-MS was used to quantify the levels of furanones in Delisea pulchra [185]. Metabolomic analyses have already led to the discovery of several new bioactive compounds from macroalgae [186]. For example, metabolic profiling of Callophycus serratus identified four bromophycolides that show activity against Plasmodium falciparum [187]. Additionally, metabolomics also helps to understand various metabolic pathways, such as defense response mechanisms in Gracilaria vermiculophylla [188]. Traditionally, metabolic engineering in macroalgae has relied on open-loop regulation, prioritizing specific biochemical pathways over system-wide efficiency [189]. This can lead to suboptimal production of desired metabolites. Closed-loop regulation offers a promising alternative, dynamically adjusting metabolic fluxes to maximize target com-pound yields [190]. Recent advancements in metabolic tools can empower researchers to implement closed-loop strategies, providing real-time feedback and optimized control over metabolic pathways.
Overall, metabolomics is a powerful tool for marine macroalgae bioprospection. By analyzing the metabolome data, researchers can:
- Identify novel bioactive compounds with potential for therapeutic or industrial applications;
- Develop new bioproduction methods for valuable compounds;
- Understand various metabolic pathways in macroalgae.
4. Macroalgal Industrial Extraction and Purification Methods
As from these resources there are many standardized procedures for the extraction and purification of the macroalgal components depending upon the industries. But the general method industrial approach is quite similar amongst all and is depicted in Figure 3.
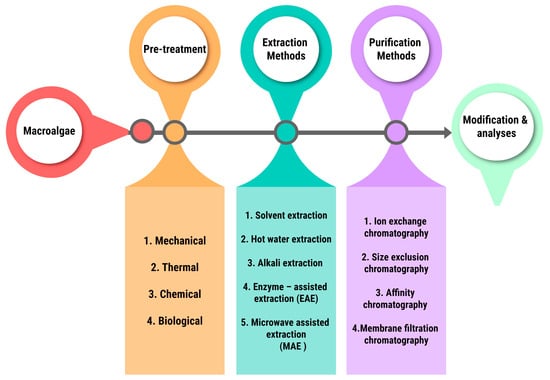
Figure 3.
General industrial method of extraction and purification.
The pretreatment methods change depending on the industry, for example the pretreatment method mostly followed for biochar production is thermal treatment, prominently pyrolysis. Macroalgal components can be extracted using a variety of methods, including solvent extraction and hot water extraction. The different advanced methods and the replaced ones are given below in Table 4. The operational principles techniques are briefly described in Table 5.

Table 4.
Advanced methods for conventional methods.

Table 5.
Operational Principle of different techniques.
5. Future Prospectives
While the potential of macroalgae as oral drugs is undeniable, their complex cell walls pose a significant hurdle. Gastric enzymes struggle to break down these barriers, limiting the bioavailability of valuable bioactive compounds [136]. However, advancements in omics technologies offer promising solutions (Figure 4). The intersection of omics technology and macroalgae research holds immense potential for unlocking a treasure trove of biopharmaceuticals and nutraceuticals.
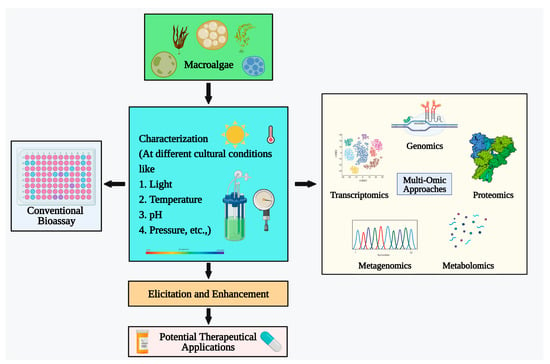
Figure 4.
Macroalgal research in enhancing the level of bioactive compounds.
By harnessing the power of genomics, researchers can engineer new macroalgae strains with weaker cell walls or even replace them with readily digestible components. Additionally, omics-driven approaches can optimize the biochemical composition of macroalgae, enriching them with specific bioactive compounds. These breakthroughs would unlock the full potential of macroalgae as a sustainable and readily available source of oral drugs.
Omics will enable the identification of novel bioactive molecules in macroalgae with specific therapeutic properties, leading to the development of targeted drugs for diseases like cancer, diabetes, and neurodegenerative disorders. Moreover, transcriptomic and metabolomics analyses provide a deeper understanding of the biochemical pathways and metabolic signatures responsible for the biosynthesis of high-value compounds. This empowers researchers to optimize cultivation and extraction processes, maximizing the yield and purity of bioactive extracts while ensuring sustainable and cost-effective production. Beyond pharmaceuticals, omics technologies hold immense potential in revolutionizing the nutraceutical landscape. By identifying and optimizing macroalgae strains with enhanced levels of essential nutrients, such as vitamins, minerals, and omega-3 fatty acids, researchers can develop highly nutritious functional foods. Furthermore, proteomic and metagenomic analyses can unveil the functional properties of macroalgae, such as anti-oxidant and anti-inflammatory activities. This enables the creation of personalized functional food formulations tailored to individual dietary needs and preferences. Omics also plays a crucial role in safeguarding consumer health by providing valuable insights into the potential side effects and interactions of macroalgae-derived products. Toxicological studies guided by omics data can ensure the safety and efficacy of these novel nutraceuticals for widespread consumption.
Looking ahead, the integration of advanced artificial intelligence and machine learning in omics data analysis holds promise. This entails predicting promising macroalgae candidates and developing environmentally friendly cultivation practices to minimize the ecological impact.
In conclusion, the integration of omics technologies with macroalgae research presents a groundbreaking opportunity to unlock a treasure trove of biopharmaceuticals and nutraceuticals. By harnessing the power of these tools, we can not only revolutionize healthcare and nutrition but also contribute to a more sustainable future. The journey towards biovalorizing macroalgae is far from over, yet the potential rewards are immeasurable. As we continue to delve deeper into the secrets of the sea, we can expect even more exciting discoveries that will illuminate the path towards a healthier and more sustainable future for all.
6. Conclusions
In conclusion, macroalgae hold significant potential as an alternative source of food and medicine due to their high nutritional and therapeutic value. However, our current knowledge and research on seaweed are insufficient to fully exploit the vast potential of this natural resource. The application of advanced scientific technologies, particularly omics techniques, has begun to unravel the deeper potentials of macroalgae. With the integration of omics technologies, researchers have made notable progress in identifying new species, elucidating the abundance of bioactive molecules within macroalgae, enhancing adaptability and resistance to global warming and climate changes, developing novel compounds of greater significance, and establishing extensive databases for future research and development. Notably, macroalgae harbor a diverse array of pharmaceutical compounds that show promise in combating conditions such as cancer, inflammation, and neurological diseases. Further research should focus on scaling up seaweed production by optimizing culture conditions to meet the growing demand. It is crucial to thoroughly investigate the potential positive and harmful effects on the human body, as well as the economic significance of seaweed. Continued advancements in extraction techniques, purification, and fractionation of bioactive components will lead to the production of more effective and safe chemicals with antibacterial, antiviral, and anticancer properties.
While this review highlights some of the commonly studied macroalgae with nutraceutical and pharmaceutical potential, it is important to note that the marine ecosystem still harbors numerous unexplored organisms with diverse consumption and medicinal significance. Further exploration and research are necessary to unlock the full spectrum of possibilities offered by macroalgae and to contribute to the development of innovative and sustainable solutions for human health and well-being.
Author Contributions
Conceptualization, P.M.; writing—original draft preparation, S.A., V.S.S.S., P.M. and K.S.N.; figures, software, S.A. and P.M.; data curation and formal analysis, P.M., M.S., L.S. and P.G.J.; writing—review and editing, P.M., M.S., L.S., B.V. and H.S.; supervision, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 3113–3137. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.C.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Rodrigues, F.; Oliveira, M.B.P.P. Macroalgae-derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef]
- Shibasaki, S.; Ueda, M. Utilization of Macroalgae for the Production of Bioactive Compounds and Bioprocesses Using Microbial Biotechnology. Microorganisms 2023, 11, 1499. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Guo, J.; Qi, M.; Chen, H.; Zhou, C.; Ruan, R.; Yan, X.; Cheng, P. Macroalgae-Derived Multifunctional Bioactive Substances: The Potential Applications for Food and Pharmaceuticals. Foods 2022, 11, 3455. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C. Seaweed as a source of natural antioxidants: Therapeutic activity and food applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Mota, C.S.C.; Pinto, O.; Sá, T.; Ferreira, M.; Delerue-Matos, C.; Cabrita, A.R.; Almeida, A.; Abreu, H.; Silva, J.; Fonseca, A.J.; et al. A Commercial Blend of Macroalgae and Microalgae Promotes Digestibility, Growth Performance, and Muscle Nutritional Value of European Seabass (Dicentrarchus labrax L.) Juveniles. Front. Nutr. 2023, 10, 1165343. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H.; Uchiyama, H. Photobioreactors for Mass Cultivation of Algae. Bioresour. Technol. 2008, 99, 4021–4023. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as nutraceuticals for health and nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef] [PubMed]
- Agregán, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.F.; Barretto, A.C.S.; Lorenzo, J.M. Fucus vesiculosus extracts as natural antioxidants for improvement of physicochemical properties and shelf life of pork patties formulated with oleogels. J. Sci. Food Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Wang, Q.; Xu, B.; Chen, H. Remarkable Natural Biological Resource of Algae for Medical Applications. Front. Mar. Sci. 2022, 9, 1060. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as Thickening and Gelling Agents in Food: A Critical Review. Food Biophys. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Rebours, C.; Marinho-Soriano, E.; Zertuche-González, J.A.; Hayashi, L.; Vásquez, J.A.; Kradolfer, P.; Soriano, G.; Ugarte, R.; Abreu, M.H.; Bay-Larsen, I. Seaweeds: An Opportunity for Wealth and Sustainable Livelihood for Coastal Communities. J. Appl. Phycol. 2014, 26, 1939–1951. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, A.; Hernández-González, M.C.; Pereda, S.V.; Gómez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Hydrobiologia 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Biological Activities and Health Benefit Effects of Natural Pigments Derived from Marine Algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential Role of Seaweed Polyphenols in Cardiovascular-Associated Disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed]
- Percival, E.; McDowell, R.H. Algal Walls—Composition and Biosynthesis. In Algae and Environment; Lobban, C.S., Wynne, M.J., Eds.; Blackwell Scientific Publications: Oxford, UK, 1981; pp. 277–316. [Google Scholar] [CrossRef]
- Hunt, D.; Dewar, A.; Dal Molin, F.; Willey, N. Enhancing Radiological Monitoring of 137Cs in Coastal Environments Using Taxonomic Signals in Brown Seaweeds. Sci. Total Environ. 2023, 268–269, 107261. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R.S.; Morrissey, M.T. Marine Biotechnology for Production of Food Ingredients. Adv. Food Nutr. Res. 2007, 52, 237–292. [Google Scholar]
- Abirami, R.G.; Kowsalya, S. Phytochemical Screening, Microbial Load, and Antimicrobial Activity of Underexploited Seaweeds. Int. Res. J. Microbiol. 2012, 3, 328–332. [Google Scholar]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness. 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Shanmugam, M.; Bhat, R. Producing Novel Edible Films from Semi Refined Carrageenan (SRC) and Ulvan Polysaccharides for Potential Food Applications. Int. J. Biol. Macromol. 2018, 112, 1164–1170. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive Potential and Structural Chracterization of Sulfated Polysaccharide from Seaweed (Gracilaria corticata). Carbohydr. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef]
- Freitas, A.C.; Rodrigues, D.; Rocha-Santos, T.; Rocha-Santos, T.; Gomes, A.M.P.; Duarte, A.C. Marine Biotechnology Advances Towards Applications in New Functional Foods. Biotechnol. Adv. 2012, 30, 1506–1515. [Google Scholar] [CrossRef]
- Healy, L.E.; Zhu, X.; Pojić, M.; Sullivan, C.; Tiwari, U.; Curtin, J.; Tiwari, B.K. Biomolecules from Macroalgae-Nutritional Profile and Bioactives for Novel Food Product Development. Biomolecules 2023, 13, 386. [Google Scholar] [CrossRef]
- Urbano, M.G.; Goñi, I. Bioavailability of Nutrients in Rats Fed on Edible Seaweeds, Nori (Porphyra tenera) and Wakame (Undaria pinnatifida), as a Source of Dietary Fibre. Food Chem. 2002, 76, 477–483. [Google Scholar] [CrossRef]
- Domínguez-González, R.; Romarís-Hortas, V.; García-Sartal, C.; Moreda-Piñeiro, A.; Barciela-Alonso, M.C.; Bermejo-Barrera, P. Evaluation of an in Vitro Method to Estimate Trace Elements Bioavailability in Edible Seaweeds. Talanta 2010, 82, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Hamann, M.T. Technology Evaluation: Kahalalide F, Pharmamar. Curr. Opin. Investig. Drugs 2004, 6, 1088–1093. [Google Scholar]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- Biancarosa, I.; Belghit, I.; Bruckner, C.G.; Liland, N.S.; Waagbø, R.; Amlund, H.; Heesch, S.; Lock, E.J. Chemical Characterization of 21 Species of Marine Macroalgae Common in Norwegian Waters: Benefits of and Limitations to Their Potential Use in Food and Feed. J. Sci. Food Agric. 2018, 98, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.-P.; Zhou, X.-F.; Yang, X.-W.; Liu, Y. Chemical Composition of Seaweeds. In Seaweeds: Diversity, Environment, Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 79–124. [Google Scholar]
- Kellogg, J.; Esposito, D.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan Seaweeds Lower Inflammation in RAW 264.7 Macrophages and Decrease Lipid Accumulation in 3T3-L1 Adipocytes. J. Funct. Foods 2015, 15, 396–407. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Carrillo-Domínguez, S.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Castillo-Domínguez, R.M. Monthly Variation in the Chemical Composition of Eisenia arborea J.E. Areschoug. J. Appl. Phycol. 2009, 21, 141–148. [Google Scholar] [CrossRef]
- Norziah, M.H.; Ching, C.Y. Nutritional Composition of Edible Seaweed Gracilaria Changgi. Food Chem. 2000, 68, 69–76. [Google Scholar] [CrossRef]
- Sarojini, Y.; Sarma, N. Vitamin c Content of Some Macroalgae of Visakhapatnam, East Coast of India. Indian J. Mar. Sci. 1999, 28, 408–412. [Google Scholar]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of Marine Carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S. Seaweed Vitamins as Nutraceuticals. Food Chem. 2011, 125, 357–369. [Google Scholar] [CrossRef]
- Zhong, R.; Wan, X.; Wang, D.; Zhao, C.; Liu, D.; Gao, L.; Wang, M.; Wu, C.; Nabavid, S.M.; Daglia, M. Polysaccharides from Marine Enteromorpha: Structure and Function. Mar. Drugs 2020, 18, 535. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Mahadevan, K. Seaweeds: A Sustainable Food Source. Biotechnol. Adv. 2015, 33, 347–364. [Google Scholar] [CrossRef]
- Bito, T.; Teng, F.; Watanabe, F. Bioactive compounds of edible purple laver Porphyra sp.(Nori). J. Agri. Food Chem. 2017, 65, 10685–10692. [Google Scholar] [CrossRef] [PubMed]
- Lafeuille, B.; Tamigneaux, É.; Berger, K.; Provencher, V.; Beaulieu, L. Impact of Harvest Month and Drying Process on the Nutritional and Bioactive Properties of Wild Palmaria palmata from Atlantic Canada. Mar. Drugs 2023, 21, 392. [Google Scholar] [CrossRef]
- Pina, A.L.; Costa, A.R.; Lage-Yusty, M.A.; López-Hernández, J. An Evaluation of Edible Red Seaweed (Chondrus crispus) Components and Their Modification during the Cooking Process. LWT-Food Sci. Technol. 2014, 56, 175–180. [Google Scholar] [CrossRef]
- Raimundo, S.C.; Pattathil, S.; Eberhard, S.; Hahn, M.G.; Popper, Z.A. β-1,3-Glucans are components of brown seaweed (Phaeophyceae) cell walls. Protoplasma 2016, 254, 997–1016. [Google Scholar] [CrossRef]
- Kadam, S.U.; O’Donnell, C.P.; Rai, D.K.; Hossain, M.B.; Burgess, C.M.; Walsh, D.; Tiwari, B.K. Laminarin from Irish Brown Seaweeds Ascophyllum nodosum and Laminaria hyperborea: Ultrasound Assisted Extraction, Characterization and Bioactivity. Mar. Drugs 2015, 13, 4270–4280. [Google Scholar] [CrossRef]
- Blunden, G.; Campbell, S.A.; Smith, J.R.; Guiry, M.D.; Hession, C.C.; Griffin, R.L. Chemical and Physical Characterization of Calcified Red Algal Deposits Known as Maërl. J. Appl. Phycol. 1997, 9, 11–17. [Google Scholar] [CrossRef]
- Pirazzoli, P.A. Geomorphological Indicators. In Sea Level Studies; Elsevier: Amsterdam, The Netherlands, 2007; pp. 2974–2983. [Google Scholar] [CrossRef]
- García-Vaquero, M.; Rajauria, G.; O’Doherty, J.V.; Sweeney, T. Polysaccharides from Macroalgae: Recent Advances, Innovative Technologies and Challenges in Extraction and Purification. Food Res Intl. 2017, 99, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Burgess, J.G.; Wu, M.; Wang, S.; Gao, K. Using Macroalgae as Biofuel: Current Opportunities and Challenges. Bot. Mar. 2020, 63, 355–370. [Google Scholar] [CrossRef]
- Rabanal, M.; Ponce, N.A.; Navarro, D.A.; Gómez, R.M.; Stortz, C.A. The System of Fucoidans from the Brown Seaweed Dictyota dichotoma: Chemical Analysis and Antiviral Activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef] [PubMed]
- D’Archino, R.; Piazzi, L. Macroalgal assemblages as indicators of the ecological status of marine coastal systems: A review. Ecol. Indic. 2021, 129, 107835. [Google Scholar] [CrossRef]
- Basak, R.; Wahid, K.A.; Dinh, A. Estimation of the Chlorophyll-a Concentration of Algae Species Using Electrical Impedance Spectroscopy. Water 2021, 13, 1223. [Google Scholar] [CrossRef]
- Milinović, J. Edible Macroalgae: Beneficial Resource of Iodine. Am. J. Biomed. Sci. Res. 2020, 8, 290–292. [Google Scholar] [CrossRef]
- Blikra, M.J.; Henjum, S.; Aakre, I. Iodine from Brown Algae in Human Nutrition, with an Emphasis on Bioaccessibility, Bioavailability, Chemistry, and Effects of Processing: A Systematic Review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1517–1536. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2016, 29, 949–982. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional Value of Proteins from Edible Seaweed Palmaria Palmata (Dulse). J Nutr Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Angell, A.; Mata, L.; de Nys, R.; Paul, N. The Protein Content of Seaweeds: A Universal Nitrogen-to-protein Conversion Factor of Five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Holdt, S.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine Macroalgae as Sources of Protein and Bioactive Compounds in Feed for Monogastric Animals. J. Agri. Food Chem. 2019, 99, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.; Sewu, D.D.; Woo, S.H. Effect of Thermal Pretreatment on the Extraction of Potassium Salt from Alga Saccharina Japonica. J. Anal. Appl. Pyrolysis 2018, 133, 68–75. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.; Jeon, Y.; Ryu, B. Bioactivities of the Edible Brown Seaweed, Undaria pinnatifida: A Review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W.; Ristivojević, P. Assessment of Antioxidant Activity in Victorian Marine Algal Extracts Using High Performance Thin-layer Chromatography and Multivariate Analysis. J. Chromatogr. A 2016, 1468, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-Assisted Fucoidan Extraction from Brown Macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296. [Google Scholar] [CrossRef]
- Circuncisão, A.R.; Catarino, M.D.; Cardoso, S.M.; Silva, A.M.S. Minerals from Macroalgae Origin: Health Benefits and Risks for Consumers. Mar. Drugs 2018, 16, 400. [Google Scholar] [CrossRef]
- Millner, G.C.; Sweeney, R.A.; Frederick, V.R. Biomass and Distribution of Cladophora Glomerata in Relation to Some Physical-Chemical Variables at Two Sites in Lake Erie. J. Great Lakes Res. 1982, 8, 35–41. [Google Scholar] [CrossRef]
- García-CasalM, N.; Pereira, A.C.; Leets, I.; Ramírez, J.; Quiroga, M.F. High Iron Content and Bioavailability in Humans from Four Species of Marine Algae. J. Nutr. 2007, 137, 2691–2695. [Google Scholar] [CrossRef]
- Geddie, A.W.; Hall, S.G. An Introduction to Copper and Zinc Pollution in Macroalgae: For Use in Remediation and Nutritional Applications. J. Appl. Phycol. 2018, 31, 691–708. [Google Scholar] [CrossRef]
- Stengel, D.B.; Macken, A.; Morrison, L.; Morley, N.J. Zinc Concentrations in Marine Macroalgae and a Lichen from Western Ireland in Relation to Phylogenetic Grouping, Habitat and Morphology. Mar. Pollut. Bull. 2004, 48, 902–909. [Google Scholar] [CrossRef] [PubMed]
- El-Agawany, N.; Kaamoush, M. Role of Zinc as an Essential Microelement for Algal Growth and Concerns About It’s a Potential Environmental Risks. Environ. Sci. Pollut. Res. 2023, 30, 71900–71911. [Google Scholar] [CrossRef] [PubMed]
- Prasetyaningrum, A.; Praptyana, I.; Nurfiningsih; Ratnawati. Carrageenan: Nutraceutical and Functional Food as Future Food. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012068. [Google Scholar] [CrossRef]
- Zaharudin, N.; Salmeán, A.A.; Dragsted, L.O. Inhibitory Effects of Edible Seaweeds, Polyphenolics and Alginates on the Activities of Porcine Pancreatic α-Amylase. Food Chem. 2018, 245, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Zargarzadeh, M.; Amaral, A.J.R.; Custódio, C.A.; Mano, J.F. Biomedical Applications of Laminarin. Carbohydr. Polym. 2020, 232, 115774. [Google Scholar] [CrossRef]
- Karuppusamy, S.; Rajauria, G.; Fitzpatrick, S.; Lyons, H.; McMahon, H.; Curtin, J.; Tiwari, B.K.; O’Donnell, C. Biological Properties and Health-Promoting Functions of Laminarin: A Comprehensive Review of Preclinical and Clinical Studies. Mar. Drugs 2022, 20, 772. [Google Scholar] [CrossRef]
- Bar-Shai, N.; Sharabani-Yosef, O.; Zollmann, M.; Lesman, A.; Golberg, A. Seaweed Cellulose Scaffolds Derived from Green Macroalgae for Tissue Engineering. Sci. Rep. 2021, 11, 11843. [Google Scholar] [CrossRef]
- Asari, F.; Kusumi, T.; Kakisawa, H. Turbinaric Acid, a Cytotoxic Secosqualene Carboxylic Acid from the Brown Alga Turbinaria ornata. J. Nat. Prod. 1989, 52, 1167–1169. [Google Scholar] [CrossRef]
- Muralidhar, P.; Radhika, P.; Krishna, N.; Rao, D.V.; Rao, C.B. Sphingolipids from Marine Organisms: A Review? Nat. Prod. Sci. 2003, 9, 117–142. [Google Scholar]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteiro, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, R.M. Valuing Bioactive Lipids from Green, Red and Brown Macroalgae from Aquaculture, to Foster Functionality and Biotechnological Applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef] [PubMed]
- Ersoy Korkmaz, N. Unlocking the Fatty Acid Profile of Macroalgae Species in Sea of Marmara, Türkiye: A Comprehensive Analysis of Extraction Methods. Int. Environ. Geoinfo. 2023, 10, 034–038. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An Overview on the Nutritional and Bioactive Components of Green Seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- de Arruda, M.C.S.; da Silva, M.R.O.B.; Cavalcanti, V.L.R.; Brandao, R.M.P.C.; de Araújo Viana Marques, D.; de Lima, L.R.A.; Porto, A.L.F.; Bezerra, R.P. Antitumor Lectins from Algae: A Systematic Review. Algal Res. 2023, 70, 102962. [Google Scholar] [CrossRef]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs. 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.-S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A. Mycosporine and Mycosporine-Like Amino Acids: A Paramount Tool Against Ultraviolet Irradiation. Pharmacogn. Rev. 2011, 5, 138. [Google Scholar] [CrossRef]
- Cikoš, A.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Kovaleski, G.; Kholany, M.; Dias, L.M.S.; Correia, S.F.H.; Ferreira, R.A.S.; Coutinho, J.A.P.; Ventura, S.P.M. Extraction and Purification of Phycobiliproteins from Algae and Their Applications. Front. Chem. 2022, 10, 1065355. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Šimat, V.; Rathod, N.B.; Hamed, I.; Čagalj, M. Algal Carotenoids: Chemistry, Sources, and Application. Foods 2023, 12, 2768. [Google Scholar] [CrossRef]
- Lunagariya, J.; Bhadja, P.; Zhong, S.; Vekariya, R.L.; Xu, S. Marine Natural Product Bis-Indole Alkaloid Caulerpin: Chemistry and Biology. Mini-Rev. Med. Chem. 2019, 19, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, Y.; Ying, Z.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid. -Based Complement. Altern. Med. 2015, 2015, 723515. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Maekawa, K.; Chen, J.R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Alves, R.C.; Harnedy, P.A.; FitzGerald, R.J.; Oliveira, M.B.P.P. Macroalgal-Derived Protein Hydrolysates and Bioactive Peptides: Enzymatic Release and Potential Health Enhancing Properties. Trends Food Sci. Technol. 2019, 93, 106–124. [Google Scholar] [CrossRef]
- Greff, S.; Zubia, M.; Genta-Jouve, G.; Massi, L.; Pérez, T.; Thomas, O.P. Mahorones, Highly Brominated Cyclopentenones from the Red Alga Asparagopsis taxiformis. J. Nat. Prod. 2014, 77, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, H.; Brinkhoff, T.; Simon, M.; Richter-Landsberg, C. Dimethylsulfoniopropionate Promotes Process Outgrowth in Neural Cells and Exerts Protective Effects against Tropodithietic Acid. Mar. Drugs 2016, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.; Rösener, H.; Müller, D. Bifuhalol und diphlorethol aus Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Popoviciu, D.R.; Anton, R.-E.; Prelipcean, A.-M. Marine Bioactive Compounds Derived from Macroalgae as New Potential Players in Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1781. [Google Scholar] [CrossRef]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and Analytical Characterization of Novel Sulfated Coumarins in the Marine Green Macroalga Dasycladus Vermicularis (Scopoli) Krasser. Molecules 2018, 23, 2735. [Google Scholar] [CrossRef]
- Yoon, M.; Cho, S. Triphlorethol A, a Dietary Polyphenol from Seaweed, Decreases Sleep Latency and Increases Non-Rapid Eye Movement Sleep in Mice. Mar. Drugs 2018, 16, 139. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 196–199. [Google Scholar] [PubMed]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- McCauley, J.I.; Winberg, P.C.; Meyer, B.J.; Skropeta, D. Effects of nutrients and processing on the nutritionally important metabolites of Ulva sp. (Chlorophyta). Algal Res. 2018, 35, 586–594. [Google Scholar] [CrossRef]
- Cho, C.-H.; Lu, Y.-A.; Kim, M.-Y.; Jeon, Y.-J.; Lee, S.-H. Therapeutic Potential of Seaweed-Derived Bioactive Compounds for Cardiovascular Disease Treatment. Appl. Sci. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Bioactive Potential and Possible Health Effects of Edible Brown Seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kwon, S.; Chun, Y.S.; Gu, M.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of AKT/NF-ΚB and MAPKS/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2016, 42, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A. Cytotoxic activity of fucoxanthin, alone and in combination with the cancer drugs imatinib and doxorubicin, in CML cell lines. Environ. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef]
- Hitoe, S.; Shimoda, H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct. Foods Health Dis. 2017, 7, 246. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.; Moon, I.S.; Hong, Y. Neuroprotective Effects of Fucoxanthin and Its Derivative Fucoxanthinol from the Phaeophyte Undaria pinnatifida Attenuate Oxidative Stress in Hippocampal Neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin during Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef]
- Bhuyar, P.; Rahim, M.H.A.; Sundararaju, S.; Maniam, G.P.; Nagarajan, G. Antioxidant and Antibacterial Activity of Red Seaweed Kappaphycus alvarezii against Pathogenic Bacteria. Glob. J. Environ. Sci. Manag. 2020, 6, 47–58. [Google Scholar] [CrossRef]
- Saleh, B.; Al-Mariri, A. Antifungal Activity of Crude Seaweed Extracts Collected from Lattakia Coast, Syria. J. Fish. Aquat. Sci. 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Smyrniotopoulos, V.; Vagias, C.; Rahman, M.M.; Gibbons, S.; Roussis, V. Ioniols I and II, Tetracyclic Diterpenes with Antibacterial Activity, from Sphaerococcus coronopifolius. Chem. Biodivers. 2010, 7, 666–676. [Google Scholar] [CrossRef]
- Chowdhury, M.M.H.; Kubra, K.; Hossain, M.B.; Mustafa, M.; Jainab, T.; Karim, M.R.; Mehedy, M.E. Screening of antibacterial and antifungal activity of freshwater and marine algae as a prominent natural antibiotic available in Bangladesh. Int. J. Pharmacol. 2015, 11, 828–833. [Google Scholar] [CrossRef]
- Mendis, E.; Kim, S. Present and Future Prospects of Seaweeds in Developing Functional Foods. Adv. Food Nutr. Res. 2011, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Morán-Santibañez, K.; Peña-Hernández, M.A.; Cruz-Suárez, L.E.; Ricque-Marie, D.; Skouta, R.; Vasquez, A.H.; Rodríguez-Padilla, C. Virucidal and Synergistic Activity of Polyphenol-Rich Extracts of Seaweeds against Measles Virus. Viruses 2018, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.M.; Prieto, J.M.; Mora, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Kendel, M.; Wielgosz-Collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid Composition, Fatty Acids and Sterols in the Seaweeds Ulva Armoricana, and Solieria Chordalis from Brittany (France): An Analysis from Nutritional, Chemotaxonomic, and Antiproliferative Activity Perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef]
- Erfani, N.; Nazemosadat, Z.; Moein, M. Cytotoxic Activity of Ten Algae from the Persian Gulf and Oman Sea on Human Breast Cancer Cell Lines; MDA-MB-231, MCF-7, and T-47D. Pharmacogn. Res. 2015, 7, 133. [Google Scholar] [CrossRef]
- Raman, M.; Doble, M. κ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Foods 2015, 15, 354–364. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.G.; De Sousa Oliveira Vanderlei, E.; De Araújo, I.W.F.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Mechanisms Involved in the Anti-Inflammatory Action of a Polysulfated Fraction from Gracilaria cornea in Rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory Effect of Sulphated Polysaccharide Porphyran on Nitric Oxide Production in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. J. Biochem. 2011, 151, 65–74. [Google Scholar] [CrossRef]
- Machado, L.P.; De Carvalho, L.R.; Young, M.C.M.; Cardoso-Lopes, E.M.; Da Cruz Centeno, D.; Zambotti-Villela, L.; Colepicolo, P.; Yokoya, N.S. Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacogn. 2015, 25, 657–662. [Google Scholar] [CrossRef]
- Paul, J.P.; Devi, S.D.K. Evaluation of Antipyretic Activity of Methanol Extract of Hypnea musciformis (Wulf) Lamouroux (Red Seaweed) in Manapad Coast, Tamil Nadu. Ind. Int. J. Med. Chem. Anal. 2015, 5, 74–78. [Google Scholar]
- Adrien, A.; Bonnet, A.; Dufour, D.; Baudouin, S.; Maugard, T.; Bridiau, N. Anticoagulant Activity of Sulfated Ulvan Isolated from the Green Macroalga Ulva rigida. Mar. Drugs 2019, 17, 291. [Google Scholar] [CrossRef]
- Holtkamp, A.D.; Kelly, S.; Ulber, R.; Lang, S. Fucoidans and fucoidanases—Focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Appl. Microbiol. Biotechnol. 2009, 82, 1–11. [Google Scholar] [CrossRef]
- Carvalhal, F.; Cristelo, R.R.; Resende, D.I.S.P.; Pinto, M.; Sousa, E.; Correia-Da-Silva, M. Antithrombotics from the Sea: Polysaccharides and Beyond. Mar. Drugs 2019, 17, 170. [Google Scholar] [CrossRef]
- Faggio, C.; Morabito, M.; Minicante, S.A.; Piano, G.L.; Pagano, M.E.; Genovese, G. Potential Use of Polysaccharides from the Brown Alga Undaria Pinnatifida as Anticoagulants. Braz Arch Biol Technol. 2015, 58, 798–804. [Google Scholar] [CrossRef]
- Chan, J.N.; Poon, B.; Salvi, J.S.; Olsen, J.B.; Emili, A.; Mekhail, K. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev. Cell 2011, 20, 867–879. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; Silva, G.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Radakovits, R.; Jinkerson, R.E.; Darzins, A.; Posewitz, M.C. Genetic Engineering of Algae for Enhanced Biofuel Production. Eukaryot. Cell 2010, 9, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Dubrasquet, H.; Reyes, J.; Sanchez, R.P.; Valdivia, N.; Guillemin, M. Molecular-Assisted Revision of Red Macroalgal Diversity and Distribution along the Western Antarctic Peninsula and South Shetland Islands. Cryptogam. Algol. 2018, 39, 409. [Google Scholar] [CrossRef]
- Steele, T.S. A Genomic Approach to Accessing and Characterizing Secondary Metabolite Biosynthetic Pathways from Marine Red Macroalgae. Available online: https://escholarship.org/uc/item/4vw303ct (accessed on 17 December 2023).
- Khatri, K.; Patel, J.; Adams, J.; Jones, H.; Phillips, D. Functional Genomic and Transformation Resources for Commercially Important Red Macroalgae (Rhodophyta). Algal Res. 2023, 74, 103227. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—the next generation. Nat. Rev. Genet. 2009, 11, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Cock, J.M.; Coelho, S.M.; Brownlee, C.; Taylor, A.R. The Ectocarpus Genome Sequence: Insights into Brown Algal Biology and the Evolutionary Diversity of the Eukaryotes. New Phytol. 2010, 188, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chandra Mohana, N.; Yashavantha Rao, H.C.; Rakshith, D.; Mithun, P.R.; Nuthan, B.R.; Satish, S. Omics-Based Approach for Biodiscovery of Microbial Natural Products in Antibiotic Resistance Era. J. Genet. Eng. Biotechnol. 2018, 16, 1–8. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, Á.S.; Sofian-Seng, N.-S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Walker, T.L.; Collet, C.; Purton, S. Algal Transgenics in the Genomic Era. J. Phycol. 2005, 41, 1077–1093. [Google Scholar] [CrossRef]
- Manat, G.; Fanuel, M.; Jouanneau, D.; Jam, M.; Mac-Bear, J.; Rogniaux, H.; Mora, T.; Larocque, R.; Lipinska, A.; Czjzek, M.; et al. Specificity of a β-porphyranase produced by the carrageenophyte red alga Chondrus crispus and implications of this unexpected activity on red algal biology. JBC. 2022, 298, 102707. [Google Scholar] [CrossRef]
- Zheng, H.; Chiang-Hsieh, Y.; Chien, C.; Hsu, B.J.; Liu, T.; Chen, C.N.; Chang, W.C. AlgaePath: Comprehensive Analysis of Metabolic Pathways Using Transcript Abundance Data from Next-Generation Sequencing in Green Algae. BMC Genom. 2014, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Vandepoele, K.; Van Bel, M.; Richard, G.; Van Landeghem, S.; Verhelst, B.; Moreau, H.; Van De Peer, Y.; Grimsley, N.; Piganeau, G. pico-PLAZA, a genome database of microbial photosynthetic eukaryotes. Environ. Microbiol. 2013, 15, 2147–2153. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, H.; Cantor, M.; Dusheyko, S.; Hua, S.Z.; Poliakov, A.; Shabalov, I.; Smirnova, T.I.; Grigoriev, I.V.; Dubchak, I. The genome portal of the Department of Energy Joint Genome Institute: 2014 updates. Nucleic Acids Res. 2014, 42, D26–D31. [Google Scholar] [CrossRef]
- Yu, L.; Li, T.; Li, L.; Lin, X.; Li, H.; Liu, C.; Guo, C.; Lin, S. SAGER: A Database of Symbiodiniaceae and Algal Genomic Resource. Database 2020, 2020, baaa051. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cui, Y.; Jia, X.; Zhang, J.; Li, R.; Yu, Y.; Jia, S.; Qu, J.; Wang, X. OGDA: A Comprehensive Organelle Genome Database for Algae. Database 2020, 2020, baaa097. [Google Scholar] [CrossRef] [PubMed]
- Karp, P.D.; Billington, R.; Caspi, R.; Fulcher, C.A.; Latendresse, M.; Kothari, A.; Keseler, I.M.; Krummenacker, M.; Midford, P.E.; Ong, Q.; et al. The BioCyc Collection of Microbial Genomes and Metabolic Pathways. Brief. Bioinform. 2017, 20, 1085–1093. [Google Scholar] [CrossRef]
- Joyce, A.; Palsson, B.Ø. The model organism as a system: Integrating “omics” data sets. Nat. Rev. Mol. Cell Biol. 2006, 7, 198–210. [Google Scholar] [CrossRef]
- Tseng, C.; Chang, C. Notes on Caloglossa, Digena, and the Other Anthelmintic Marine Algae in China. PubMed 1962, 9, 180–186. [Google Scholar]
- Shen, Y.; Motomura, T.; Ichihara, K.; Matsuda, Y.; Yoshimura, K.; Kosugi, C.; Nagasato, C. Application of CRISPR-Cas9 genome editing by microinjection of gametophytes of Saccharina japonica (Laminariales, Phaeophyceae). J. Appl. Phycol. 2023, 35, 1431–1441. [Google Scholar] [CrossRef]
- Minoda, A.; Sakagami, R.; Yagisawa, F.; Kuroiwa, T.; Tanaka, K. Improvement of Culture Conditions and Evidence for Nuclear Transformation by Homologous Recombination in a Red Alga, Cyanidioschyzon merolae 10D. Plant Cell Physiol. 2004, 45, 667–671. [Google Scholar] [CrossRef]
- Diesel, J.; Molano, G.; Montecinos, G.J.; DeWeese, K.; Calhoun, S.; Kuo, A.; Lipzen, A.; Salamov, A.; Grigoriev, I.V.; Reed, D.C.; et al. A scaffolded and annotated reference genome of giant kelp (Macrocystis pyrifera). BMC Genom. 2023, 24, 543. [Google Scholar] [CrossRef] [PubMed]
- Théodorou, I.; Kovi, M.R.; Liang, Z. Hilde-Gunn Opsahl-Sorteberg Genetic and Genomic Approaches for Improved and Sustainable Brown Algal Cultivation. In Sustainable Global Resources of Seaweeds; Springer: Cham, Switzerland, 2022; pp. 615–633. [Google Scholar] [CrossRef]
- Deng, Y.; Yao, J.; Wang, X.; Guo, H.; Duan, D. Transcriptome Sequencing and Comparative Analysis of Saccharina japonica (Laminariales, Phaeophyceae) under Blue Light Induction. PLoS ONE 2012, 7, e39704. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Chen, Y. Transcriptomics: Advances and Approaches. Sci. China Life Sci. 2013, 56, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the Neurotoxin Domoic Acid in a Bloom-Forming Diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Keeling, P.J.; Burki, F.; Wilcox, H.M.; Allam, B.; Allen, E.E.; Amaral-Zettler, L.; Armbrust, E.V.; Archibald, J.M.; Bharti, A.K.; Bell, C.J.; et al. The Marine Microbial Eukaryote Transcriptome Sequencing Project (MMETSP): Illuminating the Functional Diversity of Eukaryotic Life in the Oceans through Transcriptome Sequencing. PLOS Biol. 2014, 12, e1001889. [Google Scholar] [CrossRef] [PubMed]
- Rismani-Yazdi, H.; Haznedaroğlu, B.Z.; Hsin, C.; Peccia, J. Transcriptomic Analysis of the Oleaginous Microalga Neochloris oleoabundans Reveals Metabolic Insights into Triacylglyceride Accumulation. Biotechnol. Biofuels 2012, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.; Dittami, S.M.; Goulitquer, S.; Correa, J.A.; Boyen, C.; Potin, P.; Tonon, T. Transcriptomic and metabolomic analysis of copper stress acclimation in Ectocarpus siliculosus highlights signaling and tolerance mechanisms in brown algae. BMC Plant Biol. 2014, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Wang, D.; Mao, Y.; Kong, F.; Bi, G.; Xing, Q.; Weng, Z. Integrating Transcriptomics and Metabolomics to Characterize the Regulation of EPA Biosynthesis in Response to Cold Stress in Seaweed Bangia fuscopurpurea. PLoS ONE 2017, 12, e0186986. [Google Scholar] [CrossRef]
- Zhuo, J.; Wang, H.; Du, Y.; Shi, M.; Huan, L.; Wang, G. Transcriptomic Analysis of Ulva Prolifera in Response to Salt Stress. Water 2023, 15, 63. [Google Scholar] [CrossRef]
- Barone, R.; De Santi, C.; Palma Esposito, F.; Tedesco, P.; Galati, F.; Visone, M.; Scala, A. Donatella de Pascale Marine Metagenomics, a Valuable Tool for Enzymes and Bioactive Compounds Discovery. Front. Mar. Sci. 2014, 1, 38. [Google Scholar] [CrossRef]
- Sambles, C.; Moore, K.; Lux, T.; Jones, K.J.; Littlejohn, G.R.; Gouveia, J.D.; Aves, S.J.; Studholme, D.J.; Lee, R.J.; Love, J. Metagenomic Analysis of the Complex Microbial Consortium Associated with Cultures of the Oil-Rich Alga Botryococcus braunii. MicrobiologyOpen 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, N.; Martínez–Hernández, G.B.; Goffi, V.; Gómez, P.A.; Aguayo, E.; Artés, F.; Artés-Hernández, F. Natural Vitamin B12 and Fucose Supplementation of Green Smoothies with Edible Algae and Related Quality Changes during Their Shelf Life. J. Sci. Food Agric. 2017, 98, 2411–2421. [Google Scholar] [CrossRef]
- Miranda, L.; Hutchison, K.W.; Grossman, A.R.; Brawley, S.H. Diversity and Abundance of the Bacterial Community of the Red Macroalga Porphyra umbilicalis: Did Bacterial Farmers Produce Macroalgae? PLoS ONE 2013, 8, e58269. [Google Scholar] [CrossRef] [PubMed]
- Salaün, S.; Barre, S.; Santos-Goncalvez, M.D.; Potin, P.; Haras, D.; Bazire, A. Influence of exudates of the kelp Laminaria digitata on biofilm formation of associated and exogenous bacterial epiphytes. Microb. Ecol. 2012, 64, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Reddy, C.R.K. Unraveling the functions of the macroalgal microbiome. Front. Microbiol. 2016, 6, 1488. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L. Insights into the Regulation of Algal Proteins and Bioactive Peptides Using Proteomic and Transcriptomic Approaches. Molecules 2019, 24, 1708. [Google Scholar] [CrossRef] [PubMed]
- Patelou, M.; Infante, C.; Dardelle, F.; Randewig, D.; Kouri, E.D.; Udvardi, M.K.; Tsiplakou, E.; Mantecón, L.; Flemetakis, E. Transcriptomic and Metabolomic Adaptation of Nannochloropsis gaditana Grown under Different Light Regimes. Algal Res. 2020, 45, 101735. [Google Scholar] [CrossRef]
- Anand, V.; Singh, P.K.; Banerjee, C.; Shukla, P. Proteomic Approaches in Microalgae: Perspectives and Applications. 3 Biotech 2017, 7, 145. [Google Scholar] [CrossRef]
- Wong, P.; Tan, L.; Nawi, H.; AbuBakar, S. Proteomics of the Red Alga, Gracilaria changii (Gracilariales, Rhodophyta). J. Phycol. 2006, 42, 113–120. [Google Scholar] [CrossRef]
- Chakdar, H.; Hasan, M.T.; Pabbi, S.; Nevalainen, H.; Shukla, P. High-throughput proteomics and metabolomic studies guide re-engineering of metabolic pathways in eukaryotic microalgae: A review. Bioresour. Technol. 2021, 321, 124495. [Google Scholar] [CrossRef]
- Contreras, L.; Moenne, A.; Gaillard, F.; Potin, P.; Correa, J.A. Proteomic Analysis and Identification of Copper Stress-Regulated Proteins in the Marine Alga Scytosiphon gracilis (Phaeophyceae). Aquat. Toxicol. 2010, 96, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Begolli, R.; Chatziangelou, M.; Samiotaki, M.; Goutas, A.; Barda, S.; Goutzourelas, N.; Kevrekidis, D.P.; Malea, P.; Trachana, V.; Liu, M.; et al. Transcriptome and Proteome Analysis Reveals the Anti-Cancer Properties of Hypnea Musciformis Marine Macroalga Extract in Liver and Intestinal Cancer Cells. Hum. Genom. 2023, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Thomas, N.V.; Li, X. Anticancer Compounds from Marine Macroalgae and Their Application as Medicinal Foods. Adv. Food Nutr. Res. 2011, 64, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Belghit, I.; Rasinger, J.D.; Heesch, S.; Biancarosa, I.; Liland, N.S.; Torstensen, B.E.; Waagbø, R.; Lock, E.; Brückner, C. In-depth metabolic profiling of marine macroalgae confirms strong biochemical differences between brown, red and green algae. Algal Res. 2017, 26, 240–249. [Google Scholar] [CrossRef]
- Sanchez-Arcos, C.; Paris, D.; Mazzella, V.; Mutalipassi, M.; Costantini, M.; Buia, M.C.; Von Elert, E.; Cutignano, A.; Zupo, V. Responses of the Macroalga Ulva prolifera Müller to Ocean Acidification Revealed by Complementary NMR- and MS-Based Omics Approaches. Mar. Drugs 2022, 20, 743. [Google Scholar] [CrossRef] [PubMed]
- Verpoorte, R.; Choi, Y.H.; Mustafa, N.R.; Kim, H.K. Metabolomics: Back to Basics. Phytochem. Rev. 2008, 7, 525–537. [Google Scholar] [CrossRef]
- Jung, V.; Pohnert, G. Rapid Wound-Activated Transformation of the Green Algal Defensive Metabolite Caulerpenyne. Tetrahedron 2001, 57, 7169–7172. [Google Scholar] [CrossRef]
- Barre, S.; Weinberger, F.; Kervarec, N.; Potin, P. Monitoring defensive responses in macroalgae–limitations and perspectives. Phytochem. Rev. 2004, 3, 371–379. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Lin, A.; Stout, E.P.; Prudhomme, J.; Roch, K.L.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.G.; Hay, M.E.; Kubanek, J. Bioactive Bromophycolides R−U from the Fijian Red Alga Callophycus serratus. J. Nat. Prod. 2010, 73, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic Assessment of Induced and Activated Chemical Defence in the Invasive Red Alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, e29359. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Keasling, J.D. Engineering Cellular Metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Yoshikuni, Y. Metabolic Engineering for Valorization of Macroalgae Biomass. Metab. Eng. 2022, 71, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Pardilhó, S.; Boaventura, R.; Almeida, M.F.; Dias, J.M. Marine Macroalgae Waste: A Potential Feedstock for Biogas Production. J. Environ. Manag. 2022, 304, 114309. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Wu, X. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef] [PubMed]
- García, E.S.; Miranda, C.F.; Cesário, M.T.; Wijffels, R.H.; Van Den Berg, C.; Eppink, M. Ionic Liquid-Assisted Selective Extraction and Partitioning of Biomolecules from Macroalgae. ACS Sustain. Chem. Eng. 2023, 11, 1752–1762. [Google Scholar] [CrossRef]
- Safi, C.; Rodríguez, L.; Mulder, W.; Engelen-Smit, N.; Spekking, W.; Van Den Broek, L.; Olivieri, G.; Sijtsma, L. Energy Consumption and Water-Soluble Protein Release by Cell Wall Disruption of Nannochloropsis gaditana. Bioresour. Technol. 2017, 239, 204–210. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal Liquefaction of Macroalgae Enteromorpha prolifera to Bio-oil. Energies 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Shin, E.J.; Nimlos, M.R.; Evans, R.J. Kinetic Analysis of the Gas-Phase Pyrolysis of Carbohydrates. Fuel 2001, 80, 1697–1709. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Fakayode, O.A.; Aboagarib, E.A.A.; Zhou, C.; Ma, H. Co-pyrolysis of lignocellulosic and macroalgae biomasses for the production of biochar—A review. Bioresour. Technol. 2020, 297, 122408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, L.; Tong, D.; Hu, C. Microwave-enhanced pyrolysis of natural algae from water blooms. Bioresour. Technol. 2016, 212, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wan, Q.; Yang, N. Recent Advances of Porous Graphene: Synthesis, Functionalization, and Electrochemical Applications. Small 2019, 15, 1903780. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Usman, T.M.M.; Kumar, G. Application of Chemo-Thermal Coupled Sonic Homogenization of Marine Macroalgal Biomass for Energy Efficient Volatile Fatty Acid Recovery. Bioresour. Technol. 2020, 303, 122951. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.; Toop, T.A.; Donnison, I.S.; Gallagher, J. Seasonal Variation in Laminaria digitata and Its Impact on Biochemical Conversion Routes to Biofuels. Bioresour. Technol. 2011, 102, 9976–9984. [Google Scholar] [CrossRef]
- Motte, J.; Delenne, J.; Rouau, X.; Mayer-Laigle, C. Mineral–vegetal co-milling: An effective process to improve lignocellulosic biomass fine milling and to increase interweaving between mixed particles. Bioresour. Technol. 2015, 192, 703–710. [Google Scholar] [CrossRef]
- Bader, A.N.; Rizza, L.S.; Consolo, V.F.; Curatti, L. Efficient Saccharification of Microalgal Biomass by Trichoderma harzianum Enzymes for the Production of Ethanol. Algal Res. 2020, 48, 101926. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2021, 11, 59. [Google Scholar] [CrossRef]
- Huang, C.; Wu, S.; Yang, W.; Kuan, A.; Chen, C. Antioxidant Activities of Crude Extracts of Fucoidan Extracted from Sargassum glaucescens by a Compressional-Puffing-Hydrothermal Extraction Process. Food Chem. 2016, 197, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).