Simple Summary
The present review provides (1) an updated checklist of the Vietnamese marine flora, (2) a review of molecular-assisted alpha taxonomic efforts, (3) an analysis of marine floral biodiversity spatial distribution nationally and regionally (South China Sea), (4) a discussion on the impact of anthropogenic and environmental stressors on the Vietnamese marine flora, and (5) the efforts developed in the last decade for its conservation. The updated checklist consists of 878 species, including 439 Rhodophyta, 156 Ochrophyta, 196 Chlorophyta, 87 Cyanobacteria, and 15 seagrasses. The South Central Coast supports the highest species diversity of marine algae, which coincides with the largest density of coral reefs along the Vietnam coast. Vietnam holds one of the richest marine floras in the South China Sea owing to the country’s coastline length and associated marine habitat diversity. However, the Vietnamese marine floral biodiversity is facing critical threats, and present management efforts are yet insufficient for their conservation. A methodical molecular-assisted re-examination of Vietnam marine floral biodiversity is urgently needed, complemented with in-depth investigations of the main threats targeted against it; and finally, conservation measures should be urgently implemented.
Abstract
Part of the Indo-Chinese peninsula and located on the northwest edge of the Coral Triangle in the South China Sea, the Vietnamese coastal zone is home to a wealthy marine biodiversity associated with the regional geological setting and history, which supports a large number of marine ecosystems along a subtropical to tropical gradient. The diversity of coastal benthic marine primary producers is also a key biological factor supporting marine biological diversity. The present review provides: (1) an updated checklist of the Vietnamese marine flora, (2) a review of molecular-assisted alpha taxonomic efforts, (3) an analysis of marine floral biodiversity spatial distribution nationally and regionally (South China Sea), (4) a review of the impact of anthropogenic and environmental stressors on the Vietnamese marine flora, and (5) the efforts developed in the last decade for its conservation. Based on the studies conducted since 2013 and the nomenclatural changes that occurred during this period, an updated checklist of benthic marine algae and seagrasses consisted in a new total of 878 species, including 439 Rhodophyta, 156 Ochrophyta, 196 Chlorophyta, 87 Cyanobacteria, and 15 phanerogam seagrasses. This update contains 54 new records and 5 new species of macroalgae. The fairly poor number of new records and new species identified in the last 10 years in a “mega-diverse” country can be largely attributed to the limited efforts in exploring algal biodiversity and the limited use of genetic tools, with only 25.4% (15 species) of these new records and species made based on molecular-assisted alpha taxonomy. The South Central Coast supports the highest species diversity of marine algae, which coincides with the largest density of coral reefs along the Vietnamese coast. Vietnam holds in the South China Sea one of the richest marine floras, imputable to the country’s geographical, geological, and climatic settings. However, Vietnam marine floral biodiversity is under critical threats examined here, and current efforts are insufficient for its conservation. A methodical molecular-assisted re-examination of Vietnam marine floral biodiversity is urgently needed, complemented with in-depth investigations of the main threats targeting marine flora and vulnerable taxa, and finally, conservation measures should be urgently implemented.
1. Introduction
Located along the eastern margin of the Indo-Chinese Peninsula, on the northwest edge of the Coral Triangle biodiversity hotspot [], in the South China Sea (also known as the East Vietnam Sea or Biển Đông), the Vietnamese coastal zone is home to a remarkably rich marine biodiversity [,]. Vietnam has been listed among the top 25 most biologically diverse countries in the world [] and characterized by some authors as a “mega-diverse country” [,]. The origin of this diversity is linked to the region’s (Southeast Asia) geological and climatic history, the country’s coastline length covering some 3260 km along a north–south orientation, thus with a wide latitudinal range (stretching from 21°30′ N to 8°25′ N), spanning a subtropical–tropical transition zone, which supports no less than 20 types of marine ecosystems []. It is nonetheless important to also point to the role of coastal benthic marine primary producers (e.g., algae, corals, seagrasses) as a key biological factor supporting other forms of marine biological diversity []. Benthic marine algae occur across virtually all marine coastal systems from intertidal zone to depths of >200 m, on soft (e.g., sandy) to hard (e.g., rocky) substrates, in a variety of habitats (e.g., lagoons, bays, islands, islets, atolls, and reefs) and ecosystems (e.g., mangroves, seagrasses beds, and coral reefs).
The marine flora of Vietnam, which includes three main classes of macroalgae (Chlorophyta, Ochrophyta, Rhodophyta), marine phanerogams or seagrasses (Alismatales), and Cyanobacteria (Cyanophyceae), has attracted the attention of marine botanists since the 1800s. The first mention of Vietnamese seaweeds appeared in the Flora Cochinchinensis [], with the record of 11 names of marine macroalgal species, later referenced in the work of Agardh [,] and De Toni [,]. The French institution “Institut océanographique de l’Indochine”, corresponding today to the “Institute of Oceanography”, contributed considerably during the 1930s to the knowledge on the algal diversity of Vietnam, notably owing to the work of Pham-Hoàng Hộ [], considered to be the first Vietnamese marine algal taxonomists of Vietnam. The first collections from the Spratly Islands Archipelago, by the French naturalist Jean Marie Antoine De Lanessan in 1936 (Gouvernement Général de L’Indochine, 1936) []—currently housed at the Museum of Oceanography, Nha Trang City—are worthy of mention (Figure 1). Dawson [] published the very first checklist on the Vietnamese marine flora of Nha Trang Bay in the province of Khanh Hoa (South Central Coast region) with the “Institut Océanographique de Nha Trang”. Dawson [] reported a total of 204 species (16 Cyanophyceae, 118 Rhodophyta, 22 Ochrophyta, and 48 Chlorophyta), nearly all of which were new species records for Vietnam. Until 1967, the list of marine macroalgae in South Vietnam consisted of 517 species and subspecies []. In north of Vietnam, Nguyen et al. [] provided a list of 281 species. Subsequent studies focused mainly on understudied taxonomic groups and regions of Vietnam, for example, on the families Sargassaceae [,,] and Halymeniaceae [,] and on the genera Gracilaria and Gracilariopsis [,], Eucheuma and Kappaphycus [,], Dictyota [], and Laurencia [,]. Regions of Vietnam that were later investigated included archipelagoes or offshore islands, such as the Spratly Archipelago [,,,,], Ly Son Island [], Phu Quy Island [], and some inland coastal sites, such as Hai Van–Son Cha [], Nha Trang Bay [], Ninh Thuan [], and Con Dao Island []. Recently, Belous et al. [] published a checklist of 702 species including Rhodophyta, Ochrophyta, and Chlorophyta recorded from Southern and Central Vietnam (16°12′ southward) []. Earlier, in 2013, Nguyen et al. (2013) published the first comprehensive marine macroalgal checklist for all Vietnam based on a review of 81 books and publications []. The checklist contained a total of 827 species, including 412 Rhodophyta, 180 Chlorophyta, 147 Ochrophyta, and 88 Cyanobacteria. The authors suggested that several taxa needed further investigation to better understand their diversity in Vietnamese waters, and noted the need to combine DNA barcoding and morphological observations to resolve or clarify taxonomic uncertainties. Previously, the checklist of seagrass showed 14 species []. In addition, Halophila major (Zollinger) Miquel was newly recorded in Vietnamese waters in 2013 []. Recently, the phylogenetic analysis inferred from genetic marker and morphological observation revealed the putative hybridization form between two species, H. ovalis and H. major []. No new record or new species of Cyanobacteria were made since the work of Pham-Hoàng Hộ [].
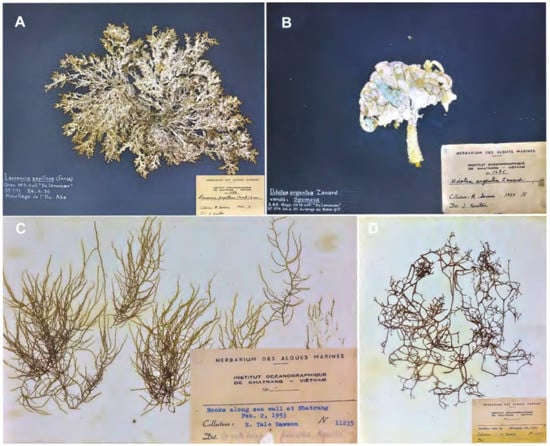
Figure 1.
Historical herbarium voucher specimens at Institution of Oceanography—Nha Trang. (A) Laurencia papillosa, (B) Udotea argentea collected at Spratly Archipelago from De Lanessan [], (C) Grateloupia filicina, and (D) Chnoospora implexa collected by Dawson [] in Nha Trang.
The aims of the present review were multifold: (1) to deliver an updated checklist of the Vietnamese marine flora, (2) to review molecular-assisted alpha taxonomic efforts implemented in its study, (3) to examine marine floral biodiversity spatial patterns across Vietnamese regions and countries in the South China Sea region, (4) to discuss anthropogenic and environmental threats directly affecting Vietnamese marine flora, and (5) the efforts deployed nationally in the last decade for its conservation.
2. Species Diversity Update
Our first objective was to thoroughly review the taxonomic work conducted on the Vietnamese marine flora in the last 10 years since the checklist of Nguyen et al. [] was published, focusing on 3 main macroalgal phyla/classes (Chlorophyta, Ochrophyta, and Rhodophyta), Cyanobacteria (Cyanophyta), and marine phanerogams or seagrasses (Alismatales). Our literature review of the past 10 years’ research on Vietnamese algae and seagrass taxonomy led to the documentation of a total of 54 new records (i.e., species newly recorded from Vietnam in the period between the last checklist and this updated checklist) and 5 new species (i.e., species newly described from Vietnam in the period between the last checklist and this updated checklist) of macroalgae (3 Rhodophyta, 1 Ochrophyta, and 1 Chlorophyta). New species and new records made during the last 10 years are listed in Table 1. No new records of Cyanobacteria or seagrasses were made since 2013. Based on these new data and the nomenclatural changes that occurred in the last 10 years (such as synonymies), we provide an updated checklist of the benthic marine flora, bringing the new total to 878 species of algae in 42 orders, 108 families, and 284 genera, and consisting of 439 Rhodophyta (21 orders, 52 families, and 161 genera), 156 Ochrophyta (7 orders, 13 families, and 36 genera), 196 Chlorophyta (6 orders, 23 families, and 44 genera), 87 Cyanobacteria (8 orders, 18 families, 42 genera), and 15 seagrasses in Alismatales (1 order, 4 families, and 10 genera) listed in Table 2. A total of 112 species of Rhodophyta are updated to the currently accepted names. Three new species and 13 new records were added to the checklist. Four species, including Gracilaria mammillaris, Dasya baillouviana, Meristotheca papulosa, and Mesophyllum erubescens, were removed from the checklist because they were misidentifications. For Chlorophyta, 17 species were updated to the corrected name; 11 species were newly recorded, mainly in 2 genera, Ulva and Caulerpa; and only 1 new species was new to science. Two species of Chlorophyta were removed from the checklist: the misspelled name (i.e., orthographic mistake in AlgaeBase) “Codium tunue” and Cladophora adhaerens Ruprecht, which is an invalidly published name. For Ochrophyta, only 1 new species and 2 new records were added to the checklist, and 7 species were updated to the currently accepted names. Finally, 21 species of Cyanobacteria were updated to currently accepted names. We compared our updated checklist with the data available from AlgaeBase [] for Cyanobacteria, Rhodophyta, Ochrophyta, and Chlorophyta. AlgaeBase [] data consisted of a lower number of species with a total of 862 species consisting of 51 Cyanobacteria, 438 Rhodophyta, 171 Ochrophyta, and 202 Chlorophyta. Among 4 phyla, Cyanobacteria showed the lowest richness (87 species, 10%). The checklist of marine macroalgae in Vietnam published by Nguyen et al. [] did not show any new species and new records of Cyanobacteria []. The checklist of marine macroalgae in South Vietnam [], Nha Trang Bay [], and Con Dao Island [] did not list any Cyanobacteria. It indicated that Cyanobacteria have not been studied in detail. Therefore, Cyanobacteria need to be included in future works.

Table 1.
List of new species (a) and new records (b) of marine macroalgae found in Vietnam with support from genetic markers and/or morphological observation since 2013. -/-, as above, na: not available. Ref.: reference.

Table 2.
Checklist of the marine flora (Alismatales, Chlorophyta, Cyanobacteria, Ochrophyta, Rhodophyta) of Vietnam. The checklist is systematically and alphabetically arranged at the phylum, ordinal, familial, generic, and species levels.
3. Molecular-Assisted Alpha Taxonomy of the Vietnamese Marine Flora
The use of molecular-assisted alpha taxonomy of marine algae is very recent in Vietnam [,]. Molecular tools are presently needed among other purposes: (1) validate previous species identification, (2) identify new records and species, and (3) detect introduced species (e.g., [,,]). Studies combining DNA-based species delimitation techniques and detailed morphological observations have refined our knowledge on Vietnamese species taxonomy and on the individual species’ biogeographical ranges. Nevertheless, such efforts have been very limited in the last decade. Among the 59 new records and species made in the last 10 years, only 25.4% (15 species) were based on molecular-assisted alpha taxonomy. Hereafter, we reviewed molecular studies conducted thus far on Vietnamese marine macroalgae, identifying the taxa studied, marker used, and taxonomic results.
3.1. Molecular-Assisted Alpha Taxonomy of Rhodophyta
Molecular-assisted alpha taxonomic studies on Rhodophyta have comprised a total of four markers, analyzed individually or combined, consisting of two chloroplast genes (large subunit of ribulose-1,5-bisphosphate-carboxylase-oxygenase (rbcL); photosystem I P700 chlorophyll a apoprotein A1 (psaA)), one mitochondrial gene (cytochrome c oxidase I (cox1)), and one nuclear gene (LSU rDNA (28S)). The plastidic rbcL gene has been mostly used. The 2006 publication by Hau et al. [] conducted one of the first molecular studies on Vietnamese Rhodophyta, analyzing the phylogenetic relationships among Gracilariaceae using rbcL, which revealed a new species of Gracilariopsis, Gracilariopsis nhatrangensis Le & Lin. Based on rbcL alone, Le et al. [] later showed that Gracilaria mammillaris (Montagne) M.Howe had been misidentified as Gracilaria phuquocensis Le, Muangmai & Zuccarello, a new species found in Vietnam; Nguyen et al. [] newly recorded the Halymeniales species Phyllymenia taiwanensis (Lin & Liang) Lin, Rodríguez-Prieto, De Clerck & Guiry in Da Nang from Central Vietnam; Nguyen et al. [] recorded Phyllymenia huangiae (Lin & Liang) Lin, Rodríguez-Prieto, De Clerck & Guiry (Figure 2E); and Duy [] reported the Rhodomelaceae species Chondrophycus tronoi (Ganzon-Fortes) Nam from Vietnam. Analyses based on cox1, psaA, and rbcL sequences allowed the discovery of the Gelidiellaceae species Perronella gracilis Boo, Nguyen, Kim & Boo from Nha Trang Bay from Southern Vietnam [], and the transfer of Gelidiella adnata Dawson to Parviphycus adnatus (Dawson) Santelices. Analyses based on the concatenated rbcL and cox1 sequences also revealed a new record of Delesseriaceae from Vietnam, Zellera tawallina Martens (Figure 2A), previously identified as Claudea batanensis Tanaka []. Analyses combining rbcL and cox1 sequences allowed the identification of a new species, Meristotheca lysonensis Nguyen, Nguyen, Kittle & McDermid, collected at Ly Son Island in the South Central Coast region of Vietnam [] (Figure 2B). A last worthy account for Rhodophyta is that of the Halymeniaceae species Halymenia dilatata Zanardini, a common species in Vietnam, previously reported in several publications [,]. Based on phylogenetic analyses using concatenated chloroplast and mitochondrial and nuclear markers (rbcL, cox1, and LSU rDNA (28S)), Vy et al. [] showed that H. dilatata may have been misidentified as Halymenia malaysiana Tan, Lim, Lin & Phang, a study that confirms new distributional records of Phycocalidia tanegashimensis along the Chinese and Vietnamese coastline in the South China Sea. The study used molecular sequence data from rbcL, COI-5P, and 18S rRNA genes to place P. tanegashimensis in a clade with P. acanthophora, P. denticulata, P. suborbiculata, and P. vietnamensis as out-groups [].
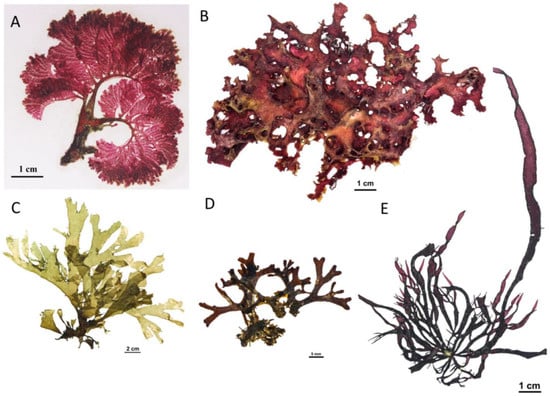
Figure 2.
Some recently newly described species and new records made of marine macroalgae from Vietnam. (A) Zellera tawallina Martens collected at Nha Trang Bay; (B) Meristotheca lysonensis Nguyen, Nguyen, Kittle & McDermid collected at Ly Son Island; (C) Dictyota hauckiana Nizamuddin collected at Ninh Thuan; (D) Dictyota grossedentata De Clerck & Coppejans collected at Nha Trang Bay; (E). Phyllymenia huangiae (Lin & Liang) Lin, Rodríguez-Prieto, De Clerck & Guiry collected at Da Nang.
3.2. Molecular-Assisted Alpha Taxonomy of Ochrophyta
Molecular-assisted alpha taxonomic studies on Ochrophyta have comprised a total of four molecular markers, analyzed individually or combined, consisting of two chloroplast genes (rbcL and the PSII thylakoid protein D1 (psbA)), one mitochondrial gene (cytochrome c oxidase subunit III (cox3)), and one nuclear encoded ribosomal cistron (ITS 2 rDNA). Tu [] used ITS2 rDNA and cox3 sequences to reassess Sargassum species diversity from Vietnam. The order Dictyotales has received particular attention in recent years. Using rbcL and psbA markers, Nguyen-Nhat et al. [] newly identified Dictyota hauckiana Nizamuddin from Ninh Thuan (Figure 2C). One additional species of Dictyota was newly recorded from Vietnam, Dictyota grossedentata De Clerck & Coppejans [] (Figure 2D). Molecular phylogenetic analyses based on concatenated rbcL and cox3 sequences led to the description of the new species Lobophora tsengii Tien & Sun from Bach Long Vy [], although morphological and molecular analyses did not conclusively rule out its conspecificity with Lobophora rosacea C.W.Vieira, Payri& De Clerck.
3.3. Molecular-Assisted Alpha Taxonomy of Chlorophyta
For Chlorophyta, the only molecular-assisted alpha taxonomic study reported until now is that by Tran et al. [], who reassessed the species diversity in Vietnam of the Ulvaceae genus Ulva based on rbcL and the elongation factor Tu (tufA). The study revealed seven new records of Ulva from Vietnam and identified a new species, U. vietnamensis L-A. T. Tran, Leliaert & De Clerck.
3.4. Molecular-Assisted Alpha Taxonomy of Cyanobacteria and Alismatales
For Alismatales (seagrasses), the concatenated rbcL and matK were applied to assess the species diversity of Halophila []. Based on the genetic marker ITS, a later study by Nguyen et al. [] showed that Halophila major was the correct name for the collections of Halophila ovalis from Nha Trang Bay. All seagrass species from Vietnam were confirmed with molecular markers, and samples previously labeled as “Halophila johnsonii” were reidentified as H. ovalis. Therefore, Halophila johnsonii was removed from the seagrass checklist of Vietnam []. Halophila major was found in most offshore islands, whereas H. ovalis occurred in lagoons in Vietnamese waters []. In contrast, no molecular-assisted alpha taxonomic study on Cyanophyceae was yet conducted in Vietnam.
3.5. Intraspecific Genetic Diversity Studies
Several DNA fingerprinting have been applied to investigate the genetic relationships among individuals within or among populations of the same species [,]. In a global study of Gracilaria salicornia (Agardh) Dawson from Southeast Asia, Yang et al. [] distinguished a lineage of the Philippines from other Southeast Asian countries (e.g., Malaysia and Thailand). For another Rhodophyta, Phycocalidia acanthophora (Oliveira & Coll) Santiañez, the dataset of rbcL indicated that there is no haplotype sharing between populations in the Philippines and other nearby areas, including Taiwan, Japan, and Hong Kong []. In Vietnam, the red algae species in Kappaphycus and Eucheuma are important economically and were widely cultivated in the South Central. So far, based on a combined cox2–3 and rbcL dataset, Zuccarelo et al. [] compared the genetic variation among cultivated Kappaphycus alvarezii (Doty) Liao farming worldwide, including a strain from Vietnam; the authors indicated that there is no genetic variation among samples collected in Vietnam and other Southeast Asian countries, such as the Philippines, Malaysia, and Indonesia. However, Kappaphycus alvarezii collected from Africa and Hawai’i showed significant differences from populations in Southeast Asian countries. By using random amplified polymorphic DNA (RAPD) markers, Hong et al. [] also revealed the genetic variation among strains of Kappaphycus spp. and Eucheuma spp. in Vietnam, Kappaphycus striatus (Schmitz) Liao. The analyses of the mitochondrial cox2–3 spacer of Kappaphycus spp. and Eucheuma spp. showed that there are two haplotypes of K. alvarezii, and an unidentified Kappaphycus sp. was also found in Vietnam and the Philippines []. A later study by Tan et al. [] indicated that the aring-aring strain was described as the new species Kappaphycus malesianus Tan, Lim & Phang. There is no evidence of occurrence of this species in Vietnamese waters. The biogeography of Halymenia malaysiana was studied in more detail. Our previous study showed that the common haplotype in Vietnam is R1, and three new haplotypes were added to H. malaysiana for Southeast Asia (Figure 3). There are statistically significant genetic differences between Sunda Shelf (Vietnam and Malaysia) populations and those in Philippine waters []. For another economic species, Gracilaria tenuistipitata Chang & Xia, Song et al. [] found that there is only one haplotype (T5) in Vietnam. Compared with other haplotypes in Thailand, Malaysia, and Singapore, a haplotype of Gracilaria tenuistipitata collected in Vietnam showed from one to eight mutational steps. Recently, the tufA gene was applied to find the haplotype and genetic diversity of the green algae Halimeda spp.; Nguyen et al. [] concluded that the genetic variation in H. macroloba Decaisne is very low, and H. opuntia (Linnaeus) J.V.Lamouroux tends to form a distinct group in Vietnamese waters.
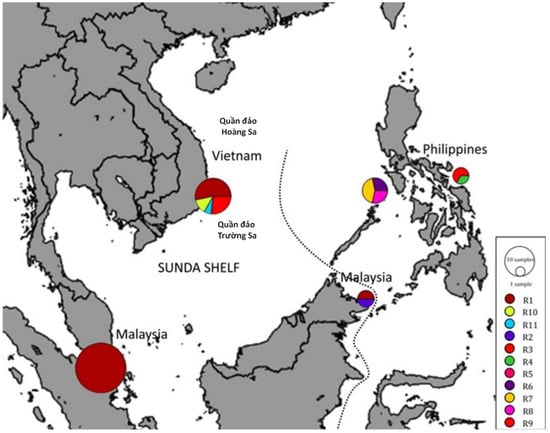
Figure 3.
Distribution of haplotypes of Halymenia malaysiana in Sunda Shelf (Malaysia and Vietnam) and the Philippines []. Adapted from Nguyen et al. [].
4. Biodiversity Distribution Patterns
4.1. Marine Floral Biodiversity across the South China Sea
Located on the northwest edge of the Coral Triangle biodiversity hotspot, the South China Sea is one of the most productive marine regions in the world [,]. The sea is bordered by twelve states and territories, including Brunei, Cambodia, (mainland) China, Hong Kong, Indonesia, Macao, Malaysia, the Philippines, Singapore, Taiwan, Thailand, and Vietnam. Phang et al. [] documented 1412 species of marine algae from the South China Sea (119 Cyanophyceae, 305 Chlorophyta, 258 Ochrophyta, and 730 Rhodophyta) from six countries bordering the South China Sea (Indonesia, Malaysia, the Philippines, Singapore, Thailand, and Vietnam). Their analyses showed similarity in the marine algal floras of Malaysia, Singapore, and Thailand and those of Vietnam and the Philippines. We present here an overview of species diversity from states and territories bordering the South China Sea based on AlgaeBase [] for four algae classes (Cyanobacteria, Rhodophyta, Ochrophyta, and Chlorophyta) and seagrasses. No data were available on AlgaeBase for Brunei, Hong Kong, Macau, Cambodia, and Taiwan. Data for seagrasses were retrieved from different sources indicated in Figure 4 and Table 3. The Gulfs of Thailand and Tonkin were included in the South China Sea. It should be noted that with the exception of Vietnam, the species numbers provided here are not restricted to the South China Sea, but are all-inclusive for each country (i.e., not restricted to the South China Sea), and retrieving data restricted to the South China Sea was not possible. In comparison with other South China Sea bordering states/territories, Vietnam supports the fourth highest marine floral diversity with 877 species, according to AlgaeBase [] (but 881 species according to our updated checklist). However, taking into account the all-inclusiveness of the number for other countries, Vietnam possibly holds the highest diversity in the South China Sea. In fact, the South China Sea coastlines of the three other species-rich states (China, the Philippines, Indonesia) represent only a fraction of these countries.
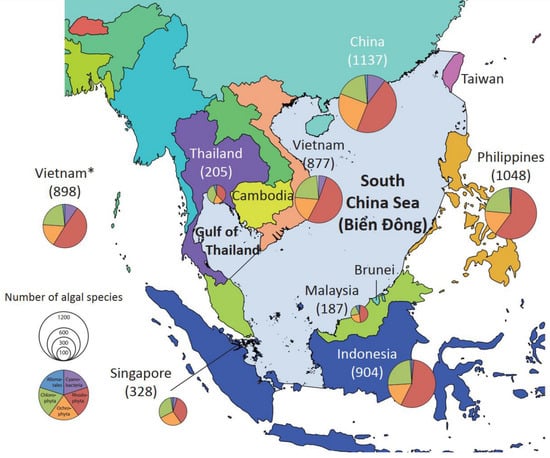
Figure 4.
Spatial variation in marine floral biodiversity (Cyanobacteria, Rhodophyta, Ochrophyta, and Chlorophyta) across countries bordering the South China Sea (East Vietnam Sea). Marine floral biodiversity data based on AlgaeBase []. * Data for the Vietnam marine flora based on the current updated checklist. Colors mean blue is Alismatales, green is Chlorophyta, red is Rhodophyta, brown is Ochrophyta, and purple is Cyanobacteria.

Table 3.
Species diversity of marine floral groups in countries bordering the South China Sea (East Vietnam Sea). Data based on AlgaeBase []. * References for seagrasses (Alismatales).
Biodiverisity numbers should nevertheless be interpreted cautiously as they may under-represent the actual floral diversity of each country and the region, since they are for the most part established on morphological-based identification, and additionally, some countries have received much lesser attention than others (e.g., Brunei, Cambodia, and Malaysia). Notwithstanding, the high floral biodiversity in the South China Sea documented so far from Vietnam can be attributed to its geographical location, situated along the southeastern margin of the Indo-Chinese Peninsula, comprising the largest area of the peninsula and the longest coastline in the South China Sea.
We examined the similarity of the marine floras in seven of the states and territories bordering the South China Sea using a Bray–Curtis similarity index [] multivariate analysis implemented in Primer V.6 software [] based on compiled data for the region. Results showed that the Vietnamese marine flora was most similar to that of China, followed by those of Indonesia and the Philippines (Figure 5), and that the marine floras of Malaysia, Singapore, and Thailand were very similar (Figure 5), consistent with previous findings by Phang et al. [].
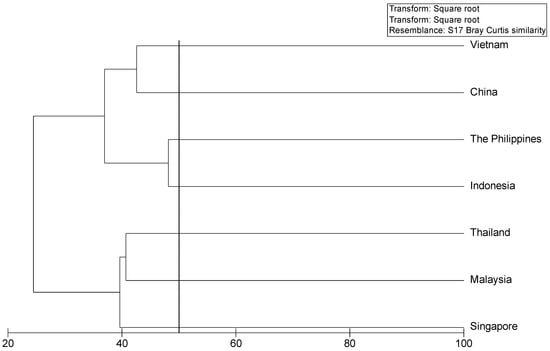
Figure 5.
Cluster analyses of similarity (Bray–Curtis index) for the marine floras of the countries bordering the South China Sea. Data for similarity analyses range between 20 and 100. Hierarchical clustering is based on square-root-transformed presence data and on a resemblance matrix calculated using S17 Bray–Curtis similarity.
4.2. Marine Floral Biodiversity across Vietnam Regions
The Vietnamese coastline is divided into three “Geographical Regions” (Northern Vietnam, Central Vietnam, Southern Vietnam) subdivided into eight “Administrative Regions” (Northeast, Northwest, Red River Delta, North Central Coast, South Central Coast, Central Highlands, Southeast, Mekong River Delta) (Figure 6A). Based on our updated checklist, we show marine floral biodiversity across the “Administrative Regions”, excluding the Northwest and Central Highlands regions, which have no coastline. The geographical distribution of the marine floral biodiversity is uneven across Vietnamese regions. The South Central Coast holds the highest diversity by far, with a total of 587 species, followed by the Southeast (243), Red River Delta (210), North Central Coast (204), and Mekong River Delta (203) regions. The Northeast region is the least species region with 160 species. The high marine floral biodiversity documented in the South Central Coast coincides with the largest coral reef density along the coastline of Vietnam (Figure 6B) and a high diversity of marine environments []. It is worth mentioning that Spalding [] proposed five marine ecoregions (Gulf of Thailand, Gulf of Tonkin, Southern Vietnam, Sunda Shelf/Java Sea, South China Sea (East Vietnam Sea) Ocean Islands) along the Vietnamese coastline (Figure 6A).
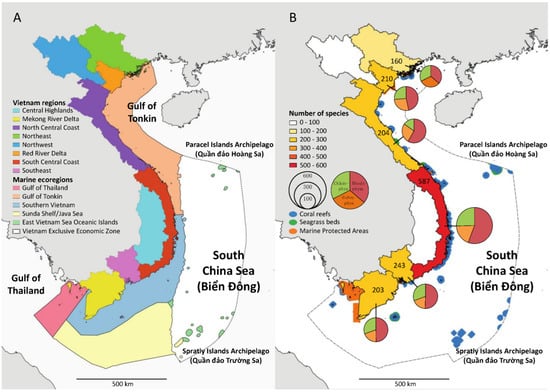
Figure 6.
Spatial variation in marine floral biodiversity across Vietnamese regions. (A) Illustration of the Vietnamese administrative regions and the marine ecoregions sensu Spalding et al. [] within the Vietnamese Exclusive Economic Zone (VEEZ). (B) Representation of species diversity across marine macroalgal classes (Cyanobacteria, Rhodophyta, Ochrophyta, and Chlorophyta) and depiction of coral reefs, seagrass beds, and marine protected area distributions within the VEEZ; numbers in each region represent the total number of species.
According to present data, with only 45 endemic species, Vietnam would seem to contain a very low level of endemism of marine flora (5.01%, 45 spp.; Table 4). However, this number most likely under-represents the actual level of endemism for the marine flora of Vietnam, since molecular-assisted alpha taxonomic efforts, needed to obtain accurate taxonomic data, have been very limited.

Table 4.
Endemic marine algae species from Vietnam.
4.3. Seaweed Biodiversity Loss, Threats, and Conservation
Some species of marine algae in Vietnam have experienced declines in their populations due to a variety of factors. Several threats to marine algae exist in Vietnam, including but not restricted to pollution, climate change, overharvesting, invasive species, and habitat destruction. Marine algae are vulnerable to pollution from a variety of sources, including agricultural runoff, industrial discharge, and sewage. Pollution can harm marine algae directly and also make their habitat less suitable for growth []. Marine algae are sensitive to changes in temperature, salinity, and other environmental conditions, and they may be negatively impacted by climate change []. Some species of marine algae are in high demand for use in food, cosmetics, and other products, and overharvesting can lead to a decline in their populations []. Non-native species of marine algae that are introduced to new areas can outcompete native species and reduce their populations []. Marine algae rely on specific types of habitat for growth, and the destruction of these habitats can negatively impact their populations []. The biodiversity (marine and terrestrial) of Vietnam has decreased quickly []. Some of the known factors in Vietnam are land conversion without a proper scientific base, quick reduction of natural forests, infrastructure developments (e.g., dams, roads, and new urban and rural human settlements), and overexploitation of natural resource/illegal exploitations in fishing, hunting, forestry [,,]. It is difficult to quantify the extent of marine algal diversity loss in Vietnam, as there are limited data available on this topic. Titlyanov et al. [] quantified seaweed community changes in Nha Trang Bay and investigated the factors associated with these changes. Collections sampled between 1953 and 1968 and 1982 and 1987 did not change significantly in either the species diversity nor the floristic composition. However, the species composition assessed between 2002 and 2010 showed changes in the species diversity composition, with an increase inf Chlorophyta and a reduction of Rhodophyta and Ochrophyta species. In Con Dao Island, significant changes in marine floral species composition were observed between 1998 and 2008, with a proportional species replacement in each taxonomic group over the last two decades []. Since the 1970s, several species have not been observed, such as Erythrocladia irregularis Rosenvinge, Acrochaetium crassipes (Børgesen) Børgesen, Metagoniolithon stelliferum (Lamarck) Ducker, and Exophyllum wentii Weber Bosse []. Similarly, the species diversity of Sargassum was previously well studied at Nha Trang Bay, with the identification of 21 species between 1950 and 1970. Between 1980 and 2000, 9 of the previously identified species were not recorded, while an additional 15 species were newly added to Sargassum. However, based on the most recent collection, in 2020 in Nha Trang Bay, Sargassum was represented by 14 species, including 7 species found in the previous two surverys and 7 new additions. Overall, 24% (149 species) of algal species in South Vietnam recorded between 1980 and 2000 could not be found between 2000 and 2020 []. A report by Vy et al. [] indicated that nearly 50% of the Sargassum beds at Hon Chong (Khanh Hoa Province) have disappeared because of loss of substratum, and the species Sargassum crassifolium, once a dominant species in this site, disappeared. In Nha Trang Bay, seawater pollution resulting from dissolved organic and inorganic compounds of nitrogen and phosphorus may lead to an increasing larger number of green algae and their biomass as well as population density. The green algae may displace fleshy and foliose forms of red and brown macroalgae from communities []. Another threat to seaweed biodiversity is harvesting of natural stocks. Local harvesters collect large quantities of Sargassum for production of alginates, Asian herbal medicine, and various human foods []. Young populations of Sargassum are commonly harvested prior to reaching sexual maturity and reproduction, thus affecting natural stock renewal (authors’ pers. obs.). Another case of overexploitation of natural stocks was reported in the edible red seaweed Betaphycus gelatinus, now very rare due to harvesting by locals at Ninh Thuan Province. The Vietnam Red data Book [] shows 8 and 5 species of Rhodophyta and Ochrophyta, respectively. Among them, Crytonemia undulata is in the critically endangered category. Six species including 5 Rhodophyta and 3 Ochrophyta are in the endangered category. The 6 remaining species are in the vulnerable category. There are 12 marine protected areas (MPAs) from 10 provinces/cites in Vietnam. Large seaweed beds in Khanh Hoa, Ninh Thuan, are out of the core zone of MPAs and therefore under threat. The natural stock of Hydropuntia eucheumatoides, Betaphycus gelatinus, and Sargassum spp. (endangered category) is still collected by local people due to lack of Red Data Book. Like seagrasses, the management models of marine macroalgal ecosystems in Vietnam are mostly integrated into coastal management models to solve the problems of weaknesses that exist in the management, exploitation, and use of natural resources and environmental protection in coastal areas.
5. Conservation Efforts
Conservation efforts are needed to protect and conserve marine floral diversity in Vietnam. It is important to address these threats in order to maintain the health and resilience of Vietnam marine environments and the economic and cultural value of these resources. This may involve measures such as habitat conservation, sustainable harvesting, pollution reduction, and invasive species management. There are several conservation efforts underway in Vietnam to protect the marine flora and the marine environments they are a part of. Some of these efforts are reviewed below.
5.1. Habitat Conservation
Many conservation efforts in Vietnam focus on protecting and preserving the habitats that support marine flora. This may involve establishing marine protected areas (MPAs) or other types of conservation zones, which are designated areas of the ocean that are set aside for the protection and conservation of marine life. Recognizing the importance of marine protected areas in the protection of marine biodiversity, the prime minister released Decision No. 742/QD-TTg on 26 May 2010, authorizing the preparation for the marine biodiversity scheme. Marine conservation in Vietnam will continue until 2020 (this deadline has been extended), with the aim of preserving habitats and marine species of economic and scientific importance. It aimed to contribute to the development of marine economy and improve the livelihoods of fishermen communities in coastal localities. The Fisheries Law of 2017 was passed by the 14th National Assembly, which includes provisions for the protection and growth of aquatic resources, including marine conservation, in the sense of sustainable fisheries development and international integration. The Communist Party of Vietnam’s Central Committee released Resolution No. 36-NQ/TW on the Strategy for Sustainable Development of Vietnam’s Marine Economy to 2030 with a Vision to 2045 on 22 October 2018. The document stated on the matter: “Sustainable development of the marine economy on the basis of green growth, biodiversity protection, and marine environment conservation; ensure harmony between economic and natural environments, conservation and development, promoting the sea’s potentials and advantages, and creating a driving force for national economic development”; and that the specific target was to “Well maintain and protect aquatic, coastal, and island ecosystems; raise the area of marine and coastal protected areas to at least 6% of the national marine area”. Currently, the Ministry of Agriculture and Rural Development and other cities have created and operationalized 12 marine protected areas (Figure 4B, Table 5). These 12 MPAs amount to a total of 243,023 ha (ca. 2430 km2), which corresponds to ca. 0.17% of the total surface (ca. 1,395,096 km2) of the Vietnamese Marine Exclusive Economic Zone. Among the 12 MPAs, 3 typically contain seaweeds and 9 seagrasses.

Table 5.
Checklist MPAs in Vietnam.
Moreover, there are other nature reserves along the coast of Vietnam [] (Table 6). The Ministry of Agriculture and Rural Development has developed comprehensive plans for the establishment of four MPAs, which have been submitted to provincial people’s committees for approval: Hon Me/Thanh Hoa, Nam Yet/Khanh Hoa, Phu Quy/Binh Thuan, and Hai Van–Son Cha/Da Nang–Hue.

Table 6.
Nature reserves and national parks in Vietnam.
Despite the fact that the Ministry of Agriculture and Rural Development has organized a mission to inspect, guide, and have several documents to direct and inform, the provincial people’s committees have not yet approved the establishment after more than 5 years of handover of Vietnam’s coastal area has high biodiversity: 13 out of 28 national parks, 22 out of 55 nature reserves, and 17 out of 34 forests of cultural, historical, and environmental significance are located in coastal areas and islands.
5.2. Sustainable Harvesting
Some species of seaweed in Vietnam are harvested for use in food, cosmetics, and other products. In order to ensure the sustainable use of these resources, there are efforts to establish sustainable harvesting practices and to manage fisheries to ensure that seaweed populations are not overharvested. Vietnam adopted international standards of the sanitary and phytosanitary (SPS) agreement–based regulation, which includes seaweeds. This established regulation covers a wide range of standards, including ensuring that the seaweeds are disease-, pathogen-, toxin-free, and furthermore that seaweeds meet permissible levels for heavy metals and other contaminants (e.g., pesticides). Vietnam has national regulations for controlling the movement of aquatic aquaculture organisms (quarantine), which also includes the import of live seaweed. For example, Vietnam provided a technical guideline for importing live seaweed, e.g., Gracilaria species [], and technical requirements for Kappaphycus alvarezii (Table 7).

Table 7.
Biosecurity components adopted in the national seaweed policies and regulations in the main Gracilaria and Kappaphycus alvarezii production.
5.3. Pollution Reduction
Seaweeds are vulnerable to pollution from a variety of sources, and efforts are being made to reduce pollution in Vietnam’s coastal waters in order to protect these ecosystems. This may involve measures such as improving wastewater treatment, regulating industrial discharge, and reducing agricultural runoff. However, no particular reports and regulations were found on pollution reduction in Vietnam’s coastal waters.
5.4. Invasive Species Management
Non-native species of seaweed that are introduced to new areas can outcompete native species and reduce their populations. To address this threat, efforts are being made to control the spread of invasive seaweed species in Vietnam. Circular No. 35/2018/TT-BTNMT dated 28 December 2018 of MONRE stipulates the criteria for the identification and promulgation of a list of invasive alien species. However, the subject only focuses on species that have been announced under the guidance of Circular 35, and the assessed ecosystems are only terrestrial and aquatic. There is almost no information about groups of marine organisms, including seaweed and seagrasses, more specifically, foreign species that are invasive in the sea and by shipping route; there has not been a specific study in Vietnam. In order to prevent the entry of alien organisms in the ballast water environment transported by ships from other sea areas, affecting the ecosystem, economy, and human health and strengthening measures to protect the marine environment, IMO ratified the BWM Convention on 13 February 2004, and the convention met the conditions to enter into force on 8 September 2017. By 8 September 2024, all ships are required to use a ballast water management system (D2). Vietnam is in the process of completing the procedures to join the convention. The basic legal documents related to the activities of dumping garbage and discharging wastewater and ballast water are specified in Article 117 of Decree 58/2017/ND-CP guiding the Vietnam Maritime Code on the management of cargo operations [].
6. Conclusions: Challenges and Future Directions
Studies conducted in the last decade effectively illustrated the need to combine molecular tools with morphological observations (i.e., habit view, vegetative and reproductive morphology) in (1) the reassessment of marine floral species diversity, (2) previous species names’ validation, (3) misidentification detection, and (4) new species discovery. However, the fairly poor number (15 taxa; 25.4% of the new records and species) of new records and species made over the last 10 years in a “mega-diverse” country raised worrying concerns on the efforts put into the study of marine floral biodiversity. Past molecular-assisted taxonomic efforts have been focused on a limited number of taxa and localities. Currently, three main institutions, including (1) the Institute of Marine Environment and Resources in the North, (2) the Institute of Oceanography in the Central, and (3) the Institute of Tropical Biology in the South, are conducting most studies on marine algae taxonomy nationally. Considering the important length of the Vietnamese coastline (>3200 km), an exhaustive exploration of the Vietnamese marine flora represents a Herculean task for these institutions alone. In addition, the limited number of algal taxonomists in Vietnam and limited funding availability represent a major challenge to the study of marine floral biodiversity. Methodical molecular-assisted re-examination of Vietnam marine floral biodiversity is urgently needed in order to get an accurate picture of biodiversity and endemism, and thereby obtain baseline data for the marine floral management and protection. In particular, future efforts will need to be directed towards specific taxa and regions of Vietnam. Data provided in this review on species diversity, groups targeted with molecular-assisted alpha taxonomic approaches, and spatial variation in biodiversity offer valuable data to orientate future efforts. Finally, a more in-depth investigation of the threats targeting the marine flora of Vietnam is needed, and urgent implementation of measures for its conservation is called for, in particular, the increase in marine protected areas across Vietnam, which represent now less than 1% of the Vietnamese Marine Exclusive Economic Zone.
Author Contributions
Conceptualization, C.V., X.-V.N. and M.-L.N.; methodology, M.-L.N., C.V. and X.-V.N.; software, M.-L.N. and C.V.; validation, C.V., M.-L.N. and X.-V.N.; formal analysis, M.-L.N. and C.V.; investigation, M.-L.N., X.-V.N. and C.V.; resources, M.-L.N.; data curation, M.-L.N., M.-S.K., N.-T.N.N., X.-T.N., V.-L.C., X.-V.N. and C.V.; writing—original draft preparation, C.V., M.-L.N. and X.-V.N.; writing—review and editing, C.V., X.-V.N. and M.-L.N.; visualization, C.V., X.-V.N. and M.-L.N.; supervision, C.V. and X.-V.N.; project administration, C.V., X.-V.N. and M.-S.K.; funding acquisition, V.-L.C., X.-V.N., C.V. and M.-S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Vietnam Academy of Science and Technology, grant number VAST06.01/22-23; TĐĐTB0.04/21-23. This research was supported by the Basic Science Research Program (2019R1A6A1A10072987) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article is part of the results of a PhD program at the Graduate University of Science and Technology so we are deeply indebted to Graduate University of Science and Technology, Vietnam Academy of Science and Technology (VAST), Ha Noi, Vietnam; and all staff at the Institute of Marine Environment and Resources, VAST, Hai Phong, Vietnam. We thank project ĐL000.01/23-24; QTRU04.03/18-19; QTRU02.08/21-22 of Vietnam Academy of Science and Technology for sharing information; Vingroup Innovation Foundation—VinIF grant number VINIF.2022.DA00079 and JSPS CREPSUM collaborative research and education project in Southeast Asia for sustainable use of marine ecosystems. This paper is a contribution to celebrate the 65th anniversary of the Institute of Marine Environment and Resources, Hai Phong, VAST.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoeksema, B.W. Delineation of the Indo-Malayan Centre of Maximum Marine Biodiversity: The Coral Triangle. In Biogeography, Time, and Place: Distributions, Barriers, and Islands; Renema, W., Ed.; Springer: Dordrecht, The Netherlands, 2007; Volume 29, pp. 117–178. ISBN 978-1-4020-6374-9. [Google Scholar]
- Do Cong, T. Research Results of Marine Biodiversity and Biological Resources in Vietnam: Current Status, Threats, Proposed Solutions for Sustainable Use. Vietnam J. Mar. Sci. Technol. 2022, 22, 1–20. [Google Scholar] [CrossRef]
- Nhung, N.; Armstrong, C.W.; Kim Anh, N.T.; Ngoc, Q.T.K.; Hai Anh, N. Incorporating Fisheries Management into Biodiversity Conservation Policies to Enhance Effectiveness of MPAs: A Case Study in Cu Lao Cham MPA, Vietnam. Fish People 2011, 9, 39–49. [Google Scholar]
- Groombridge, B.; Jenkins, M.D. World Atlas of Biodiversity Earth’s Living Resources; University of California Press: Berkeley, CA, USA, 2002; ISBN 0520236688. [Google Scholar]
- Larsen, P.B. Linking Livelihoods and Protected Area Conservation in Vietnam:Phong Nha Kẻ Bàng World Heritage, Local Futures? In People, Protected Areas, and Global Change: Participatory Conservation in Latin America, Africa, Asia and Europe; Galvin, M., Haller, T., Larsen, P.B., Eds.; NCCR North-South: Bern, Switzerland, 2008; Volume 3, pp. 431–470. ISBN 978-3-905835-06-9. [Google Scholar]
- Ziegler, T.; Vu, T.N. Ten Years of Herpetodiversity Research in Phong Nha—Ke Bang National Park, Central Vietnam. In Phong Nha—Ke Bang National Park and Cologne Zoo, 10 Years of Cooperation (1999–2009); Phong Nha–Ke Bang National Park and Cologne Zoo: Laos, Vietnam, 2009; pp. 103–124. [Google Scholar]
- Hung, N.Q.; Chieu, H.D.; Dung, D.T.; Son, L.T.; Duc, V.T.; Duy, D.A. Climate Change Impacts on Marine Ecosystems in Vietnam. In Environmental Management of Marine Ecosystems; CRC Press: Boca Raton, FL, USA, 2018; pp. 209–235. ISBN 9781315153933. [Google Scholar]
- Chavez, F.P.; Messié, M.; Pennington, J.T. Marine Primary Production in Relation to Climate Variability and Change. Annu. Rev. Mar. Sci. 2011, 3, 227–260. [Google Scholar] [CrossRef]
- de Loureiro, J. Flora Cochinchinensi: Sistens Plantas in Regno Cochinchina Nascentes: Quibus Accedunt Aliae Observatae in Sinensi Imperio, Africa Orientali, Indiaeque Locis Variis: Omnes Dispositae Secundum Systema Sexuale Linnaeanum; Typis, et expensis Academicis: Ulyssipone; 1790; Volume 1. [Google Scholar] [CrossRef]
- Georg, A.J. Species, Genera et Ordines Algarum: Seu Descriptiones Succinctae Specierum, Generum et Rodinum, Quibus Algarum Regnum Constituitur, Lundae, apud C.W.K. Gleerum, 1848–1901; BHL: Washington, DC, USA, 1851; Volume 2. [Google Scholar]
- S., F.A.; Agardh, J.G. Species Genera et Ordines Algarum. Taxon 1978, 27, 101. [Google Scholar] [CrossRef]
- Toni, G.B.d.; De’ Toni, E.; Boston Society of Natural History. Sylloge Algarum Omnium Hucusque Cognitarum; Patavii, Sumptibus auctoris, 1889–1924; 1895; Volume 1. [Google Scholar] [CrossRef]
- De Toni, G.B. Sylloge Algarum Omnium Hucusque Cognitarum. Volume IV. Florideae. Sectio IV.Pdf. Patavii 1905, 4, 476. [Google Scholar]
- Ho, P.H. Vietnam Seaweed-Southern Part; Ministry of Education and Youth, Saigon Learning Resource Center: Ho Chi Minh City, Vietnam, 1969. [Google Scholar]
- Peñailillo Núñez, T.C.; Medina Valmaseda, A.A.; Pomponi, S.A.; Rodríguez, S.; Ross, N.A.; Aagaard, J.; Aagaard, J.; Aahangarzadeh, M.; Aahmadi, B.; Aakbarinasab, M.; et al. Campagnes Du «De Lanessan» (1925–1929): Liste Des Stations; Gouvernement Général de L’Indochine: New Delhi, India, 1936; Volume 17. [Google Scholar]
- Dawson, E.Y. Marine Plants in the Vicinity of the Institute Oceanographique de Nhatrang, Vietnam. Pac. Sc. 1954, 8, 373–481. [Google Scholar]
- Dinh, N.H.; Nang, H.Q.; But, T.N.; Van Tien, N. Vietnam Seaweed—Northern Part; Hanoi Science and Technology Publishing: Ha Noi, Vietnam, 1993. [Google Scholar]
- Dai, N.H. Sargassum (Sargassacea) Vietnam; Agriculture Publishing House: Ho Chi Minh City, Vietnam, 1997. [Google Scholar]
- Nang, H.Q. Current Status and Economic Resources of Seaweed in the South Coast of Vietnam; ELMAR: Cam Giang District, Vietnam, 1999. [Google Scholar]
- Van Tu, N.; Boo, S.M. Distribution Patterns and Biogeography of Sargassum (Fucales, Phaeophyceae) along the Coast of Vietnam. Bot. Mar. 2020, 63, 463–468. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Dinh, N.H. Taxonomic Studies of the Halymeniaceae (Halymeniales, Rhodophyta) from Vietnam, I. A Transfer of Carpopeltis Formosana Okamura to Prionitis. Bot. Mar. 1998, 41, 391–397. [Google Scholar] [CrossRef]
- Kawaguchi, S. Morphology of Halymenia Maculata J. Agardh from Vietnam. Section V. Halymenia Species. In Taxonomy of Economic Seaweeds with Reference to Some Pacific Species; University of California: California, CA, USA, 2002; pp. 259–266. [Google Scholar]
- Dinh, N.H. Vietnamese Species of Gracilaria and Gracilariopsis. In Species, Taxonomy of Economic Seaweeds with Reference to Some Pacific Species; Abbott, I.A., Ed.; California Sea Grant College Program: California, CA, USA, 1992; pp. 207–210. [Google Scholar]
- Hau, L.N.; Lin, S. Gracilariopsis nhatrangensis (Gracilariaceae, Rhodophyta), a New Marine Red Alga from Nhatrang, Southern Vietnam. Bot. Stud. 2006, 47, 329–337. [Google Scholar]
- Dai, N.H.; Tri, P.H. Some New Records of Marine Algae From Vietnam. Collect. Mar. Res. Work. 2002, Volume XII, 149–158. [Google Scholar]
- Dinh, N.H.; Nang, H.Q. Species of Eucheuma and Kappaphycus in Vietnam. In Proceedings of the Taxonomy of Economic Seaweeds with Reference to Some Pacific Species; Abbott, I.A., Ed.; California Sea Grant College Program: California, CA, USA, 1995; pp. 43–51. [Google Scholar]
- Abbott, I.A.; Fisher, J.; McDermid, K.J. Newly Reported and Revised Marine Algae from the Vicinity of Nha Trang, Vietnam. Taxon. Econ. Seaweeds Ref. Some Pac. Species 2002, 8, 291–321. [Google Scholar]
- Masuda, M.; Kawaguchi, S.; Takahashi, Y.; Matsuo, Y.; Susuki, M. A Taxonomic Study of the Genus Laurencia (Ceramiales, Rhodophyta) from Vietnam. I. Laurencia Caduciramulosa Masunda et Kawaguchi, Sp. Nov. Cryptogam. Algol. 1997, 18, 1–10. [Google Scholar]
- Masuda, M.; Takahashi, Y.; Matsuo, Y.; Suzuki, M. A Taxonomic Study of the Genus Laurencia (Ceramiales, Rhodophyta) from Vietnam. II. Laurencia lageniformis Sp. Nov. Cryptogam. Algol. 1997, 18, 163–174. [Google Scholar]
- Dam, D.T.; Nguyen, V.T. Species Composition and Distribution of Seaweed in Truong Sa Island-Spratly Islands. Collect. Mar. Res. Work. 1996, 3, 243–252. [Google Scholar]
- Tri, P.H. Contributing to the Study of Seaweed in Truong Sa Archipelago. Collect. Mar. Res. Work. 1996, 147–162. [Google Scholar]
- Tri, P.H. Marine Macroalgae Resources at Son Ca and Song Tu Tay Island—Spratly Islands. Collect. Mar. Res. Work. 1999, 205–215. [Google Scholar]
- Hau, L.N. Notes on Some New Records of Marine Algae from Truong Sa Islands (Vietnam). Collect. Mar. Res. Work. 2001, 11, 115–120. [Google Scholar]
- Tien, D.D.; Cuong, D.H. Species Composition and Distribution of Seaweeds From Some Small Islands (Nam Yet, Son Ca, Song Tu Tay, Sinh Ton) of Truong Sa Archipelago. J. Mar. Sci. Technol. 2016, 16, 297–305. [Google Scholar] [CrossRef]
- Dai, N.H.; Tri, P.H. Seaweed Resources of Ly Son Island. Collect. Mar. Res. Work. 2001, 11, 121–134. [Google Scholar]
- Dai, N.H.; Tri, P.H.; Vy, N.X. Species Composition and Distribution of Seaweed, Segrass in Phu Quy Island (Cu Lao Thu), Binh Thuan Provine. Collect. Mar. Res. Work. 2009, 225–243. [Google Scholar]
- Tien, D.D. Seaweed Resources of Hai Van—Son Cha. In Proceedings of the Scientific, Technological and Economic Conference Serving the Cause of Industrialization and Modernization of the Country; Vietnam Union of Science and Technology Associations: Ha Noi, Vietnam, 2006; pp. 164–169. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S. Checklist of the Marine Flora of Nha Trang Bay (Vietnam, South China Sea) and Decadal Changes in the Species Diversity Composition between 1953 and 2010. Bot. Mar. 2015, 58, 367–377. [Google Scholar] [CrossRef]
- Hau, L.N. Notes on Some New Species of Marine Algae from Ninh Thuan Province (South Vietnam). Collect. Mar. Res. Work. 2000, Volume X, 141–148. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V.; Belous, O.S.; Pham, T.H.; Dam, D.T. An Inventory and Decadal Changes of the Benthic Marine Flora on the Con Dao Islands, South China Sea, Vietnam. Russ. J. Mar. Biol. 2020, 46, 166–180. [Google Scholar] [CrossRef]
- Belous, O.S.; Titlyanov, E.A.; Titlyanova, T.V. Decadal Comparison (1950–2020) of Benthic Marine Flora from Central and Southern Vietnam; 2021; Volume 521, ISBN ISBN 0000000329. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Le, N.H.; Lin, S.M.; Steen, F.; De Clerck, O. Checklist of the Marine Macroalgae of Vietnam. Bot. Mar. 2013, 56, 207–227. [Google Scholar] [CrossRef]
- Tien, N.V.; Thanh, D.N.; Dai, N.H. Seagrasses in Viet Nam; Ha Noi Science and Technology Publishing House: Ha Noi, Vietnam, 2002. [Google Scholar]
- Vy, N.X.; Holzmeyer, L.; Papenbrock, J. New Record of the Seagrass Species Halophila major (Zoll.) Miquel in Vietnam: Evidence from Leaf Morphology and ITS Analysis. Bot. Mar. 2013, 56, 313–321. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Lau, V.-K.; Nguyen-Nhat, N.-T.; Nguyen, T.-H.; Phan, K.-H.; Dao, V.-H.; Ho-Dinh, D.; Hayashizaki, K.; Fortes, M.D.; Papenbrock, J. Update of Seagrass Cover and Species Diversity in Southern Viet Nam Using Remote Sensing Data and Molecular Analyses. Reg. Stud. Mar. Sci. 2021, 44, 101803. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G. M AlgaeBase. World-Wide Electronic Publication. National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 6 January 2023).
- Duy, D.A. Research on Seaweed Resources and Biodiversity in Nam Du Archipelago, Vietnam. Ph.D. Thesis, Research Institute for Marine Fisheries, 2022. [Google Scholar]
- Hau, L.N.; Ly, B.M.; Huynh, T.V.; Trung, V.T. Some New Records of Marine Algae From Vietnam. J. Mar. Sci. Technol. 2015, 15, 35–44. [Google Scholar] [CrossRef]
- Le, H.N.; Muangmai, N.; Kheauthong, S.; Sun, Z.; Zuccarello, G.C. Gracilaria phuquocensis Sp. Nov., a New Flattened Gracilaria Species (Gracilariales, Rhodophyta), Previously Recognized as G. mammillaris, from the Southern Coast of Vietnam. Phycol. Res. 2020, 68, 50–56. [Google Scholar] [CrossRef]
- Vy, N.X.; Thuy, N.N.N.; Thuy, N.X.; Hieu, N.T.; Thanh, T.S.H.; Ha, D.V.; Duy, D.A.; McDermid, K.J. New Record of Halymenia malaysiana (Halymeniaceae, Rhodophyta) from Viet Nam, and Its Genetic Diversity in the Western Pacific. Bot. Mar. 2023, 66, 113–123. [Google Scholar] [CrossRef]
- Binh, D.T.; An, K.T.; Cam, V.H.; Tuan, T.V. New Records and Evolutionary Relationship of Epiphyte (Melanothamnus thailandicus) on Red Algae (Kappaphycus alvarezii) in Khanh Hoa. J. Fish Sci. Technol. 2020, 2, 2–10. [Google Scholar]
- Vy, N.X.; Thuy, N.X.; Ronald, P.K.I.; McDermid, K.J. Meristotheca lysonensis sp. nov. (Solieriaceae, Rhodophyta), a New Flattened Species from Vietnamese Waters. Phytotaxa 2022, 574, 137–148. [Google Scholar] [CrossRef]
- Boo, G.H.; Van Nguyen, T.; Kim, J.Y.; Gall, L.L.; Rico, J.M.; Bottalico, A.; Boo, S.M. A Revised Classification of the Gelidiellaceae (Rhodophyta) with Descriptions of Three New Genera: Huismaniella, Millerella and Perronella. Taxon 2016, 65, 965–979. [Google Scholar] [CrossRef]
- Zhao, W.; Dong, L.; Hong, D.D.; Brodie, J.; Chen, W.-Z.; Tien, D.D.; Zhou, W.; Lu, Q.-Q.; Zhang, M.-R.; Yang, L.-E. Haplotype Networks of Phycocalidia Tanegashimensis (Bangiales, Rhodophyta) Indicate a Probable Invasion from the South China Sea to Brazil. Mar. Biodivers. 2021, 51, 33. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Nguyen-Nhat, N.-T.; Nguyen, X.-T.T.; Nguyen, M.-N.T.; Dao, V.-H.; McDermid, K.J. Three New Records of Marine Macroalgae from Viet Nam Based on Morphological Observations and Molecular Analyses. Pac. Sci. 2021, 75, 497–512. [Google Scholar] [CrossRef]
- Nguyen, X.V.; Nguyen, T.H.; Dao, V.H.; Liao, L. New Record of Grateloupia taiwanensis S.-M. Lin et H.-Y. Liang in Vietnam: Evidence of Morphological Observation and RbcL Sequence Analysis. Biodiversitas 2019, 20, 688–695. [Google Scholar] [CrossRef]
- Boo, G.H.; Leliaert, F.; Le Gall, L.; Coppejans, E.; De Clerck, O.; Van Nguyen, T.; Payri, C.E.; Miller, K.A.; Yoon, H.S. Ancient Tethyan Vicariance and Long-Distance Dispersal Drive Global Diversification and Cryptic Speciation in the Red Seaweed Pterocladiella. Front. Plant Sci. 2022, 13, 849476. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Nhat, N.T.; Dao, V.H.; Nguyen, X.V. New Record of the Rare Brown Alga Dictyota hauckiana from Vietnam. Bot. Mar. 2019, 30, 599–603. [Google Scholar] [CrossRef]
- Van Tu, N.; Trang, L.T.; Phuong Thao, T.T. Diversity and Distribution of the Brown Macroalgae (Phaeophyceae Kjellman, 1891) in Cham Islands, Quang Nam Province, Vietnam. Acad. J. Biol. 2021, 43, 37–45. [Google Scholar] [CrossRef]
- Sun, Z.; Dao, M.D.; Tran, Q.T.; Dam, D.T. A New Species Lobophora tsengii sp. nov. (Dictyotales; Phaeophyceae ) from Bach Long Vy (Bailongwei) Island. J. Oceanol. Limnol. 2021, 39, 2363–2369. [Google Scholar] [CrossRef]
- Tran, L.T.; Leliaert, F.; Vieira, C.; Tran, T.V.; Nguyen, T.V.; Dam, T.D.; De Clerck, O. Molecular Assessment of Ulva (Ulvales, Chlorophyta) Diversity in Vietnam Including the New Species U. vietnamensis. Phycol. Res. 2022, 71, 3–24. [Google Scholar] [CrossRef]
- Nguyet, P.M.; Tan, H.T.W.; Mitrovic, S.; Yeo, H.H.T. A Checklist of the Algae of Singapore; Raffles Museum of Biodiversity Research: Singapore, 2011; ISBN 9789810701833. [Google Scholar]
- Phang, S.M.; Yeong, H.Y.; Ganzon-Fortes, E.T.; Lewmanomont, K.; Prathep, A.; Hau, L.N.; Gerung, G.S.; Tan, K.S. Marine Algae of the South China Sea Bordered by Indonesia, Malaysia, Philippines, Singapore, Thailand and Vietnam. Raffles Bull. Zool. 2016, 2016, 13–59. [Google Scholar] [CrossRef]
- Kristiansen, J.; Lind, J.F. On the Taxonomic Relation between Synura curtispina and S. favus (Synurophyceae). Nord. J. Bot. 1995, 15, 443–448. [Google Scholar] [CrossRef]
- Tanaka, T.; Ho, P.H. Notes on Some Marine Algae from Viet Nam—I. Mem. Fac. Fish. 1962, 11, 23–40. [Google Scholar]
- Garbary, D.J. The Acrochaetiaceae (Rhodophyta): An Annotated Bibliography; Koeltz Botanical Books: Darmstadt, Germany, 1987; Volume 77, ISBN 978-3-443-60004-4. [Google Scholar]
- Tsutsui, I.; Nang, H.Q.; Dinh, N.H.; Airai, S.; Yoshida, T. The Common Marine Plants of Southern Vietnam. Umi no Kenkyu (Oceanography in Japan) 2006, 15, 299. [Google Scholar]
- Titlyanov, E.A.; Titlyanova, T.V. Marine Plants of the Asian Pacific Region Countries, Their Use and Cultivation; Dalnauka: Vladivostok, Russia, 2012. [Google Scholar]
- Sutherland, J.E.; Lindstrom, S.C.; Nelson, W.A.; Brodie, J.; Lynch, M.D.J.; Hwang, M.S.; Choi, H.-G.; Miyata, M.; Kikuchi, N.; Oliveira, M.C.; et al. A New Look at an Ancient Order: Generic Revision of the Bangiales (Rhodophyta). J. Phycol. 2011, 47, 1131–1151. [Google Scholar] [CrossRef]
- Gropman, A.L. Neuroimaging in Mitochondrial Disorders. NeuroTherapeutics 2013, 10, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ní Chualáin, F.; Maggs, C.A.; Saunders, G.W.; Guiry, M.D. The Invasive Genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): Molecular Systematics, Morphology, and Ecophysiology of Falkenbergia Isolates. J. Phycol. 2004, 40, 1112–1126. [Google Scholar] [CrossRef]
- Titlyanov, E.A.; Kiyashko, S.I.; Titlyanova, T.V.; Huyen, P.V.; Yakovleva, I.M. Identifying Nitrogen Sources for Macroalgal Growth in Variously Polluted Coastal Areas of Southern Vietnam. Bot. Mar. 2011, 54, 367–376. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Kamiya, M.; Ootsuki, R.; De Goër, S.L.; Pedroche, F.F.; West, J.A. New Records of Red Algae from Mangroves in El Salvador and Pacific Mexico, Combining Culture and Molecular Observations. Bot. Mar. 2012, 55, 101–111. [Google Scholar] [CrossRef]
- Wynne, M. The Red Algal Families Delesseriaceae and Sarcomeniaceae; Koeltz Scientific Books: Königstein, Germany, 2014; ISBN 978-3-87429-443-0. [Google Scholar]
- Yeurt, A.D.R.N. A Revision of Amansia glomerata C. Agardh, Amansia rhodantha (Harvey) J. Agardh and Melanamansia glomerata (C. Agardh) R. E. Norris (Rhodophyta: Rhodomelaceae). Bot. Mar. 2002, 45, 231–242. [Google Scholar] [CrossRef]
- Boo, G.H.; Qiu, Y.-X.; Kim, J.Y.; Ang, P.O.J.; Bosch, S.; De Clerck, O.; He, P.; Higa, A.; Huang, B.; Kogame, K.; et al. Contrasting Patterns of Genetic Structure and Phylogeography in the Marine Agarophytes Gelidiophycus divaricatus and G. freshwateri (Gelidiales, Rhodophyta) from East Asia. J. Phycol. 2019, 55, 1319–1334. [Google Scholar] [CrossRef] [PubMed]
- Santelices, B. Parviphycus, a New Genus in the Gelidiellaceae (Gelidiales, Rhodophyta). Cryptogam. Algol. 2004, 25, 313–326. [Google Scholar]
- Kawaguchi, S.; Shimada, S.; Wang, H.W.; Masuda, M. The New Genus Yonagunia Kawaguchi et Masuda (Halymeniaceae, Rhodophyta), Based on Y. tenuifolia Kawaguchi et Masuda Sp. Nov. from Southern Japan and Including Y. formosana (Okamura) Kawaguchi et Masuda Comb. Nov. from Southeast Asia. J. Phycol. 2004, 40, 180–192. [Google Scholar] [CrossRef]
- Tseng, C.K.; Xia, B.-M. On the Gracilaria in the Western Pacific and the Southeastern Asia Region. Bot. Mar. 1999, 42, 209–218. [Google Scholar] [CrossRef]
- Ohno, M.; Terada, R.; Yamamoto, H. The Species of Gracilaria from Vietnam. In Taxonomy of Economic Seaweeds with Reference to Some Pacific Species Vol. VII; Abbott, I.A., Ed.; California Sea Grant College System: La Jolla, CA, USA, 1999; Volume 7, pp. 99–111. [Google Scholar]
- Vo, T.D.; Nishihara, G.N.; Kitamura, Y.; Shimada, S.; Kawaguchi, S.; Terada, R. The Effect of Irradiance and Temperature on the Photosynthesis of Hydropuntia edulis and Hydropuntia eucheumatoides (Gracilariaceae, Rhodophyta) from Vietnam. Phycologia 2015, 54, 24–31. [Google Scholar] [CrossRef]
- Vy, N.X.; Thu, N.N.N.; Hieu, N.T.; Thuy, N.T.X. Selection of Suitable Fragment from RbcL Gene for DNA Barcode Analysis of Family Halymeniaceae, Rhodophyta. Vietnam J. Mar. Sci. Technol. 2019, 19, 201–213. [Google Scholar] [CrossRef]
- Titlyanova, T.V.; Titlyanov, E.A.; Xia, B.; Bartsch, I. New Records of Benthic Marine Green Algae (Chlorophyta) for the Island of Hainan (China). Nova Hedwig. 2012, 94, 441–470. [Google Scholar] [CrossRef]
- Lee, K.M.; Hong, D.D.; Boo, S.M. Phylogenetic Relationships of Rosenvingea (Scytosiphonaceae, Phaeophyceae) from Vietnam Based on Cox3 and PsaA Sequences. Algae 2014, 29, 289–297. [Google Scholar] [CrossRef]
- Dai, N.H. Sargasseae. In Vietnamese Plants; Hanoi Science and Technology Publishing: Ha Noi, Vietnam, 2007; pp. 1–117. [Google Scholar]
- Yoshida, T.; Dai, N.H.; Ajisaka, T.; Noro, T. Verification of Sargassum Species Identified with Subgenera Bactrophycus, Phyllotrichia and Schizophycus in Vietnam. In Taxonomy of Economic Seaweeds with Reference to Some Pacific Species. Vol. VIII; Abbott, I.A., Mcdermid, K.J., Eds.; California Sea Grant College: La Jolla, CA, USA, 2002; Volume 8, pp. 95–102. [Google Scholar]
- Van Tien, N. Chlorophyta Pascher (Marine Taxons); Hanoi Science and Technology Publishing: Ha Noi, Vietnam, 2007. [Google Scholar]
- Linh, N.M.; Quang, P.V.; Luong, C.V.; Hung, V.M.; Tien, D.D. Diversity of Green Macroalgae Genus Ulva (Chlorophyta) in Hai Phong. VNU J. Sci. Nat. Sci. Technol. 2022, 38, 61–69. [Google Scholar] [CrossRef]
- Fortes, M.D.; Ooi, J.L.S.; Tan, Y.M.; Prathep, A.; Bujang, J.S.; Yaakub, S.M. Seagrass in Southeast Asia: A Review of Status and Knowledge Gaps, and a Road Map for Conservation. Bot. Mar. 2018, 61, 269–288. [Google Scholar] [CrossRef]
- Phan, T.T.H.; Stiers, I.; Nguyen, T.T.H.; Pham, T.T.; Ton, T.P.; Luong, Q.D.; Triest, L. Spatial and Temporal Distribution of Submerged Aquatic Vegetation in a Tropical Coastal Lagoon Habitat in Viet Nam. Bot. Mar. 2018, 61, 213–224. [Google Scholar] [CrossRef]
- Novak, A.B.; Short, F.T. Leaf Reddening in Seagrasses. Bot. Mar. 2010, 53, 93–97. [Google Scholar] [CrossRef]
- Luong, C.V.; Thao, N.V.; Komatsu, T.; Ve, N.D.; Tien, D.D. Status and Threats on Seagrass Beds Using GIS in Vietnam. Remote Sens. Mar. Environ. II 2012, 8525, 852512. [Google Scholar] [CrossRef]
- Hong, D.D.; Bach, N.D.; Hai, L.Q.; Thu, N.H.; Tien, D.D.; Van Dong, N. Phylogenestic Characterization of Some Vietnamese Algae (Gracilaria, Hypnea, Caulerpa, Scenedesmus) Based on Their ITS-1 Sequences. In Proceedings of the Basic Research Issues in Life Sciences: Scientific Report of the Second National Conference, Basic Research in Biology, Agriculture, Medicine; 2003; pp. 913–916. Available online: https://www.researchgate.net/publication/285816725_Phylogenetic_characterization_of_some_Vietnamese_algae_based_on_their_ITS-1_sequences (accessed on 6 January 2023).
- Bringloe, T.T.; Sjøtun, K.; Saunders, G.W. A DNA Barcode Survey of Marine Macroalgae from Bergen (Norway). Mar. Biol. Res. 2019, 15, 580–589. [Google Scholar] [CrossRef]
- Vieira, C.; De Ramon N’Yeurt, A.; Rasoamanendrika, F.A.; D’Hondt, S.; Tran, L.-A.T.; Van den Spiegel, D.; Kawai, H.; De Clerck, O. Marine Macroalgal Biodiversity of Northern Madagascar: Morpho-Genetic Systematics and Implications of Anthropic Impacts for Conservation. Biodivers. Conserv. 2021, 30, 1501–1546. [Google Scholar] [CrossRef]
- Saunders, G.W. Routine DNA Barcoding of Canadian Gracilariales (Rhodophyta) Reveals the Invasive Species Gracilaria vermiculophylla in British Columbia. Mol. Ecol. Resour. 2009, 9 (Suppl. S1), 140–150. [Google Scholar] [CrossRef] [PubMed]
- Van Tu, N. Seaweed Diversity in Vietnam, with an Emphasis on the Brown Algal Genus Sargassum. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2014. Available online: http://hdl.handle.net/1854/LU-6940554 (accessed on 6 January 2023).
- Vy, N.X.; Bujang, J.S.; Papenbrock, J. Variability of Leaf Morphology and Marker Genes of Members of the Halophila Complex Collected in Viet Nam. Aquat. Bot. 2013, 110, 6–15. [Google Scholar] [CrossRef]
- Nguyen, X.-V.; Höfler, S.; Glasenapp, Y.; Thangaradjou, T.; Lucas, C.; Papenbrock, J. New Insights into DNA Barcoding of Seagrasses. Syst. Biodivers. 2015, 13, 496–508. [Google Scholar] [CrossRef]
- Nguyen, X.V.; Nguyen-Nhat, N.T.; Nguyen, X.T.; Dao, V.H.; Liao, L.M.; Papenbrock, J. Analysis of RDNA Reveals a High Genetic Diversity of Halophila major in the Wallacea Region. PLoS ONE 2021, 16, e0258956. [Google Scholar] [CrossRef]
- Trivedi, S.; Ansari, A.A.; Ghosh, S.; Rehman, H. DNA Barcoding in Marine Perspectives: Assessment and Conservation of Biodiversity; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Trivedi, S.; Aloufi, A.A.; Ansari, A.A.; Ghosh, S.K. Role of DNA Barcoding in Marine Biodiversity Assessment and Conservation: An Update. Saudi J. Biol. Sci. 2016, 23, 161–171. [Google Scholar] [CrossRef]
- Yang, M.Y.; Geraldino, P.J.L.; Kim, M.S. DNA Barcode Assessment of Gracilaria salicornia (Gracilariaceae, Rhodophyta) from Southeast Asia. Bot. Stud. 2013, 54, 27. [Google Scholar] [CrossRef] [PubMed]
- Dumilag, R.V.; Aguinaldo, Z.-Z.A. Genetic Differentiation and Distribution of Pyropia, Acanthophora (Bangiales, Rhodophyta) in the Philippines. Eur. J. Phycol. 2017, 52, 104–115. [Google Scholar] [CrossRef]
- Zuccarello, G.C.; Critchley, A.T.; Smith, J.; Sieber, V.; Lhonneur, G.B.; West, J.A. Systematics and Genetic Variation in Commercial Shape Kappaphycus and Shape Eucheuma (Solieriaceae, Rhodophyta). J. Appl. Phycol. 2006, 18, 643–651. [Google Scholar] [CrossRef]
- Hong, D.D.; Hien, H.T.M.; Thu, N.H.; Nam, H.S.; Nang, H.Q.; Duc, T.M. Taxonomy of Southeast Asian seaweeds. Phycologia 2008, 51, 475–476. [Google Scholar] [CrossRef]
- Lim, P.E.; Tan, J.; Phang, S.M.; Nikmatullah, A.; Hong, D.D.; Sunarpi, H.; Hurtado, A.Q. Genetic Diversity of Kappaphycus Doty and Eucheuma J. Agardh (Solieriaceae, Rhodophyta) in Southeast Asia. J. Appl. Phycol. 2014, 26, 1253–1272. [Google Scholar] [CrossRef]
- Tan, J.; Lim, P.E.; Phang, S.M.; Rahiman, A.; Nikmatullah, A.; Sunarpi, H.; Hurtado, A.Q. Kappaphycus malesianus Sp. Nov.: A New Species of Kappaphycus (Gigartinales, Rhodophyta) from Southeast Asia. J. Appl. Phycol. 2014, 26, 1273–1285. [Google Scholar] [CrossRef]
- Song, S.L.; Lim, P.E.; Poong, S.W.; Phang, S.M. Genetic Variation in Gracilaria tenuistipitata (Rhodophyta) from Northern Singapore and Neighbouring Countries. Raffles Bull. Zool. 2015, 2015, 16–23. [Google Scholar]
- Nguyen, T.H.; Nguyen Nhat, N.T.; Nguyen, X.T.; Nguyen, V.X. Morphological Variation and Haplotype Diversity of Halimeda macroloba and H. opuntia (Chlorophyta: Halimedaceae) from Southern Vietnam. Vietnam J. Mar. Sci. Technol. 2022, 22, 165–176. [Google Scholar] [CrossRef]
- Sherman, K.; Hempel, G. The UNEP Large Marine Ecosystem Report: A Perspective on Changing Conditions in LMEs of the World’s Regional Seas. In Proceedings of the UNEP Regional Seas Report and Studies; United Nations Environment Programme: Nairobi, Kenya, 2008; pp. 297–308. [Google Scholar]
- Morton, B.; Blackmore, G. South China Sea. Mar. Pollut. Bull. 2001, 42, 1236–1263. [Google Scholar] [CrossRef]
- Zheng, F.; Qiu, G.; Fan, H.; Zhang, W. Diversity, distribution and conservation of Chinese seagrass species. Biodivers. Sci. 2013, 21, 517–526. [Google Scholar] [CrossRef]
- Tanto, T.A.; Putra, A.; Hermon, D.; Damanhuri, H. Suitability of Seagrass Ecosystem for Marine Ecotourism in Padang City, West Sumatera Province. Forum Geogr. 2018, 32, 88–95. [Google Scholar] [CrossRef]
- Bujang, J.S.; Zakaria, M.H.; Short, F.T. Seagrass in Malaysia: Issues and Challenges Ahead. In The Wetland Book: II: Distribution, Description, and Conservation; Finlayson, C.M., Milton, G.R., Prentice, R.C., Davidson, N.C., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 1875–1883. ISBN 978-94-007-4001-3. [Google Scholar]
- Fortes, M.D. A Review: Biodiversity, Distribution and Conservation of Philippine Seagrasses. Philipp. J. Sci. 2013, 142, 95–111. [Google Scholar]
- McKenzie, L.J.; Yaakub, S.M.; Tan, R.; Seymour, J.; Yoshida, R.L. Seagrass Habitats of Singapore: Environmental Drivers and Key Processes. Raffles Bull. Zool. 2016, 34, 60–77. [Google Scholar]
- UNEP. National Action Plan for Seagrass Thailand. In Proceedings of the Sixth Meeting of the Regional Working Group for the Seagrass Sub-component of the UNEP/GEF Project: “Reversing Environmental Degradation Trends in the South China Sea and Gulf of Thailand”, Philippines, 2005; pp. 1–8. Available online: http://www.unepscs.org/remository/startdown/270.html (accessed on 6 January 2023).
- Van Luong, C. Study on Characteristics of Seagrass Communities and Their Carbon Storage Capacity in Some Typical Lagoons in Central Vietnam. Ph.D. Thesis, Institute of Ecology and Biology Resources, Ha Noi, Vietnam, 2019. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research), PRIMER-e, Plymouth 2006, 192. Available online: https://www.researchgate.net/publication/285668711_PRIMER_v6_user_manualtutorial_PRIMER-E_Plymouth (accessed on 6 January 2023).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Contreras-Porcia, L.; Meynard, A.; López-Cristoffanini, C.; Latorre, N.; Kumar, M. Marine Metal Pollution and Effects on Seaweed Species. In Proceedings of the Systems Biology of Marine Ecosystems; Springer: Cham, Switzerland, 2017; pp. 35–48. [Google Scholar]
- Harley, C.D.G.; Anderson, K.M.; Demes, K.W.; Jorve, J.P.; Kordas, R.L.; Coyle, T.A.; Graham, M.H. Effects Of Climate Change On Global Seaweed Communities. J. Phycol. 2012, 48, 1064–1078. [Google Scholar] [CrossRef]
- Tano, S.A.; Halling, C.; Lind, E.; Buriyo, A.; Wikström, S.A. Extensive Spread of Farmed Seaweeds Causes a Shift from Native to Non-Native Haplotypes in Natural Seaweed Beds. Mar. Biol. 2015, 162, 1983–1992. [Google Scholar] [CrossRef]
- Schaffelke, B.; Hewitt, C.L. Impacts of Introduced Seaweeds. Bot. Mar. 2007, 50, 397–417. [Google Scholar] [CrossRef]
- Walker, D.I.; Kendrick, G.A. Threats to Macroalgal Diversity: Marine Habitat Destruction and Fragmentation, Pollution and Introduced Species. Bot. Mar. 1998, 41, 105–112. [Google Scholar] [CrossRef]
- Loc, P.K.; Yen, M.D.; Averyanov, L. Biodiversity in Vietnam. In Global Biodiversity. Selected Countries in Asia; Pullaiah, T., Ed.; Apple Academic Press: Ottawa, Canada, 2018; Volume 1, p. 30. ISBN 9780429487743. [Google Scholar]
- Ca, V.T.; Hieu, P.V.; Luong, C.V.; Tien, D.D. Application of a Quantitative Assessment Method for the Onservation Potential of the Seagrass Ecosystem in a Pilot Study at Ly Son Islands, Quang Ngai. J. Mar. Sci. Technol. 2011, 11, 47–56. [Google Scholar] [CrossRef]
- WWF. Root Causes of Biodiversity Loss in Vietnam Summary; WWF: New York, NY, USA, 1998. [Google Scholar]
- Vy, N.X.; Dai, N.H. Re-Assessment of Sargassum Beds at Hon Chong Area, Nha Trang Bay, Vietnam. Seto Mar. Biol. Lab. 2011, 41, 63–69. [Google Scholar]
- Hong, D.D.; Ha, N.C. Seaweeds of Vietnam: Opportunities for Commercial Production. In Sustainable Global Resources of Seaweeds Volume 1: Bioresources, Cultivation, Trade and Multifarious Applications; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 109–127. ISBN 978-3-030-91955-9. [Google Scholar]
- Averyanov, L.V.; Anh, T.; Tran, B.; Ban, N.; Bich, L.; Bien, L.; Binh, N.; Chieu, N.; Duc, P.; Dung, V.; et al. Vietnam Red Data Book. Part II. Plants. Orchidaceae; Natural Science and Technology Publishing House: Ha Noi, Vietnam, 2007; p. 611. [Google Scholar]
- PEMSEA. Viet Nam Administration for Seas and Islands (VASI) and Ministry of Environment and Natural Resources (MONRE). In Proceedings of the National State of Oceans and Coasts 2018: Blue Economy Growth of Viet Nam. Partnerships in Environmental Management for the Seas of East Asia (PEMSEA); PEMSEA: Quezon City, Philippines, 2018; p. 232. [Google Scholar]
- Mantri, V.A.; Kambey, C.S.B.; Cottier-Cook, E.J.; Usandizaga, S.; Buschmann, A.H.; Chung, I.K.; Liu, T.; Sondak, C.F.A.; Qi, Z.; Lim, P.E.; et al. Overview of Global Gracilaria Production, the Role of Biosecurity Policies and Regulations in the Sustainable Development of This Industry. Rev. Aquac. 2023, 15, 801–819. [Google Scholar] [CrossRef]
- News. Vietnam Maritime Administration. Available online: https://www.vinamarine.gov.vn/en (accessed on 10 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).